 Found 435 hits with Last Name = 'berry' and Initial = 'd'
Found 435 hits with Last Name = 'berry' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50010161
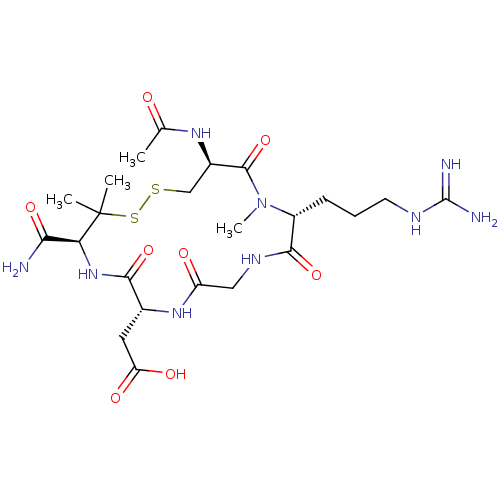
(CHEMBL2370397 | [16-Acetylamino-4-carbamoyl-13-(3-...)Show SMILES [#6]-[#7]-1-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6](-[#7])=O)C([#6])([#6])[#16]-[#16]-[#6]-[#6@@H](-[#7]-[#6](-[#6])=O)-[#6]-1=O Show InChI InChI=1S/C23H39N9O8S2/c1-11(33)29-13-10-41-42-23(2,3)17(18(24)37)31-19(38)12(8-16(35)36)30-15(34)9-28-20(39)14(32(4)21(13)40)6-5-7-27-22(25)26/h12-14,17H,5-10H2,1-4H3,(H2,24,37)(H,28,39)(H,29,33)(H,30,34)(H,31,38)(H,35,36)(H4,25,26,27)/t12-,13-,14-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of canine platelet-rich plasma agregation induced by ADP |
J Med Chem 34: 3114-25 (1991)
BindingDB Entry DOI: 10.7270/Q2445KF0 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50010161

(CHEMBL2370397 | [16-Acetylamino-4-carbamoyl-13-(3-...)Show SMILES [#6]-[#7]-1-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6](-[#7])=O)C([#6])([#6])[#16]-[#16]-[#6]-[#6@@H](-[#7]-[#6](-[#6])=O)-[#6]-1=O Show InChI InChI=1S/C23H39N9O8S2/c1-11(33)29-13-10-41-42-23(2,3)17(18(24)37)31-19(38)12(8-16(35)36)30-15(34)9-28-20(39)14(32(4)21(13)40)6-5-7-27-22(25)26/h12-14,17H,5-10H2,1-4H3,(H2,24,37)(H,28,39)(H,29,33)(H,30,34)(H,31,38)(H,35,36)(H4,25,26,27)/t12-,13-,14-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of canine platelet-rich plasma agregation induced by ADP |
J Med Chem 34: 3114-25 (1991)
BindingDB Entry DOI: 10.7270/Q2445KF0 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50040751
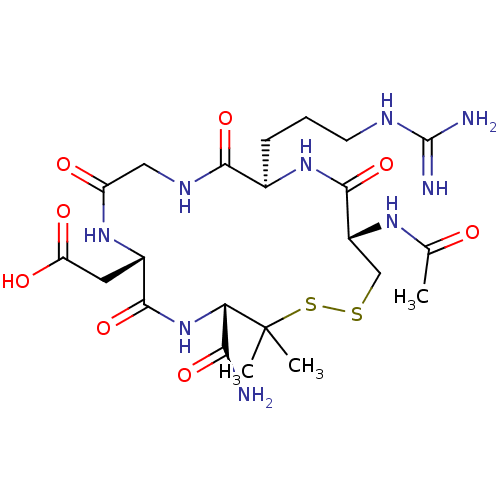
(CHEMBL351938 | [(4S,7S,13R,16S)-16-Acetylamino-4-c...)Show SMILES CC(=O)N[C@@H]1CSSC(C)(C)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](CCCNC(N)=N)NC1=O)C(N)=O Show InChI InChI=1S/C22H37N9O8S2/c1-10(32)28-13-9-40-41-22(2,3)16(17(23)36)31-19(38)12(7-15(34)35)29-14(33)8-27-18(37)11(30-20(13)39)5-4-6-26-21(24)25/h11-13,16H,4-9H2,1-3H3,(H2,23,36)(H,27,37)(H,28,32)(H,29,33)(H,30,39)(H,31,38)(H,34,35)(H4,24,25,26)/t11-,12+,13-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of 125[I] Fibrinogen binding to isolated purified human fibrinogen receptor |
