 Found 219 hits with Last Name = 'ito' and Initial = 'b'
Found 219 hits with Last Name = 'ito' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]T3 from recombinant thyroid hormone receptor alpha (unknown origin) expressed in sf9 cells by scintillation proximity assay |
Proc Natl Acad Sci U S A 104: 15490-5 (2007)
Article DOI: 10.1073/pnas.0702759104
BindingDB Entry DOI: 10.7270/Q2JW8DPH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18869
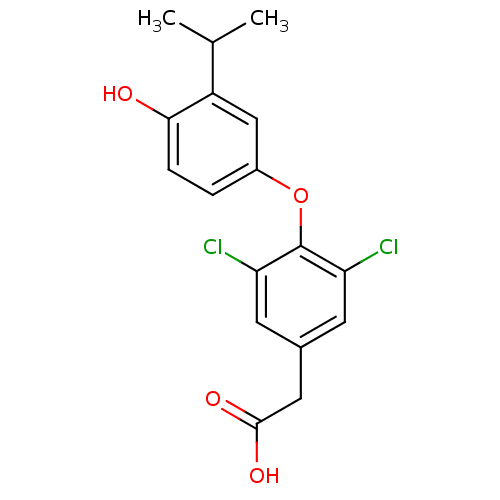
(2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C17H16Cl2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]T3 from recombinant thyroid hormone receptor beta (unknown origin) expressed in sf9 cells by scintillation proximity assay |
Proc Natl Acad Sci U S A 104: 15490-5 (2007)
Article DOI: 10.1073/pnas.0702759104
BindingDB Entry DOI: 10.7270/Q2JW8DPH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]T3 from recombinant thyroid hormone receptor beta (unknown origin) expressed in sf9 cells by scintillation proximity assay |
Proc Natl Acad Sci U S A 104: 15490-5 (2007)
Article DOI: 10.1073/pnas.0702759104
BindingDB Entry DOI: 10.7270/Q2JW8DPH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50242403
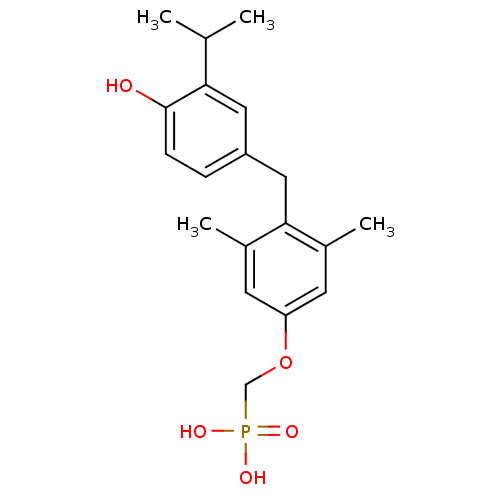
((3,5-dimethyl-4-(4-hydroxy-3-isopropylbenzyl)pheno...)Show InChI InChI=1S/C19H25O5P/c1-12(2)17-9-15(5-6-19(17)20)10-18-13(3)7-16(8-14(18)4)24-11-25(21,22)23/h5-9,12,20H,10-11H2,1-4H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]T3 from recombinant thyroid hormone receptor beta (unknown origin) expressed in sf9 cells by scintillation proximity assay |
Proc Natl Acad Sci U S A 104: 15490-5 (2007)
Article DOI: 10.1073/pnas.0702759104
BindingDB Entry DOI: 10.7270/Q2JW8DPH |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18869

(2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C17H16Cl2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 7.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]T3 from recombinant thyroid hormone receptor alpha (unknown origin) expressed in sf9 cells by scintillation proximity assay |
Proc Natl Acad Sci U S A 104: 15490-5 (2007)
Article DOI: 10.1073/pnas.0702759104
BindingDB Entry DOI: 10.7270/Q2JW8DPH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50242403

((3,5-dimethyl-4-(4-hydroxy-3-isopropylbenzyl)pheno...)Show InChI InChI=1S/C19H25O5P/c1-12(2)17-9-15(5-6-19(17)20)10-18-13(3)7-16(8-14(18)4)24-11-25(21,22)23/h5-9,12,20H,10-11H2,1-4H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 35.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]T3 from recombinant thyroid hormone receptor alpha (unknown origin) expressed in sf9 cells by scintillation proximity assay |
Proc Natl Acad Sci U S A 104: 15490-5 (2007)
Article DOI: 10.1073/pnas.0702759104
BindingDB Entry DOI: 10.7270/Q2JW8DPH |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50170601
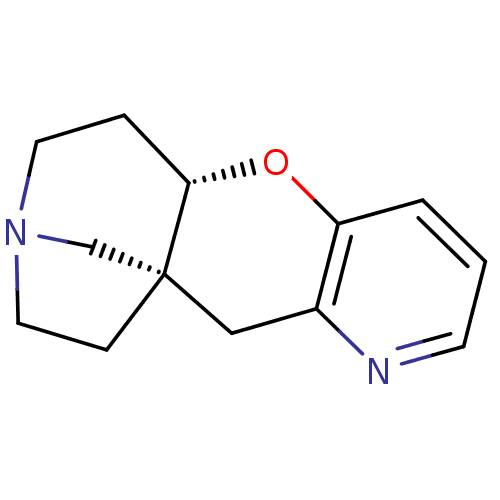
