 Found 366 hits with Last Name = 'kostlan' and Initial = 'cr'
Found 366 hits with Last Name = 'kostlan' and Initial = 'cr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Caspase-1
(Homo sapiens (Human)) | BDBM50139695

(3-((S)-2-{(S)-2-[2-((S)-Acetylamino)-3-(4-hydroxy-...)Show SMILES C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)NC(CC(O)=O)C(=O)CNS(=O)(=O)CCc1ccccc1 Show InChI InChI=1S/C32H41N5O12S/c1-19(30(45)37-25(17-29(43)44)27(40)18-33-50(48,49)15-14-21-6-4-3-5-7-21)34-31(46)24(12-13-28(41)42)36-32(47)26(35-20(2)38)16-22-8-10-23(39)11-9-22/h3-11,19,24-26,33,39H,12-18H2,1-2H3,(H,34,46)(H,35,38)(H,36,47)(H,37,45)(H,41,42)(H,43,44)/t19-,24-,25?,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against caspase-1 |
Bioorg Med Chem Lett 14: 809-12 (2004)
BindingDB Entry DOI: 10.7270/Q2FB52CR |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50139702
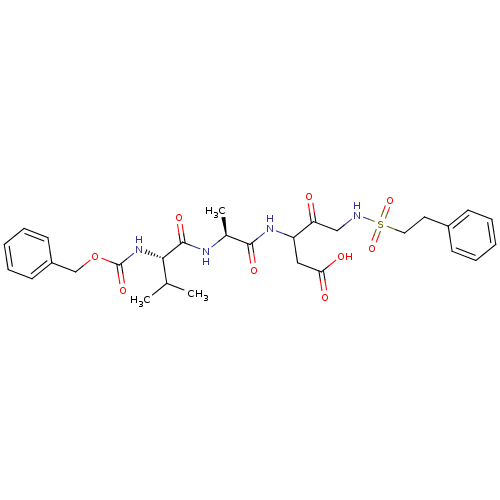
(3-[(S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-bu...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)CNS(=O)(=O)CCc1ccccc1 Show InChI InChI=1S/C29H38N4O9S/c1-19(2)26(33-29(39)42-18-22-12-8-5-9-13-22)28(38)31-20(3)27(37)32-23(16-25(35)36)24(34)17-30-43(40,41)15-14-21-10-6-4-7-11-21/h4-13,19-20,23,26,30H,14-18H2,1-3H3,(H,31,38)(H,32,37)(H,33,39)(H,35,36)/t20-,23?,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against caspase-1 |
Bioorg Med Chem Lett 14: 809-12 (2004)
BindingDB Entry DOI: 10.7270/Q2FB52CR |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM12197
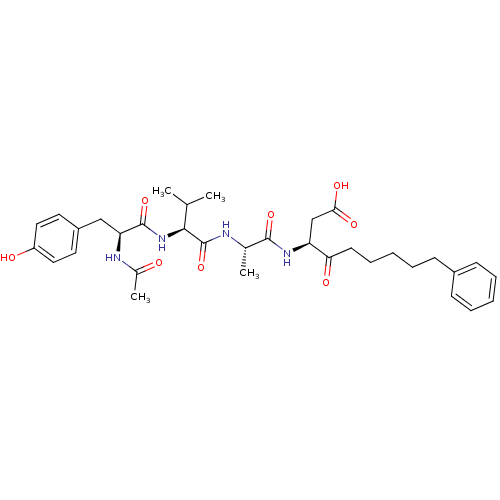
((3S)-3-[(2S)-2-[(2S)-2-[(2S)-2-acetamido-3-(4-hydr...)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)CCCCCc1ccccc1 |r| Show InChI InChI=1S/C34H46N4O8/c1-21(2)31(38-33(45)28(36-23(4)39)19-25-15-17-26(40)18-16-25)34(46)35-22(3)32(44)37-27(20-30(42)43)29(41)14-10-6-9-13-24-11-7-5-8-12-24/h5,7-8,11-12,15-18,21-22,27-28,31,40H,6,9-10,13-14,19-20H2,1-4H3,(H,35,46)(H,36,39)(H,37,44)(H,38,45)(H,42,43)/t22-,27-,28-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | -47.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer
| Assay Description
The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... |
Bioorg Med Chem 10: 31-40 (2002)
Article DOI: 10.1016/s0968-0896(01)00250-4
BindingDB Entry DOI: 10.7270/Q2BP011G |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50091585

(3-((S)-2-{(S)-2-[2-((S)-Acetylamino)-3-(4-hydroxy-...)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)CCCCCc1ccccc1 Show InChI InChI=1S/C34H46N4O8/c1-21(2)31(38-33(45)28(36-23(4)39)19-25-15-17-26(40)18-16-25)34(46)35-22(3)32(44)37-27(20-30(42)43)29(41)14-10-6-9-13-24-11-7-5-8-12-24/h5,7-8,11-12,15-18,21-22,27-28,31,40H,6,9-10,13-14,19-20H2,1-4H3,(H,35,46)(H,36,39)(H,37,44)(H,38,45)(H,42,43)/t22-,27?,28-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against caspase-1 |
Bioorg Med Chem Lett 14: 809-12 (2004)
BindingDB Entry DOI: 10.7270/Q2FB52CR |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50139699
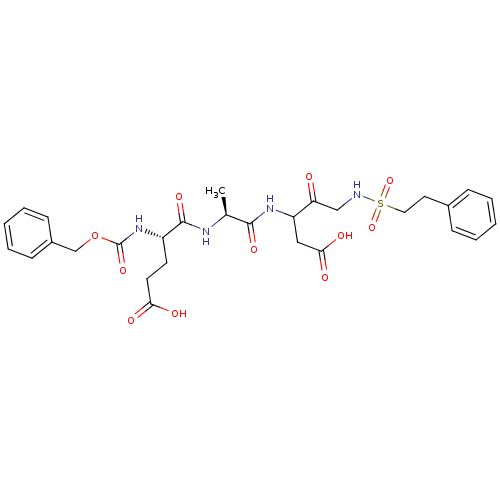
(3-[(S)-2-((S)-2-Benzyloxycarbonylamino-4-carboxy-b...)Show SMILES C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CNS(=O)(=O)CCc1ccccc1 Show InChI InChI=1S/C29H36N4O11S/c1-19(31-28(40)22(12-13-25(35)36)33-29(41)44-18-21-10-6-3-7-11-21)27(39)32-23(16-26(37)38)24(34)17-30-45(42,43)15-14-20-8-4-2-5-9-20/h2-11,19,22-23,30H,12-18H2,1H3,(H,31,40)(H,32,39)(H,33,41)(H,35,36)(H,37,38)/t19-,22-,23?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against caspase-1 |
Bioorg Med Chem Lett 14: 809-12 (2004)
BindingDB Entry DOI: 10.7270/Q2FB52CR |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50005795
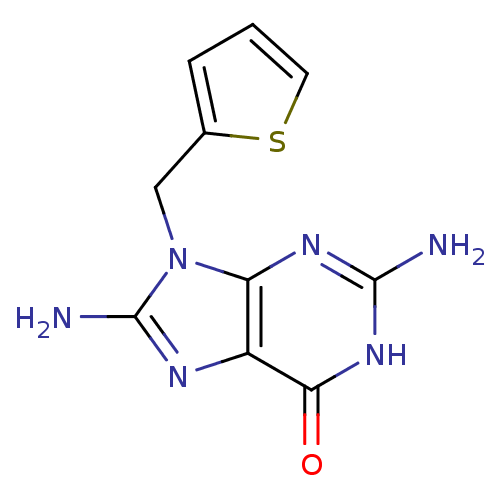
(2,8-Diamino-9-thiophen-2-ylmethyl-1,9-dihydro-puri...)Show InChI InChI=1S/C10H10N6OS/c11-9-14-7-6(8(17)15-9)13-10(12)16(7)4-5-2-1-3-18-5/h1-3H,4H2,(H2,12,13)(H3,11,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit purine nucleoside phosphorylase (PNP) |
J Med Chem 35: 1605-9 (1992)
BindingDB Entry DOI: 10.7270/Q2KW5GPH |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50139690

