 Found 100 hits with Last Name = 'kim' and Initial = 'by'
Found 100 hits with Last Name = 'kim' and Initial = 'by' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311254
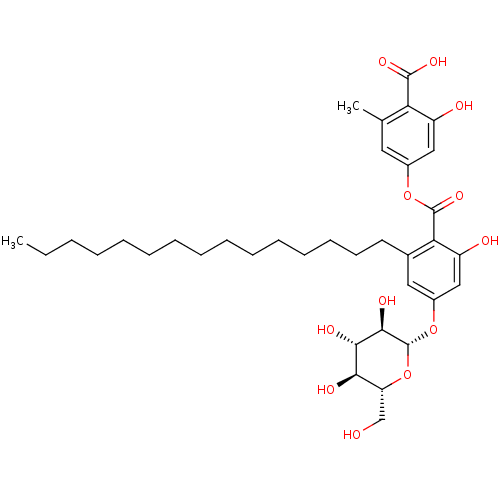
(2-hydroxy-4-(2-hydroxy-6-pentadecyl-4-((2S,3R,4S,5...)Show SMILES CCCCCCCCCCCCCCCc1cc(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc(O)c1C(=O)Oc1cc(C)c(C(O)=O)c(O)c1 |r| Show InChI InChI=1S/C36H52O12/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-23-18-25(47-36-33(42)32(41)31(40)28(21-37)48-36)20-27(39)30(23)35(45)46-24-17-22(2)29(34(43)44)26(38)19-24/h17-20,28,31-33,36-42H,3-16,21H2,1-2H3,(H,43,44)/t28-,31-,32+,33-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Competitive inhibition of PTP1B mediated pNPP hydrolysis by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 19: 6095-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.025
BindingDB Entry DOI: 10.7270/Q22J6BZQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50104694
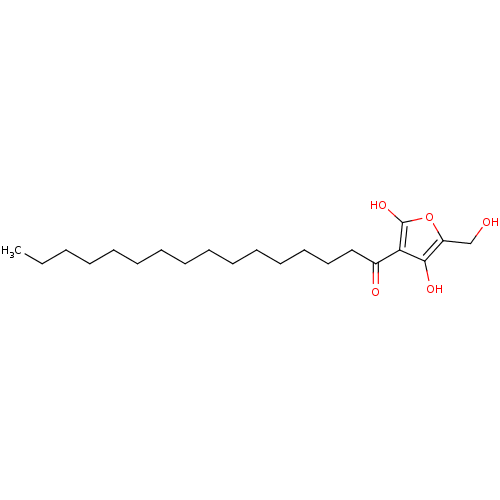
((R)-4-hydroxy-5-(hydroxymethyl)-3-palmitoylfuran-2...)Show InChI InChI=1S/C21H36O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-17(23)19-20(24)18(16-22)26-21(19)25/h22,24-25H,2-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3061-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.053
BindingDB Entry DOI: 10.7270/Q29024KF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50433414
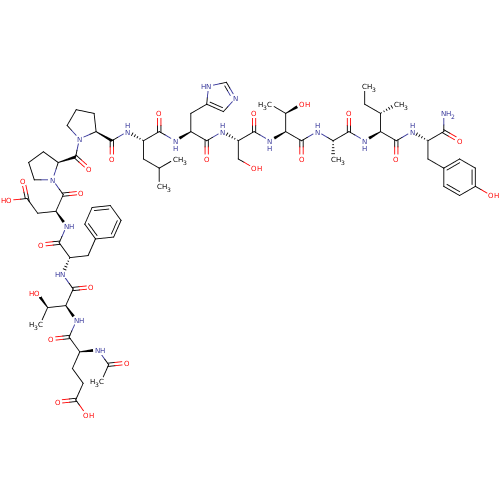
(CHEMBL2380650)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(C)=O)[C@@H](C)O)[C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O |r| Show InChI InChI=1S/C71H102N16O22/c1-9-36(4)56(67(105)77-46(59(72)97)28-42-19-21-44(92)22-20-42)83-60(98)37(5)75-68(106)57(38(6)89)85-65(103)51(33-88)82-64(102)49(30-43-32-73-34-74-43)78-62(100)47(27-35(2)3)79-66(104)52-17-13-25-86(52)71(109)53-18-14-26-87(53)70(108)50(31-55(95)96)81-63(101)48(29-41-15-11-10-12-16-41)80-69(107)58(39(7)90)84-61(99)45(76-40(8)91)23-24-54(93)94/h10-12,15-16,19-22,32,34-39,45-53,56-58,88-90,92H,9,13-14,17-18,23-31,33H2,1-8H3,(H2,72,97)(H,73,74)(H,75,106)(H,76,91)(H,77,105)(H,78,100)(H,79,104)(H,80,107)(H,81,101)(H,82,102)(H,83,98)(H,84,99)(H,85,103)(H,93,94)(H,95,96)/t36-,37-,38+,39+,45-,46-,47-,48-,49-,50-,51-,52-,53-,56-,57-,58-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Basic Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of HA-EGFP tagged Plk1 polo-box domain (unknown origin) expressed in 293A cells assessed as inhibition of biotin-p-T78 binding after 1 hr ... |
Bioorg Med Chem 21: 2623-34 (2013)
Article DOI: 10.1016/j.bmc.2013.02.020
BindingDB Entry DOI: 10.7270/Q2J38TZ4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311254

(2-hydroxy-4-(2-hydroxy-6-pentadecyl-4-((2S,3R,4S,5...)Show SMILES CCCCCCCCCCCCCCCc1cc(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc(O)c1C(=O)Oc1cc(C)c(C(O)=O)c(O)c1 |r| Show InChI InChI=1S/C36H52O12/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-23-18-25(47-36-33(42)32(41)31(40)28(21-37)48-36)20-27(39)30(23)35(45)46-24-17-22(2)29(34(43)44)26(38)19-24/h17-20,28,31-33,36-42H,3-16,21H2,1-2H3,(H,43,44)/t28-,31-,32+,33-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B mediated pNPP hydrolysis |
Bioorg Med Chem Lett 19: 6095-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.025
BindingDB Entry DOI: 10.7270/Q22J6BZQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311256
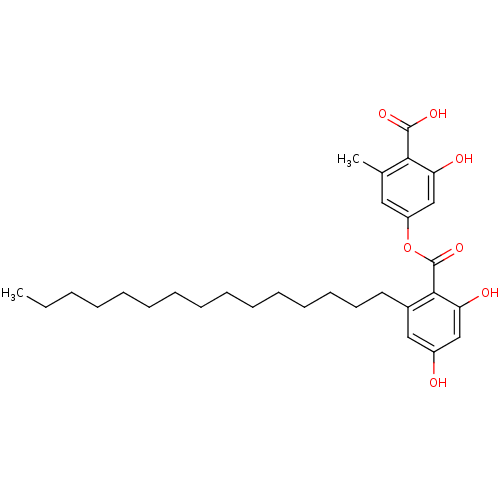
(4-(2,4-dihydroxy-6-pentadecylbenzoyloxy)-2-hydroxy...)Show SMILES CCCCCCCCCCCCCCCc1cc(O)cc(O)c1C(=O)Oc1cc(C)c(C(O)=O)c(O)c1 Show InChI InChI=1S/C30H42O7/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-22-18-23(31)19-25(32)28(22)30(36)37-24-17-21(2)27(29(34)35)26(33)20-24/h17-20,31-33H,3-16H2,1-2H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B mediated pNPP hydrolysis |
Bioorg Med Chem Lett 19: 6095-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.025
BindingDB Entry DOI: 10.7270/Q22J6BZQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50554673
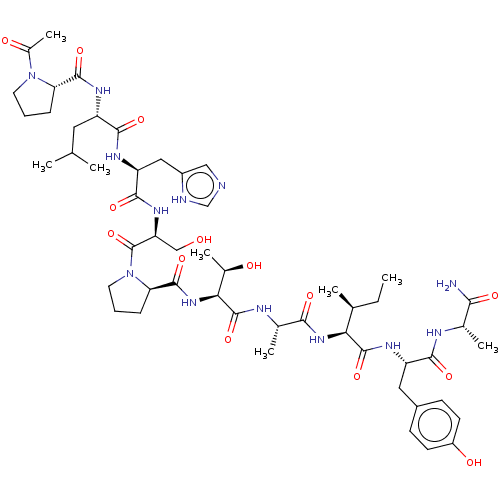
(CHEMBL4760204)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H]1CCCN1C(=O)[C@H](CO)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1C(C)=O)[C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 5-carboxyfluorescein-labeled PBD-binding peptide from PLk1 PBD (unknown origin) expressed in bacterial expression system incubated fo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01451
BindingDB Entry DOI: 10.7270/Q2W38105 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM24778