((1R,10S)-9-oxa-4,13-diazatetracyclo[11.2.1.0^{1,10...)Show InChI InChI=1S/C13H16N2O/c1-2-11-10(14-5-1)8-13-4-7-15(9-13)6-3-12(13)16-11/h1-2,5,12H,3-4,6-9H2/t12-,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50170601

((1R,10S)-9-oxa-4,13-diazatetracyclo[11.2.1.0^{1,10...)Show InChI InChI=1S/C13H16N2O/c1-2-11-10(14-5-1)8-13-4-7-15(9-13)6-3-12(13)16-11/h1-2,5,12H,3-4,6-9H2/t12-,13+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50170601

((1R,10S)-9-oxa-4,13-diazatetracyclo[11.2.1.0^{1,10...)Show InChI InChI=1S/C13H16N2O/c1-2-11-10(14-5-1)8-13-4-7-15(9-13)6-3-12(13)16-11/h1-2,5,12H,3-4,6-9H2/t12-,13+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(Homo sapiens (Human)) | BDBM50170601

((1R,10S)-9-oxa-4,13-diazatetracyclo[11.2.1.0^{1,10...)Show InChI InChI=1S/C13H16N2O/c1-2-11-10(14-5-1)8-13-4-7-15(9-13)6-3-12(13)16-11/h1-2,5,12H,3-4,6-9H2/t12-,13+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50242404
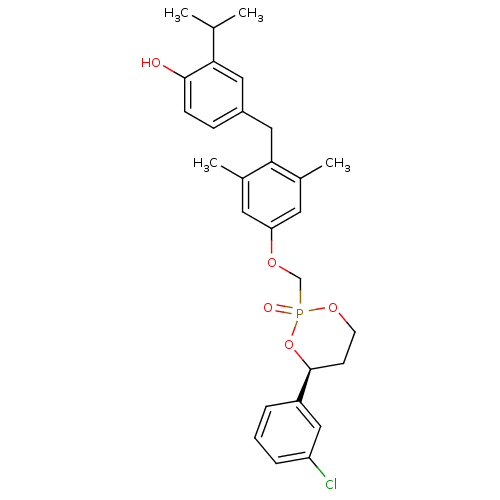
((4S)-4-(3-chlorophenyl)-2-[(3,5-dimethyl-4-(4-hydr...)Show SMILES CC(C)c1cc(Cc2c(C)cc(OCP3(=O)OCC[C@H](O3)c3cccc(Cl)c3)cc2C)ccc1O |r| Show InChI InChI=1S/C28H32ClO5P/c1-18(2)25-14-21(8-9-27(25)30)15-26-19(3)12-24(13-20(26)4)32-17-35(31)33-11-10-28(34-35)22-6-5-7-23(29)16-22/h5-9,12-14,16,18,28,30H,10-11,15,17H2,1-4H3/t28-,35?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]T3 from recombinant thyroid hormone receptor alpha (unknown origin) expressed in sf9 cells by scintillation proximity assay |
Proc Natl Acad Sci U S A 104: 15490-5 (2007)
Article DOI: 10.1073/pnas.0702759104
BindingDB Entry DOI: 10.7270/Q2JW8DPH |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50242404

((4S)-4-(3-chlorophenyl)-2-[(3,5-dimethyl-4-(4-hydr...)Show SMILES CC(C)c1cc(Cc2c(C)cc(OCP3(=O)OCC[C@H](O3)c3cccc(Cl)c3)cc2C)ccc1O |r| Show InChI InChI=1S/C28H32ClO5P/c1-18(2)25-14-21(8-9-27(25)30)15-26-19(3)12-24(13-20(26)4)32-17-35(31)33-11-10-28(34-35)22-6-5-7-23(29)16-22/h5-9,12-14,16,18,28,30H,10-11,15,17H2,1-4H3/t28-,35?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]T3 from recombinant thyroid hormone receptor beta (unknown origin) expressed in sf9 cells by scintillation proximity assay |
Proc Natl Acad Sci U S A 104: 15490-5 (2007)
Article DOI: 10.1073/pnas.0702759104
BindingDB Entry DOI: 10.7270/Q2JW8DPH |
More data for this
Ligand-Target Pair | |
Serine palmitoyltransferase 2
(Homo sapiens (Human)) | BDBM50461646
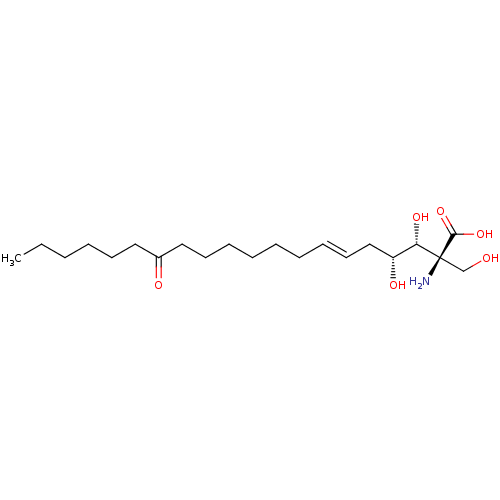
(CHEBI:582124 | Myriocin)Show SMILES CCCCCCC(=O)CCCCCC\C=C\C[C@@H](O)[C@H](O)[C@@](N)(CO)C(O)=O |r| Show InChI InChI=1S/C21H39NO6/c1-2-3-4-10-13-17(24)14-11-8-6-5-7-9-12-15-18(25)19(26)21(22,16-23)20(27)28/h9,12,18-19,23,25-26H,2-8,10-11,13-16,22H2,1H3,(H,27,28)/b12-9+/t18-,19+,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... |
Bioorg Med Chem 26: 2452-2465 (2018)
Article DOI: 10.1016/j.bmc.2018.04.008
BindingDB Entry DOI: 10.7270/Q2RF5XNH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine palmitoyltransferase 2
