 Found 1042 hits with Last Name = 'gude' and Initial = 'c'
Found 1042 hits with Last Name = 'gude' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aromatase
(Homo sapiens (Human)) | BDBM10044
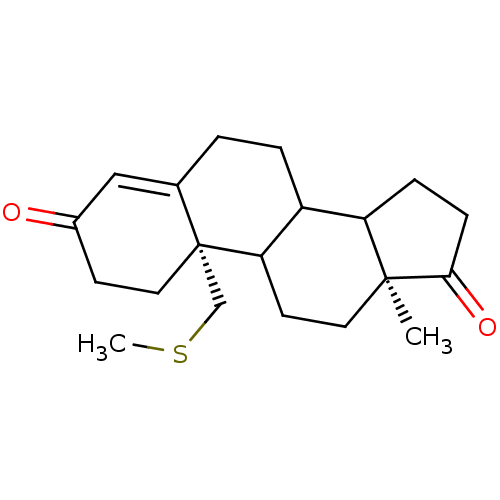
((2S,15S)-15-methyl-2-[(methylsulfanyl)methyl]tetra...)Show SMILES CSC[C@]12CCC(=O)C=C1CCC1C3CCC(=O)[C@@]3(C)CCC21 |r,c:8| Show InChI InChI=1S/C20H28O2S/c1-19-9-8-17-15(16(19)5-6-18(19)22)4-3-13-11-14(21)7-10-20(13,17)12-23-2/h11,15-17H,3-10,12H2,1-2H3/t15?,16?,17?,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Aromatase inhibitor potency as iron-binding-related type II difference spectrum |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsome cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50283607
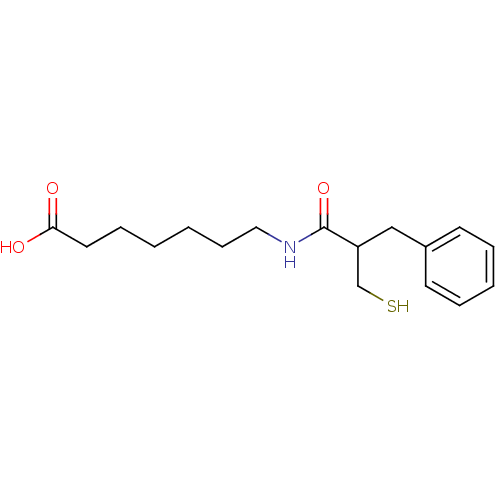
(7-(2-Mercaptomethyl-3-phenyl-propionylamino)-hepta...)Show InChI InChI=1S/C17H25NO3S/c19-16(20)10-6-1-2-7-11-18-17(21)15(13-22)12-14-8-4-3-5-9-14/h3-5,8-9,15,22H,1-2,6-7,10-13H2,(H,18,21)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition against neutral endopeptidase 24.11 (NEP) in rat kidney cortex membrane |
Bioorg Med Chem Lett 4: 2673-2676 (1994)
Article DOI: 10.1016/S0960-894X(01)80694-6
BindingDB Entry DOI: 10.7270/Q2X34XXT |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Competitive inhibition of human placental Cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
Aromatase inhibitor potency as iron-binding-related type II difference spectrum |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50149491
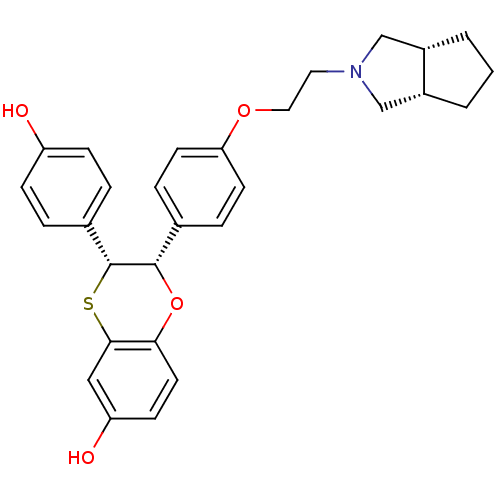
((2S,3R)-2-{4-[(3aS,6aR)-2-(Hexahydro-cyclopenta[c]...)Show SMILES Oc1ccc(cc1)[C@H]1Sc2cc(O)ccc2O[C@H]1c1ccc(OCCN2C[C@@H]3CCC[C@@H]3C2)cc1 Show InChI InChI=1S/C29H31NO4S/c31-23-8-4-20(5-9-23)29-28(34-26-13-10-24(32)16-27(26)35-29)19-6-11-25(12-7-19)33-15-14-30-17-21-2-1-3-22(21)18-30/h4-13,16,21-22,28-29,31-32H,1-3,14-15,17-18H2/t21-,22+,28-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding potency for human ER alpha |
Bioorg Med Chem Lett 14: 3861-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.074
BindingDB Entry DOI: 10.7270/Q2C24VW7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50149496

((2S,3R)-2-{4-[2-(2-Aza-spiro[3.3]hept-2-yl)-ethoxy...)Show SMILES Oc1ccc(cc1)[C@H]1Sc2cc(O)ccc2O[C@H]1c1ccc(OCCN2CC3(CCC3)C2)cc1 Show InChI InChI=1S/C28H29NO4S/c30-21-6-2-20(3-7-21)27-26(33-24-11-8-22(31)16-25(24)34-27)19-4-9-23(10-5-19)32-15-14-29-17-28(18-29)12-1-13-28/h2-11,16,26-27,30-31H,1,12-15,17-18H2/t26-,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding potency for human ER alpha |
Bioorg Med Chem Lett 14: 3861-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.074
BindingDB Entry DOI: 10.7270/Q2C24VW7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50149488

