

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50051007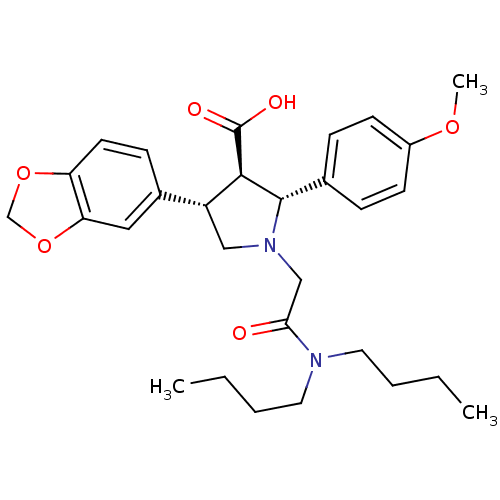 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human Endothelin A receptor in chinese hamster ovary cells | J Med Chem 39: 1039-48 (1996) Article DOI: 10.1021/jm9505369 BindingDB Entry DOI: 10.7270/Q29G5KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50106407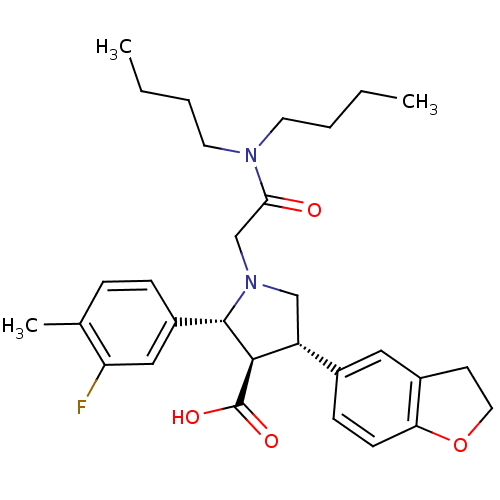 (1-Dibutylcarbamoylmethyl-4-(2,3-dihydro-benzofuran...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Endothelin A receptor | J Med Chem 44: 3978-84 (2001) BindingDB Entry DOI: 10.7270/Q2JS9PR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50051007 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description binding affinity against human Endothelin A receptor | J Med Chem 44: 3978-84 (2001) BindingDB Entry DOI: 10.7270/Q2JS9PR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50050976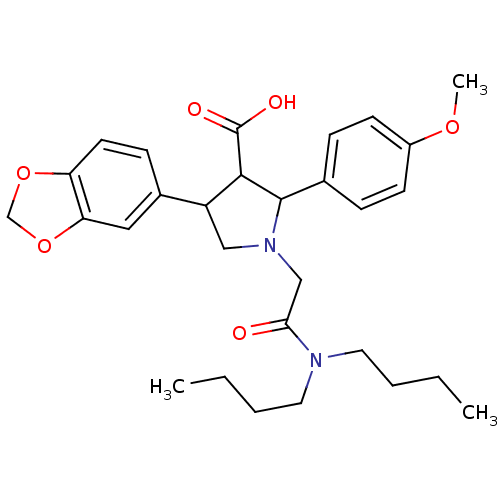 (4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbamoylmethyl-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human Endothelin A receptor in chinese hamster ovary cells | J Med Chem 39: 1039-48 (1996) Article DOI: 10.1021/jm9505369 BindingDB Entry DOI: 10.7270/Q29G5KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50050964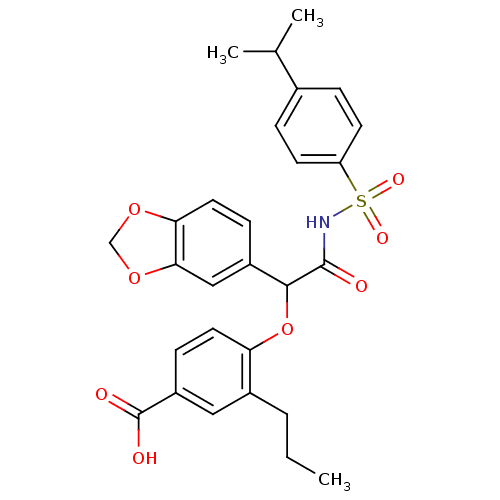 (4-[1-Benzo[1,3]dioxol-5-yl-2-(4-isopropyl-benzenes...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human Endothelin A receptor in chinese hamster ovary cells | J Med Chem 39: 1039-48 (1996) Article DOI: 10.1021/jm9505369 BindingDB Entry DOI: 10.7270/Q29G5KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50034267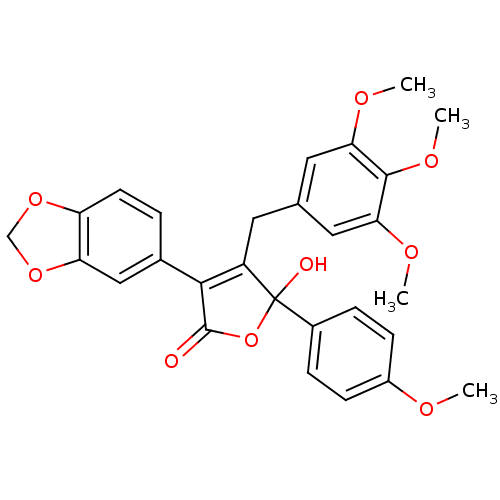 (3-(benzo[d][1,3]dioxol-5-yl)-5-hydroxy-5-(4-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human Endothelin A receptor in chinese hamster ovary cells | J Med Chem 39: 1039-48 (1996) Article DOI: 10.1021/jm9505369 