(2-methyl-1,4-dihydronaphthalene-1,4-dione | 2-meth...)Show InChI InChI=1S/C11H8O2/c1-7-6-10(12)8-4-2-3-5-9(8)11(7)13/h2-6H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of ascorbic acid/methylene blue activated recombinant human IDO expressed in Escherichia coli using L-Tryptophan as substrate after 60 min... |
J Nat Prod 80: 1378-1386 (2017)
Article DOI: 10.1021/acs.jnatprod.6b01059
BindingDB Entry DOI: 10.7270/Q2902687 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50269626

(Naphthomycin A)Show SMILES C[C@H]1\C=C\C=C/C=C(C)\C(=O)NC2=C(Cl)C(=O)c3c(cc(C)c(O)c3C(=O)\C(C)=C\[C@H](C)[C@@H](O)[C@@H](C)\C=C\[C@@H](O)C\C=C(C)\C(=O)C[C@@H]1O)C2=O |r,c:4,12,42,t:2,6,29,37| Show InChI InChI=1S/C40H46ClNO9/c1-20-11-9-8-10-12-23(4)40(51)42-34-33(41)39(50)31-28(38(34)49)18-26(7)37(48)32(31)36(47)25(6)17-24(5)35(46)22(3)14-16-27(43)15-13-21(2)30(45)19-29(20)44/h8-14,16-18,20,22,24,27,29,35,43-44,46,48H,15,19H2,1-7H3,(H,42,51)/b10-8-,11-9+,16-14+,21-13+,23-12-,25-17+/t20-,22-,24-,27-,29-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of ascorbic acid/methylene blue activated recombinant human IDO expressed in Escherichia coli using L-Tryptophan as substrate after 60 min... |
J Nat Prod 80: 1378-1386 (2017)
Article DOI: 10.1021/acs.jnatprod.6b01059
BindingDB Entry DOI: 10.7270/Q2902687 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50311254

(2-hydroxy-4-(2-hydroxy-6-pentadecyl-4-((2S,3R,4S,5...)Show SMILES CCCCCCCCCCCCCCCc1cc(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc(O)c1C(=O)Oc1cc(C)c(C(O)=O)c(O)c1 |r| Show InChI InChI=1S/C36H52O12/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-23-18-25(47-36-33(42)32(41)31(40)28(21-37)48-36)20-27(39)30(23)35(45)46-24-17-22(2)29(34(43)44)26(38)19-24/h17-20,28,31-33,36-42H,3-16,21H2,1-2H3,(H,43,44)/t28-,31-,32+,33-,36-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of TCPTP |
Bioorg Med Chem Lett 19: 6095-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.025
BindingDB Entry DOI: 10.7270/Q22J6BZQ |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50269632

(CHEMBL4126044)Show SMILES C[C@@H]1O[C@@H](Oc2cc3C(=O)C=C(NC(C)=O)C(=O)c3cc2C)[C@H](O)[C@H](O)[C@@H]1O |r,t:10| Show InChI InChI=1S/C19H21NO8/c1-7-4-11-10(13(22)6-12(16(11)24)20-9(3)21)5-14(7)28-19-18(26)17(25)15(23)8(2)27-19/h4-6,8,15,17-19,23,25-26H,1-3H3,(H,20,21)/t8-,15+,17+,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of ascorbic acid/methylene blue activated recombinant human IDO expressed in Escherichia coli using L-Tryptophan as substrate after 60 min... |
J Nat Prod 80: 1378-1386 (2017)
Article DOI: 10.1021/acs.jnatprod.6b01059
BindingDB Entry DOI: 10.7270/Q2902687 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311257