(Homo sapiens (Human)) | BDBM50461644
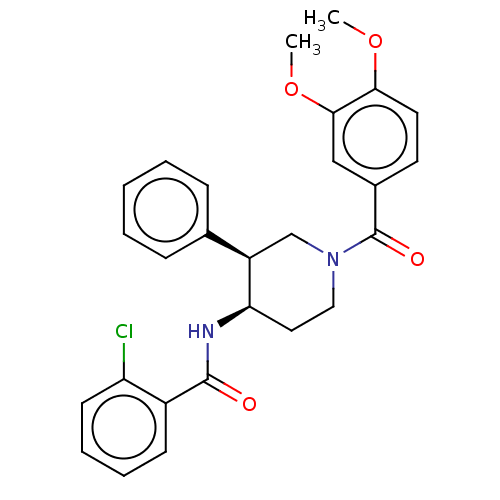
(CHEMBL4228416)Show SMILES COc1ccc(cc1OC)C(=O)N1CC[C@@H](NC(=O)c2ccccc2Cl)[C@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C27H27ClN2O4/c1-33-24-13-12-19(16-25(24)34-2)27(32)30-15-14-23(21(17-30)18-8-4-3-5-9-18)29-26(31)20-10-6-7-11-22(20)28/h3-13,16,21,23H,14-15,17H2,1-2H3,(H,29,31)/t21-,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... |
Bioorg Med Chem 26: 2452-2465 (2018)
Article DOI: 10.1016/j.bmc.2018.04.008
BindingDB Entry DOI: 10.7270/Q2RF5XNH |
More data for this
Ligand-Target Pair | |
Serine palmitoyltransferase 2
(Homo sapiens (Human)) | BDBM50461649
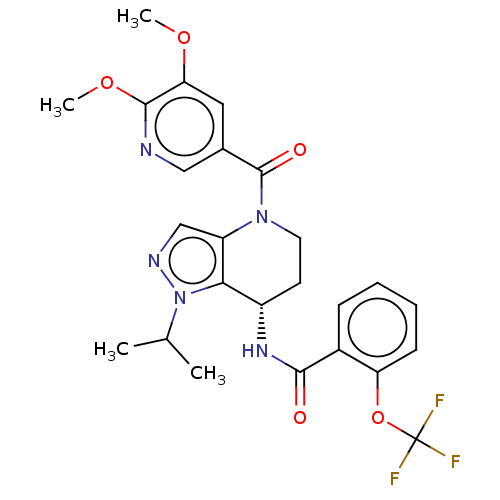
(CHEMBL4225519)Show SMILES COc1cc(cnc1OC)C(=O)N1CC[C@H](NC(=O)c2ccccc2OC(F)(F)F)c2c1cnn2C(C)C |r| Show InChI InChI=1S/C25H26F3N5O5/c1-14(2)33-21-17(31-22(34)16-7-5-6-8-19(16)38-25(26,27)28)9-10-32(18(21)13-30-33)24(35)15-11-20(36-3)23(37-4)29-12-15/h5-8,11-14,17H,9-10H2,1-4H3,(H,31,34)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... |
Bioorg Med Chem 26: 2452-2465 (2018)
Article DOI: 10.1016/j.bmc.2018.04.008
BindingDB Entry DOI: 10.7270/Q2RF5XNH |
More data for this
Ligand-Target Pair | |
Serine palmitoyltransferase 2
(Homo sapiens (Human)) | BDBM50461645
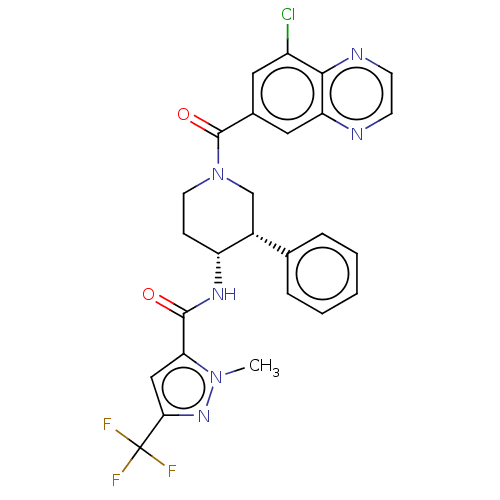
(CHEMBL4228472)Show SMILES Cn1nc(cc1C(=O)N[C@@H]1CCN(C[C@@H]1c1ccccc1)C(=O)c1cc(Cl)c2nccnc2c1)C(F)(F)F |r| Show InChI InChI=1S/C26H22ClF3N6O2/c1-35-21(13-22(34-35)26(28,29)30)24(37)33-19-7-10-36(14-17(19)15-5-3-2-4-6-15)25(38)16-11-18(27)23-20(12-16)31-8-9-32-23/h2-6,8-9,11-13,17,19H,7,10,14H2,1H3,(H,33,37)/t17-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... |
Bioorg Med Chem 26: 2452-2465 (2018)
Article DOI: 10.1016/j.bmc.2018.04.008
BindingDB Entry DOI: 10.7270/Q2RF5XNH |
More data for this
Ligand-Target Pair | |
Serine palmitoyltransferase 2
(Homo sapiens (Human)) | BDBM50461647
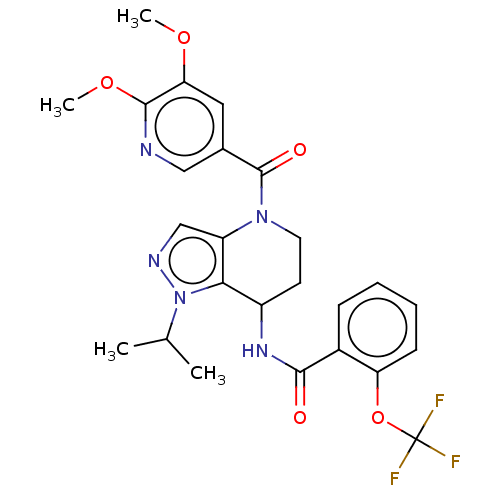
(CHEMBL4225462)Show SMILES COc1cc(cnc1OC)C(=O)N1CCC(NC(=O)c2ccccc2OC(F)(F)F)c2c1cnn2C(C)C Show InChI InChI=1S/C25H26F3N5O5/c1-14(2)33-21-17(31-22(34)16-7-5-6-8-19(16)38-25(26,27)28)9-10-32(18(21)13-30-33)24(35)15-11-20(36-3)23(37-4)29-12-15/h5-8,11-14,17H,9-10H2,1-4H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... |
Bioorg Med Chem 26: 2452-2465 (2018)
Article DOI: 10.1016/j.bmc.2018.04.008
BindingDB Entry DOI: 10.7270/Q2RF5XNH |
More data for this
Ligand-Target Pair | |
Serine palmitoyltransferase 2
(Homo sapiens (Human)) | BDBM50461643
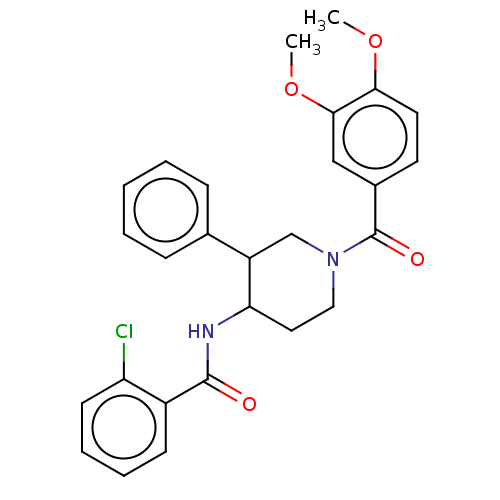
(CHEMBL4225764)Show SMILES COc1ccc(cc1OC)C(=O)N1CCC(NC(=O)c2ccccc2Cl)C(C1)c1ccccc1 Show InChI InChI=1S/C27H27ClN2O4/c1-33-24-13-12-19(16-25(24)34-2)27(32)30-15-14-23(21(17-30)18-8-4-3-5-9-18)29-26(31)20-10-6-7-11-22(20)28/h3-13,16,21,23H,14-15,17H2,1-2H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... |
Bioorg Med Chem 26: 2452-2465 (2018)