BindingDB Entry DOI: 10.7270/Q29G5KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50041617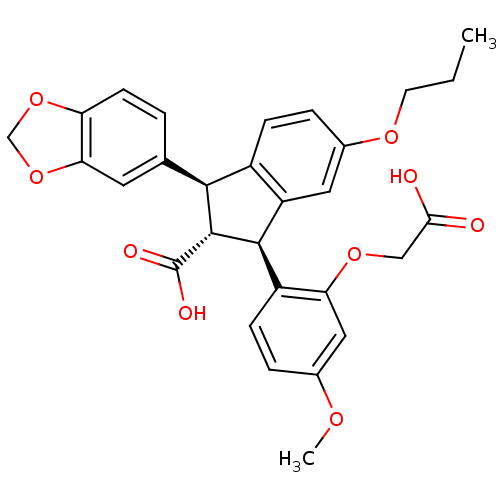 ((1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-(2-carboxymet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human Endothelin A receptor in chinese hamster ovary cells | J Med Chem 39: 1039-48 (1996) Article DOI: 10.1021/jm9505369 BindingDB Entry DOI: 10.7270/Q29G5KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50066391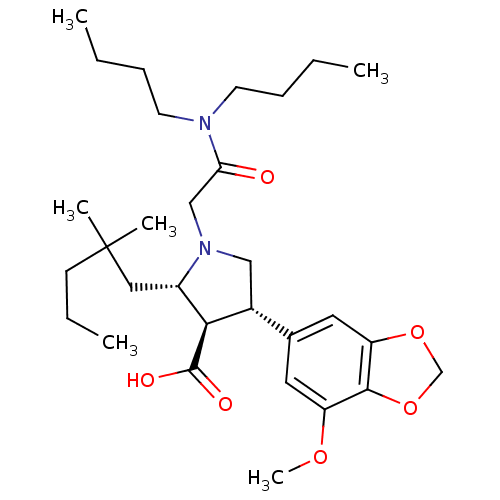 ((2S,3R,4S)-1-Dibutylcarbamoylmethyl-2-(2,2-dimethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against endothelin A receptor in MMQ cells in rat | J Med Chem 44: 3978-84 (2001) BindingDB Entry DOI: 10.7270/Q2JS9PR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM217096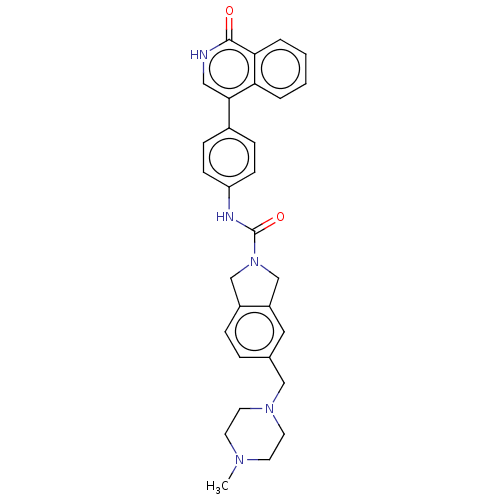 (US9302989, 391) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description HTRF assay: This assay used the CisBio HTRF KinEASE kit (kit 62ST2PEZ) and the kinase reaction containing 0.2 uM biotinylated substrate peptide (S2, ... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50089414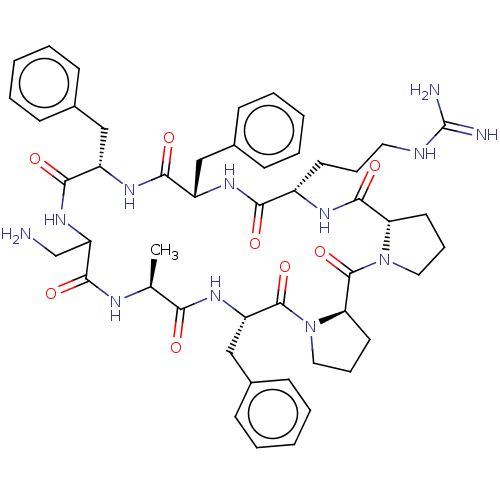 (CHEMBL3577996) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of MTII from mouse MC4R expressed in HEK293 cells | J Med Chem 58: 4638-47 (2015) Article DOI: 10.1021/acs.jmedchem.5b00184 BindingDB Entry DOI: 10.7270/Q2T155C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217112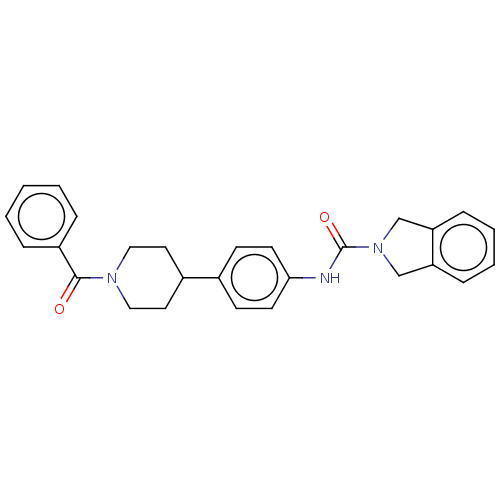 (US9302989, 407) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM217092 (US9302989, 387) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description HTRF assay: This assay used the CisBio HTRF KinEASE kit (kit 62ST2PEZ) and the kinase reaction containing 0.2 uM biotinylated substrate peptide (S2, ... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50254198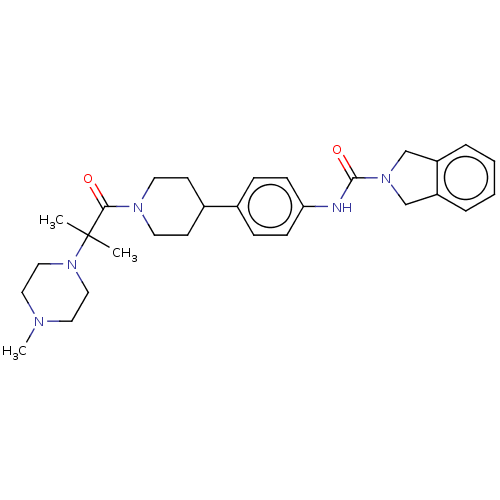 (CHEMBL4060799) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217057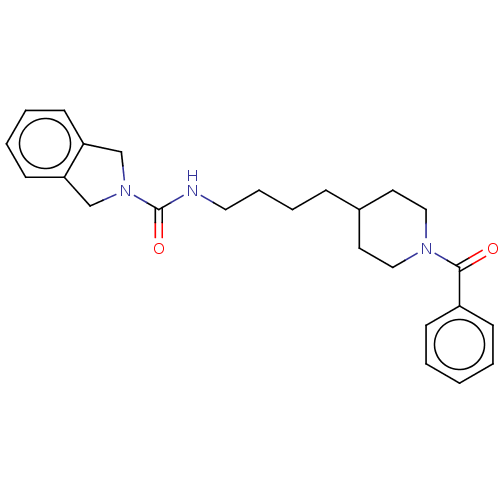 (US9302989, 349) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50254199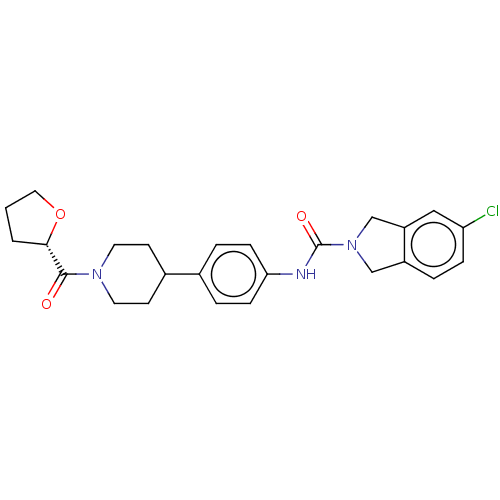 (CHEMBL4083505) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM217094 (US9302989, 389) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description HTRF assay: This assay used the CisBio HTRF KinEASE kit (kit 62ST2PEZ) and the kinase reaction containing 0.2 uM biotinylated substrate peptide (S2, ... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50254216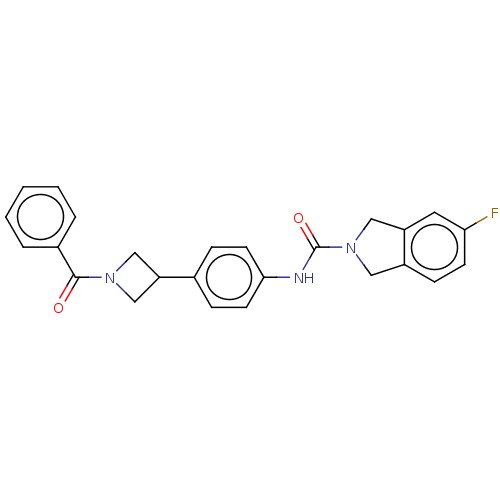 (CHEMBL4096471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50197418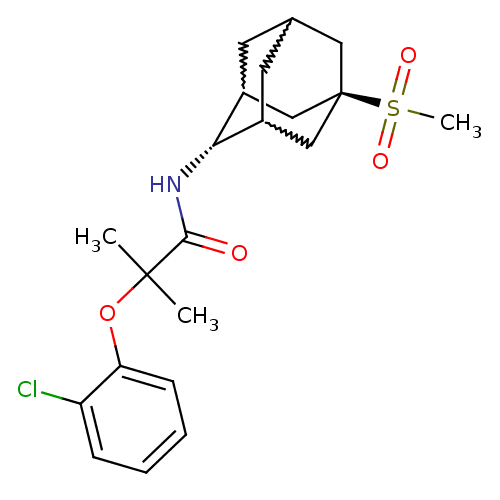 (2-(2-chloro-phenoxy)-N-(5-methanesulfonyl-adamanta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol | Bioorg Med Chem Lett 17: 527-32 (2007) Article DOI: 10.1016/j.bmcl.2006.10.008 BindingDB Entry DOI: 10.7270/Q2BZ65PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50195289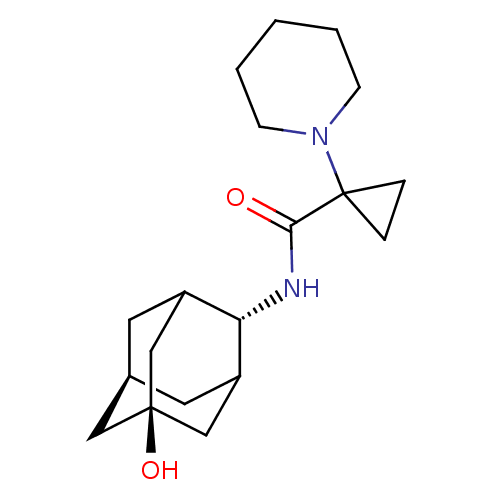 (1-piperidin-1-yl-cyclopropanecarboxylic acid (5-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human truncated 11beta-HSD1 assessed as inhibition of radiolabeled cortisone to cortisol conversion by SPA | Bioorg Med Chem Lett 16: 5958-62 (2006) Article DOI: 10.1016/j.bmcl.2006.08.129 BindingDB Entry DOI: 10.7270/Q23R0SJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50195289 (1-piperidin-1-yl-cyclopropanecarboxylic acid (5-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse truncated 11beta-HSD1 assessed as inhibition of radiolabeled cortisone to cortisol conversion by SPA | Bioorg Med Chem Lett 16: 5958-62 (2006) Article DOI: 10.1016/j.bmcl.2006.08.129 BindingDB Entry DOI: 10.7270/Q23R0SJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50197404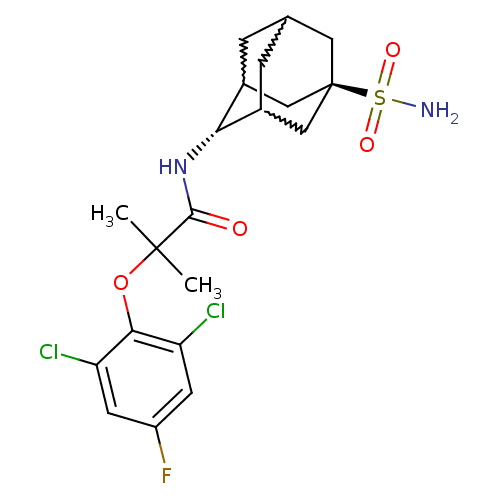 (2-(2,6-dichloro-4-fluoro-phenoxy)-2-methyl-N-(5-su...