(3-[(S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-bu...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)CCCCCc1ccccc1 Show InChI InChI=1S/C31H41N3O7/c1-21(2)28(34-31(40)41-20-24-16-10-5-11-17-24)30(39)32-22(3)29(38)33-25(19-27(36)37)26(35)18-12-6-9-15-23-13-7-4-8-14-23/h4-5,7-8,10-11,13-14,16-17,21-22,25,28H,6,9,12,15,18-20H2,1-3H3,(H,32,39)(H,33,38)(H,34,40)(H,36,37)/t22-,25?,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against caspase-1 |
Bioorg Med Chem Lett 14: 809-12 (2004)
BindingDB Entry DOI: 10.7270/Q2FB52CR |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50368609

(CHEMBL604660)Show SMILES O[C@@H]1[C@@H](CI)OC([C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H12IN3O4/c12-1-5-8(16)9(17)10(19-5)4-2-13-7-6(4)14-3-15-11(7)18/h2-3,5,8-10,13,16-17H,1H2,(H,14,15,18)/t5-,8-,9-,10?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 176 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit purine nucleoside phosphorylase (PNP) |
J Med Chem 35: 1605-9 (1992)
BindingDB Entry DOI: 10.7270/Q2KW5GPH |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50368611
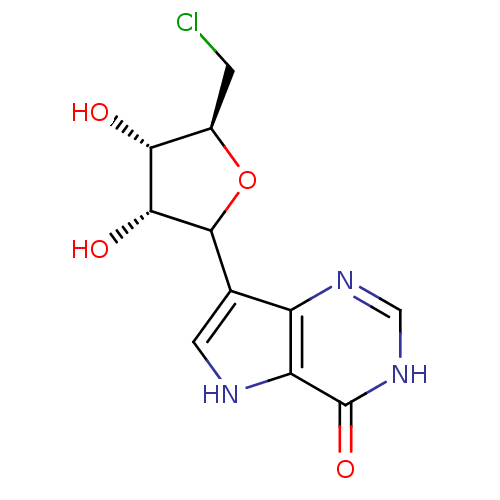
(CHEMBL604864)Show SMILES O[C@@H]1[C@@H](CCl)OC([C@@H]1O)c1c[nH]c2c1nc[nH]c2=O |r| Show InChI InChI=1S/C11H12ClN3O4/c12-1-5-8(16)9(17)10(19-5)4-2-13-7-6(4)14-3-15-11(7)18/h2-3,5,8-10,13,16-17H,1H2,(H,14,15,18)/t5-,8-,9-,10?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit purine nucleoside phosphorylase (PNP) |
J Med Chem 35: 1605-9 (1992)
BindingDB Entry DOI: 10.7270/Q2KW5GPH |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50005916

(2,6-diamino-7-(thien-3-ylmethyl)-3,5-dihydro-4H-py...)Show InChI InChI=1S/C11H11N5OS/c12-9-6(3-5-1-2-18-4-5)7-8(14-9)10(17)16-11(13)15-7/h1-2,4,14H,3,12H2,(H3,13,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit purine nucleoside phosphorylase (PNP) |
J Med Chem 35: 1605-9 (1992)
BindingDB Entry DOI: 10.7270/Q2KW5GPH |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM12055
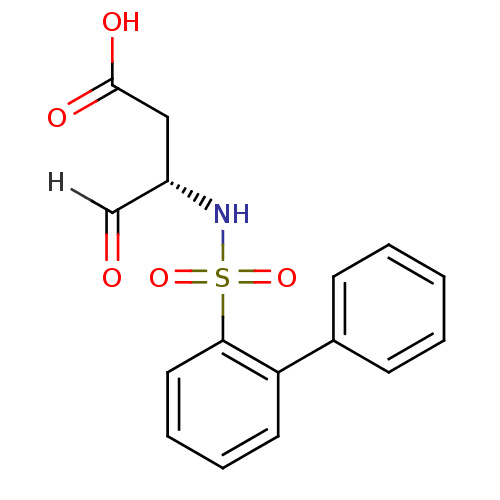
((3S)-4-oxo-3-[(2-phenylbenzene)sulfonamido]butanoi...)Show SMILES OC(=O)C[C@H](NS(=O)(=O)c1ccccc1-c1ccccc1)C=O |r| Show InChI InChI=1S/C16H15NO5S/c18-11-13(10-16(19)20)17-23(21,22)15-9-5-4-8-14(15)12-6-2-1-3-7-12/h1-9,11,13,17H,10H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | -34.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer
| Assay Description
The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... |
Bioorg Med Chem 10: 31-40 (2002)
Article DOI: 10.1016/s0968-0896(01)00250-4
BindingDB Entry DOI: 10.7270/Q2BP011G |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM12049

((3S)-3-[(3-methyl-2-phenylbenzene)sulfonamido]-4-o...)Show SMILES Cc1cccc(c1-c1ccccc1)S(=O)(=O)N[C@@H](CC(O)=O)C=O |r| Show InChI InChI=1S/C17H17NO5S/c1-12-6-5-9-15(17(12)13-7-3-2-4-8-13)24(22,23)18-14(11-19)10-16(20)21/h2-9,11,14,18H,10H2,1H3,(H,20,21)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | -34.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer
| Assay Description
The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... |
Bioorg Med Chem 10: 31-40 (2002)
Article DOI: 10.1016/s0968-0896(01)00250-4
BindingDB Entry DOI: 10.7270/Q2BP011G |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50230691