(2-hydroxy-6-pentadecyl-4-((2S,3R,4S,5S,6R)-3,4,5-t...)Show SMILES CCCCCCCCCCCCCCCc1cc(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc(O)c1C(O)=O |r| Show InChI InChI=1S/C28H46O9/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-19-16-20(17-21(30)23(19)27(34)35)36-28-26(33)25(32)24(31)22(18-29)37-28/h16-17,22,24-26,28-33H,2-15,18H2,1H3,(H,34,35)/t22-,24-,25+,26-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B mediated pNPP hydrolysis |
Bioorg Med Chem Lett 19: 6095-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.025
BindingDB Entry DOI: 10.7270/Q22J6BZQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50433415
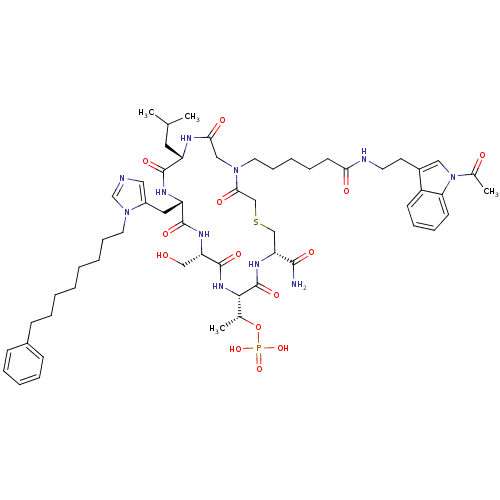
(CHEMBL2380764)Show SMILES CC(C)C[C@@H]1NC(=O)CN(CCCCCC(=O)NCCc2cn(C(C)=O)c3ccccc23)C(=O)CSC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2cncn2CCCCCCCCc2ccccc2)NC1=O)[C@@H](C)OP(O)(O)=O)C(N)=O |r| Show InChI InChI=1S/C58H84N11O14PS/c1-38(2)29-45-55(76)63-46(30-43-31-60-37-68(43)28-17-8-6-5-7-11-19-41-20-12-9-13-21-41)56(77)64-47(34-70)57(78)66-53(39(3)83-84(80,81)82)58(79)65-48(54(59)75)35-85-36-52(74)67(33-51(73)62-45)27-18-10-14-24-50(72)61-26-25-42-32-69(40(4)71)49-23-16-15-22-44(42)49/h9,12-13,15-16,20-23,31-32,37-39,45-48,53,70H,5-8,10-11,14,17-19,24-30,33-36H2,1-4H3,(H2,59,75)(H,61,72)(H,62,73)(H,63,76)(H,64,77)(H,65,79)(H,66,78)(H2,80,81,82)/t39-,45+,46+,47+,48-,53+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Basic Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of HA-EGFP tagged Plk1 polo-box domain (unknown origin) expressed in 293A cells assessed as inhibition of biotin-p-T78 binding after 1 hr ... |
Bioorg Med Chem 21: 2623-34 (2013)
Article DOI: 10.1016/j.bmc.2013.02.020
BindingDB Entry DOI: 10.7270/Q2J38TZ4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50179012
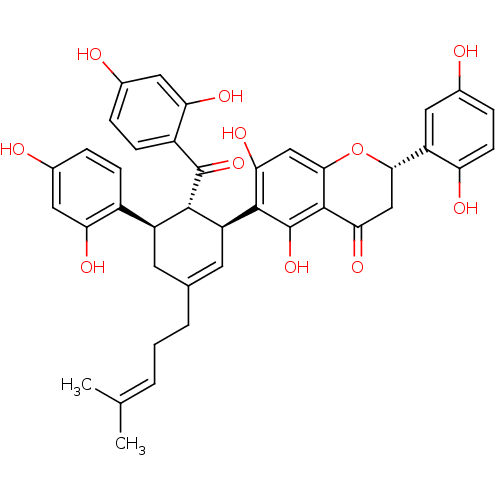
(CHEMBL382338 | Sanggenon G)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]-[#6]-1=[#6]-[#6@@H](-[#6@H](-[#6@@H](-[#6]-1)-c1ccc(-[#8])cc1-[#8])-[#6](=O)-c1ccc(-[#8])cc1-[#8])-c1c(-[#8])cc2-[#8]-[#6@@H](-[#6]-[#6](=O)-c2c1-[#8])-c1cc(-[#8])ccc1-[#8] |t:6| Show InChI InChI=1S/C40H38O11/c1-19(2)4-3-5-20-12-26(24-9-6-22(42)15-30(24)45)36(39(49)25-10-7-23(43)16-31(25)46)28(13-20)37-32(47)18-35-38(40(37)50)33(48)17-34(51-35)27-14-21(41)8-11-29(27)44/h4,6-11,13-16,18,26,28,34,36,41-47,50H,3,5,12,17H2,1-2H3/t26-,28-,34-,36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology (KRIBB)
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 16: 1426-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.071
BindingDB Entry DOI: 10.7270/Q2JQ10KT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B mediated pNPP hydrolysis |
Bioorg Med Chem Lett 19: 6095-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.025
BindingDB Entry DOI: 10.7270/Q22J6BZQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50433416
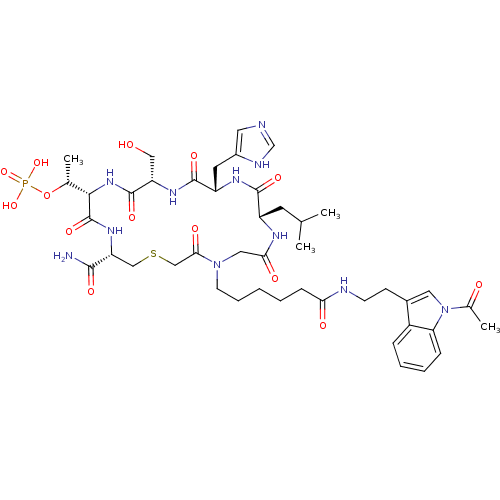
(CHEMBL2380660)Show SMILES CC(C)C[C@@H]1NC(=O)CN(CCCCCC(=O)NCCc2cn(C(C)=O)c3ccccc23)C(=O)CSC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)[C@@H](C)OP(O)(O)=O)C(N)=O |r| Show InChI InChI=1S/C44H64N11O14PS/c1-25(2)16-31-41(62)50-32(17-29-18-46-24-48-29)42(63)51-33(21-56)43(64)53-39(26(3)69-70(66,67)68)44(65)52-34(40(45)61)22-71-23-38(60)54(20-37(59)49-31)15-9-5-6-12-36(58)47-14-13-28-19-55(27(4)57)35-11-8-7-10-30(28)35/h7-8,10-11,18-19,24-26,31-34,39,56H,5-6,9,12-17,20-23H2,1-4H3,(H2,45,61)(H,46,48)(H,47,58)(H,49,59)(H,50,62)(H,51,63)(H,52,65)(H,53,64)(H2,66,67,68)/t26-,31+,32+,33+,34-,39+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Basic Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of HA-EGFP tagged Plk1 polo-box domain (unknown origin) expressed in 293A cells assessed as inhibition of biotin-p-T78 binding after 1 hr ... |
Bioorg Med Chem 21: 2623-34 (2013)
Article DOI: 10.1016/j.bmc.2013.02.020
BindingDB Entry DOI: 10.7270/Q2J38TZ4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50179008

(CHEMBL204813 | sanggenon C)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6][C@]12[#8]-c3cc(-[#8])c(-[#6@H]-4-[#6]=[#6](-[#6])-[#6]-[#6@@H](-[#6@H]-4-[#6](=O)-c4ccc(-[#8])cc4-[#8])-c4ccc(-[#8])cc4-[#8])c(-[#8])c3-[#6](=O)[C@@]1([#8])[#8]-c1cc(-[#8])ccc21 |r,t:13| Show InChI InChI=1S/C40H36O12/c1-18(2)10-11-39-27-9-6-22(43)16-31(27)52-40(39,50)38(49)35-32(51-39)17-30(46)34(37(35)48)26-13-19(3)12-25(23-7-4-20(41)14-28(23)44)33(26)36(47)24-8-5-21(42)15-29(24)45/h4-10,13-17,25-26,33,41-46,48,50H,11-12H2,1-3H3/t25-,26+,33-,39-,40-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology (KRIBB)
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 16: 1426-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.071
BindingDB Entry DOI: 10.7270/Q2JQ10KT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50148911

((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| Show InChI InChI=1S/C30H48O3/c1-18-10-15-30(25(32)33)17-16-28(6)20(24(30)19(18)2)8-9-22-27(5)13-12-23(31)26(3,4)21(27)11-14-29(22,28)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21+,22-,23+,24+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Nat Prod 69: 1572-6 (2006)
Article DOI: 10.1021/np0601861
BindingDB Entry DOI: 10.7270/Q2B8591J |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50346601

(NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2C1)C(O)=O |r,c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21-,22+,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50104694

((R)-4-hydroxy-5-(hydroxymethyl)-3-palmitoylfuran-2...)Show InChI InChI=1S/C21H36O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-17(23)19-20(24)18(16-22)26-21(19)25/h22,24-25H,2-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology (KRIBB)
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 16: 1426-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.071
BindingDB Entry DOI: 10.7270/Q2JQ10KT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50104694

((R)-4-hydroxy-5-(hydroxymethyl)-3-palmitoylfuran-2...)Show InChI InChI=1S/C21H36O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-17(23)19-20(24)18(16-22)26-21(19)25/h22,24-25H,2-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50104694