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol | Bioorg Med Chem Lett 17: 527-32 (2007) Article DOI: 10.1016/j.bmcl.2006.10.008 BindingDB Entry DOI: 10.7270/Q2BZ65PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50197399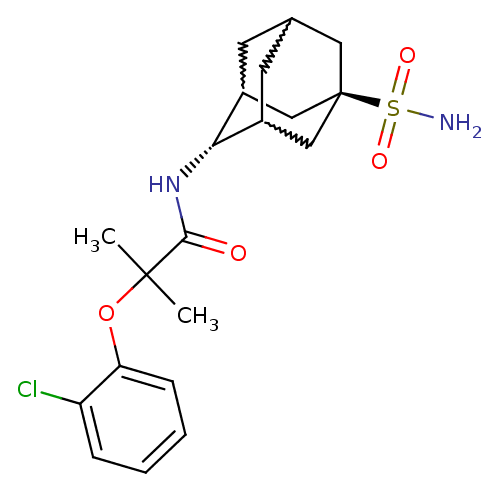 (2-(2-chloro-phenoxy)-2-methyl-N-(5-sulfamoyl-adama...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol | Bioorg Med Chem Lett 17: 527-32 (2007) Article DOI: 10.1016/j.bmcl.2006.10.008 BindingDB Entry DOI: 10.7270/Q2BZ65PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50202094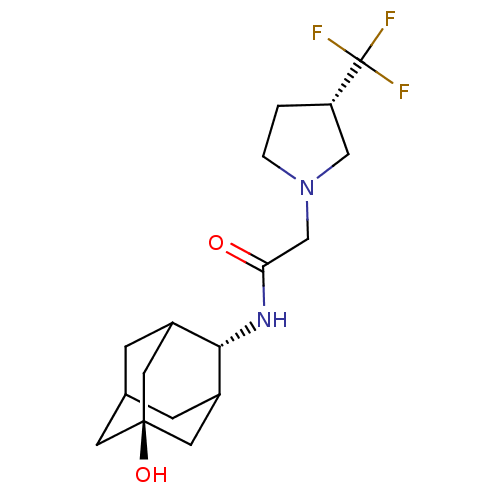 (CHEMBL375341 | N-(5-hydroxy-adamantan-2-yl)-2-(3-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in E. coli by SPA | J Med Chem 50: 149-64 (2007) Article DOI: 10.1021/jm0609364 BindingDB Entry DOI: 10.7270/Q2Z60NQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM217103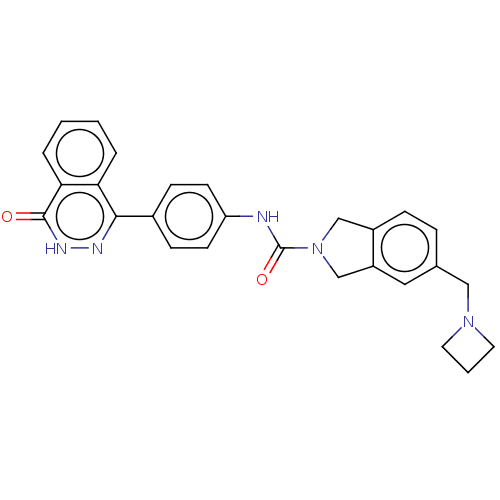 (US9302989, 398) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description HTRF assay: This assay used the CisBio HTRF KinEASE kit (kit 62ST2PEZ) and the kinase reaction containing 0.2 uM biotinylated substrate peptide (S2, ... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM217085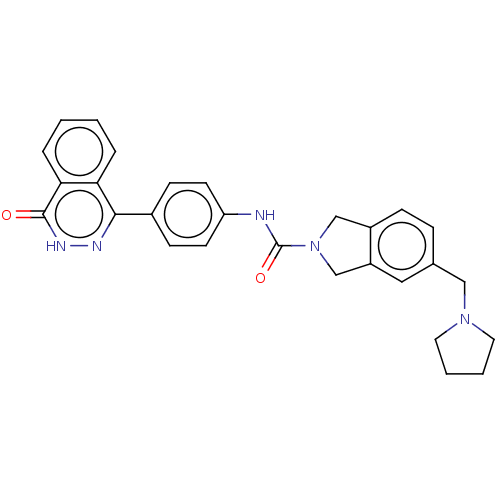 (US9302989, 380) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description HTRF assay: This assay used the CisBio HTRF KinEASE kit (kit 62ST2PEZ) and the kinase reaction containing 0.2 uM biotinylated substrate peptide (S2, ... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM217083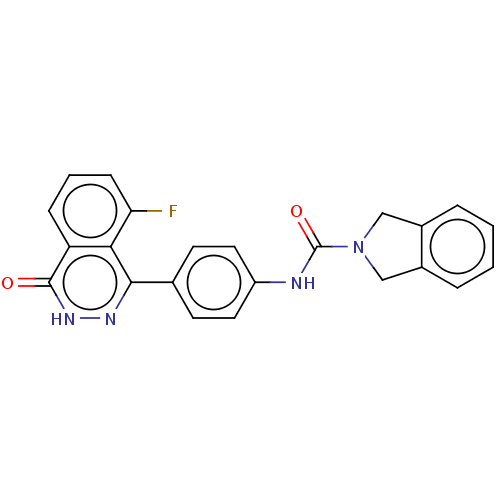 (US9302989, 378) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description HTRF assay: This assay used the CisBio HTRF KinEASE kit (kit 62ST2PEZ) and the kinase reaction containing 0.2 uM biotinylated substrate peptide (S2, ... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM217084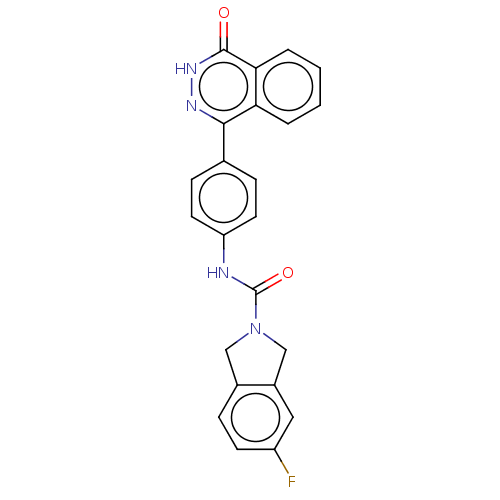 (US9302989, 379) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description HTRF assay: This assay used the CisBio HTRF KinEASE kit (kit 62ST2PEZ) and the kinase reaction containing 0.2 uM biotinylated substrate peptide (S2, ... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM217095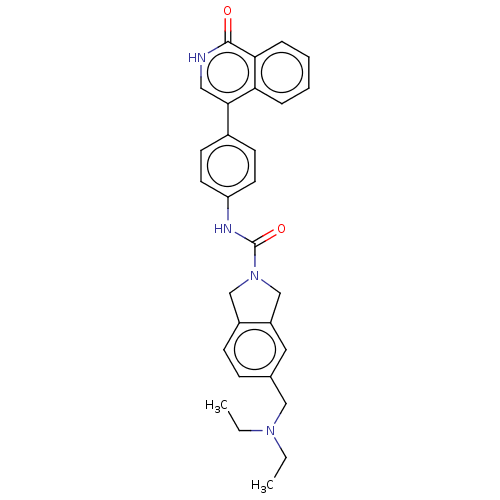 (US9302989, 390) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description HTRF assay: This assay used the CisBio HTRF KinEASE kit (kit 62ST2PEZ) and the kinase reaction containing 0.2 uM biotinylated substrate peptide (S2, ... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50254215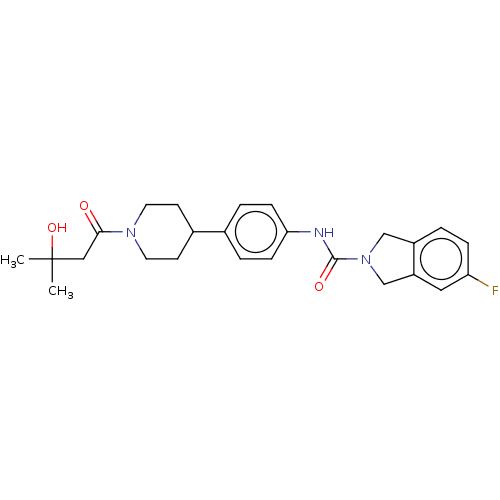 (CHEMBL4089003) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50195299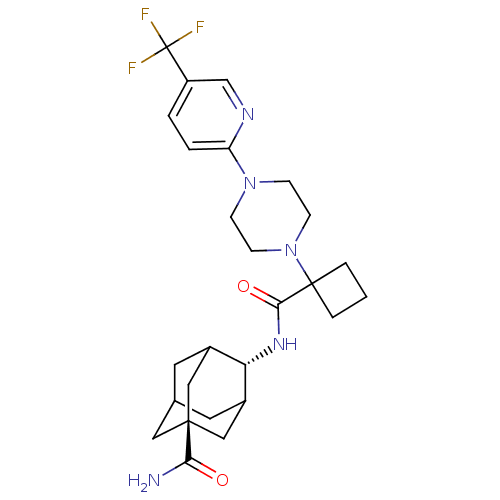 (4-({1-[4-(5-trifluoromethyl-pyridin-2-yl)-piperazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse truncated 11beta-HSD1 assessed as inhibition of radiolabeled cortisone to cortisol conversion by SPA | Bioorg Med Chem Lett 16: 5958-62 (2006) Article DOI: 10.1016/j.bmcl.2006.08.129 BindingDB Entry DOI: 10.7270/Q23R0SJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50195298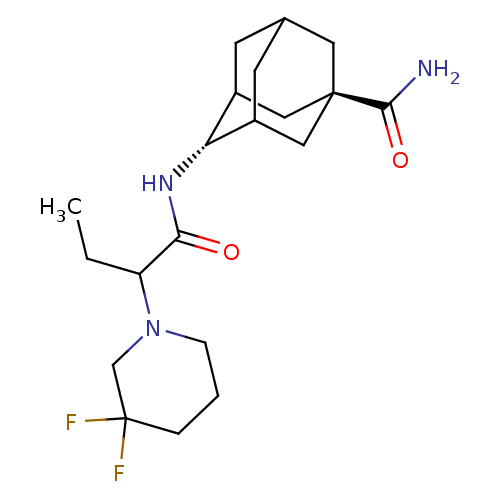 (4-[2-(3,3-difluoro-piperidin-1-yl)-butyrylamino]-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse truncated 11beta-HSD1 assessed as inhibition of radiolabeled cortisone to cortisol conversion by SPA | Bioorg Med Chem Lett 16: 5958-62 (2006) Article DOI: 10.1016/j.bmcl.2006.08.129 BindingDB Entry DOI: 10.7270/Q23R0SJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50144020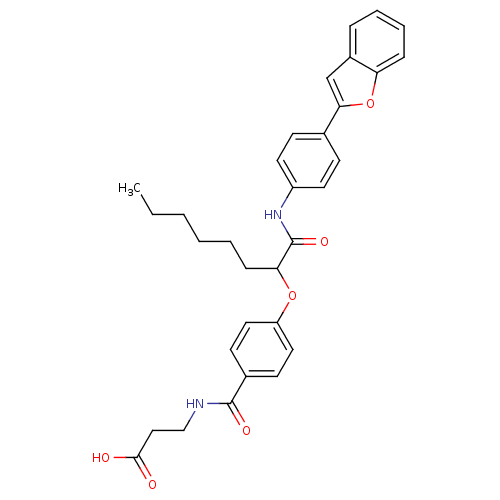 (3-{4-[1-(4-Benzofuran-2-yl-phenylcarbamoyl)-heptyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity against human glucagon receptor (h-GlucR) was determined | Bioorg Med Chem Lett 14: 2047-50 (2004) Article DOI: 10.1016/j.bmcl.2004.02.056 BindingDB