(CHEMBL3143996)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)C1=CNC2C1=NC=NC2=O |r,c:17,t:10,15| Show InChI InChI=1S/C11H13N3O5/c15-2-5-8(16)9(17)10(19-5)4-1-12-7-6(4)13-3-14-11(7)18/h1,3,5,7-10,12,15-17H,2H2/t5-,7?,8-,9-,10+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit purine nucleoside phosphorylase (PNP) |
J Med Chem 35: 1605-9 (1992)
BindingDB Entry DOI: 10.7270/Q2KW5GPH |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM12054
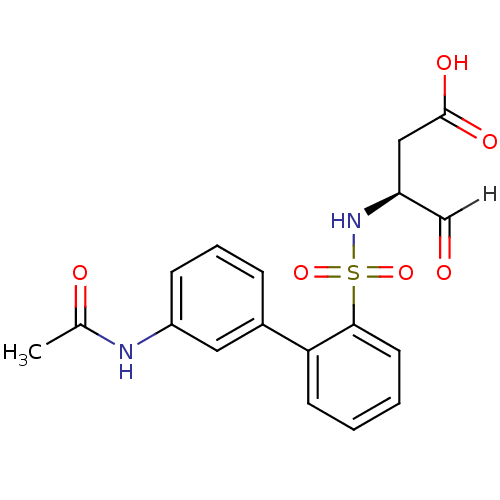
((3S)-3-{[2-(3-acetamidophenyl)benzene]sulfonamido}...)Show SMILES CC(=O)Nc1cccc(c1)-c1ccccc1S(=O)(=O)N[C@@H](CC(O)=O)C=O |r| Show InChI InChI=1S/C18H18N2O6S/c1-12(22)19-14-6-4-5-13(9-14)16-7-2-3-8-17(16)27(25,26)20-15(11-21)10-18(23)24/h2-9,11,15,20H,10H2,1H3,(H,19,22)(H,23,24)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer
| Assay Description
The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... |
Bioorg Med Chem 10: 31-40 (2002)
Article DOI: 10.1016/s0968-0896(01)00250-4
BindingDB Entry DOI: 10.7270/Q2BP011G |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Homo sapiens (Human)) | BDBM50230690
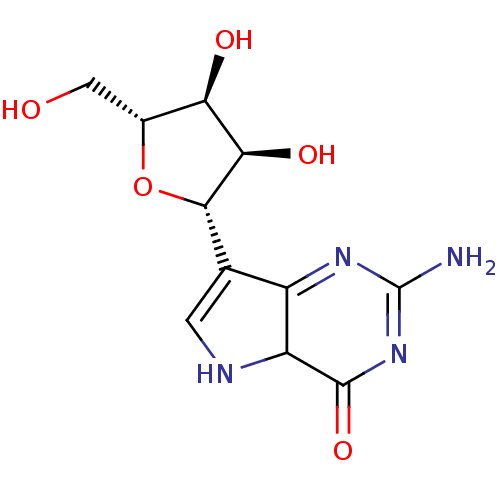
(CHEMBL3143997)Show SMILES NC1=NC(=O)C2NC=C([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)C2=N1 |r,c:20,t:1,7| Show InChI InChI=1S/C11H14N4O5/c12-11-14-5-3(1-13-6(5)10(19)15-11)9-8(18)7(17)4(2-16)20-9/h1,4,6-9,13,16-18H,2H2,(H2,12,15,19)/t4-,6?,7-,8-,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Ability to inhibit purine nucleoside phosphorylase (PNP) |
J Med Chem 35: 1605-9 (1992)
BindingDB Entry DOI: 10.7270/Q2KW5GPH |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM12051

((3S)-3-{[2-(3-methylphenyl)benzene]sulfonamido}-4-...)Show SMILES Cc1cccc(c1)-c1ccccc1S(=O)(=O)N[C@@H](CC(O)=O)C=O |r| Show InChI InChI=1S/C17H17NO5S/c1-12-5-4-6-13(9-12)15-7-2-3-8-16(15)24(22,23)18-14(11-19)10-17(20)21/h2-9,11,14,18H,10H2,1H3,(H,20,21)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10E+3 | -32.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer
| Assay Description
The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... |
Bioorg Med Chem 10: 31-40 (2002)
Article DOI: 10.1016/s0968-0896(01)00250-4
BindingDB Entry DOI: 10.7270/Q2BP011G |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM12050
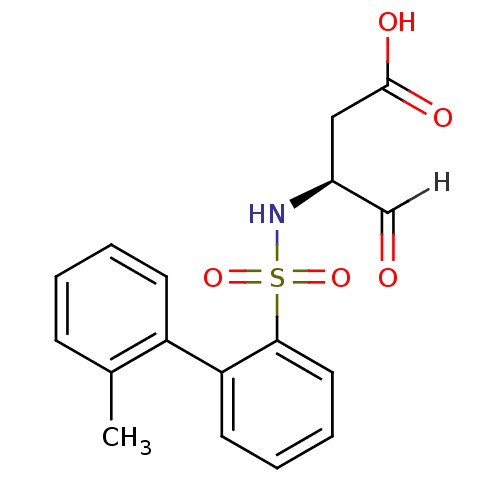
((3S)-3-{[2-(2-methylphenyl)benzene]sulfonamido}-4-...)Show SMILES Cc1ccccc1-c1ccccc1S(=O)(=O)N[C@@H](CC(O)=O)C=O |r| Show InChI InChI=1S/C17H17NO5S/c1-12-6-2-3-7-14(12)15-8-4-5-9-16(15)24(22,23)18-13(11-19)10-17(20)21/h2-9,11,13,18H,10H2,1H3,(H,20,21)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80E+3 | -30.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer
| Assay Description
The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... |
Bioorg Med Chem 10: 31-40 (2002)
Article DOI: 10.1016/s0968-0896(01)00250-4
BindingDB Entry DOI: 10.7270/Q2BP011G |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM12053
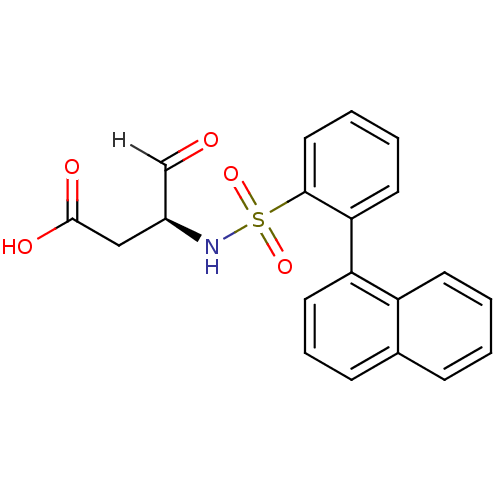
((3S)-3-{[2-(naphthalen-1-yl)benzene]sulfonamido}-4...)Show SMILES OC(=O)C[C@H](NS(=O)(=O)c1ccccc1-c1cccc2ccccc12)C=O |r| Show InChI InChI=1S/C20H17NO5S/c22-13-15(12-20(23)24)21-27(25,26)19-11-4-3-9-18(19)17-10-5-7-14-6-1-2-8-16(14)17/h1-11,13,15,21H,12H2,(H,23,24)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90E+3 | -30.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer
| Assay Description
The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... |
Bioorg Med Chem 10: 31-40 (2002)
Article DOI: 10.1016/s0968-0896(01)00250-4
BindingDB Entry DOI: 10.7270/Q2BP011G |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM12052
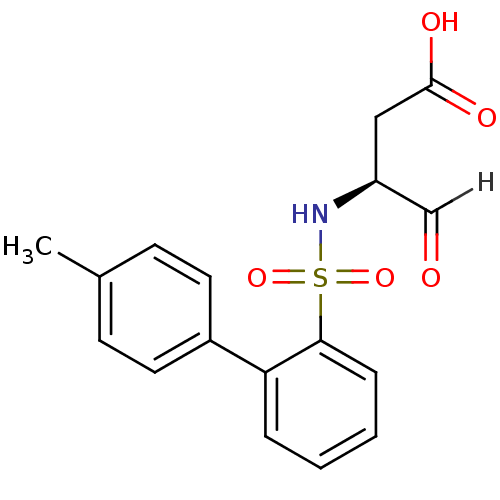
((3S)-3-{[2-(4-methylphenyl)benzene]sulfonamido}-4-...)Show SMILES Cc1ccc(cc1)-c1ccccc1S(=O)(=O)N[C@@H](CC(O)=O)C=O |r| Show InChI InChI=1S/C17H17NO5S/c1-12-6-8-13(9-7-12)15-4-2-3-5-16(15)24(22,23)18-14(11-19)10-17(20)21/h2-9,11,14,18H,10H2,1H3,(H,20,21)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.20E+3 | -30.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer
| Assay Description
The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... |
Bioorg Med Chem 10: 31-40 (2002)
Article DOI: 10.1016/s0968-0896(01)00250-4
BindingDB Entry DOI: 10.7270/Q2BP011G |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM12047