((R)-4-hydroxy-5-(hydroxymethyl)-3-palmitoylfuran-2...)Show InChI InChI=1S/C21H36O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-17(23)19-20(24)18(16-22)26-21(19)25/h22,24-25H,2-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Nat Prod 69: 1572-6 (2006)
Article DOI: 10.1021/np0601861
BindingDB Entry DOI: 10.7270/Q2B8591J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50433413
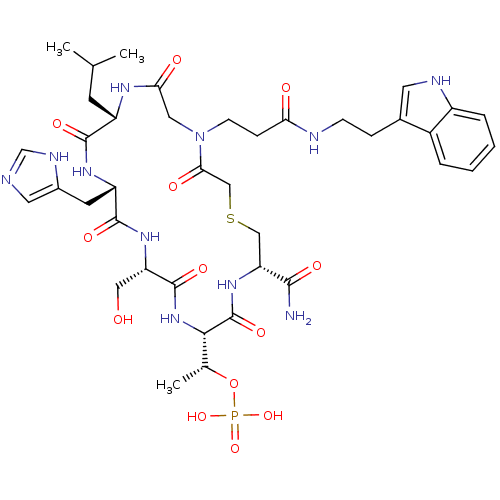
(CHEMBL2380651)Show SMILES CC(C)C[C@@H]1NC(=O)CN(CCC(=O)NCCc2c[nH]c3ccccc23)C(=O)CSC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)[C@@H](C)OP(O)(O)=O)C(N)=O |r| Show InChI InChI=1S/C39H56N11O13PS/c1-21(2)12-27-36(56)46-28(13-24-15-41-20-44-24)37(57)47-29(17-51)38(58)49-34(22(3)63-64(60,61)62)39(59)48-30(35(40)55)18-65-19-33(54)50(16-32(53)45-27)11-9-31(52)42-10-8-23-14-43-26-7-5-4-6-25(23)26/h4-7,14-15,20-22,27-30,34,43,51H,8-13,16-19H2,1-3H3,(H2,40,55)(H,41,44)(H,42,52)(H,45,53)(H,46,56)(H,47,57)(H,48,59)(H,49,58)(H2,60,61,62)/t22-,27+,28+,29+,30-,34+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Basic Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of HA-EGFP tagged Plk1 polo-box domain (unknown origin) expressed in 293A cells assessed as inhibition of biotin-p-T78 binding after 1 hr ... |
Bioorg Med Chem 21: 2623-34 (2013)
Article DOI: 10.1016/j.bmc.2013.02.020
BindingDB Entry DOI: 10.7270/Q2J38TZ4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50179010
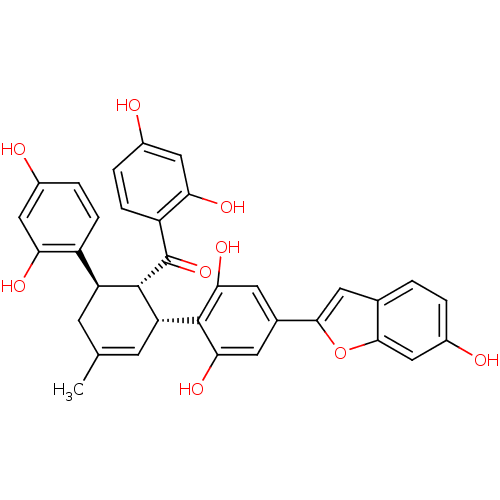
(CHEMBL378806 | CVD-0019900 | Mulberrofuran C)Show SMILES CC1=C[C@H]([C@H]([C@@H](C1)c1ccc(O)cc1O)C(=O)c1ccc(O)cc1O)c1c(O)cc(cc1O)-c1cc2ccc(O)cc2o1 |t:1| Show InChI InChI=1S/C34H28O9/c1-16-8-24(22-6-4-19(35)13-26(22)38)32(34(42)23-7-5-20(36)14-27(23)39)25(9-16)33-28(40)10-18(11-29(33)41)30-12-17-2-3-21(37)15-31(17)43-30/h2-7,9-15,24-25,32,35-41H,8H2,1H3/t24-,25+,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology (KRIBB)
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 16: 1426-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.071
BindingDB Entry DOI: 10.7270/Q2JQ10KT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50185123
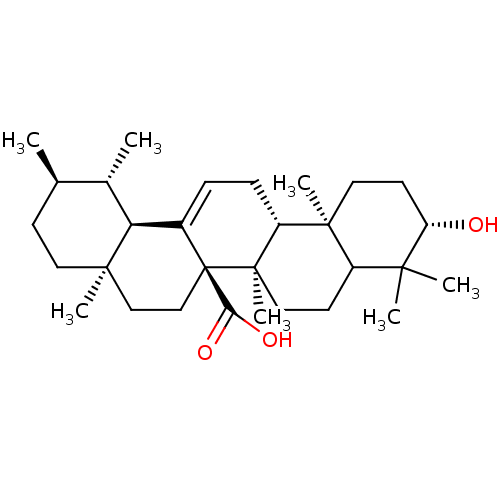
(3beta-hydroxyurs-12-en-27-oic acid | CHEMBL380467)Show SMILES C[C@@H]1CC[C@]2(C)CC[C@]3(C(O)=O)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)C5CC[C@@]34C)[C@@H]2[C@H]1C |c:12| Show InChI InChI=1S/C30H48O3/c1-18-10-13-27(5)16-17-30(25(32)33)20(24(27)19(18)2)8-9-22-28(6)14-12-23(31)26(3,4)21(28)11-15-29(22,30)7/h8,18-19,21-24,31H,9-17H2,1-7H3,(H,32,33)/t18-,19+,21?,22-,23+,24+,27-,28+,29-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50185131
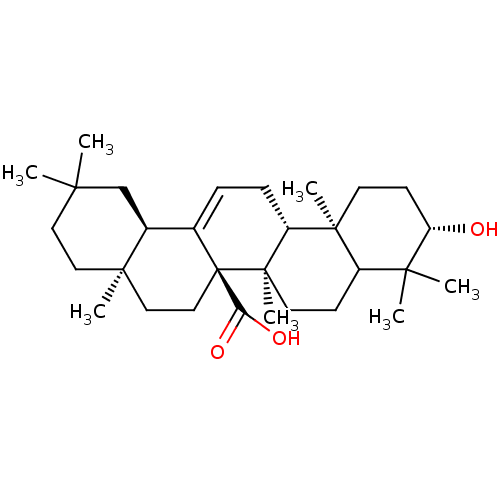
(3beta-hydroxyolean-12-en-27-oic acid | CHEMBL21097...)Show SMILES CC1(C)CC[C@]2(C)CC[C@]3(C(O)=O)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)C5CC[C@@]34C)[C@@H]2C1 |c:13| Show InChI InChI=1S/C30H48O3/c1-25(2)14-15-27(5)16-17-30(24(32)33)19(20(27)18-25)8-9-22-28(6)12-11-23(31)26(3,4)21(28)10-13-29(22,30)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21?,22+,23-,27+,28-,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50370716