Article DOI: 10.1016/j.bmc.2018.04.008
BindingDB Entry DOI: 10.7270/Q2RF5XNH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50425719
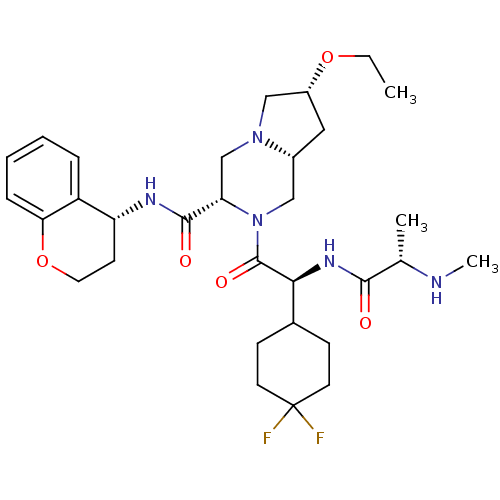
(CHEMBL2316217)Show SMILES CCO[C@@H]1C[C@@H]2CN([C@@H](CN2C1)C(=O)N[C@@H]1CCOc2ccccc12)C(=O)[C@@H](NC(=O)[C@H](C)NC)C1CCC(F)(F)CC1 |r| Show InChI InChI=1S/C31H45F2N5O5/c1-4-42-22-15-21-16-38(30(41)27(36-28(39)19(2)34-3)20-9-12-31(32,33)13-10-20)25(18-37(21)17-22)29(40)35-24-11-14-43-26-8-6-5-7-23(24)26/h5-8,19-22,24-25,27,34H,4,9-18H2,1-3H3,(H,35,40)(H,36,39)/t19-,21+,22+,24+,25-,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... |
J Med Chem 56: 1228-46 (2013)
Article DOI: 10.1021/jm301674z
BindingDB Entry DOI: 10.7270/Q23N24QX |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50425721
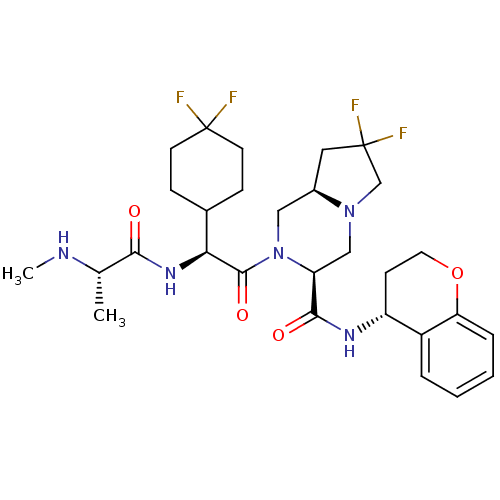
(CHEMBL2311586)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCC(F)(F)CC1)C(=O)N1C[C@H]2CC(F)(F)CN2C[C@H]1C(=O)N[C@@H]1CCOc2ccccc12 |r| Show InChI InChI=1S/C29H39F4N5O4/c1-17(34-2)25(39)36-24(18-7-10-28(30,31)11-8-18)27(41)38-14-19-13-29(32,33)16-37(19)15-22(38)26(40)35-21-9-12-42-23-6-4-3-5-20(21)23/h3-6,17-19,21-22,24,34H,7-16H2,1-2H3,(H,35,40)(H,36,39)/t17-,19+,21+,22-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... |
J Med Chem 56: 1228-46 (2013)
Article DOI: 10.1021/jm301674z
BindingDB Entry DOI: 10.7270/Q23N24QX |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50425728
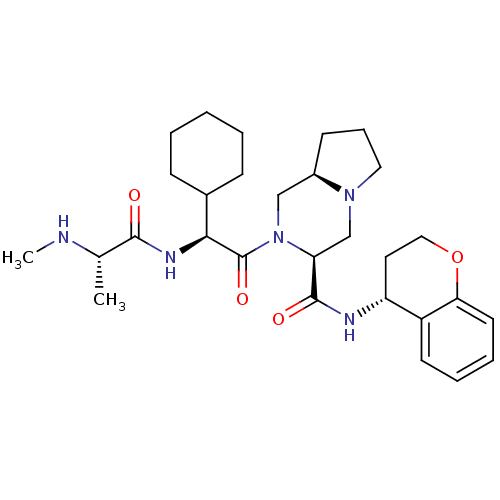
(CHEMBL2365533)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1C[C@H]2CCCN2C[C@H]1C(=O)N[C@@H]1CCOc2ccccc12 |r| Show InChI InChI=1S/C29H43N5O4/c1-19(30-2)27(35)32-26(20-9-4-3-5-10-20)29(37)34-17-21-11-8-15-33(21)18-24(34)28(36)31-23-14-16-38-25-13-7-6-12-22(23)25/h6-7,12-13,19-21,23-24,26,30H,3-5,8-11,14-18H2,1-2H3,(H,31,36)(H,32,35)/t19-,21+,23+,24-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... |
J Med Chem 56: 1228-46 (2013)
Article DOI: 10.1021/jm301674z
BindingDB Entry DOI: 10.7270/Q23N24QX |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50425722
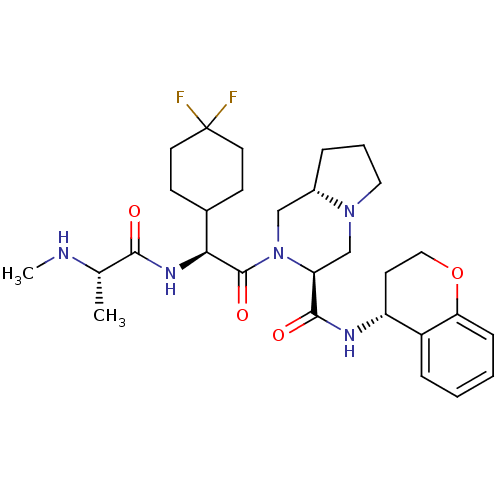
(CHEMBL2316215)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCC(F)(F)CC1)C(=O)N1C[C@@H]2CCCN2C[C@H]1C(=O)N[C@@H]1CCOc2ccccc12 |r| Show InChI InChI=1S/C29H41F2N5O4/c1-18(32-2)26(37)34-25(19-9-12-29(30,31)13-10-19)28(39)36-16-20-6-5-14-35(20)17-23(36)27(38)33-22-11-15-40-24-8-4-3-7-21(22)24/h3-4,7-8,18-20,22-23,25,32H,5-6,9-17H2,1-2H3,(H,33,38)(H,34,37)/t18-,20-,22+,23-,25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... |
J Med Chem 56: 1228-46 (2013)
Article DOI: 10.1021/jm301674z
BindingDB Entry DOI: 10.7270/Q23N24QX |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50425725
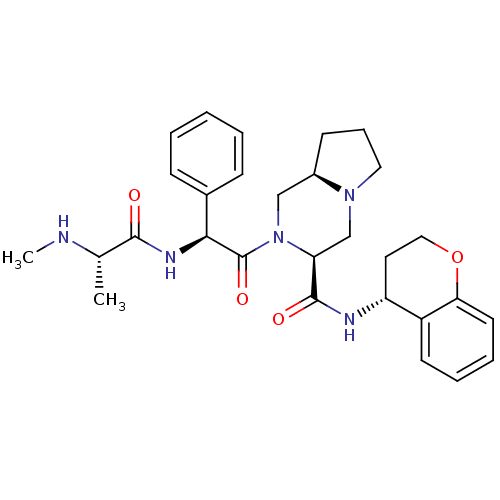
(CHEMBL2316224)Show SMILES CN[C@@H](C)C(=O)N[C@H](C(=O)N1C[C@H]2CCCN2C[C@H]1C(=O)N[C@@H]1CCOc2ccccc12)c1ccccc1 |r| Show InChI InChI=1S/C29H37N5O4/c1-19(30-2)27(35)32-26(20-9-4-3-5-10-20)29(37)34-17-21-11-8-15-33(21)18-24(34)28(36)31-23-14-16-38-25-13-7-6-12-22(23)25/h3-7,9-10,12-13,19,21,23-24,26,30H,8,11,14-18H2,1-2H3,(H,31,36)(H,32,35)/t19-,21+,23+,24-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... |
