

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (2019-nCoV) | BDBM423466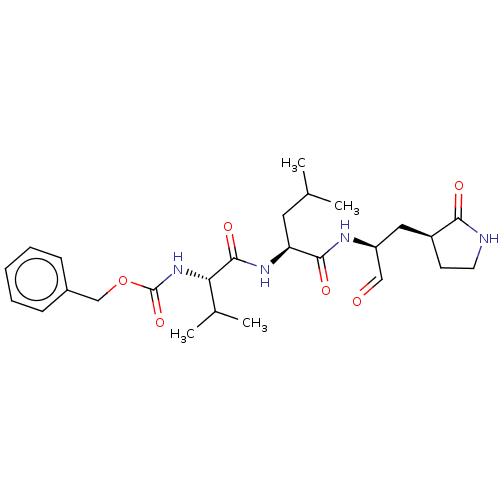 (WO2006061714, P39.1 | WO2006061714-ID-11 | cmdc.20...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.30 | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM442754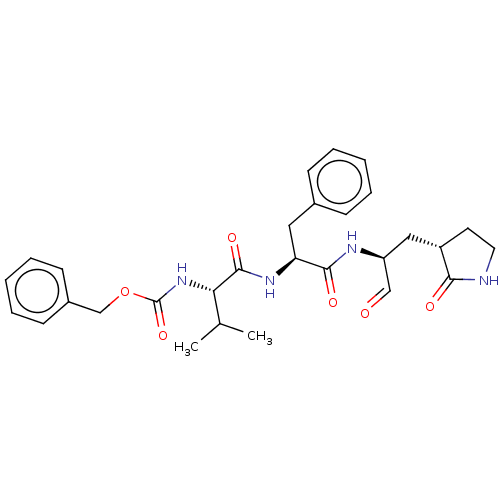 (MPI4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM420296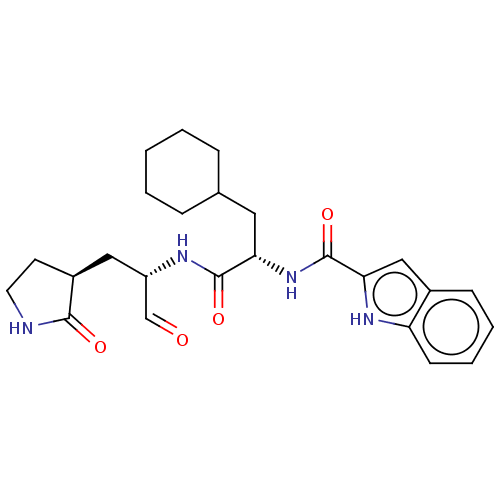 (Advanced SARS-CoV-2 Inhibitor 11a | MPI10 | acs.jm...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 30 | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM419133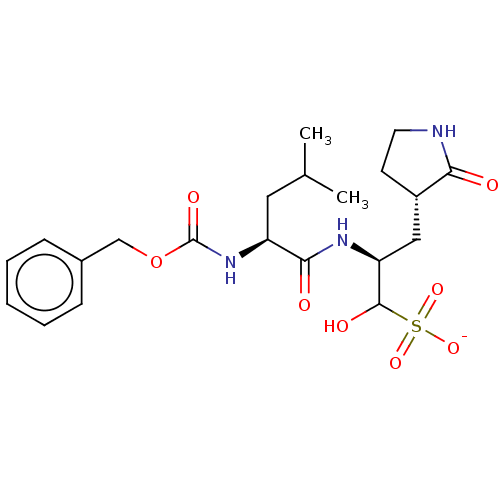 (BDBM429386 | GC376) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 30 | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM442755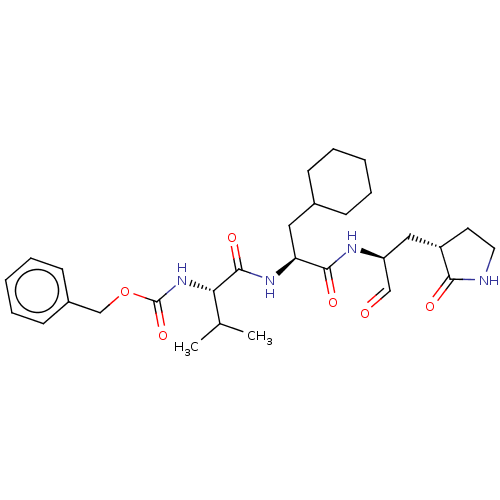 (MPI5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM442792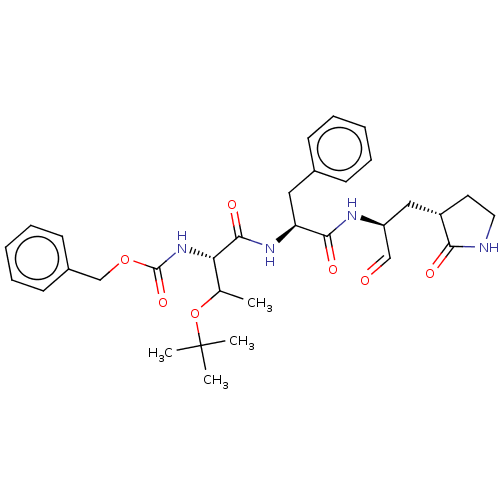 (MPI7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 46 | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM442794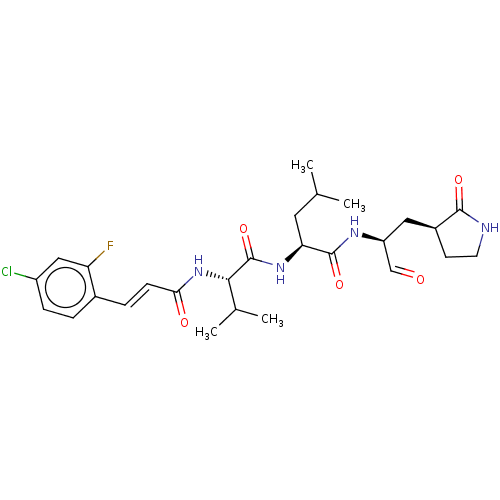 (MPI9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 55 | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM442762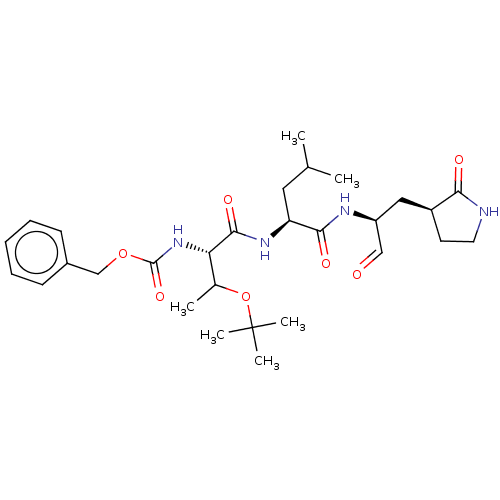 (MPI6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 59 | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM442746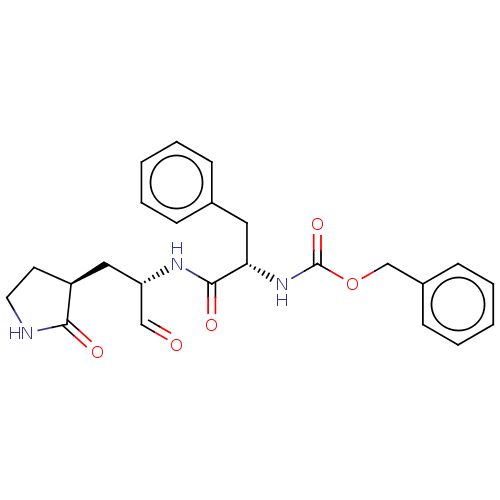 (MPI1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 98 | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM429372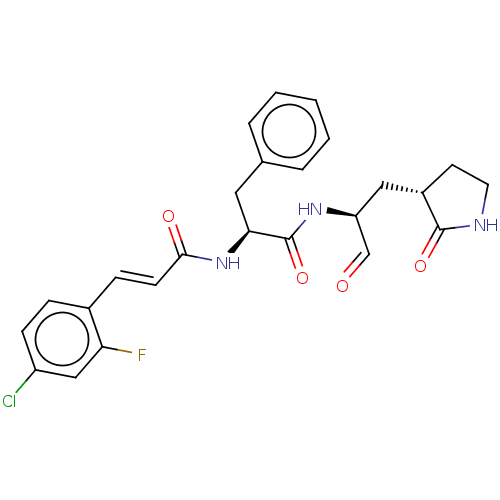 (MPI2 | med.21724, Compound 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 101 | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM429100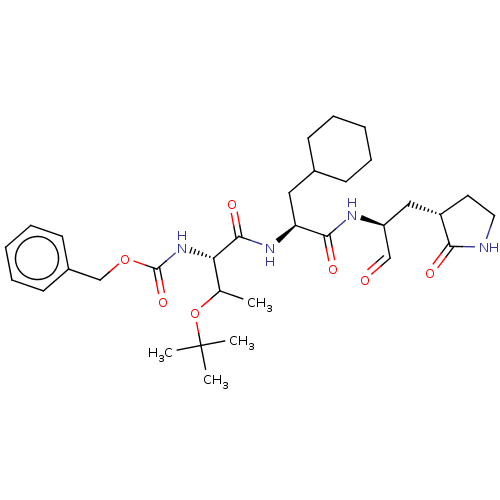 (MPI8 | jm5b01461, Compound 45) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 103 | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University | Assay Description The assays were carried out with 20 nM enzyme (except for MPI3, for which 10 nM enzyme was used) and 10 μM substrate at 37oC with continuous sha... | ChemMedChem (2020) Article DOI: 10.1002/cmdc.202000924 BindingDB Entry DOI: 10.7270/Q2G163V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50089657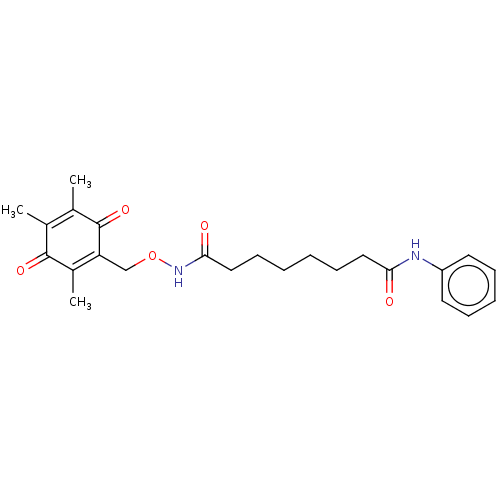 (CHEMBL3577298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity to human Zn2+-HDAC8 assessed as loss of activity by Fluor-de-Lys activity assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50089657 (CHEMBL3577298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Reversible-time dependent inhibition of human wild type HDAC8 by Fluor-de-Lys activity assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using MAZ1600 as fluorogenic substrate measured every 5 mins by optimized homogenous fluorescence based assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50089657 (CHEMBL3577298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using MAZ1600 as fluorogenic substrate measured every 5 mins by optimized homogenous fluorescence based assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using MAZ1600 as fluorogenic substrate measured every 5 mins by optimized homogenous fluorescence based assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC3 using MAZ1600 as fluorogenic substrate measured every 5 mins by optimized homogenous fluorescence based assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using MAZ1600 as fluorogenic substrate measured every 5 mins by optimized homogenous fluorescence based assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50089657 (CHEMBL3577298) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC3 using MAZ1600 as fluorogenic substrate measured every 5 mins by optimized homogenous fluorescence based assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50089657 (CHEMBL3577298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 255 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using MAZ1600 as fluorogenic substrate measured every 5 mins by optimized homogenous fluorescence based assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50089657 (CHEMBL3577298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 371 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using MAZ1600 as fluorogenic substrate measured every 5 mins by optimized homogenous fluorescence based assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC8 using MAZ1675 as fluorogenic substrate measured every 5 mins by optimized homogenous fluorescence based assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50074938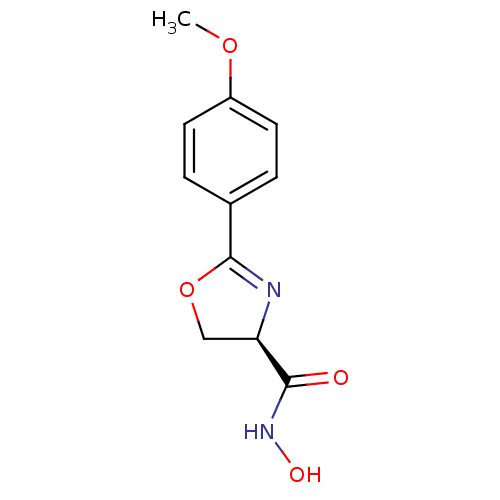 ((R)-2-(4-Methoxy-phenyl)-4,5-dihydro-oxazole-4-car...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50089657 (CHEMBL3577298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC8 using MAZ1675 as fluorogenic substrate measured every 5 mins by optimized homogenous fluorescence based assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50118596 ((R/S)-[3-(4-Methoxy-phenyl)-4,5-dihydro-isoxazol-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50409600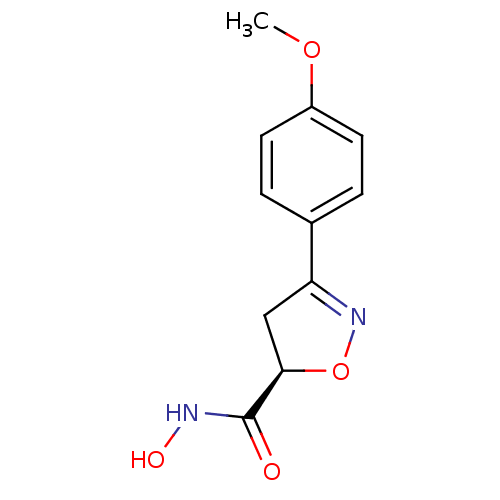 (CHEMBL2111931) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50089658 (CHEMBL3577299) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using MAZ1600 as fluorogenic substrate measured every 5 mins by optimized homogenous fluorescence based assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50089658 (CHEMBL3577299) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 using MAZ1600 as fluorogenic substrate measured every 5 mins by optimized homogenous fluorescence based assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50089658 (CHEMBL3577299) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC3 using MAZ1600 as