((3S)-3-benzenesulfonamido-4-oxobutanoic acid | (S)...)Show InChI InChI=1S/C10H11NO5S/c12-7-8(6-10(13)14)11-17(15,16)9-4-2-1-3-5-9/h1-5,7-8,11H,6H2,(H,13,14)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.40E+3 | -29.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer
| Assay Description
The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... |
Bioorg Med Chem 10: 31-40 (2002)
Article DOI: 10.1016/s0968-0896(01)00250-4
BindingDB Entry DOI: 10.7270/Q2BP011G |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50139698

(3-Benzyloxycarbonylamino-4-oxo-5-(2-phenyl-ethanes...)Show SMILES OC(=O)CC(NC(=O)OCc1ccccc1)C(=O)CNS(=O)(=O)CCc1ccccc1 Show InChI InChI=1S/C21H24N2O7S/c24-19(14-22-31(28,29)12-11-16-7-3-1-4-8-16)18(13-20(25)26)23-21(27)30-15-17-9-5-2-6-10-17/h1-10,18,22H,11-15H2,(H,23,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against caspase-1 |
Bioorg Med Chem Lett 14: 809-12 (2004)
BindingDB Entry DOI: 10.7270/Q2FB52CR |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50139701
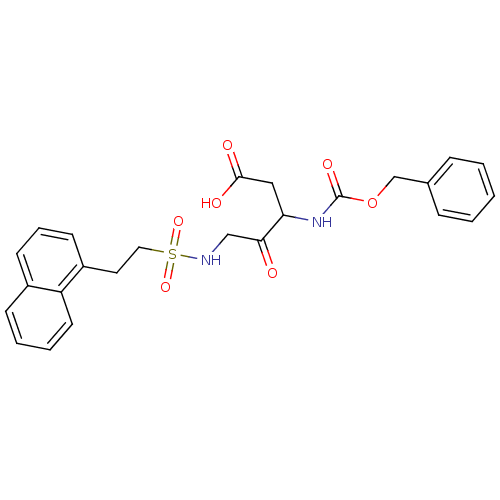
(3-Benzyloxycarbonylamino-5-(2-naphthalen-1-yl-etha...)Show SMILES OC(=O)CC(NC(=O)OCc1ccccc1)C(=O)CNS(=O)(=O)CCc1cccc2ccccc12 Show InChI InChI=1S/C25H26N2O7S/c28-23(22(15-24(29)30)27-25(31)34-17-18-7-2-1-3-8-18)16-26-35(32,33)14-13-20-11-6-10-19-9-4-5-12-21(19)20/h1-12,22,26H,13-17H2,(H,27,31)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against caspase-1 |
Bioorg Med Chem Lett 14: 809-12 (2004)
BindingDB Entry DOI: 10.7270/Q2FB52CR |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50139694

(3-Benzyloxycarbonylamino-5-(naphthalene-1-sulfonyl...)Show SMILES OC(=O)CC(NC(=O)OCc1ccccc1)C(=O)CNS(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C23H22N2O7S/c26-20(14-24-33(30,31)21-12-6-10-17-9-4-5-11-18(17)21)19(13-22(27)28)25-23(29)32-15-16-7-2-1-3-8-16/h1-12,19,24H,13-15H2,(H,25,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against caspase-1 |
Bioorg Med Chem Lett 14: 809-12 (2004)
BindingDB Entry DOI: 10.7270/Q2FB52CR |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM12045

((3S)-3-benzenesulfonamido-4-oxo-9-phenylnonanoic a...)Show SMILES OC(=O)C[C@H](NS(=O)(=O)c1ccccc1)C(=O)CCCCCc1ccccc1 |r| Show InChI InChI=1S/C21H25NO5S/c23-20(15-9-2-6-12-17-10-4-1-5-11-17)19(16-21(24)25)22-28(26,27)18-13-7-3-8-14-18/h1,3-5,7-8,10-11,13-14,19,22H,2,6,9,12,15-16H2,(H,24,25)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40E+4 | -27.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer
| Assay Description
The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... |
Bioorg Med Chem 10: 31-40 (2002)
Article DOI: 10.1016/s0968-0896(01)00250-4
BindingDB Entry DOI: 10.7270/Q2BP011G |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM12046
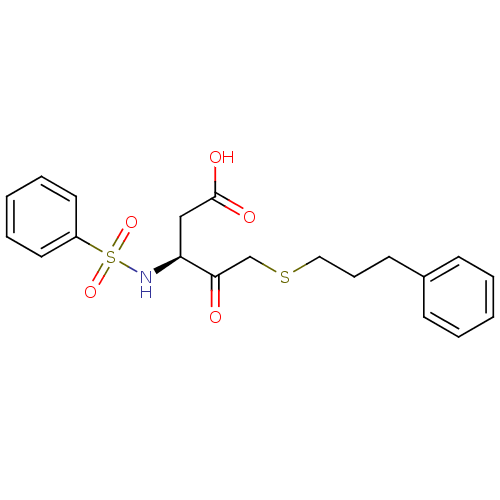
((3S)-3-benzenesulfonamido-4-oxo-5-[(3-phenylpropyl...)Show SMILES OC(=O)C[C@H](NS(=O)(=O)c1ccccc1)C(=O)CSCCCc1ccccc1 |r| Show InChI InChI=1S/C20H23NO5S2/c22-19(15-27-13-7-10-16-8-3-1-4-9-16)18(14-20(23)24)21-28(25,26)17-11-5-2-6-12-17/h1-6,8-9,11-12,18,21H,7,10,13-15H2,(H,23,24)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40E+4 | -27.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer
| Assay Description
The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... |
Bioorg Med Chem 10: 31-40 (2002)
Article DOI: 10.1016/s0968-0896(01)00250-4
BindingDB Entry DOI: 10.7270/Q2BP011G |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM12044