J Med Chem 56: 1228-46 (2013)
Article DOI: 10.1021/jm301674z
BindingDB Entry DOI: 10.7270/Q23N24QX |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50425730
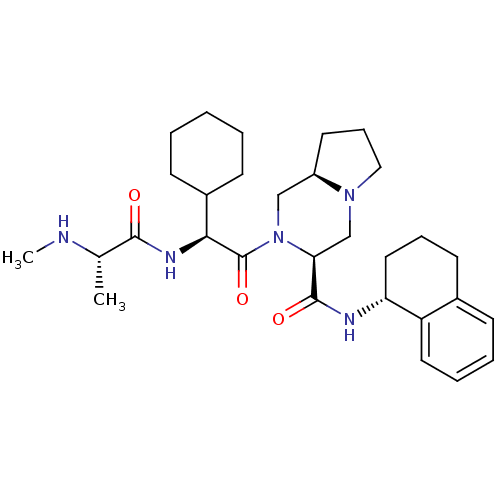
(CHEMBL2316219)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1C[C@H]2CCCN2C[C@H]1C(=O)N[C@@H]1CCCc2ccccc12 |r| Show InChI InChI=1S/C30H45N5O3/c1-20(31-2)28(36)33-27(22-11-4-3-5-12-22)30(38)35-18-23-14-9-17-34(23)19-26(35)29(37)32-25-16-8-13-21-10-6-7-15-24(21)25/h6-7,10,15,20,22-23,25-27,31H,3-5,8-9,11-14,16-19H2,1-2H3,(H,32,37)(H,33,36)/t20-,23+,25+,26-,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... |
J Med Chem 56: 1228-46 (2013)
Article DOI: 10.1021/jm301674z
BindingDB Entry DOI: 10.7270/Q23N24QX |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50425724
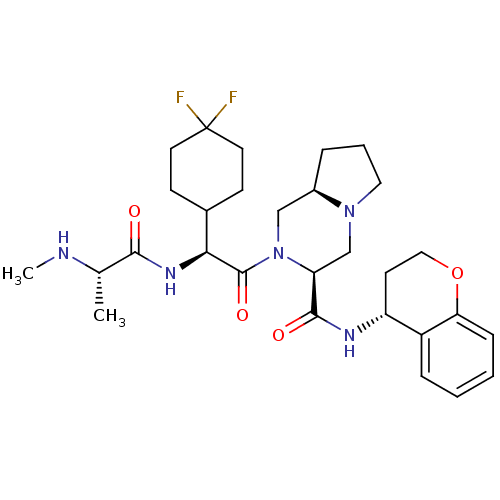
(CHEMBL2316213)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCC(F)(F)CC1)C(=O)N1C[C@H]2CCCN2C[C@H]1C(=O)N[C@@H]1CCOc2ccccc12 |r| Show InChI InChI=1S/C29H41F2N5O4/c1-18(32-2)26(37)34-25(19-9-12-29(30,31)13-10-19)28(39)36-16-20-6-5-14-35(20)17-23(36)27(38)33-22-11-15-40-24-8-4-3-7-21(22)24/h3-4,7-8,18-20,22-23,25,32H,5-6,9-17H2,1-2H3,(H,33,38)(H,34,37)/t18-,20+,22+,23-,25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... |
J Med Chem 56: 1228-46 (2013)
Article DOI: 10.1021/jm301674z
BindingDB Entry DOI: 10.7270/Q23N24QX |
More data for this
Ligand-Target Pair | |
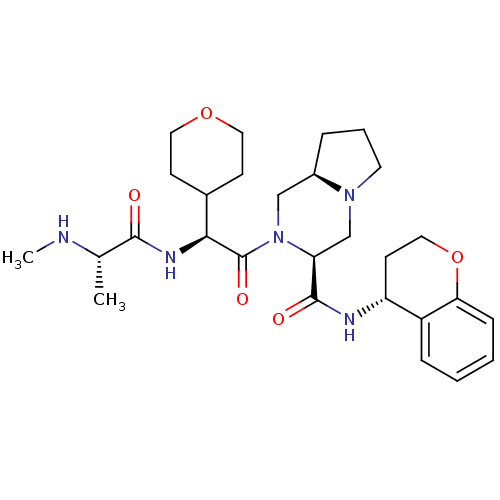
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50425723
(CHEMBL2316214)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCOCC1)C(=O)N1C[C@H]2CCCN2C[C@H]1C(=O)N[C@@H]1CCOc2ccccc12 |r| Show InChI InChI=1S/C28H41N5O5/c1-18(29-2)26(34)31-25(19-9-13-37-14-10-19)28(36)33-16-20-6-5-12-32(20)17-23(33)27(35)30-22-11-15-38-24-8-4-3-7-21(22)24/h3-4,7-8,18-20,22-23,25,29H,5-6,9-17H2,1-2H3,(H,30,35)(H,31,34)/t18-,20+,22+,23-,25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... |
J Med Chem 56: 1228-46 (2013)
Article DOI: 10.1021/jm301674z
BindingDB Entry DOI: 10.7270/Q23N24QX |
More data for this
Ligand-Target Pair | |
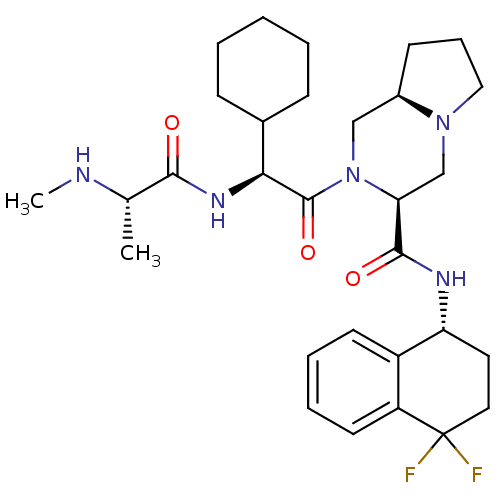
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50425727
(CHEMBL2316222)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1C[C@H]2CCCN2C[C@H]1C(=O)N[C@@H]1CCC(F)(F)c2ccccc12 |r| Show InChI InChI=1S/C30H43F2N5O3/c1-19(33-2)27(38)35-26(20-9-4-3-5-10-20)29(40)37-17-21-11-8-16-36(21)18-25(37)28(39)34-24-14-15-30(31,32)23-13-7-6-12-22(23)24/h6-7,12-13,19-21,24-26,33H,3-5,8-11,14-18H2,1-2H3,(H,34,39)(H,35,38)/t19-,21+,24+,25-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... |
J Med Chem 56: 1228-46 (2013)
Article DOI: 10.1021/jm301674z
BindingDB Entry DOI: 10.7270/Q23N24QX |
More data for this