((2S,3R)-2-{4-[(R)-2-(2-Aza-bicyclo[3.1.0]hex-2-yl)...)Show SMILES Oc1ccc(cc1)[C@H]1Sc2cc(O)ccc2O[C@H]1c1ccc(OCCN2CC[C@@H]3C[C@H]23)cc1 Show InChI InChI=1S/C27H27NO4S/c29-20-5-1-18(2-6-20)27-26(32-24-10-7-21(30)16-25(24)33-27)17-3-8-22(9-4-17)31-14-13-28-12-11-19-15-23(19)28/h1-10,16,19,23,26-27,29-30H,11-15H2/t19-,23+,26+,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding potency for human ER alpha |
Bioorg Med Chem Lett 14: 3861-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.074
BindingDB Entry DOI: 10.7270/Q2C24VW7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50149493
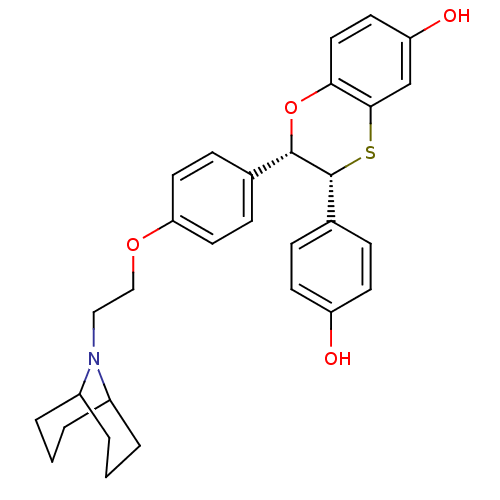
((2S,3R)-2-{4-[2-(9-Aza-bicyclo[3.3.1]non-9-yl)-eth...)Show SMILES Oc1ccc(cc1)[C@H]1Sc2cc(O)ccc2O[C@H]1c1ccc(OCCN2C3CCCC2CCC3)cc1 |THB:24:25:28.27.29:32.33.31| Show InChI InChI=1S/C30H33NO4S/c32-24-11-7-21(8-12-24)30-29(35-27-16-13-25(33)19-28(27)36-30)20-9-14-26(15-10-20)34-18-17-31-22-3-1-4-23(31)6-2-5-22/h7-16,19,22-23,29-30,32-33H,1-6,17-18H2/t22?,23?,29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding potency for human ER alpha |
Bioorg Med Chem Lett 14: 3861-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.074
BindingDB Entry DOI: 10.7270/Q2C24VW7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50149489
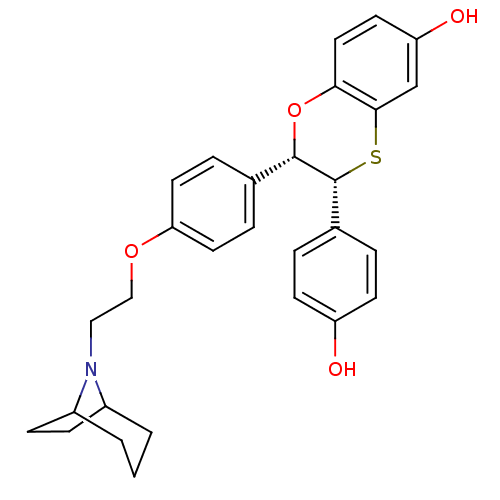
((2S,3R)-2-{4-[2-(8-Aza-bicyclo[3.2.1]oct-8-yl)-eth...)Show SMILES Oc1ccc(cc1)[C@H]1Sc2cc(O)ccc2O[C@H]1c1ccc(OCCN2C3CCC2CCC3)cc1 |TLB:24:25:27.28:31.30.32| Show InChI InChI=1S/C29H31NO4S/c31-23-10-4-20(5-11-23)29-28(34-26-15-12-24(32)18-27(26)35-29)19-6-13-25(14-7-19)33-17-16-30-21-2-1-3-22(30)9-8-21/h4-7,10-15,18,21-22,28-29,31-32H,1-3,8-9,16-17H2/t21?,22?,28-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding potency for human ER alpha |
Bioorg Med Chem Lett 14: 3861-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.074
BindingDB Entry DOI: 10.7270/Q2C24VW7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50149492
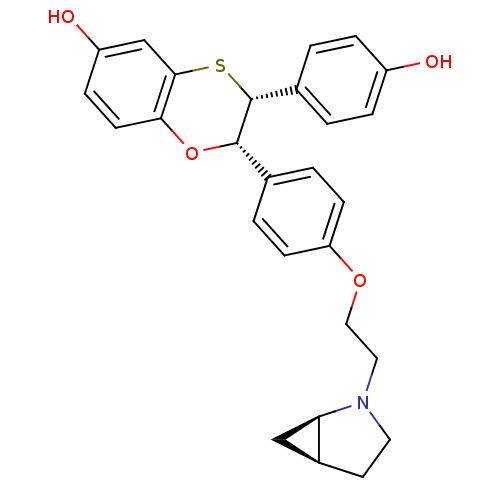
((2S,3R)-2-{4-[(1R,5S)-2-(2-Aza-bicyclo[3.1.0]hex-2...)Show SMILES Oc1ccc(cc1)[C@H]1Sc2cc(O)ccc2O[C@H]1c1ccc(OCCN2CC[C@H]3C[C@@H]23)cc1 Show InChI InChI=1S/C27H27NO4S/c29-20-5-1-18(2-6-20)27-26(32-24-10-7-21(30)16-25(24)33-27)17-3-8-22(9-4-17)31-14-13-28-12-11-19-15-23(19)28/h1-10,16,19,23,26-27,29-30H,11-15H2/t19-,23+,26-,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding potency for human ER alpha |
Bioorg Med Chem Lett 14: 3861-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.074
BindingDB Entry DOI: 10.7270/Q2C24VW7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50149484

((2S,3R)-2-{4-[2-(4-Aza-tricyclo[4.3.1.1*3,8*]undec...)Show SMILES Oc1ccc(cc1)[C@H]1Sc2cc(O)ccc2O[C@H]1c1ccc(OCCN2CC3CC4CC(C3)CC2C4)cc1 |TLB:35:34:32:29.28.30,THB:35:29:25.34.26.33:32,25:34:27.28.32:30,26:27:34.33.35:30,24:25:32:29.28.30| Show InChI InChI=1S/C32H35NO4S/c34-26-5-1-24(2-6-26)32-31(37-29-10-7-27(35)18-30(29)38-32)23-3-8-28(9-4-23)36-12-11-33-19-22-14-20-13-21(15-22)17-25(33)16-20/h1-10,18,20-22,25,31-32,34-35H,11-17,19H2/t20?,21?,22?,25?,31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding potency for human ER alpha |
Bioorg Med Chem Lett 14: 3861-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.074
BindingDB Entry DOI: 10.7270/Q2C24VW7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50149497