(CHEMBL1081157)Show SMILES CC1(C)CC[C@]2(C)CC[C@]3(C(O)=O)C(=CC[C@@H]4[C@@]5(C)CCC(=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2C1 |r,c:13| Show InChI InChI=1S/C30H46O3/c1-25(2)14-15-27(5)16-17-30(24(32)33)19(20(27)18-25)8-9-22-28(6)12-11-23(31)26(3,4)21(28)10-13-29(22,30)7/h8,20-22H,9-18H2,1-7H3,(H,32,33)/t20-,21-,22+,27+,28-,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50184543
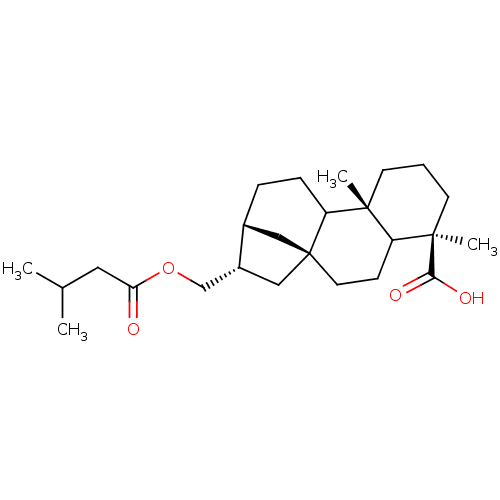
(16-alphaH,17-isovaleryloxy-ent-kauran-19-oic acid ...)Show SMILES CC(C)CC(=O)OC[C@H]1C[C@]23C[C@H]1CCC2[C@]1(C)CCC[C@](C)(C1CC3)C(O)=O Show InChI InChI=1S/C25H40O4/c1-16(2)12-21(26)29-15-18-14-25-11-8-19-23(3,20(25)7-6-17(18)13-25)9-5-10-24(19,4)22(27)28/h16-20H,5-15H2,1-4H3,(H,27,28)/t17?,18-,19?,20?,23-,24-,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3061-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.053
BindingDB Entry DOI: 10.7270/Q29024KF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50185122

(3beta-acetoxyolean-12-en-27-oic acid | CHEMBL20863...)Show SMILES CC(=O)O[C@H]1CC[C@@]2(C)C(CC[C@]3(C)[C@@H]2CC=C2[C@@H]4CC(C)(C)CC[C@]4(C)CC[C@@]32C(O)=O)C1(C)C |t:17| Show InChI InChI=1S/C32H50O4/c1-20(33)36-25-12-13-30(7)23(28(25,4)5)11-14-31(8)24(30)10-9-21-22-19-27(2,3)15-16-29(22,6)17-18-32(21,31)26(34)35/h9,22-25H,10-19H2,1-8H3,(H,34,35)/t22-,23?,24+,25-,29+,30-,31+,32+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50433417
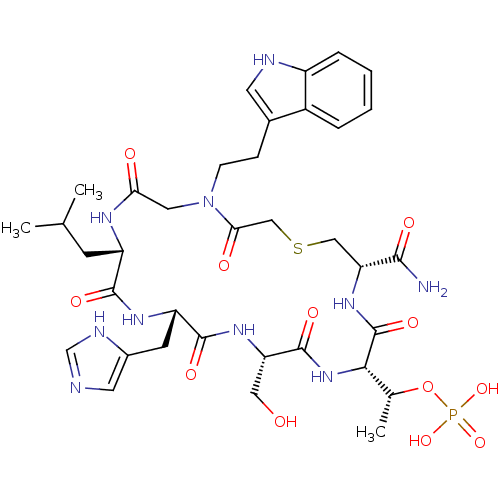
(CHEMBL2380653)Show SMILES CC(C)C[C@@H]1NC(=O)CN(CCc2c[nH]c3ccccc23)C(=O)CSC[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)[C@@H](C)OP(O)(O)=O)C(N)=O |r| Show InChI InChI=1S/C36H51N10O12PS/c1-19(2)10-25-33(51)42-26(11-22-13-38-18-40-22)34(52)43-27(15-47)35(53)45-31(20(3)58-59(55,56)57)36(54)44-28(32(37)50)16-60-17-30(49)46(14-29(48)41-25)9-8-21-12-39-24-7-5-4-6-23(21)24/h4-7,12-13,18-20,25-28,31,39,47H,8-11,14-17H2,1-3H3,(H2,37,50)(H,38,40)(H,41,48)(H,42,51)(H,43,52)(H,44,54)(H,45,53)(H2,55,56,57)/t20-,25+,26+,27+,28-,31+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Basic Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of HA-EGFP tagged Plk1 polo-box domain (unknown origin) expressed in 293A cells assessed as inhibition of biotin-p-T78 binding after 1 hr ... |
Bioorg Med Chem 21: 2623-34 (2013)
Article DOI: 10.1016/j.bmc.2013.02.020
BindingDB Entry DOI: 10.7270/Q2J38TZ4 |
More data for this
Ligand-Target Pair | |
1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase gamma-1
(Homo sapiens (Human)) | BDBM50269158

(3-beta-hydroxy-27-p-(E)-coumaroyloxyurs-12-en-28-o...)Show SMILES C[C@@H]1CC[C@@]2(CC[C@]3(CC(=O)C=Cc4ccc(O)cc4)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,w:11.10,c:21| Show InChI InChI=1S/C39H54O5/c1-24-15-20-38(34(43)44)21-22-39(23-28(41)12-9-26-7-10-27(40)11-8-26)29(33(38)25(24)2)13-14-31-36(5)18-17-32(42)35(3,4)30(36)16-19-37(31,39)6/h7-13,24-25,30-33,40,42H,14-23H2,1-6H3,(H,43,44)/t24-,25+,30+,31-,32+,33+,36+,37-,38+,39+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of PLCgamma1 (unknown origin) |
J Nat Prod 63: 753-6 (2000)
BindingDB Entry DOI: 10.7270/Q2JS9Q69 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50433418
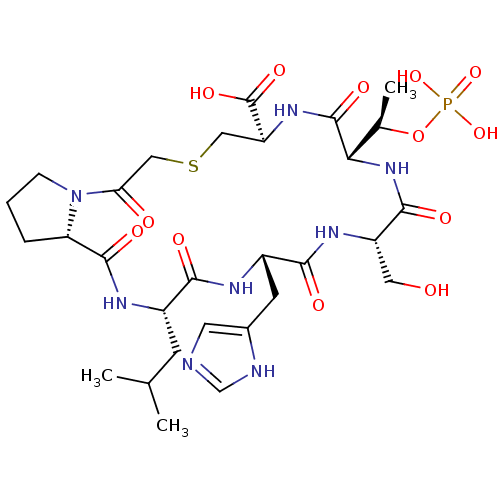
(CHEMBL2380765)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)CSC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)[C@@H](C)OP(O)(O)=O)C(O)=O |r| Show InChI InChI=1S/C29H45N8O13PS/c1-14(2)7-17-24(40)32-18(8-16-9-30-13-31-16)25(41)34-19(10-38)26(42)36-23(15(3)50-51(47,48)49)28(44)35-20(29(45)46)11-52-12-22(39)37-6-4-5-21(37)27(43)33-17/h9,13-15,17-21,23,38H,4-8,10-12H2,1-3H3,(H,30,31)(H,32,40)(H,33,43)(H,34,41)(H,35,44)(H,36,42)(H,45,46)(H2,47,48,49)/t15-,17+,18+,19+,20+,21+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Basic Science Institute
Curated by ChEMBL
| Assay Description
Inhibition of HA-EGFP tagged Plk1 polo-box domain (unknown origin) expressed in 293A cells assessed as inhibition of biotin-p-T78 binding after 1 hr ... |
Bioorg Med Chem 21: 2623-34 (2013)
Article DOI: 10.1016/j.bmc.2013.02.020
BindingDB Entry DOI: 10.7270/Q2J38TZ4 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50104694