Ligand-Target Pair | |
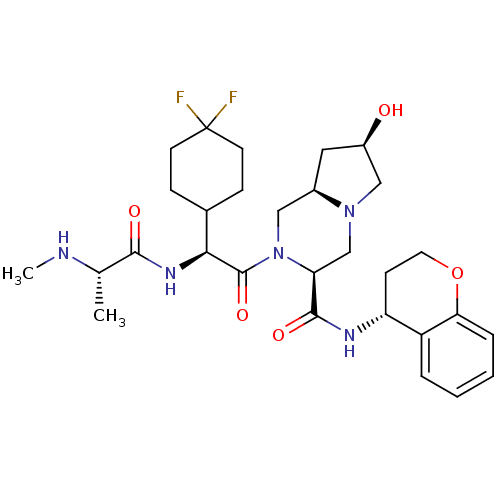
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50425720
(CHEMBL2316216)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCC(F)(F)CC1)C(=O)N1C[C@H]2C[C@@H](O)CN2C[C@H]1C(=O)N[C@@H]1CCOc2ccccc12 |r| Show InChI InChI=1S/C29H41F2N5O5/c1-17(32-2)26(38)34-25(18-7-10-29(30,31)11-8-18)28(40)36-14-19-13-20(37)15-35(19)16-23(36)27(39)33-22-9-12-41-24-6-4-3-5-21(22)24/h3-6,17-20,22-23,25,32,37H,7-16H2,1-2H3,(H,33,39)(H,34,38)/t17-,19+,20+,22+,23-,25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... |
J Med Chem 56: 1228-46 (2013)
Article DOI: 10.1021/jm301674z
BindingDB Entry DOI: 10.7270/Q23N24QX |
More data for this
Ligand-Target Pair | |
Serine palmitoyltransferase 2
(Homo sapiens (Human)) | BDBM50461639
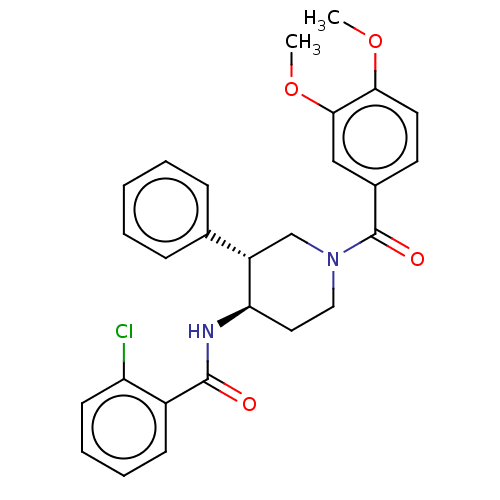
(CHEMBL4229253)Show SMILES COc1ccc(cc1OC)C(=O)N1CC[C@@H](NC(=O)c2ccccc2Cl)[C@@H](C1)c1ccccc1 |r| Show InChI InChI=1S/C27H27ClN2O4/c1-33-24-13-12-19(16-25(24)34-2)27(32)30-15-14-23(21(17-30)18-8-4-3-5-9-18)29-26(31)20-10-6-7-11-22(20)28/h3-13,16,21,23H,14-15,17H2,1-2H3,(H,29,31)/t21-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... |
Bioorg Med Chem 26: 2452-2465 (2018)
Article DOI: 10.1016/j.bmc.2018.04.008
BindingDB Entry DOI: 10.7270/Q2RF5XNH |
More data for this
Ligand-Target Pair | |
Serine palmitoyltransferase 2
(Homo sapiens (Human)) | BDBM50461641
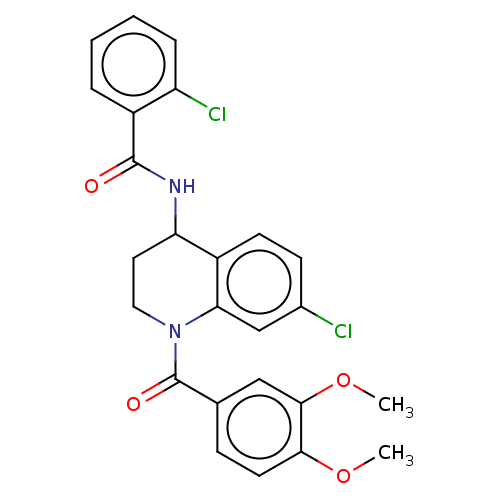
(CHEMBL4225192)Show SMILES COc1ccc(cc1OC)C(=O)N1CCC(NC(=O)c2ccccc2Cl)c2ccc(Cl)cc12 Show InChI InChI=1S/C25H22Cl2N2O4/c1-32-22-10-7-15(13-23(22)33-2)25(31)29-12-11-20(18-9-8-16(26)14-21(18)29)28-24(30)17-5-3-4-6-19(17)27/h3-10,13-14,20H,11-12H2,1-2H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... |
Bioorg Med Chem 26: 2452-2465 (2018)
Article DOI: 10.1016/j.bmc.2018.04.008
BindingDB Entry DOI: 10.7270/Q2RF5XNH |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50425726
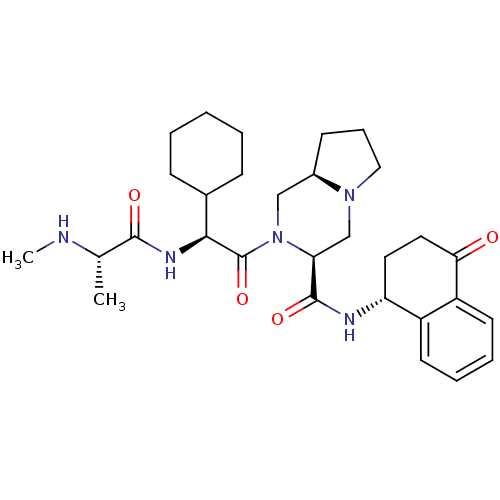
(CHEMBL2316223)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1C[C@H]2CCCN2C[C@H]1C(=O)N[C@@H]1CCC(=O)c2ccccc12 |r| Show InChI InChI=1S/C30H43N5O4/c1-19(31-2)28(37)33-27(20-9-4-3-5-10-20)30(39)35-17-21-11-8-16-34(21)18-25(35)29(38)32-24-14-15-26(36)23-13-7-6-12-22(23)24/h6-7,12-13,19-21,24-25,27,31H,3-5,8-11,14-18H2,1-2H3,(H,32,38)(H,33,37)/t19-,21+,24+,25-,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... |
J Med Chem 56: 1228-46 (2013)
Article DOI: 10.1021/jm301674z
BindingDB Entry DOI: 10.7270/Q23N24QX |
More data for this
Ligand-Target Pair | |
Serine palmitoyltransferase 2
(Homo sapiens (Human)) | BDBM50461638
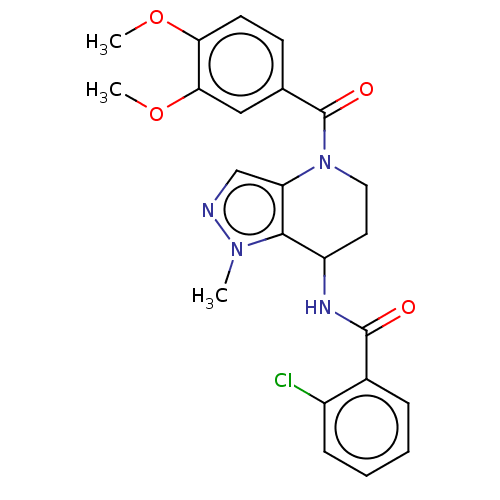
(CHEMBL4226297)Show SMILES COc1ccc(cc1OC)C(=O)N1CCC(NC(=O)c2ccccc2Cl)c2c1cnn2C Show InChI InChI=1S/C23H23ClN4O4/c1-27-21-17(26-22(29)15-6-4-5-7-16(15)24)10-11-28(18(21)13-25-27)23(30)14-8-9-19(31-2)20(12-14)32-3/h4-9,12-13,17H,10-11H2,1-3H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra... |