((2S,3R)-2-{4-[2-(3-Aza-bicyclo[3.1.0]hex-3-yl)-eth...)Show SMILES Oc1ccc(cc1)[C@H]1Sc2cc(O)ccc2O[C@H]1c1ccc(OCCN2CC3CC3C2)cc1 Show InChI InChI=1S/C27H27NO4S/c29-21-5-1-18(2-6-21)27-26(32-24-10-7-22(30)14-25(24)33-27)17-3-8-23(9-4-17)31-12-11-28-15-19-13-20(19)16-28/h1-10,14,19-20,26-27,29-30H,11-13,15-16H2/t19?,20?,26-,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding potency for human ER alpha |
Bioorg Med Chem Lett 14: 3861-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.074
BindingDB Entry DOI: 10.7270/Q2C24VW7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50149490
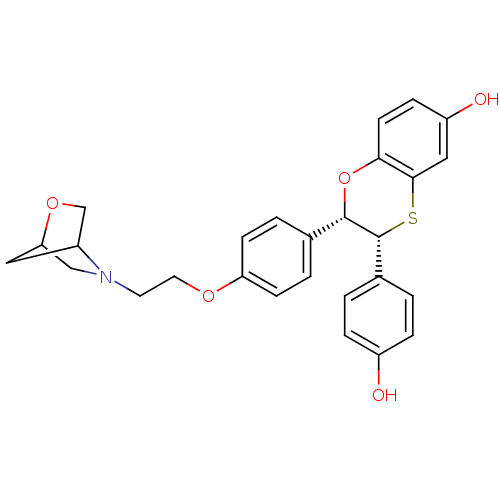
((2S,3R)-3-(4-Hydroxy-phenyl)-2-{4-[2-(2-oxa-5-aza-...)Show SMILES Oc1ccc(cc1)[C@H]1Sc2cc(O)ccc2O[C@H]1c1ccc(OCCN2CC3CC2CO3)cc1 |THB:24:25:28:31.30| Show InChI InChI=1S/C27H27NO5S/c29-20-5-1-18(2-6-20)27-26(33-24-10-7-21(30)14-25(24)34-27)17-3-8-22(9-4-17)31-12-11-28-15-23-13-19(28)16-32-23/h1-10,14,19,23,26-27,29-30H,11-13,15-16H2/t19?,23?,26-,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding potency for human ER alpha |
Bioorg Med Chem Lett 14: 3861-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.074
BindingDB Entry DOI: 10.7270/Q2C24VW7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50144849
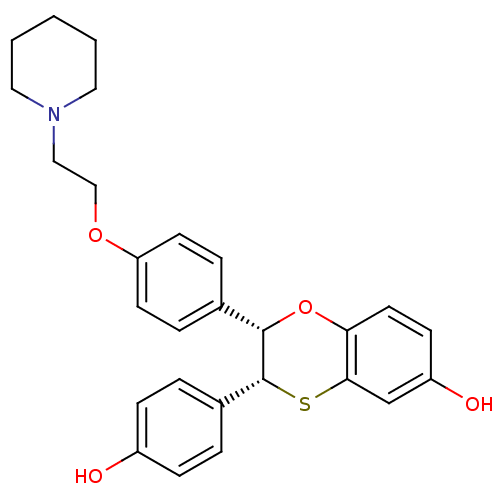
((2S,3R)-2-(4-(2-(PIPERIDIN-1-YL)ETHOXY)PHENYL)-2,3...)Show SMILES Oc1ccc(cc1)[C@H]1Sc2cc(O)ccc2O[C@H]1c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C27H29NO4S/c29-21-8-4-20(5-9-21)27-26(32-24-13-10-22(30)18-25(24)33-27)19-6-11-23(12-7-19)31-17-16-28-14-2-1-3-15-28/h4-13,18,26-27,29-30H,1-3,14-17H2/t26-,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding potency for human ER alpha |
Bioorg Med Chem Lett 14: 3861-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.074
BindingDB Entry DOI: 10.7270/Q2C24VW7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-lactamase
(Pseudomonas aeruginosa) | BDBM98812
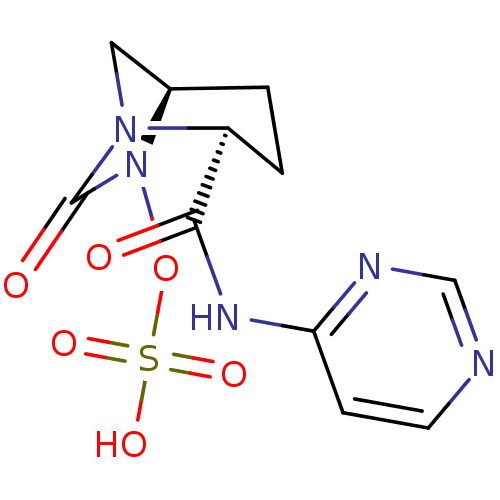
(US8487093, 204)Show SMILES OS(=O)(=O)ON1[C@H]2CN([C@@H](CC2)C(=O)Nc2ccncn2)C1=O |r| Show InChI InChI=1S/C11H13N5O6S/c17-10(14-9-3-4-12-6-13-9)8-2-1-7-5-15(8)11(18)16(7)22-23(19,20)21/h3-4,6-8H,1-2,5H2,(H,19,20,21)(H,12,13,14,17)/t7-,8+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. |
US Patent US8487093 (2013)
BindingDB Entry DOI: 10.7270/Q2057DKK |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50149487
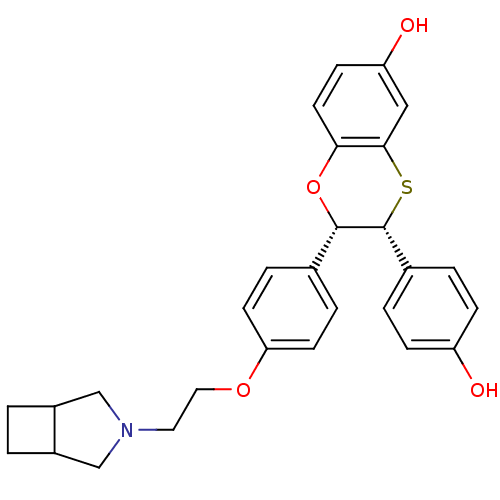
((2S,3R)-2-{4-[2-(3-Aza-bicyclo[3.2.0]hept-3-yl)-et...)Show SMILES Oc1ccc(cc1)[C@H]1Sc2cc(O)ccc2O[C@H]1c1ccc(OCCN2CC3CCC3C2)cc1 Show InChI InChI=1S/C28H29NO4S/c30-22-7-3-19(4-8-22)28-27(33-25-12-9-23(31)15-26(25)34-28)18-5-10-24(11-6-18)32-14-13-29-16-20-1-2-21(20)17-29/h3-12,15,20-21,27-28,30-31H,1-2,13-14,16-17H2/t20?,21?,27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding potency for human ER alpha |
Bioorg Med Chem Lett 14: 3861-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.074
BindingDB Entry DOI: 10.7270/Q2C24VW7 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Pseudomonas aeruginosa) | BDBM98802