((3S)-3-{[(benzyloxy)carbonyl]amino}-4-oxo-5-[(3-ph...)Show SMILES OC(=O)C[C@H](NC(=O)OCc1ccccc1)C(=O)CSCCCc1ccccc1 |r| Show InChI InChI=1S/C22H25NO5S/c24-20(16-29-13-7-12-17-8-3-1-4-9-17)19(14-21(25)26)23-22(27)28-15-18-10-5-2-6-11-18/h1-6,8-11,19H,7,12-16H2,(H,23,27)(H,25,26)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+4 | -27.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer
| Assay Description
The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... |
Bioorg Med Chem 10: 31-40 (2002)
Article DOI: 10.1016/s0968-0896(01)00250-4
BindingDB Entry DOI: 10.7270/Q2BP011G |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50139697

(3-Benzyloxycarbonylamino-5-(2-cyclohexyl-ethanesul...)Show SMILES OC(=O)CC(NC(=O)OCc1ccccc1)C(=O)CNS(=O)(=O)CCC1CCCCC1 Show InChI InChI=1S/C21H30N2O7S/c24-19(14-22-31(28,29)12-11-16-7-3-1-4-8-16)18(13-20(25)26)23-21(27)30-15-17-9-5-2-6-10-17/h2,5-6,9-10,16,18,22H,1,3-4,7-8,11-15H2,(H,23,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against caspase-1 |
Bioorg Med Chem Lett 14: 809-12 (2004)
BindingDB Entry DOI: 10.7270/Q2FB52CR |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50139693
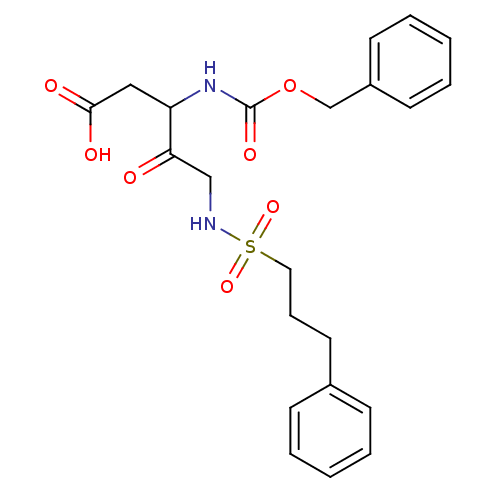
(3-Benzyloxycarbonylamino-4-oxo-5-(3-phenyl-propane...)Show SMILES OC(=O)CC(NC(=O)OCc1ccccc1)C(=O)CNS(=O)(=O)CCCc1ccccc1 Show InChI InChI=1S/C22H26N2O7S/c25-20(15-23-32(29,30)13-7-12-17-8-3-1-4-9-17)19(14-21(26)27)24-22(28)31-16-18-10-5-2-6-11-18/h1-6,8-11,19,23H,7,12-16H2,(H,24,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against caspase-1 |
Bioorg Med Chem Lett 14: 809-12 (2004)
BindingDB Entry DOI: 10.7270/Q2FB52CR |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50139691
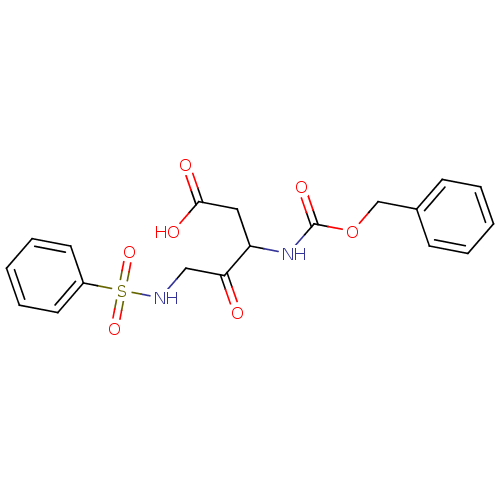
(5-Benzenesulfonylamino-3-benzyloxycarbonylamino-4-...)Show SMILES OC(=O)CC(NC(=O)OCc1ccccc1)C(=O)CNS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C19H20N2O7S/c22-17(12-20-29(26,27)15-9-5-2-6-10-15)16(11-18(23)24)21-19(25)28-13-14-7-3-1-4-8-14/h1-10,16,20H,11-13H2,(H,21,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against caspase-1 |
Bioorg Med Chem Lett 14: 809-12 (2004)
BindingDB Entry DOI: 10.7270/Q2FB52CR |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50139703
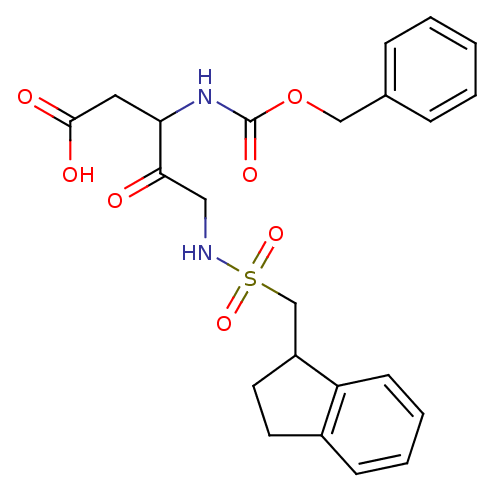
(3-Benzyloxycarbonylamino-5-(indan-1-ylmethanesulfo...)Show SMILES OC(=O)CC(NC(=O)OCc1ccccc1)C(=O)CNS(=O)(=O)CC1CCc2ccccc12 Show InChI InChI=1S/C23H26N2O7S/c26-21(13-24-33(30,31)15-18-11-10-17-8-4-5-9-19(17)18)20(12-22(27)28)25-23(29)32-14-16-6-2-1-3-7-16/h1-9,18,20,24H,10-15H2,(H,25,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against caspase-1 |
Bioorg Med Chem Lett 14: 809-12 (2004)
BindingDB Entry DOI: 10.7270/Q2FB52CR |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50139696
(3-Benzyloxycarbonylamino-4-oxo-9-phenyl-nonanoic a...)Show SMILES OC(=O)CC(NC(=O)OCc1ccccc1)C(=O)CCCCCc1ccccc1 Show InChI InChI=1S/C23H27NO5/c25-21(15-9-3-6-12-18-10-4-1-5-11-18)20(16-22(26)27)24-23(28)29-17-19-13-7-2-8-14-19/h1-2,4-5,7-8,10-11,13-14,20H,3,6,9,12,15-17H2,(H,24,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against caspase-1 |
Bioorg Med Chem Lett 14: 809-12 (2004)
BindingDB Entry DOI: 10.7270/Q2FB52CR |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM12043
((3S)-3-{[(benzyloxy)carbonyl]amino}-4-oxo-9-phenyl...)Show SMILES OC(=O)C[C@H](NC(=O)OCc1ccccc1)C(=O)CCCCCc1ccccc1 |r| Show InChI InChI=1S/C23H27NO5/c25-21(15-9-3-6-12-18-10-4-1-5-11-18)20(16-22(26)27)24-23(28)29-17-19-13-7-2-8-14-19/h1-2,4-5,7-8,10-11,13-14,20H,3,6,9,12,15-17H2,(H,24,28)(H,26,27)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.19E+5 | -23.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 37 |
Pfizer
| Assay Description
The substrate peptides terminating in AMC are processed by caspases with or without inhibitors, and the accumulation of AMC was assessed with a Cytof... |
Bioorg Med Chem 10: 31-40 (2002)
Article DOI: 10.1016/s0968-0896(01)00250-4
BindingDB Entry DOI: 10.7270/Q2BP011G |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50139692
(3-Benzyloxycarbonylamino-5-methanesulfonylamino-4-...)Show SMILES CS(=O)(=O)NCC(=O)C(CC(O)=O)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C14H18N2O7S/c1-24(21,22)15-8-12(17)11(7-13(18)19)16-14(20)23-9-10-5-3-2-4-6-10/h2-6,11,15H,7-9H2,1H3,(H,16,20)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against caspase-1 |
Bioorg Med Chem Lett 14: 809-12 (2004)
BindingDB Entry DOI: 10.7270/Q2FB52CR |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230906
(CHEMBL308261)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1c(Cl)ccc1Cl |(11.28,-11.6,;12.74,-12.07,;13.89,-11.03,;15.36,-11.5,;15.83,-12.96,;17.36,-12.96,;17.84,-11.47,;19.3,-11.03,;20.44,-12.04,;19.61,-9.53,;16.59,-10.6,;16.59,-9.05,;15.25,-8.29,;15.23,-6.73,;13.89,-5.99,;12.58,-6.76,;12.58,-8.29,;13.91,-9.05,;11.24,-6,;9.91,-6.76,;8.57,-6,;8.57,-4.44,;9.91,-3.67,;11.24,-4.44,;12.55,-3.66,;12.71,-2.13,;14.21,-1.79,;14.99,-3.13,;13.95,-4.28,;18.28,-14.2,;19.81,-14.18,;21.14,-13.41,;20.29,-15.64,;19.05,-16.57,;17.81,-15.66,;16.33,-16.06,)| Show InChI InChI=1S/C25H21Cl2N7O2/c1-2-5-21-28-24(34-19(26)12-13-20(34)27)22(25(35)36)33(21)14-15-8-10-16(11-9-15)17-6-3-4-7-18(17)23-29-31-32-30-23/h3-4,6-13H,2,5,14H2,1H3,(H,35,36)(H,29,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230919
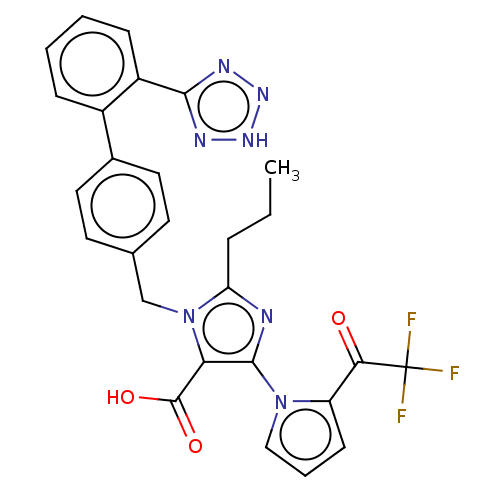
(CHEMBL307844 | CI-996)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1C(=O)C(F)(F)F Show InChI InChI=1S/C27H22F3N7O3/c1-2-6-21-31-25(36-14-5-9-20(36)23(38)27(28,29)30)22(26(39)40)37(21)15-16-10-12-17(13-11-16)18-7-3-4-8-19(18)24-32-34-35-33-24/h3-5,7-14H,2,6,15H2,1H3,(H,39,40)(H,32,33,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230891