Bioorg Med Chem 26: 2452-2465 (2018)
Article DOI: 10.1016/j.bmc.2018.04.008
BindingDB Entry DOI: 10.7270/Q2RF5XNH |
More data for this
Ligand-Target Pair | |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50612294
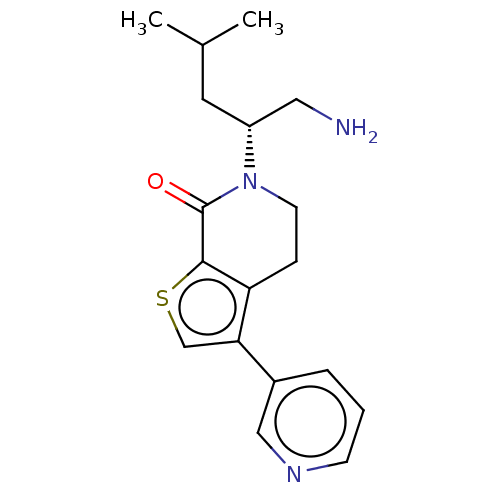
(CHEMBL5281395)Show SMILES CC(C)C[C@H](CN)N1CCc2c(csc2C1=O)-c1cccnc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50612293
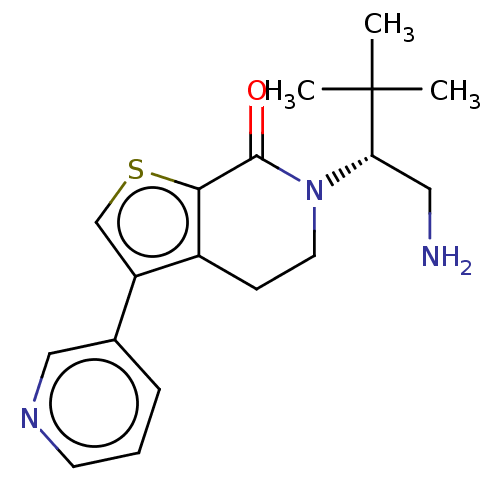
(CHEMBL5265996)Show SMILES CC(C)(C)[C@H](CN)N1CCc2c(csc2C1=O)-c1cccnc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50612290
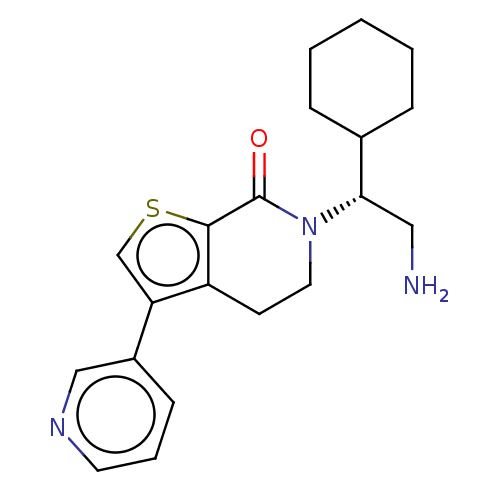
(CHEMBL5288132)Show SMILES NC[C@@H](C1CCCCC1)N1CCc2c(csc2C1=O)-c1cccnc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50612285

(CHEMBL5269564)Show SMILES NC[C@H](N1CCc2c(csc2C1=O)-c1cccnc1)c1ccccc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50425731

(CHEMBL2316218)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1C[C@H]2CCCN2C[C@H]1C(=O)N[C@H](C)c1ccccc1 |r| Show InChI InChI=1S/C28H43N5O3/c1-19(21-11-6-4-7-12-21)30-27(35)24-18-32-16-10-15-23(32)17-33(24)28(36)25(22-13-8-5-9-14-22)31-26(34)20(2)29-3/h4,6-7,11-12,19-20,22-25,29H,5,8-10,13-18H2,1-3H3,(H,30,35)(H,31,34)/t19-,20+,23-,24+,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged cIAP1 protein BIR3 domain (250 to 350 amino acid residues) using biotinylated-Smac as substrate after over... |
J Med Chem 56: 1228-46 (2013)
Article DOI: 10.1021/jm301674z
BindingDB Entry DOI: 10.7270/Q23N24QX |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50443808

(CHEMBL3094405)Show SMILES CC[C@H](NC)C(=O)N[C@@H](C1CCCCC1)C(=O)N1C[C@@H]2Cc3cc(ccc3N2C[C@H]1C(=O)N[C@@H]1CCOc2ccccc12)C#N |r| Show InChI InChI=1S/C35H44N6O4/c1-3-27(37-2)33(42)39-32(23-9-5-4-6-10-23)35(44)41-20-25-18-24-17-22(19-36)13-14-29(24)40(25)21-30(41)34(43)38-28-15-16-45-31-12-8-7-11-26(28)31/h7-8,11-14,17,23,25,27-28,30,32,37H,3-6,9-10,15-16,18,20-21H2,1-2H3,(H,38,43)(H,39,42)/t25-,27-,28+,30-,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged XIAP BIR3 domain (252 to 356) using AVPIAQ-K(biotin)-NH2 as substrate after overnight incubatio... |
Bioorg Med Chem 21: 7938-54 (2013)
Article DOI: 10.1016/j.bmc.2013.09.067
BindingDB Entry DOI: 10.7270/Q2833TGG |
More data for this
Ligand-Target Pair | |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50612287
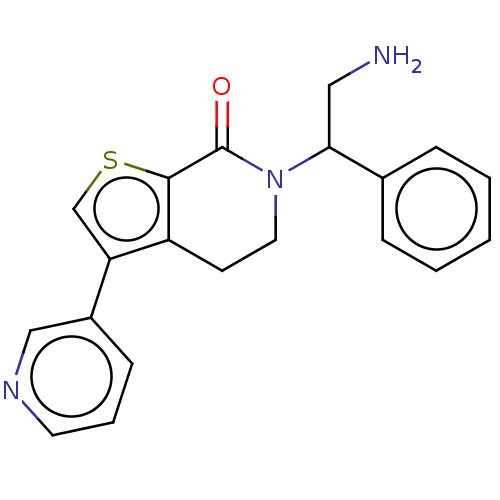
(CHEMBL5274333) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50443812

(CHEMBL3094408)Show SMILES CC[C@H](NC)C(=O)N[C@@H](C1CCCCC1)C(=O)N1C[C@@H]2Cc3ccccc3N2C[C@H]1C(=O)N[C@@H]1CCOc2ccccc12 |r| Show InChI InChI=1S/C34H45N5O4/c1-3-26(35-2)32(40)37-31(22-11-5-4-6-12-22)34(42)39-20-24-19-23-13-7-9-15-28(23)38(24)21-29(39)33(41)36-27-17-18-43-30-16-10-8-14-25(27)30/h7-10,13-16,22,24,26-27,29,31,35H,3-6,11-12,17-21H2,1-2H3,(H,36,41)(H,37,40)/t24-,26-,27+,29-,31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged XIAP BIR3 domain (252 to 356) using AVPIAQ-K(biotin)-NH2 as substrate after overnight incubatio... |