(US8487093, 192)Show SMILES OS(=O)(=O)ON1[C@H]2CN([C@@H](CC2)C(=O)Nc2cc(ccn2)C2CCNCC2)C1=O |r| Show InChI InChI=1S/C17H23N5O6S/c23-16(20-15-9-12(5-8-19-15)11-3-6-18-7-4-11)14-2-1-13-10-21(14)17(24)22(13)28-29(25,26)27/h5,8-9,11,13-14,18H,1-4,6-7,10H2,(H,19,20,23)(H,25,26,27)/t13-,14+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. |
US Patent US8487093 (2013)
BindingDB Entry DOI: 10.7270/Q2057DKK |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50149494

((2S,3R)-2-{4-[2-(7-Aza-bicyclo[2.2.1]hept-7-yl)-et...)Show SMILES Oc1ccc(cc1)[C@H]1Sc2cc(O)ccc2O[C@H]1c1ccc(OCCN2C3CCC2CC3)cc1 |TLB:24:25:27.28:31.30| Show InChI InChI=1S/C28H29NO4S/c30-22-9-1-19(2-10-22)28-27(33-25-14-11-23(31)17-26(25)34-28)18-3-12-24(13-4-18)32-16-15-29-20-5-6-21(29)8-7-20/h1-4,9-14,17,20-21,27-28,30-31H,5-8,15-16H2/t20?,21?,27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding potency for human ER alpha |
Bioorg Med Chem Lett 14: 3861-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.074
BindingDB Entry DOI: 10.7270/Q2C24VW7 |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50003792
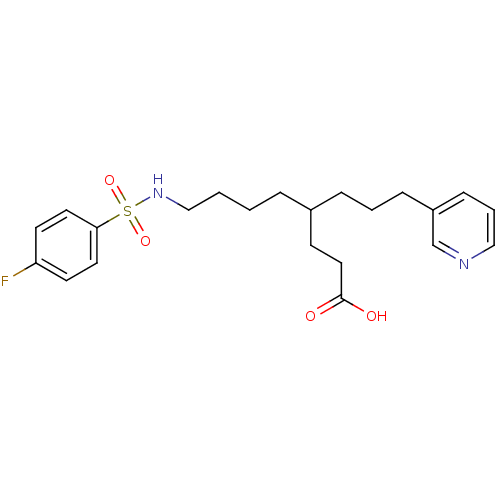
(8-(4-Fluoro-benzenesulfonylamino)-4-(3-pyridin-3-y...)Show SMILES OC(=O)CCC(CCCCNS(=O)(=O)c1ccc(F)cc1)CCCc1cccnc1 Show InChI InChI=1S/C22H29FN2O4S/c23-20-10-12-21(13-11-20)30(28,29)25-16-2-1-5-18(9-14-22(26)27)6-3-7-19-8-4-15-24-17-19/h4,8,10-13,15,17-18,25H,1-3,5-7,9,14,16H2,(H,26,27) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of Thromboxane A2 synthase |
J Med Chem 35: 4373-83 (1992)
BindingDB Entry DOI: 10.7270/Q2F47N3W |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50003784