fluorogenic substrate measured every 5 mins by optimized homogenous fluorescence based assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50089658 (CHEMBL3577299) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC8 using MAZ1675 as fluorogenic substrate measured every 5 mins by optimized homogenous fluorescence based assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50089658 (CHEMBL3577299) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using MAZ1600 as fluorogenic substrate measured every 5 mins by optimized homogenous fluorescence based assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50118597 ((R/S)-Thioacetic acid S-{2-[3-(4-methoxy-phenyl)-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50118582 ((R)3-(4-Methoxy-phenyl)-4,5-dihydro-isoxazole-5-ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50118590 ((R/S)-[3-(4-Methoxy-phenyl)-4,5-dihydro-isoxazol-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50118594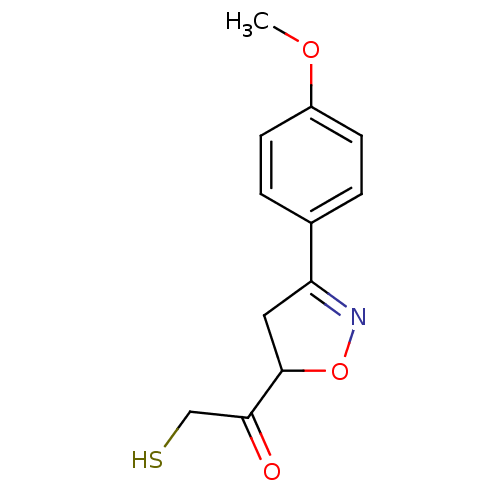 ((R/S)-2-Mercapto-1-[3-(4-methoxy-phenyl)-4,5-dihyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50118584 ((R/S)-[3-(4-Methoxy-phenyl)-4,5-dihydro-isoxazol-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50118581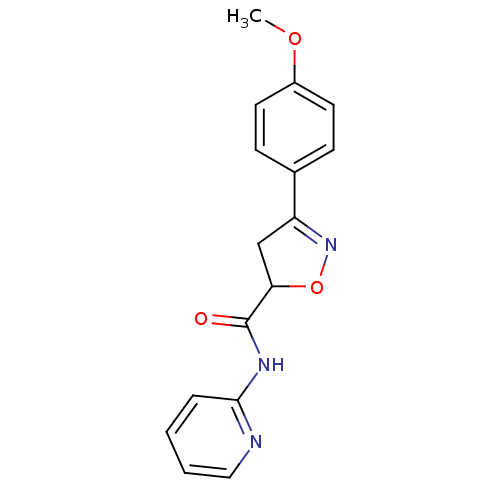 ((R/S)-3-(4-Methoxy-phenyl)-4,5-dihydro-isoxazole-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >3.