Bioorg Med Chem 21: 7938-54 (2013)
Article DOI: 10.1016/j.bmc.2013.09.067
BindingDB Entry DOI: 10.7270/Q2833TGG |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50443807

(CHEMBL3094404)Show SMILES CC[C@H](NC)C(=O)N[C@@H](C1CCCCC1)C(=O)N1C[C@@H]2Cc3cc(Cl)ccc3N2C[C@H]1C(=O)N[C@@H]1CCOc2ccccc12 |r| Show InChI InChI=1S/C34H44ClN5O4/c1-3-26(36-2)32(41)38-31(21-9-5-4-6-10-21)34(43)40-19-24-18-22-17-23(35)13-14-28(22)39(24)20-29(40)33(42)37-27-15-16-44-30-12-8-7-11-25(27)30/h7-8,11-14,17,21,24,26-27,29,31,36H,3-6,9-10,15-16,18-20H2,1-2H3,(H,37,42)(H,38,41)/t24-,26-,27+,29-,31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged XIAP BIR3 domain (252 to 356) using AVPIAQ-K(biotin)-NH2 as substrate after overnight incubatio... |
Bioorg Med Chem 21: 7938-54 (2013)
Article DOI: 10.1016/j.bmc.2013.09.067
BindingDB Entry DOI: 10.7270/Q2833TGG |
More data for this
Ligand-Target Pair | |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50612292
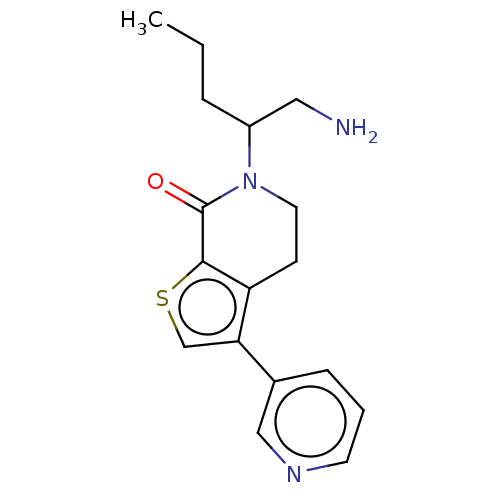
(CHEMBL5268272) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50612295
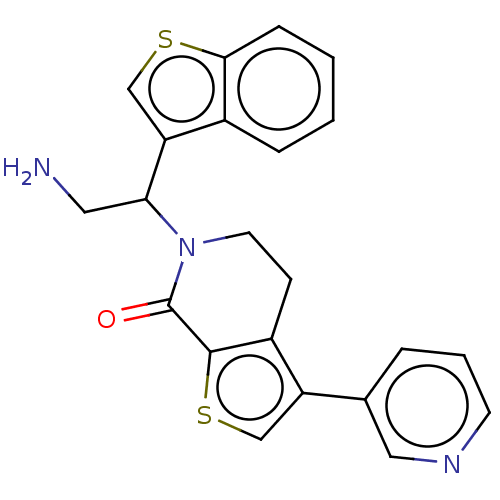
(CHEMBL5267547)Show SMILES NCC(N1CCc2c(csc2C1=O)-c1cccnc1)c1csc2ccccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50612291

(CHEMBL5278471) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
N-formyl peptide receptor 2
(Homo sapiens (Human)) | BDBM50559829
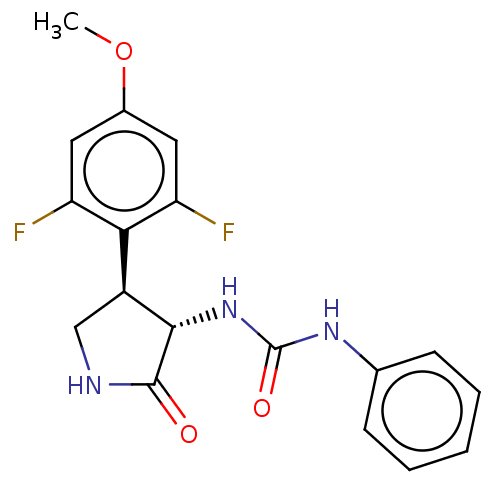
(CHEMBL4784510)Show SMILES COc1cc(F)c([C@@H]2CNC(=O)[C@H]2NC(=O)Nc2ccccc2)c(F)c1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at FPR2 in human HL-60 cells assessed as reduction in chemoattractant induced chemotaxis by luminescence cell viability assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02101
BindingDB Entry DOI: 10.7270/Q2V98CSC |
More data for this
Ligand-Target Pair | |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50514710
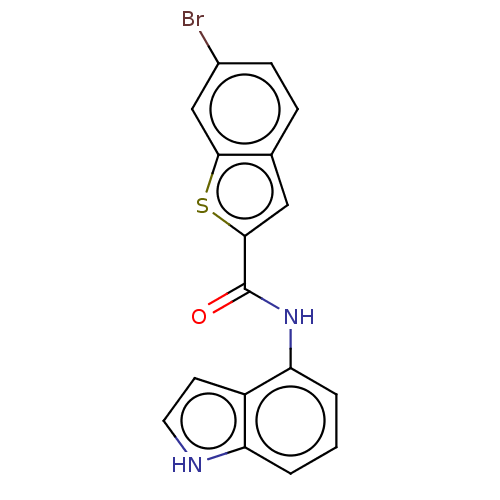
(CHEMBL4555677)Show InChI InChI=1S/C17H11BrN2OS/c18-11-5-4-10-8-16(22-15(10)9-11)17(21)20-14-3-1-2-13-12(14)6-7-19-13/h1-9,19H,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50443806
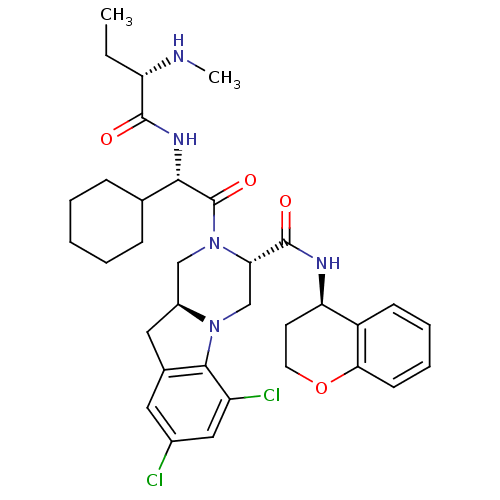
(CHEMBL3094403)Show SMILES CC[C@H](NC)C(=O)N[C@@H](C1CCCCC1)C(=O)N1C[C@@H]2Cc3cc(Cl)cc(Cl)c3N2C[C@H]1C(=O)N[C@@H]1CCOc2ccccc12 |r| Show InChI InChI=1S/C34H43Cl2N5O4/c1-3-26(37-2)32(42)39-30(20-9-5-4-6-10-20)34(44)41-18-23-16-21-15-22(35)17-25(36)31(21)40(23)19-28(41)33(43)38-27-13-14-45-29-12-8-7-11-24(27)29/h7-8,11-12,15,17,20,23,26-28,30,37H,3-6,9-10,13-14,16,18-19H2,1-2H3,(H,38,43)(H,39,42)/t23-,26-,27+,28-,30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged XIAP BIR3 domain (252 to 356) using AVPIAQ-K(biotin)-NH2 as substrate after overnight incubatio... |
Bioorg Med Chem 21: 7938-54 (2013)
Article DOI: 10.1016/j.bmc.2013.09.067
BindingDB Entry DOI: 10.7270/Q2833TGG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data