(8-(4-Chloro-benzenesulfonylamino)-4-[3-(4-methyl-p...)Show SMILES Cc1ccncc1CCCC(CCCCNS(=O)(=O)c1ccc(Cl)cc1)CCC(O)=O Show InChI InChI=1S/C23H31ClN2O4S/c1-18-14-16-25-17-20(18)7-4-6-19(8-13-23(27)28)5-2-3-15-26-31(29,30)22-11-9-21(24)10-12-22/h9-12,14,16-17,19,26H,2-8,13,15H2,1H3,(H,27,28) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of Thromboxane A2 synthase |
J Med Chem 35: 4373-83 (1992)
BindingDB Entry DOI: 10.7270/Q2F47N3W |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50003780
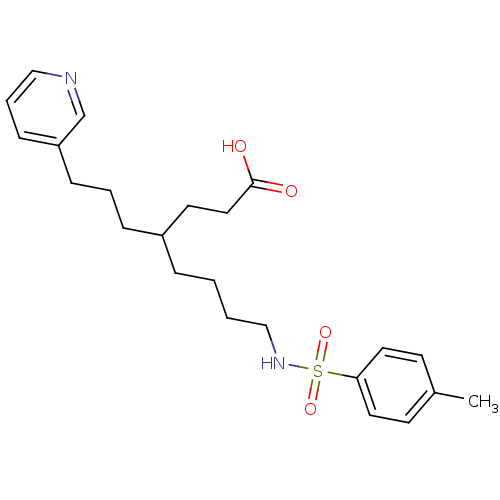
(4-(3-Pyridin-3-yl-propyl)-8-(toluene-4-sulfonylami...)Show SMILES Cc1ccc(cc1)S(=O)(=O)NCCCCC(CCCc1cccnc1)CCC(O)=O Show InChI InChI=1S/C23H32N2O4S/c1-19-10-13-22(14-11-19)30(28,29)25-17-3-2-6-20(12-15-23(26)27)7-4-8-21-9-5-16-24-18-21/h5,9-11,13-14,16,18,20,25H,2-4,6-8,12,15,17H2,1H3,(H,26,27) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of Thromboxane A2 synthase |
J Med Chem 35: 4373-83 (1992)
BindingDB Entry DOI: 10.7270/Q2F47N3W |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50149485
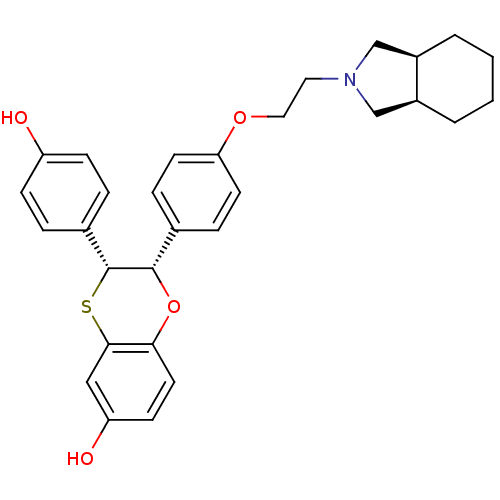
((2S,3R)-3-(4-Hydroxy-phenyl)-2-{4-[(6R,7S)-2-(octa...)Show SMILES Oc1ccc(cc1)[C@H]1Sc2cc(O)ccc2O[C@H]1c1ccc(OCCN2C[C@H]3CCCC[C@H]3C2)cc1 Show InChI InChI=1S/C30H33NO4S/c32-24-9-5-21(6-10-24)30-29(35-27-14-11-25(33)17-28(27)36-30)20-7-12-26(13-8-20)34-16-15-31-18-22-3-1-2-4-23(22)19-31/h5-14,17,22-23,29-30,32-33H,1-4,15-16,18-19H2/t22-,23+,29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding potency for human ER alpha |
Bioorg Med Chem Lett 14: 3861-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.074
BindingDB Entry DOI: 10.7270/Q2C24VW7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding potency for human ER beta |
Bioorg Med Chem Lett 14: 3861-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.074
BindingDB Entry DOI: 10.7270/Q2C24VW7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-lactamase
(Pseudomonas aeruginosa) | BDBM98861
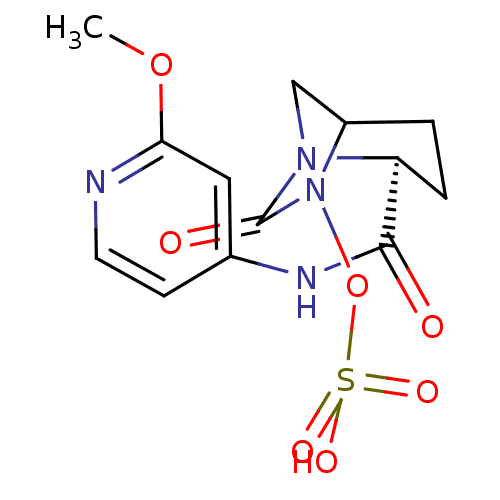
(US8487093, 7)Show SMILES COc1cc(NC(=O)[C@@H]2CCC3CN2C(=O)N3OS(O)(=O)=O)ccn1 |r| Show InChI InChI=1S/C13H16N4O7S/c1-23-11-6-8(4-5-14-11)15-12(18)10-3-2-9-7-16(10)13(19)17(9)24-25(20,21)22/h4-6,9-10H,2-3,7H2,1H3,(H,14,15,18)(H,20,21,22)/t9?,10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. |
US Patent US8487093 (2013)
BindingDB Entry DOI: 10.7270/Q2057DKK |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERbeta |
Bioorg Med Chem Lett 16: 834-8 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.014
BindingDB Entry DOI: 10.7270/Q2R78G0P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50017129

((S)-1-((S)-2-((R)-1-ethoxy-1-oxo-4-phenylbutan-2-y...)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C20H28N2O5/c1-3-27-20(26)16(12-11-15-8-5-4-6-9-15)21-14(2)18(23)22-13-7-10-17(22)19(24)25/h4-6,8-9,14,16-17,21H,3,7,10-13H2,1-2H3,(H,24,25)/t14-,16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition against angiotensin converting enzyme (ACE) |
Bioorg Med Chem Lett 4: 2673-2676 (1994)
Article DOI: 10.1016/S0960-894X(01)80694-6
BindingDB Entry DOI: 10.7270/Q2X34XXT |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant ERbeta by scintillation proximity assay |
Bioorg Med Chem Lett 17: 2944-8 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.053
BindingDB Entry DOI: 10.7270/Q2D50NS7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-lactamase
(Pseudomonas aeruginosa) | BDBM98792
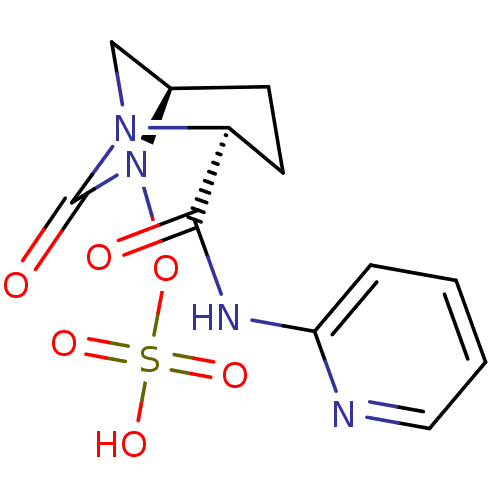
(US8487093, 180)Show SMILES OS(=O)(=O)ON1[C@H]2CN([C@@H](CC2)C(=O)Nc2ccccn2)C1=O |r| Show InChI InChI=1S/C12H14N4O6S/c17-11(14-10-3-1-2-6-13-10)9-5-4-8-7-15(9)12(18)16(8)22-23(19,20)21/h1-3,6,8-9H,4-5,7H2,(H,13,14,17)(H,19,20,21)/t8-,9+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. |
US Patent US8487093 (2013)
BindingDB Entry DOI: 10.7270/Q2057DKK |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity at human recombinant ERbeta |
Bioorg Med Chem Lett 17: 6295-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.001
BindingDB Entry DOI: 10.7270/Q2JQ11VT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50149486
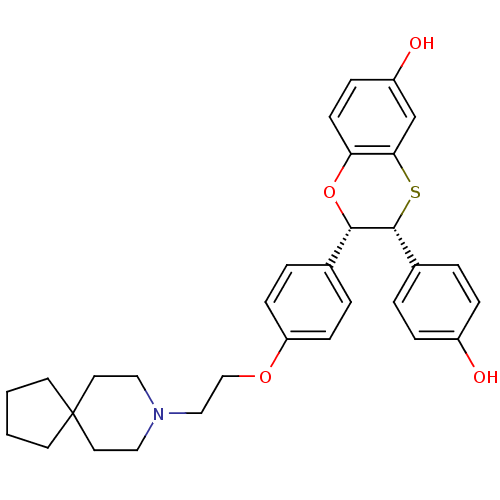
((2S,3R)-2-{4-[2-(8-Aza-spiro[4.5]dec-8-yl)-ethoxy]...)Show SMILES Oc1ccc(cc1)[C@H]1Sc2cc(O)ccc2O[C@H]1c1ccc(OCCN2CCC3(CCCC3)CC2)cc1 Show InChI InChI=1S/C31H35NO4S/c33-24-7-3-23(4-8-24)30-29(36-27-12-9-25(34)21-28(27)37-30)22-5-10-26(11-6-22)35-20-19-32-17-15-31(16-18-32)13-1-2-14-31/h3-12,21,29-30,33-34H,1-2,13-20H2/t29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding potency for human ER alpha |
Bioorg Med Chem Lett 14: 3861-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.074
BindingDB Entry DOI: 10.7270/Q2C24VW7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50149495