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50118595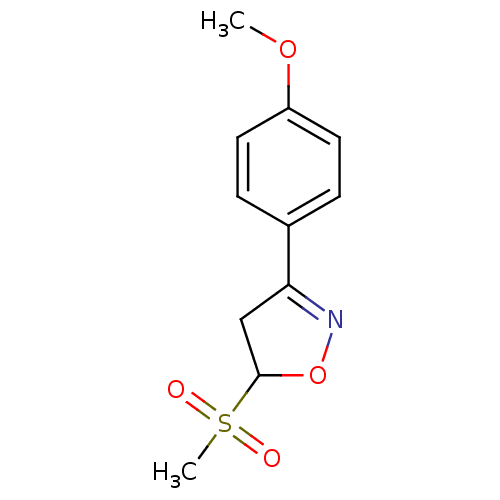 ((R/S)-5-Methanesulfonyl-3-(4-methoxy-phenyl)-4,5-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50118587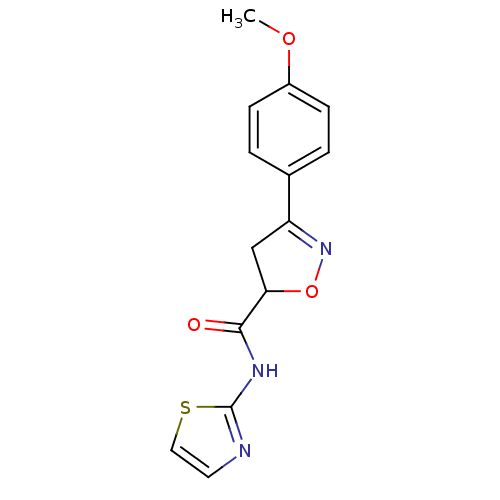 ((R/S)-3-(4-Methoxy-phenyl)-4,5-dihydro-isoxazole-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >3.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50118585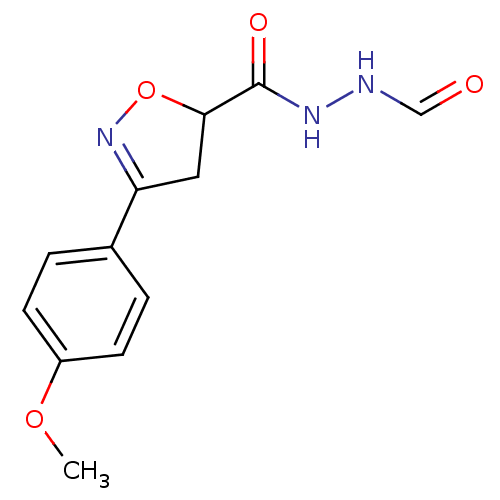 ((R/S)-3-(4-Methoxy-phenyl)-4,5-dihydro-isoxazole-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50118593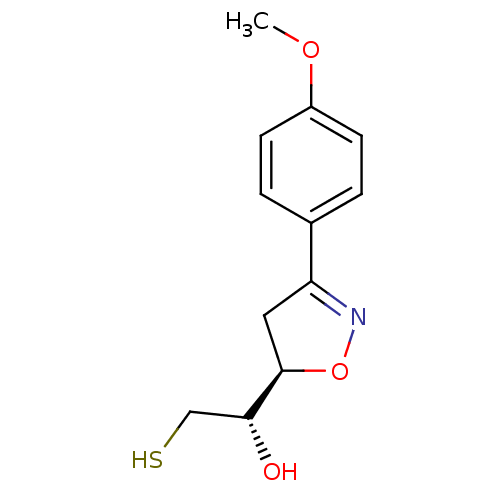 ((R*,R*)2-Mercapto-1-[3-(4-methoxy-phenyl)-4,5-dihy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50118586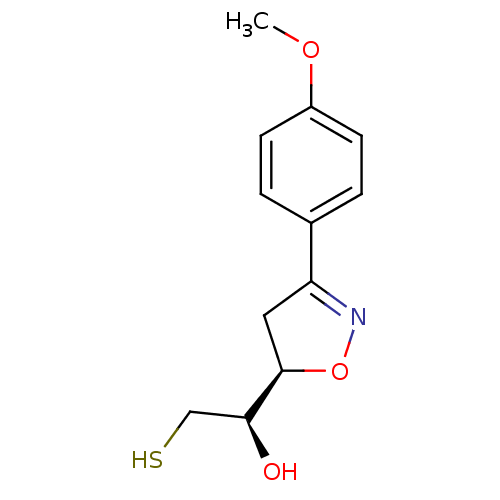 ((R*,S*)-2-Mercapto-1-[3-(4-methoxy-phenyl)-4,5-dih...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50118583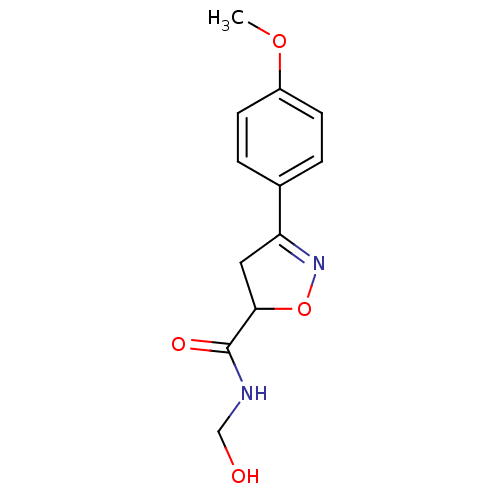 ((R/S)-3-(4-Methoxy-phenyl)-4,5-dihydro-isoxazole-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50118592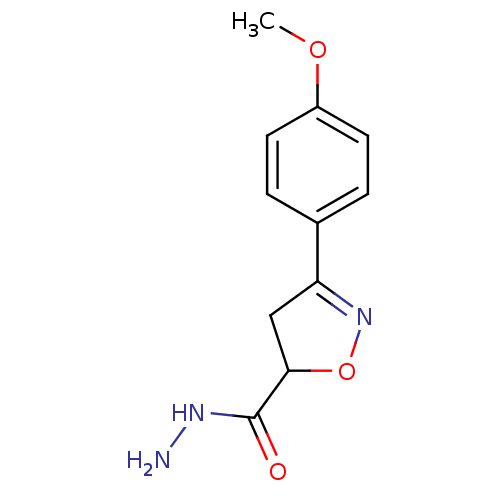 ((R/S)-3-(4-Methoxy-phenyl)-4,5-dihydro-isoxazole-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >4.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50118591 ((R/S)-3-(4-Methoxy-phenyl)-4,5-dihydro-isoxazole-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >4.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50118580 ((R/S)-3-(4-Methoxy-phenyl)-4,5-dihydro-isoxazole-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||