Entry DOI: 10.7270/Q2XS5TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50435350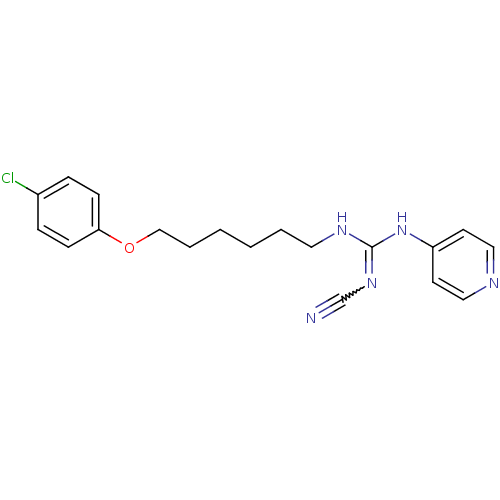 (CHEMBL17289) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM217040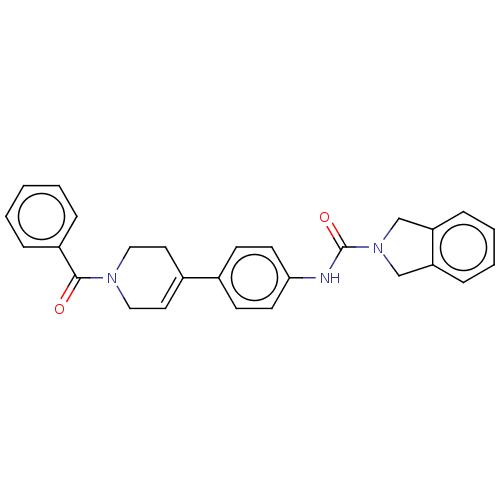 (US9302989, 332) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50202094 (CHEMBL375341 | N-(5-hydroxy-adamantan-2-yl)-2-(3-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against rat 11beta-HSD1 | J Med Chem 50: 149-64 (2007) Article DOI: 10.1021/jm0609364 BindingDB Entry DOI: 10.7270/Q2Z60NQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50202094 (CHEMBL375341 | N-(5-hydroxy-adamantan-2-yl)-2-(3-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in E. coli by SPA | J Med Chem 50: 149-64 (2007) Article DOI: 10.1021/jm0609364 BindingDB Entry DOI: 10.7270/Q2Z60NQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM81395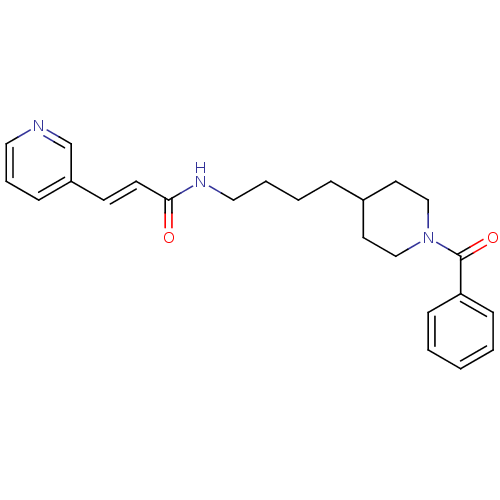 (APO-866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50197403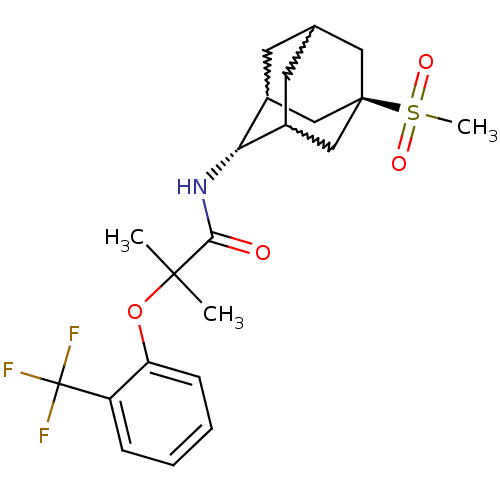 (CHEMBL393167 | N-(5-methanesulfonyl-adamantan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol | Bioorg Med Chem Lett 17: 527-32 (2007) Article DOI: 10.1016/j.bmcl.2006.10.008 BindingDB Entry DOI: 10.7270/Q2BZ65PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50197410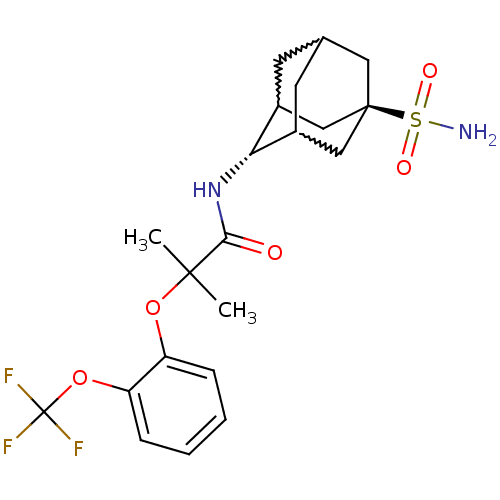 (2-methyl-N-(5-sulfamoyl-adamantan-2-yl)-2-(2-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol | Bioorg Med Chem Lett 17: 527-32 (2007) Article DOI: 10.1016/j.bmcl.2006.10.008 BindingDB Entry DOI: 10.7270/Q2BZ65PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50197404 (2-(2,6-dichloro-4-fluoro-phenoxy)-2-methyl-N-(5-su...