((2S,3R)-2-{4-[2-(2-Aza-bicyclo[2.2.2]oct-2-yl)-eth...)Show SMILES Oc1ccc(cc1)[C@H]1Sc2cc(O)ccc2O[C@H]1c1ccc(OCCN2CC3CCC2CC3)cc1 |wD:7.7,17.20,(2.33,3.78,;.99,3,;1.01,1.46,;-.33,.7,;-1.64,1.46,;-1.66,2.98,;-.35,3.76,;-2.98,.7,;-4.32,1.46,;-5.66,.67,;-6.97,1.43,;-8.31,.67,;-9.65,1.43,;-8.31,-.87,;-6.97,-1.63,;-5.63,-.87,;-4.32,-1.63,;-2.98,-.87,;-1.64,-1.63,;-.33,-.84,;1.01,-1.63,;1.01,-3.14,;2.35,-3.9,;3.68,-3.14,;3.68,-1.6,;5.07,-.79,;5.85,.55,;7.67,1.46,;9.38,.53,;8.56,-.73,;6.81,.23,;6.75,1.71,;7.59,2.65,;-.32,-3.93,;-1.64,-3.17,)| Show InChI InChI=1S/C29H31NO4S/c31-23-9-3-21(4-10-23)29-28(34-26-14-11-24(32)17-27(26)35-29)20-5-12-25(13-6-20)33-16-15-30-18-19-1-7-22(30)8-2-19/h3-6,9-14,17,19,22,28-29,31-32H,1-2,7-8,15-16,18H2/t19?,22?,28-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding potency for human ER alpha |
Bioorg Med Chem Lett 14: 3861-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.074
BindingDB Entry DOI: 10.7270/Q2C24VW7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding potency for human ER alpha |
Bioorg Med Chem Lett 14: 3861-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.074
BindingDB Entry DOI: 10.7270/Q2C24VW7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity at human recombinant ERalpha |
Bioorg Med Chem Lett 17: 6295-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.001
BindingDB Entry DOI: 10.7270/Q2JQ11VT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant ERalpha by scintillation proximity assay |
Bioorg Med Chem Lett 17: 2944-8 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.053
BindingDB Entry DOI: 10.7270/Q2D50NS7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human ERalpha |
Bioorg Med Chem Lett 16: 834-8 (2006)
Article DOI: 10.1016/j.bmcl.2005.11.014
BindingDB Entry DOI: 10.7270/Q2R78G0P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50003803

(8-(Naphthalene-2-sulfonylamino)-4-(3-pyridin-3-yl-...)Show SMILES OC(=O)CCC(CCCCNS(=O)(=O)c1ccc2ccccc2c1)CCCc1cccnc1 Show InChI InChI=1S/C26H32N2O4S/c29-26(30)16-13-21(8-5-9-22-10-6-17-27-20-22)7-3-4-18-28-33(31,32)25-15-14-23-11-1-2-12-24(23)19-25/h1-2,6,10-12,14-15,17,19-21,28H,3-5,7-9,13,16,18H2,(H,29,30) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of Thromboxane A2 synthase |
J Med Chem 35: 4373-83 (1992)
BindingDB Entry DOI: 10.7270/Q2F47N3W |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM272063

(US10065945, Example 57)Show SMILES C[C@@H](N1CCc2ccc(cc2C1)S(=O)(=O)Nc1nc(no1)C(=O)N1CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C24H25F2N5O4S/c1-15(17-10-19(25)13-20(26)11-17)31-9-6-16-4-5-21(12-18(16)14-31)36(33,34)29-24-27-22(28-35-24)23(32)30-7-2-3-8-30/h4-5,10-13,15H,2-3,6-9,14H2,1H3,(H,27,28,29)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
A solution of 1 M LiHMDS in THF (51.7 ml, 51.7 mmol) was added dropwise to a mixture of Intermediate 3 (7.5 g, 17.2 mmol) and 5-(tert-butyl)-4-methyl... |
US Patent US10065945 (2018)
BindingDB Entry DOI: 10.7270/Q24X59TJ |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Klebsiella pneumoniae) | BDBM98862
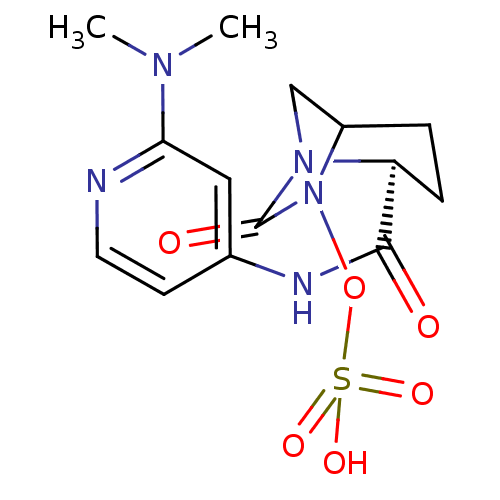
(US8487093, 8)Show SMILES CN(C)c1cc(NC(=O)[C@@H]2CCC3CN2C(=O)N3OS(O)(=O)=O)ccn1 |r| Show InChI InChI=1S/C14H19N5O6S/c1-17(2)12-7-9(5-6-15-12)16-13(20)11-4-3-10-8-18(11)14(21)19(10)25-26(22,23)24/h5-7,10-11H,3-4,8H2,1-2H3,(H,15,16,20)(H,22,23,24)/t10?,11-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. |
US Patent US8487093 (2013)
BindingDB Entry DOI: 10.7270/Q2057DKK |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Pseudomonas aeruginosa) | BDBM98862