((R)-4-hydroxy-5-(hydroxymethyl)-3-palmitoylfuran-2...)Show InChI InChI=1S/C21H36O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-17(23)19-20(24)18(16-22)26-21(19)25/h22,24-25H,2-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology (KRIBB)
Curated by ChEMBL
| Assay Description
Inhibition of VHR DS-PTP |
Bioorg Med Chem Lett 16: 1426-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.071
BindingDB Entry DOI: 10.7270/Q2JQ10KT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50185120
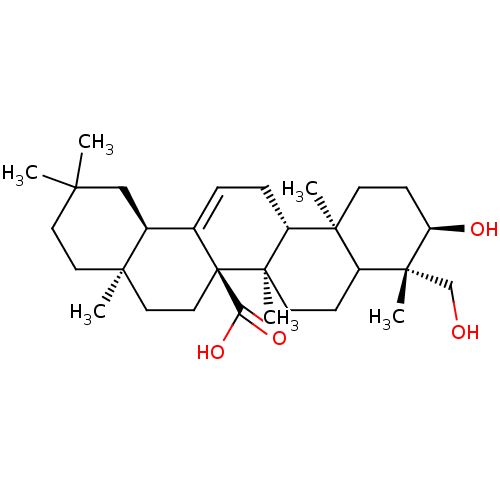
(3alpha,24-dihydroxyolean-12-en-27-oic acid | CHEMB...)Show SMILES CC1(C)CC[C@]2(C)CC[C@]3(C(O)=O)C(=CC[C@@H]4[C@@]5(C)CC[C@@H](O)[C@](C)(CO)C5CC[C@@]34C)[C@@H]2C1 |c:13| Show InChI InChI=1S/C30H48O4/c1-25(2)13-14-26(3)15-16-30(24(33)34)19(20(26)17-25)7-8-22-27(4)11-10-23(32)28(5,18-31)21(27)9-12-29(22,30)6/h7,20-23,31-32H,8-18H2,1-6H3,(H,33,34)/t20-,21?,22+,23+,26+,27-,28+,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50185125

(3beta,6beta-dihydroxyolean-12-en-27-oic acid | CHE...)Show SMILES CC1(C)CC[C@]2(C)CC[C@]3(C(O)=O)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)C5[C@H](O)C[C@@]34C)[C@@H]2C1 |c:13| Show InChI InChI=1S/C30H48O4/c1-25(2)12-13-27(5)14-15-30(24(33)34)18(19(27)16-25)8-9-21-28(6)11-10-22(32)26(3,4)23(28)20(31)17-29(21,30)7/h8,19-23,31-32H,9-17H2,1-7H3,(H,33,34)/t19-,20+,21+,22-,23?,27+,28+,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50009248
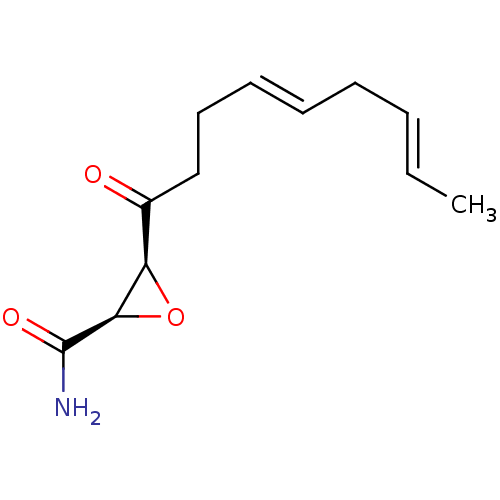
((2R,3S)-3-((4E,7E)-Nona-4,7-dienoyl)-oxirane-2-car...)Show InChI InChI=1S/C12H17NO3/c1-2-3-4-5-6-7-8-9(14)10-11(16-10)12(13)15/h2-3,5-6,10-11H,4,7-8H2,1H3,(H2,13,15)/b3-2+,6-5+/t10-,11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of FAS |
Bioorg Med Chem Lett 16: 4738-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.018
BindingDB Entry DOI: 10.7270/Q2QV3NB7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM23195
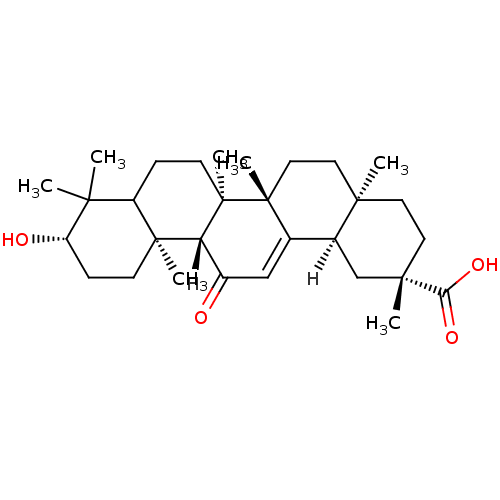
((2S,4aS,6aS,6bR,10S,12aS,12bR,14bR)-10-hydroxy-2,4...)Show SMILES [H][C@@]12C[C@](C)(CC[C@]1(C)CC[C@]1(C)C2=CC(=O)[C@]2([H])[C@@]3(C)CC[C@H](O)C(C)(C)C3CC[C@@]12C)C(O)=O |r,t:15| Show InChI InChI=1S/C30H46O4/c1-25(2)21-8-11-30(7)23(28(21,5)10-9-22(25)32)20(31)16-18-19-17-27(4,24(33)34)13-12-26(19,3)14-15-29(18,30)6/h16,19,21-23,32H,8-15,17H2,1-7H3,(H,33,34)/t19-,21?,22-,23+,26+,27-,28-,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50185124

(CHEMBL381707 | methyl 3beta-hydroxyolean-12-en-28-...)Show SMILES COC(=O)[C@@]12CCC3C(=CC[C@H]4[C@@]3(C)CCC3C(C)(C)[C@@H](O)CC[C@]43C)[C@@H]1CC(C)(C)CC2 |c:8| Show InChI InChI=1S/C30H48O3/c1-26(2)16-17-30(25(32)33-7)15-10-20-19(21(30)18-26)8-9-23-28(20,5)13-11-22-27(3,4)24(31)12-14-29(22,23)6/h8,20-24,31H,9-18H2,1-7H3/t20?,21-,22?,23-,24-,28-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase gamma-1
(Homo sapiens (Human)) | BDBM50269154

(3-beta-hydroxy-27-(E)-feruloyloxyurs-12-en-28-oic ...)Show SMILES COc1cc(C=CC(=O)C[C@@]23CC[C@]4(CC[C@@H](C)[C@H](C)[C@H]4C2=CC[C@@H]2[C@@]4(C)CC[C@H](O)C(C)(C)[C@@H]4CC[C@@]32C)C(O)=O)ccc1O |r,w:6.6,c:23| Show InChI InChI=1S/C40H56O6/c1-24-14-19-39(35(44)45)20-21-40(23-27(41)10-8-26-9-12-29(42)30(22-26)46-7)28(34(39)25(24)2)11-13-32-37(5)17-16-33(43)36(3,4)31(37)15-18-38(32,40)6/h8-12,22,24-25,31-34,42-43H,13-21,23H2,1-7H3,(H,44,45)/t24-,25+,31+,32-,33+,34+,37+,38-,39+,40+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of PLCgamma1 (unknown origin) |
J Nat Prod 63: 753-6 (2000)
BindingDB Entry DOI: 10.7270/Q2JS9Q69 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50241807