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human HSD1 assessed as interconversion of cortisone to cortisol | Bioorg Med Chem Lett 17: 527-32 (2007) Article DOI: 10.1016/j.bmcl.2006.10.008 BindingDB Entry DOI: 10.7270/Q2BZ65PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50197416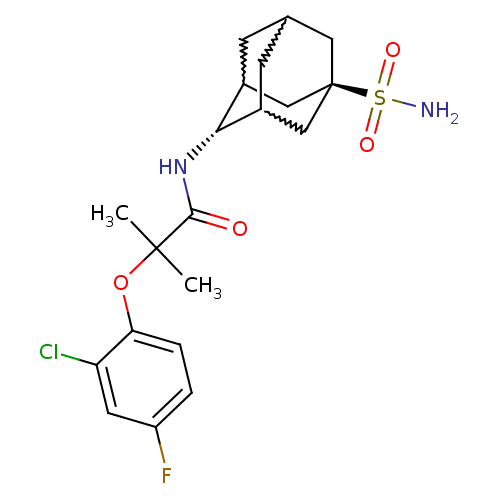 (2-(2-chloro-4-fluoro-phenoxy)-2-methyl-N-(5-sulfam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol | Bioorg Med Chem Lett 17: 527-32 (2007) Article DOI: 10.1016/j.bmcl.2006.10.008 BindingDB Entry DOI: 10.7270/Q2BZ65PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM217324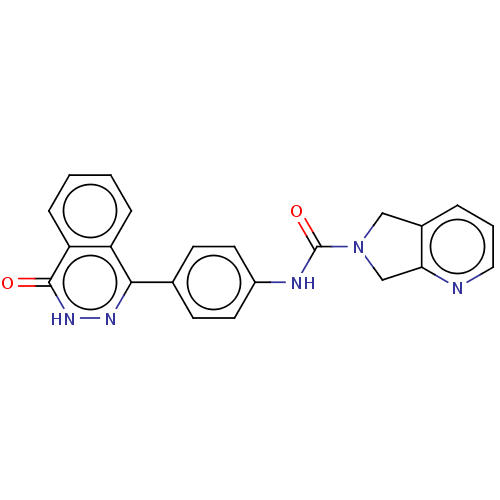 (US9302989, 549) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description HTRF assay: This assay used the CisBio HTRF KinEASE kit (kit 62ST2PEZ) and the kinase reaction containing 0.2 uM biotinylated substrate peptide (S2, ... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM217320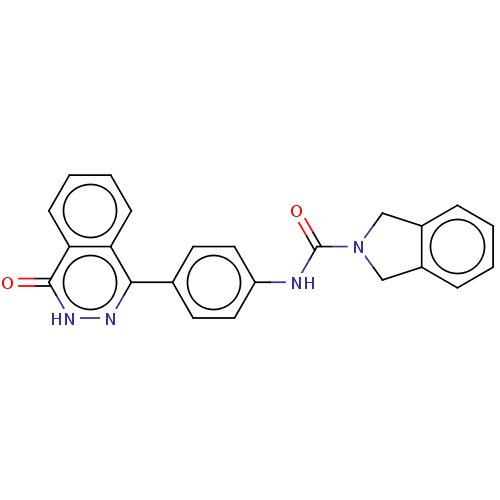 (US9302989, 374) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description HTRF assay: This assay used the CisBio HTRF KinEASE kit (kit 62ST2PEZ) and the kinase reaction containing 0.2 uM biotinylated substrate peptide (S2, ... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM217088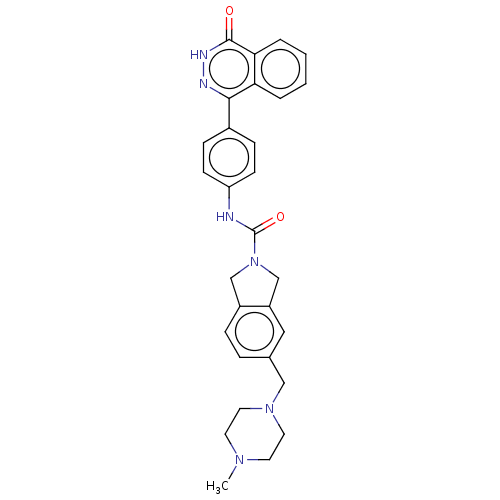 (US9302989, 383) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description HTRF assay: This assay used the CisBio HTRF KinEASE kit (kit 62ST2PEZ) and the kinase reaction containing 0.2 uM biotinylated substrate peptide (S2, ... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nicotinamide phosphoribosyltransferase (Homo sapiens (Human)) | BDBM50254212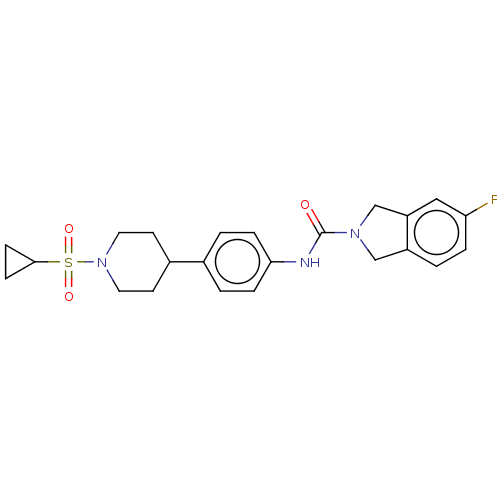 (CHEMBL4076064) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc, 1 North Waukegan Rd., North Chicago, IL 60064, United States. Electronic address: mike.curtin@abbvie.com. Curated by ChEMBL | Assay Description Inhibition of C-terminal His-tagged human recombinant NAMPT using FK866 or isoindoline urea-based Oregon green (488) probe incubated for 3 hrs by TR-... | Bioorg Med Chem Lett 27: 3317-3325 (2017) Article DOI: 10.1016/j.bmcl.2017.06.018 BindingDB Entry DOI: 10.7270/Q2D22123 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50197399 (2-(2-chloro-phenoxy)-2-methyl-N-(5-sulfamoyl-adama...