(US8487093, 8)Show SMILES CN(C)c1cc(NC(=O)[C@@H]2CCC3CN2C(=O)N3OS(O)(=O)=O)ccn1 |r| Show InChI InChI=1S/C14H19N5O6S/c1-17(2)12-7-9(5-6-15-12)16-13(20)11-4-3-10-8-18(11)14(21)19(10)25-26(22,23)24/h5-7,10-11H,3-4,8H2,1-2H3,(H,15,16,20)(H,22,23,24)/t10?,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. |
US Patent US8487093 (2013)
BindingDB Entry DOI: 10.7270/Q2057DKK |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19441

(2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...)Show SMILES Oc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H27NO4S/c30-21-8-4-20(5-9-21)28-26(24-13-10-22(31)18-25(24)34-28)27(32)19-6-11-23(12-7-19)33-17-16-29-14-2-1-3-15-29/h4-13,18,30-31H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding potency for human ER alpha |
Bioorg Med Chem Lett 14: 3861-4 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.074
BindingDB Entry DOI: 10.7270/Q2C24VW7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-lactamase
(Pseudomonas aeruginosa) | BDBM98819
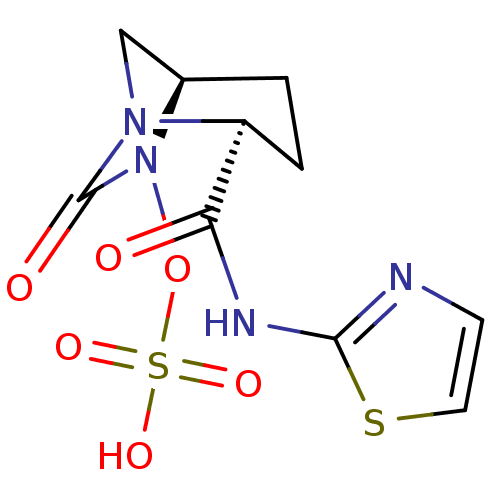
(US8487093, 212)Show SMILES OS(=O)(=O)ON1[C@H]2CN([C@@H](CC2)C(=O)Nc2nccs2)C1=O |r| Show InChI InChI=1S/C10H12N4O6S2/c15-8(12-9-11-3-4-21-9)7-2-1-6-5-13(7)10(16)14(6)20-22(17,18)19/h3-4,6-7H,1-2,5H2,(H,11,12,15)(H,17,18,19)/t6-,7+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. |
US Patent US8487093 (2013)
BindingDB Entry DOI: 10.7270/Q2057DKK |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50003781
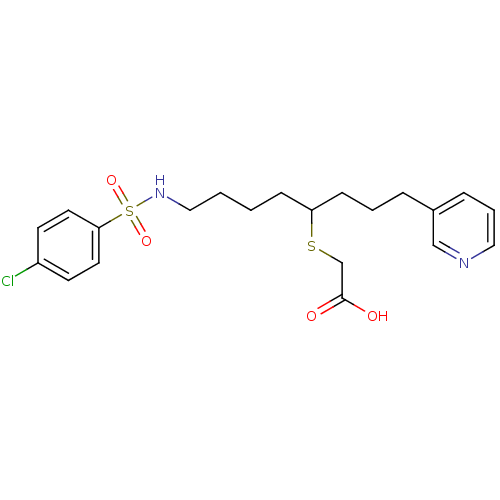
(CHEMBL341686 | [5-(4-Chloro-benzenesulfonylamino)-...)Show SMILES OC(=O)CSC(CCCCNS(=O)(=O)c1ccc(Cl)cc1)CCCc1cccnc1 Show InChI InChI=1S/C21H27ClN2O4S2/c22-18-9-11-20(12-10-18)30(27,28)24-14-2-1-7-19(29-16-21(25)26)8-3-5-17-6-4-13-23-15-17/h4,6,9-13,15,19,24H,1-3,5,7-8,14,16H2,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of Thromboxane A2 synthase |
J Med Chem 35: 4373-83 (1992)
BindingDB Entry DOI: 10.7270/Q2F47N3W |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50003776
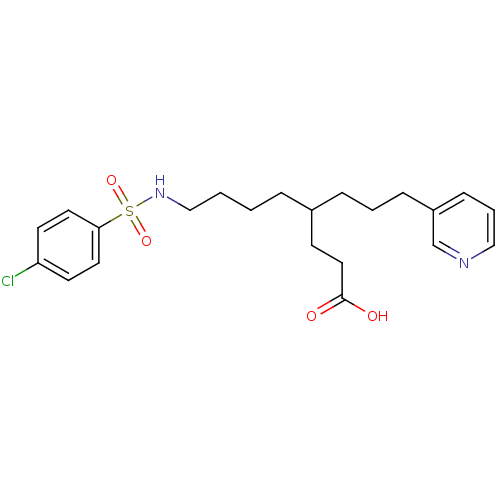
(8-(4-Chloro-benzenesulfonylamino)-4-(3-pyridin-3-y...)Show SMILES OC(=O)CCC(CCCCNS(=O)(=O)c1ccc(Cl)cc1)CCCc1cccnc1 Show InChI InChI=1S/C22H29ClN2O4S/c23-20-10-12-21(13-11-20)30(28,29)25-16-2-1-5-18(9-14-22(26)27)6-3-7-19-8-4-15-24-17-19/h4,8,10-13,15,17-18,25H,1-3,5-7,9,14,16H2,(H,26,27) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of thromboxane synthasase in microsomal platelet preparation |
J Med Chem 36: 205-10 (1993)
BindingDB Entry DOI: 10.7270/Q2XS5TFK |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Klebsiella pneumoniae) | BDBM98847
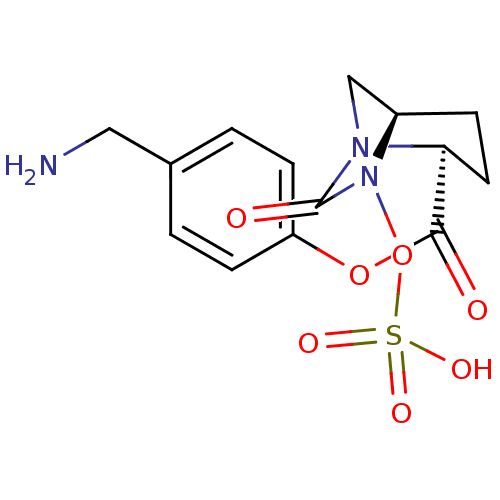
(US8487093, 266)Show SMILES NCc1ccc(OC(=O)[C@@H]2CC[C@@H]3CN2C(=O)N3OS(O)(=O)=O)cc1 |r| Show InChI InChI=1S/C14H17N3O7S/c15-7-9-1-4-11(5-2-9)23-13(18)12-6-3-10-8-16(12)14(19)17(10)24-25(20,21)22/h1-2,4-5,10,12H,3,6-8,15H2,(H,20,21,22)/t10-,12+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. |
US Patent US8487093 (2013)
BindingDB Entry DOI: 10.7270/Q2057DKK |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Pseudomonas aeruginosa) | BDBM98799
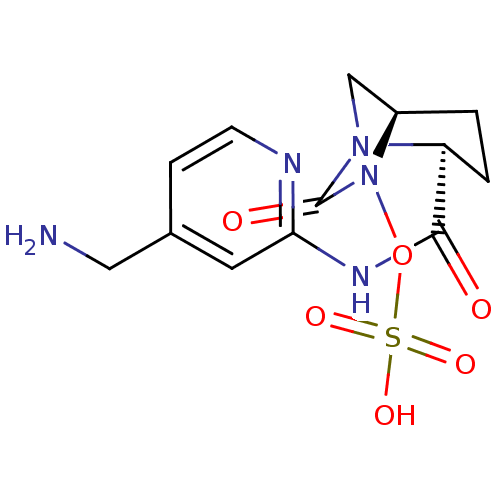
(US8487093, 188)Show SMILES NCc1ccnc(NC(=O)[C@@H]2CC[C@@H]3CN2C(=O)N3OS(O)(=O)=O)c1 |r| Show InChI InChI=1S/C13H17N5O6S/c14-6-8-3-4-15-11(5-8)16-12(19)10-2-1-9-7-17(10)13(20)18(9)24-25(21,22)23/h3-5,9-10H,1-2,6-7,14H2,(H,15,16,19)(H,21,22,23)/t9-,10+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. |
US Patent US8487093 (2013)
BindingDB Entry DOI: 10.7270/Q2057DKK |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Pseudomonas aeruginosa) | BDBM98806
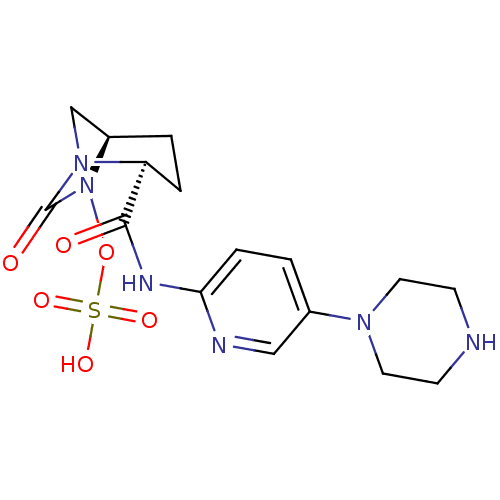
(US8487093, 196)Show SMILES OS(=O)(=O)ON1[C@H]2CN([C@@H](CC2)C(=O)Nc2ccc(cn2)N2CCNCC2)C1=O |r| Show InChI InChI=1S/C16H22N6O6S/c23-15(19-14-4-2-11(9-18-14)20-7-5-17-6-8-20)13-3-1-12-10-21(13)16(24)22(12)28-29(25,26)27/h2,4,9,12-13,17H,1,3,5-8,10H2,(H,18,19,23)(H,25,26,27)/t12-,13+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. |
US Patent US8487093 (2013)
BindingDB Entry DOI: 10.7270/Q2057DKK |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50368174