(CHEMBL470865 | abyssinone-VI-4-O-methyl ether)Show SMILES [#6]-[#8]-c1c(-[#6]\[#6]=[#6](\[#6])-[#6])cc(\[#6]=[#6]\[#6](=O)-c2ccc(-[#8])cc2-[#8])cc1-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C26H30O4/c1-17(2)6-9-20-14-19(15-21(26(20)30-5)10-7-18(3)4)8-13-24(28)23-12-11-22(27)16-25(23)29/h6-8,11-16,27,29H,9-10H2,1-5H3/b13-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Nat Prod 69: 1572-6 (2006)
Article DOI: 10.1021/np0601861
BindingDB Entry DOI: 10.7270/Q2B8591J |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50185128
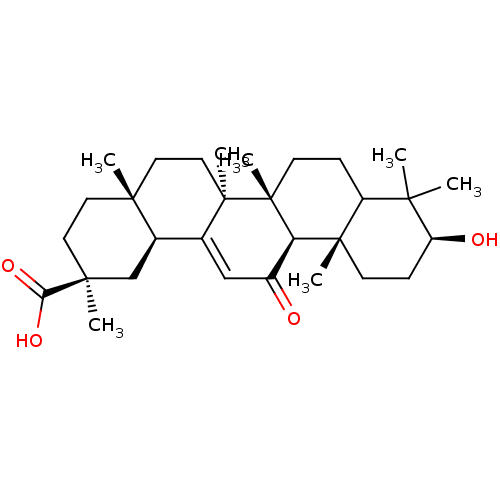
(18alpha-Glycyrrhetic acid | CHEMBL378653)Show SMILES CC1(C)[C@@H](O)CC[C@@]2(C)C1CC[C@]1(C)[C@@H]2C(=O)C=C2[C@H]3C[C@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O |t:19| Show InChI InChI=1S/C30H46O4/c1-25(2)21-8-11-30(7)23(28(21,5)10-9-22(25)32)20(31)16-18-19-17-27(4,24(33)34)13-12-26(19,3)14-15-29(18,30)6/h16,19,21-23,32H,8-15,17H2,1-7H3,(H,33,34)/t19-,21?,22+,23-,26-,27+,28+,29-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50241806
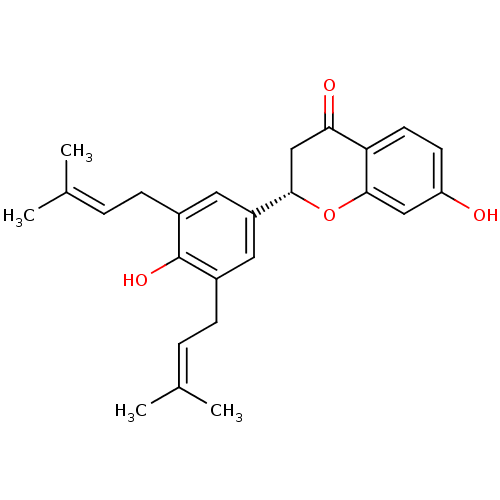
(CHEMBL470454 | abyssinone IV | abyssinone-IV)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(cc(-[#6]\[#6]=[#6](\[#6])-[#6])c1-[#8])-[#6@@H]-1-[#6]-[#6](=O)-c2ccc(-[#8])cc2-[#8]-1 |r| Show InChI InChI=1S/C25H28O4/c1-15(2)5-7-17-11-19(12-18(25(17)28)8-6-16(3)4)23-14-22(27)21-10-9-20(26)13-24(21)29-23/h5-6,9-13,23,26,28H,7-8,14H2,1-4H3/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Nat Prod 69: 1572-6 (2006)
Article DOI: 10.1021/np0601861
BindingDB Entry DOI: 10.7270/Q2B8591J |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50185130
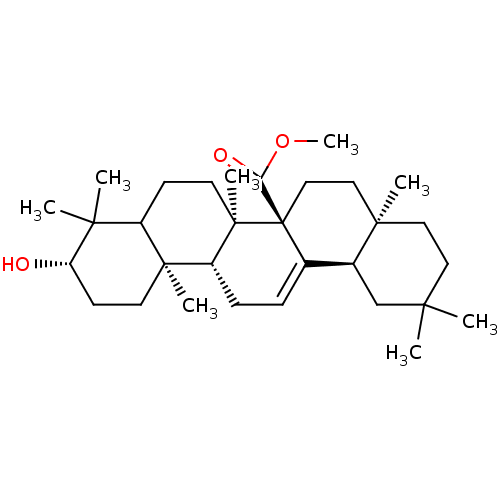
(CHEMBL207494 | methyl3beta-hydroxyolean-12-en-27-o...)Show SMILES COC(=O)[C@@]12CC[C@@]3(C)CCC(C)(C)C[C@H]3C1=CC[C@@H]1[C@@]3(C)CC[C@H](O)C(C)(C)C3CC[C@@]21C |c:18| Show InChI InChI=1S/C31H50O3/c1-26(2)15-16-28(5)17-18-31(25(33)34-8)20(21(28)19-26)9-10-23-29(6)13-12-24(32)27(3,4)22(29)11-14-30(23,31)7/h9,21-24,32H,10-19H2,1-8H3/t21-,22?,23+,24-,28+,29-,30+,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3273-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.036
BindingDB Entry DOI: 10.7270/Q2ZP46WV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50554676
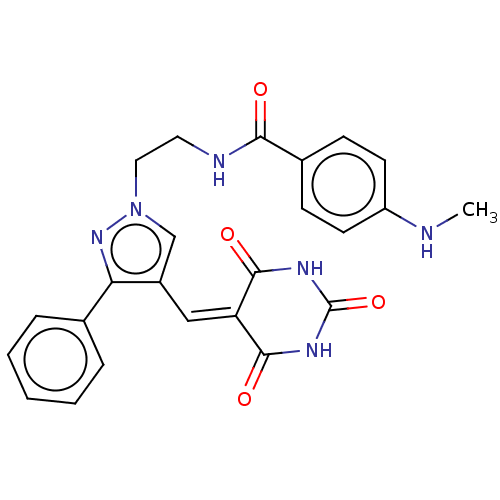
(CHEMBL4788427)Show SMILES [#6]-[#7]-c1ccc(cc1)-[#6](=O)-[#7]-[#6]-[#6]-n1cc(\[#6]=[#6]-2\[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-2=O)c(n1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 5-carboxyfluorescein-labeled PBD-binding peptide from PLk1 PBD (unknown origin) expressed in bacterial expression system incubated fo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01451
BindingDB Entry DOI: 10.7270/Q2W38105 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50179009
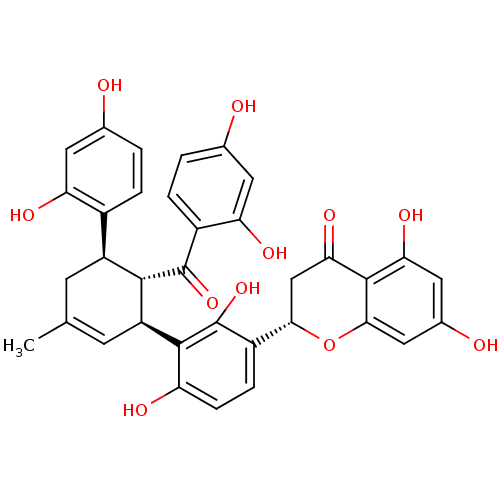
(CHEMBL377937 | Kuwanon L)Show SMILES CC1=C[C@@H]([C@H]([C@@H](C1)c1ccc(O)cc1O)C(=O)c1ccc(O)cc1O)c1c(O)ccc([C@@H]2CC(=O)c3c(O)cc(O)cc3O2)c1O |t:1| Show InChI InChI=1S/C35H30O11/c1-15-8-22(19-4-2-16(36)10-25(19)40)31(34(44)20-5-3-17(37)11-26(20)41)23(9-15)32-24(39)7-6-21(35(32)45)29-14-28(43)33-27(42)12-18(38)13-30(33)46-29/h2-7,9-13,22-23,29,31,36-42,45H,8,14H2,1H3/t22-,23-,29-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology (KRIBB)
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
Bioorg Med Chem Lett 16: 1426-9 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.071
BindingDB Entry DOI: 10.7270/Q2JQ10KT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50311255