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human HSD1 assessed as interconversion of cortisone to cortisol | Bioorg Med Chem Lett 17: 527-32 (2007) Article DOI: 10.1016/j.bmcl.2006.10.008 BindingDB Entry DOI: 10.7270/Q2BZ65PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50197412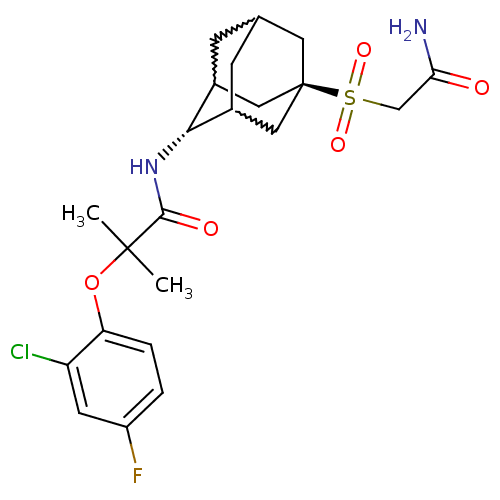 (CHEMBL241711 | N-(5-carbamoylmethanesulfonyl-adama...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse HSD1 assessed as interconversion of cortisone to cortisol | Bioorg Med Chem Lett 17: 527-32 (2007) Article DOI: 10.1016/j.bmcl.2006.10.008 BindingDB Entry DOI: 10.7270/Q2BZ65PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50195481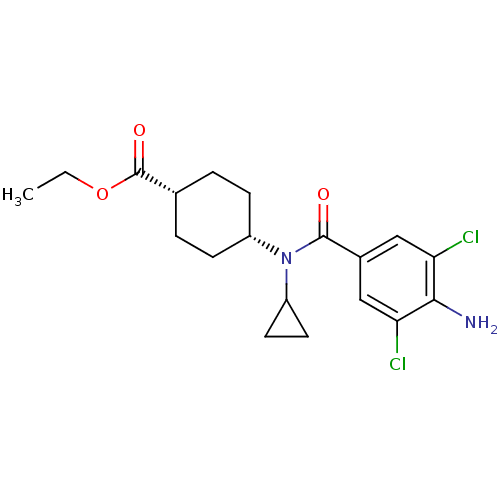 ((1s,4s)-ethyl 4-(4-amino-3,5-dichloro-N-cyclopropy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of rat 11beta-HSD1 | Bioorg Med Chem Lett 16: 6241-5 (2006) Article DOI: 10.1016/j.bmcl.2006.09.035 BindingDB Entry DOI: 10.7270/Q2B857R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50195506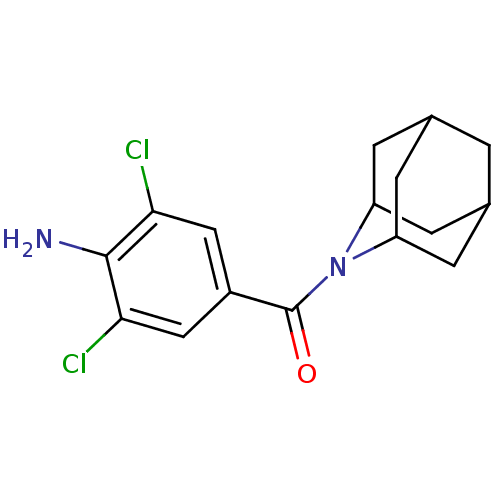 ((4-amino-3,5-dichloro-phenyl)-(2-aza-tricyclo[3.3....) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of rat 11beta-HSD1 | Bioorg Med Chem Lett 16: 6241-5 (2006) Article DOI: 10.1016/j.bmcl.2006.09.035 BindingDB Entry DOI: 10.7270/Q2B857R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50195504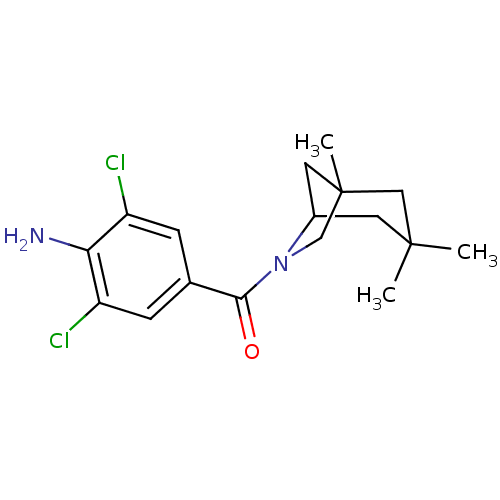 ((4-amino-3,5-dichlorophenyl)(1,3,3-trimethyl-6-aza...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in Escherichia coli by SPA | Bioorg Med Chem Lett 16: 6241-5 (2006) Article DOI: 10.1016/j.bmcl.2006.09.035 BindingDB Entry DOI: 10.7270/Q2B857R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3217 total ) | Next | Last >> |