(CHEMBL76166)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1C=O Show InChI InChI=1S/C26H23N7O3/c1-2-6-22-27-25(32-14-5-7-19(32)16-34)23(26(35)36)33(22)15-17-10-12-18(13-11-17)20-8-3-4-9-21(20)24-28-30-31-29-24/h3-5,7-14,16H,2,6,15H2,1H3,(H,35,36)(H,28,29,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230883
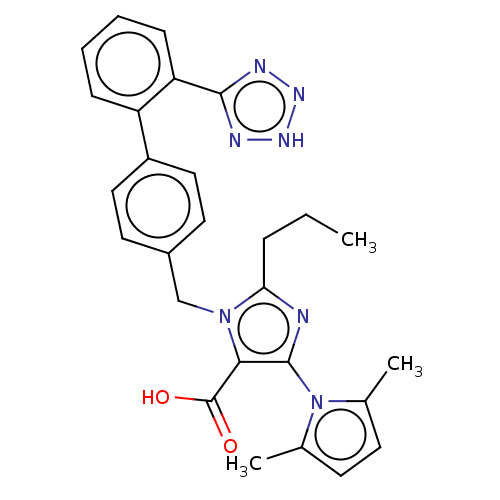
(CHEMBL309089)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1c(C)ccc1C |(11.28,-11.6,;12.74,-12.07,;13.89,-11.03,;15.36,-11.5,;15.83,-12.96,;17.36,-12.96,;17.84,-11.47,;19.3,-11.03,;20.44,-12.04,;19.61,-9.53,;16.59,-10.6,;16.59,-9.05,;15.25,-8.29,;15.23,-6.73,;13.89,-5.99,;12.58,-6.76,;12.58,-8.29,;13.91,-9.05,;11.24,-6,;9.91,-6.76,;8.57,-6,;8.57,-4.44,;9.91,-3.67,;11.24,-4.44,;12.55,-3.66,;12.71,-2.13,;14.21,-1.79,;14.99,-3.13,;13.95,-4.28,;18.28,-14.2,;17.81,-15.66,;16.36,-16.14,;19.05,-16.57,;20.29,-15.64,;19.81,-14.18,;20.89,-13.6,)| Show InChI InChI=1S/C27H27N7O2/c1-4-7-23-28-26(34-17(2)10-11-18(34)3)24(27(35)36)33(23)16-19-12-14-20(15-13-19)21-8-5-6-9-22(21)25-29-31-32-30-25/h5-6,8-15H,4,7,16H2,1-3H3,(H,35,36)(H,29,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230915
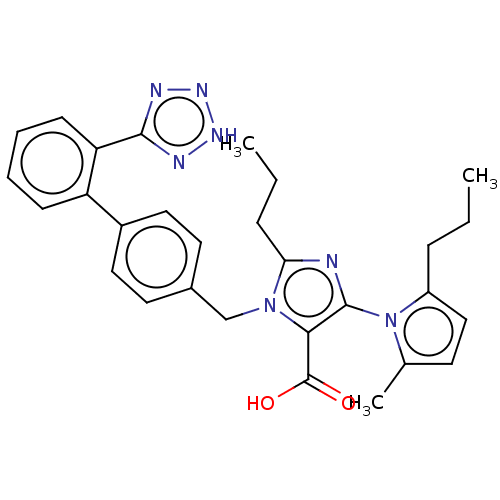
(CHEMBL76169)Show SMILES CCCc1ccc(C)n1-c1nc(CCC)n(Cc2ccc(cc2)-c2ccccc2-c2nn[nH]n2)c1C(O)=O |(13.76,-15.58,;15.23,-15.1,;16.36,-16.14,;17.81,-15.66,;19.05,-16.57,;20.29,-15.64,;19.81,-14.18,;20.89,-13.6,;18.28,-14.2,;17.36,-12.96,;15.83,-12.96,;15.36,-11.5,;13.89,-11.03,;12.74,-12.07,;11.28,-11.6,;16.59,-10.6,;16.59,-9.05,;15.25,-8.29,;15.23,-6.73,;13.89,-5.99,;12.58,-6.76,;12.58,-8.29,;13.91,-9.05,;11.24,-6,;9.91,-6.76,;8.57,-6,;8.57,-4.44,;9.91,-3.67,;11.24,-4.44,;12.55,-3.66,;12.71,-2.13,;14.21,-1.79,;14.99,-3.13,;13.95,-4.28,;17.84,-11.47,;19.3,-11.03,;20.44,-12.04,;19.61,-9.53,)| Show InChI InChI=1S/C29H31N7O2/c1-4-8-22-17-12-19(3)36(22)28-26(29(37)38)35(25(30-28)9-5-2)18-20-13-15-21(16-14-20)23-10-6-7-11-24(23)27-31-33-34-32-27/h6-7,10-17H,4-5,8-9,18H2,1-3H3,(H,37,38)(H,31,32,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230887
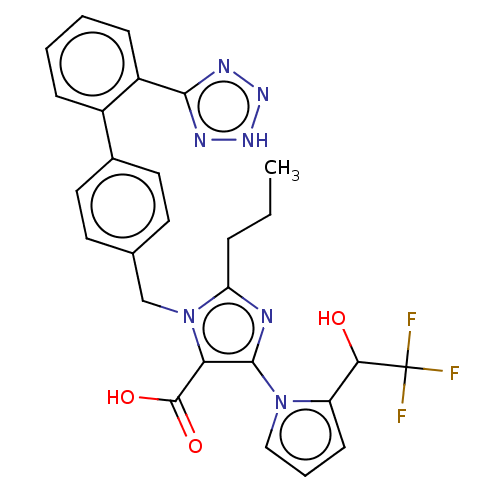