(3-hydroxy-4-(methoxycarbonyl)-5-methylphenyl 2-hyd...)Show SMILES CCCCCCCCCCCCCCCc1cc(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc(O)c1C(=O)Oc1cc(C)c(C(=O)OC)c(O)c1 |r| Show InChI InChI=1S/C37H54O12/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17-24-19-26(48-37-34(43)33(42)32(41)29(22-38)49-37)21-28(40)31(24)36(45)47-25-18-23(2)30(27(39)20-25)35(44)46-3/h18-21,29,32-34,37-43H,4-17,22H2,1-3H3/t29-,32-,33+,34-,37-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Silla University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B mediated pNPP hydrolysis |
Bioorg Med Chem Lett 19: 6095-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.025
BindingDB Entry DOI: 10.7270/Q22J6BZQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50370742

(KAURENOIC ACID)Show SMILES C[C@@]12CCC[C@](C)([C@H]1CC[C@@]13C[C@H](CC[C@@H]21)C(=C)C3)C(O)=O |r| Show InChI InChI=1S/C20H30O2/c1-13-11-20-10-7-15-18(2,16(20)6-5-14(13)12-20)8-4-9-19(15,3)17(21)22/h14-16H,1,4-12H2,2-3H3,(H,21,22)/t14?,15-,16-,18+,19+,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3061-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.053
BindingDB Entry DOI: 10.7270/Q29024KF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50241797
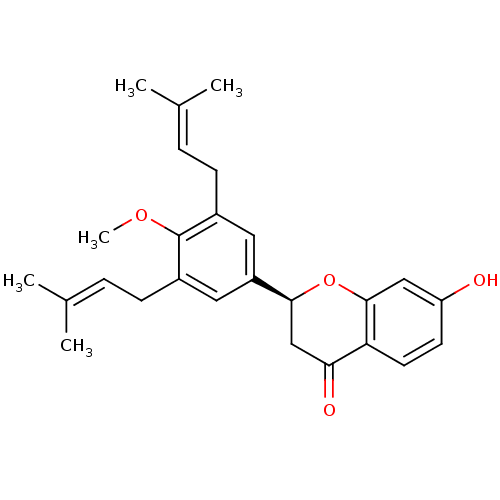
(CHEMBL470452 | abyssinone-IV-4'-O-methyl ether)Show SMILES [#6]-[#8]-c1c(-[#6]\[#6]=[#6](\[#6])-[#6])cc(cc1-[#6]\[#6]=[#6](\[#6])-[#6])-[#6@@H]-1-[#6]-[#6](=O)-c2ccc(-[#8])cc2-[#8]-1 |r| Show InChI InChI=1S/C26H30O4/c1-16(2)6-8-18-12-20(13-19(26(18)29-5)9-7-17(3)4)24-15-23(28)22-11-10-21(27)14-25(22)30-24/h6-7,10-14,24,27H,8-9,15H2,1-5H3/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
J Nat Prod 69: 1572-6 (2006)
Article DOI: 10.1021/np0601861
BindingDB Entry DOI: 10.7270/Q2B8591J |
More data for this
Ligand-Target Pair | |
1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase gamma-1
(Homo sapiens (Human)) | BDBM50269156

(3-beta-hydroxy-27-p-(E)-coumaroyloxyolean-12-en-28...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(CC(=O)C=Cc4ccc(O)cc4)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2C1)C(O)=O |r,w:12.11,c:22| Show InChI InChI=1S/C39H54O5/c1-34(2)19-20-38(33(43)44)21-22-39(23-27(41)12-9-25-7-10-26(40)11-8-25)28(29(38)24-34)13-14-31-36(5)17-16-32(42)35(3,4)30(36)15-18-37(31,39)6/h7-13,29-32,40,42H,14-24H2,1-6H3,(H,43,44)/t29-,30-,31+,32-,36-,37+,38-,39-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of PLCgamma1 (unknown origin) |
J Nat Prod 63: 753-6 (2000)
BindingDB Entry DOI: 10.7270/Q2JS9Q69 |
More data for this
Ligand-Target Pair | |
Fatty acid synthase
(Homo sapiens (Human)) | BDBM50191684
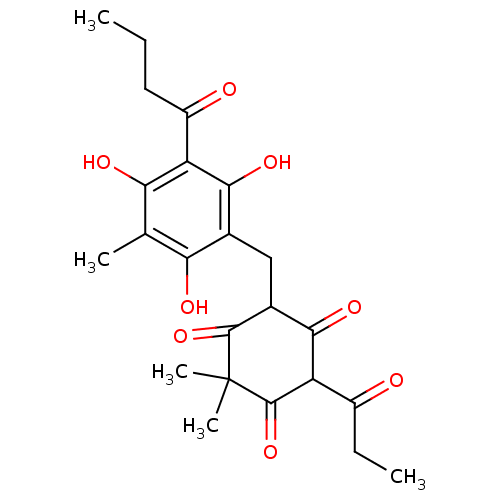
(CHEMBL213433 | flavaspidic acid-PB)Show SMILES CCCC(=O)c1c(O)c(C)c(O)c(CC2C(=O)C(C(=O)CC)C(=O)C(C)(C)C2=O)c1O Show InChI InChI=1S/C23H28O8/c1-6-8-14(25)15-18(27)10(3)17(26)11(19(15)28)9-12-20(29)16(13(24)7-2)22(31)23(4,5)21(12)30/h12,16,26-28H,6-9H2,1-5H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of FAS |
Bioorg Med Chem Lett 16: 4738-42 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.018
BindingDB Entry DOI: 10.7270/Q2QV3NB7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50097211

((1R,4aR,7S)-1,4a,7-Trimethyl-7-vinyl-1,2,3,4,4a,6,...)Show SMILES C[C@@]1(CC=C2C(CCC3[C@@](C)(CCC[C@@]23C)C(O)=O)C1)C=C |c:3| Show InChI InChI=1S/C20H30O2/c1-5-18(2)12-9-15-14(13-18)7-8-16-19(15,3)10-6-11-20(16,4)17(21)22/h5,9,14,16H,1,6-8,10-13H2,2-4H3,(H,21,22)/t14?,16?,18-,19-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3061-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.053
BindingDB Entry DOI: 10.7270/Q29024KF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data