(CHEMBL1203762)Show InChI InChI=1S/C13H11N3/c14-7-10-1-3-11(4-2-10)13-6-5-12-8-15-9-16(12)13/h1-4,8-9,13H,5-6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of cytochrome P450 19A1 |
J Med Chem 34: 725-36 (1991)
BindingDB Entry DOI: 10.7270/Q2SB46BP |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50003776

(8-(4-Chloro-benzenesulfonylamino)-4-(3-pyridin-3-y...)Show SMILES OC(=O)CCC(CCCCNS(=O)(=O)c1ccc(Cl)cc1)CCCc1cccnc1 Show InChI InChI=1S/C22H29ClN2O4S/c23-20-10-12-21(13-11-20)30(28,29)25-16-2-1-5-18(9-14-22(26)27)6-3-7-19-8-4-15-24-17-19/h4,8,10-13,15,17-18,25H,1-3,5-7,9,14,16H2,(H,26,27) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for its inhibitory activity against thromboxane synthase A2 (TXA2) |
J Med Chem 35: 4366-72 (1992)
BindingDB Entry DOI: 10.7270/Q2JW8CVB |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50003801

(4-(3-Pyridin-3-yl-propyl)-8-(4-trifluoromethyl-ben...)Show SMILES OC(=O)CCC(CCCCNS(=O)(=O)c1ccc(cc1)C(F)(F)F)CCCc1cccnc1 Show InChI InChI=1S/C23H29F3N2O4S/c24-23(25,26)20-10-12-21(13-11-20)33(31,32)28-16-2-1-5-18(9-14-22(29)30)6-3-7-19-8-4-15-27-17-19/h4,8,10-13,15,17-18,28H,1-3,5-7,9,14,16H2,(H,29,30) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation
Curated by ChEMBL
| Assay Description
In vitro inhibition of Thromboxane A2 synthase |
J Med Chem 35: 4373-83 (1992)
BindingDB Entry DOI: 10.7270/Q2F47N3W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data