(CHEMBL306259)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1C(O)C(F)(F)F Show InChI InChI=1S/C27H24F3N7O3/c1-2-6-21-31-25(36-14-5-9-20(36)23(38)27(28,29)30)22(26(39)40)37(21)15-16-10-12-17(13-11-16)18-7-3-4-8-19(18)24-32-34-35-33-24/h3-5,7-14,23,38H,2,6,15H2,1H3,(H,39,40)(H,32,33,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382321
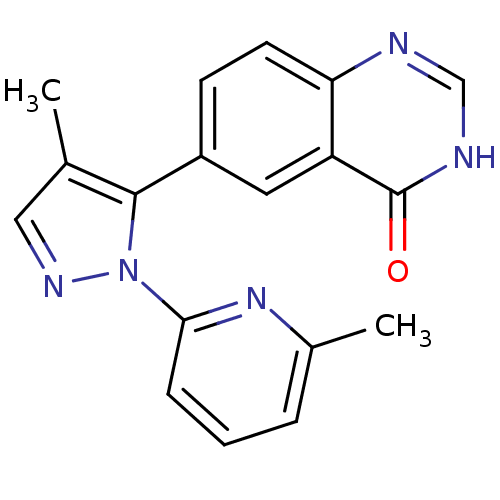
(CHEMBL2024688)Show SMILES Cc1cnn(c1-c1ccc2nc[nH]c(=O)c2c1)-c1cccc(C)n1 Show InChI InChI=1S/C18H15N5O/c1-11-9-21-23(16-5-3-4-12(2)22-16)17(11)13-6-7-15-14(8-13)18(24)20-10-19-15/h3-10H,1-2H3,(H,19,20,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382322
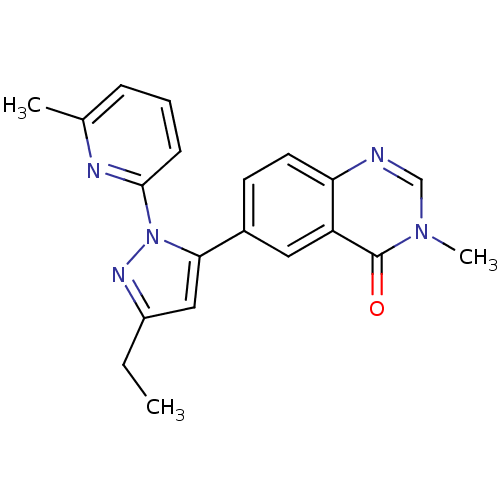
(CHEMBL2024689)Show SMILES CCc1cc(-c2ccc3ncn(C)c(=O)c3c2)n(n1)-c1cccc(C)n1 Show InChI InChI=1S/C20H19N5O/c1-4-15-11-18(25(23-15)19-7-5-6-13(2)22-19)14-8-9-17-16(10-14)20(26)24(3)12-21-17/h5-12H,4H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230912
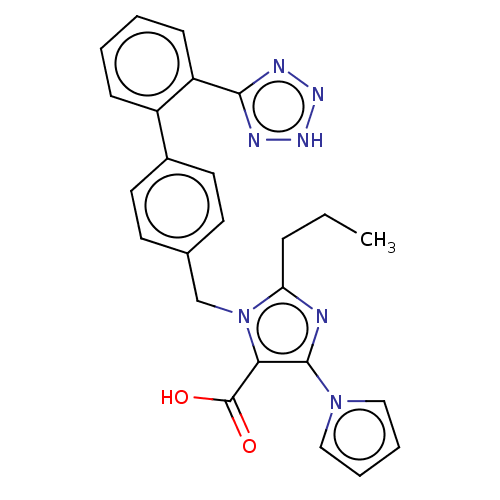
(CHEMBL72922)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1 Show InChI InChI=1S/C25H23N7O2/c1-2-7-21-26-24(31-14-5-6-15-31)22(25(33)34)32(21)16-17-10-12-18(13-11-17)19-8-3-4-9-20(19)23-27-29-30-28-23/h3-6,8-15H,2,7,16H2,1H3,(H,33,34)(H,27,28,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230886
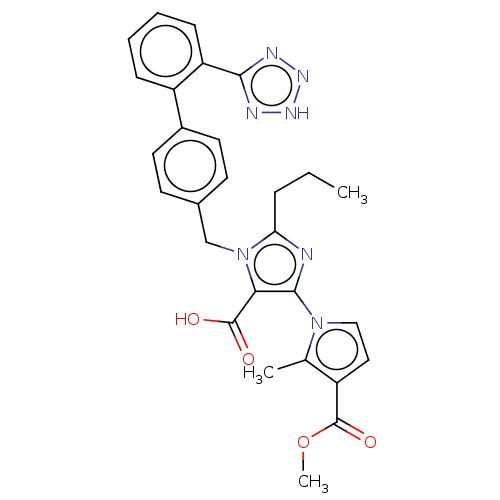
(CHEMBL307455)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1ccc(C(=O)OC)c1C Show InChI InChI=1S/C28H27N7O4/c1-4-7-23-29-26(34-15-14-20(17(34)2)28(38)39-3)24(27(36)37)35(23)16-18-10-12-19(13-11-18)21-8-5-6-9-22(21)25-30-32-33-31-25/h5-6,8-15H,4,7,16H2,1-3H3,(H,36,37)(H,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230882
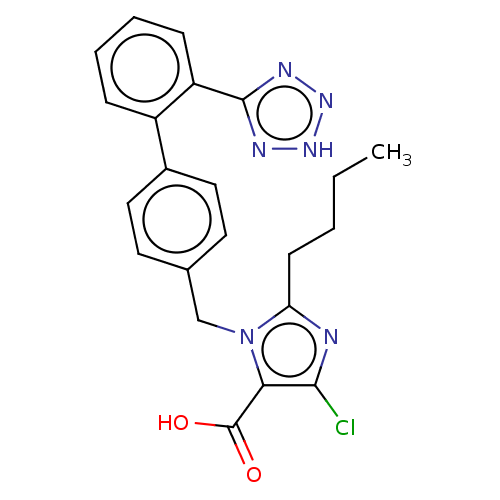
(CARBOXYLIC ACID METABOLITE | CHEBI:74125 | E-3174)Show SMILES CCCCc1nc(Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1 Show InChI InChI=1S/C22H21ClN6O2/c1-2-3-8-18-24-20(23)19(22(30)31)29(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-25-27-28-26-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Tested for inhibitory concentration against AT1 receptor binding affinity in rat liver |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230910

(CHEMBL306066)Show SMILES CCCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1 Show InChI InChI=1S/C26H25N7O2/c1-2-3-10-22-27-25(32-15-6-7-16-32)23(26(34)35)33(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)24-28-30-31-29-24/h4-9,11-16H,2-3,10,17H2,1H3,(H,34,35)(H,28,29,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230882

(CARBOXYLIC ACID METABOLITE | CHEBI:74125 | E-3174)Show SMILES CCCCc1nc(Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1 Show InChI InChI=1S/C22H21ClN6O2/c1-2-3-8-18-24-20(23)19(22(30)31)29(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-25-27-28-26-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1/Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50230882

(CARBOXYLIC ACID METABOLITE | CHEBI:74125 | E-3174)Show SMILES CCCCc1nc(Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1 Show InChI InChI=1S/C22H21ClN6O2/c1-2-3-8-18-24-20(23)19(22(30)31)29(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-25-27-28-26-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against [125I]angiotensin II(AII) induced contraction in rabbit aorta by 50% |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50382324

(CHEMBL2024691)Show SMILES CCc1cnn(c1-c1ccc2nc[nH]c(=O)c2c1)-c1cccc(C)n1 Show InChI InChI=1S/C19H17N5O/c1-3-13-10-22-24(17-6-4-5-12(2)23-17)18(13)14-7-8-16-15(9-14)19(25)21-11-20-16/h4-11H,3H2,1-2H3,(H,20,21,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ALK5 |
Bioorg Med Chem Lett 22: 3392-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.013
BindingDB Entry DOI: 10.7270/Q2QC04H9 |
More data for this
Ligand-Target Pair | |
Type-1/Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50230883

(CHEMBL309089)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1c(C)ccc1C |(11.28,-11.6,;12.74,-12.07,;13.89,-11.03,;15.36,-11.5,;15.83,-12.96,;17.36,-12.96,;17.84,-11.47,;19.3,-11.03,;20.44,-12.04,;19.61,-9.53,;16.59,-10.6,;16.59,-9.05,;15.25,-8.29,;15.23,-6.73,;13.89,-5.99,;12.58,-6.76,;12.58,-8.29,;13.91,-9.05,;11.24,-6,;9.91,-6.76,;8.57,-6,;8.57,-4.44,;9.91,-3.67,;11.24,-4.44,;12.55,-3.66,;12.71,-2.13,;14.21,-1.79,;14.99,-3.13,;13.95,-4.28,;18.28,-14.2,;17.81,-15.66,;16.36,-16.14,;19.05,-16.57,;20.29,-15.64,;19.81,-14.18,;20.89,-13.6,)| Show InChI InChI=1S/C27H27N7O2/c1-4-7-23-28-26(34-17(2)10-11-18(34)3)24(27(35)36)33(23)16-19-12-14-20(15-13-19)21-8-5-6-9-22(21)25-29-31-32-30-25/h5-6,8-15H,4,7,16H2,1-3H3,(H,35,36)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against [125I]angiotensin II(AII) induced contraction in rabbit aorta by 50% |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50230877
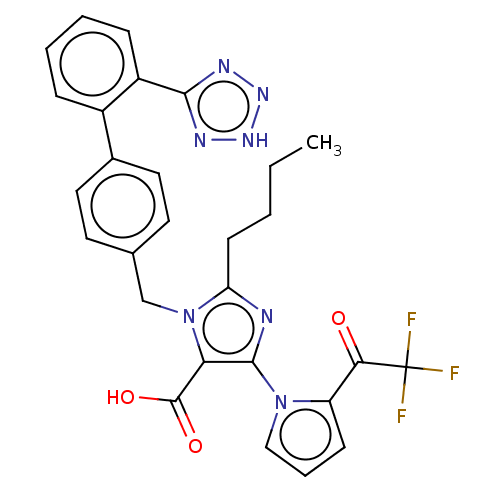
(CHEMBL70371)Show SMILES CCCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nn[nH]n1)-n1cccc1C(=O)C(F)(F)F Show InChI InChI=1S/C28H24F3N7O3/c1-2-3-10-22-32-26(37-15-6-9-21(37)24(39)28(29,30)31)23(27(40)41)38(22)16-17-11-13-18(14-12-17)19-7-4-5-8-20(19)25-33-35-36-34-25/h4-9,11-15H,2-3,10,16H2,1H3,(H,40,41)(H,33,34,35,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding of [125I]angiotensin II to rat liver expressing Angiotensin II receptor |
J Med Chem 36: 2253-65 (1993)
Article DOI: 10.1021/jm00068a002
BindingDB Entry DOI: 10.7270/Q25H7JGW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data