J Med Chem 34: 3114-25 (1991)
BindingDB Entry DOI: 10.7270/Q2445KF0 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50010176
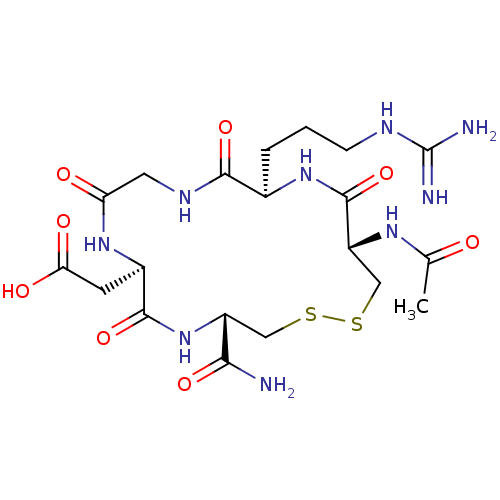
(CHEMBL107009 | [(4S,7R,13R,16S)-16-Acetylamino-4-c...)Show SMILES CC(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](CCCNC(N)=N)NC1=O)C(N)=O Show InChI InChI=1S/C20H33N9O8S2/c1-9(30)26-13-8-39-38-7-12(16(21)34)29-18(36)11(5-15(32)33)27-14(31)6-25-17(35)10(28-19(13)37)3-2-4-24-20(22)23/h10-13H,2-8H2,1H3,(H2,21,34)(H,25,35)(H,26,30)(H,27,31)(H,28,37)(H,29,36)(H,32,33)(H4,22,23,24)/t10-,11-,12-,13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of canine platelet-rich plasma agregation induced by ADP |
J Med Chem 34: 3114-25 (1991)
BindingDB Entry DOI: 10.7270/Q2445KF0 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50003326
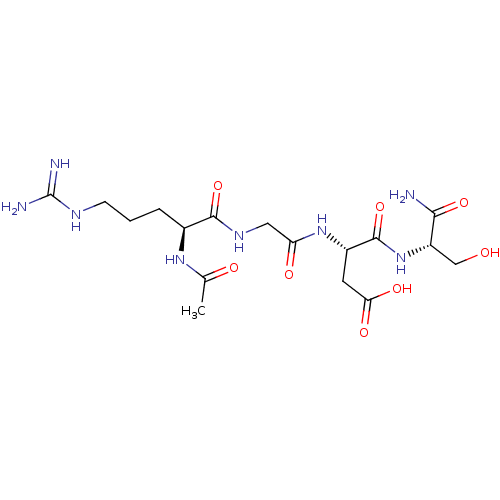
(3-[2-(2-Acetylamino-5-guanidino-pentanoylamino)-ac...)Show SMILES CC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(N)=O Show InChI InChI=1S/C17H30N8O8/c1-8(27)23-9(3-2-4-21-17(19)20)15(32)22-6-12(28)24-10(5-13(29)30)16(33)25-11(7-26)14(18)31/h9-11,26H,2-7H2,1H3,(H2,18,31)(H,22,32)(H,23,27)(H,24,28)(H,25,33)(H,29,30)(H4,19,20,21)/t9-,10-,11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of 125[I] Fibrinogen binding to isolated purified human fibrinogen receptor |
J Med Chem 34: 3114-25 (1991)
BindingDB Entry DOI: 10.7270/Q2445KF0 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50010187

(CHEMBL320288 | [19-Acetylamino-4-carbamoyl-16-(3-g...)Show SMILES CC(=O)N[C@@H]1CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](CCCNC(N)=N)NC1=O)C(N)=O Show InChI InChI=1S/C23H38N10O10S2/c1-10(35)29-15-9-45-44-8-14(18(24)39)33-21(42)13(7-34)32-20(41)12(5-17(37)38)30-16(36)6-28-19(40)11(31-22(15)43)3-2-4-27-23(25)26/h11-15,34H,2-9H2,1H3,(H2,24,39)(H,28,40)(H,29,35)(H,30,36)(H,31,43)(H,32,41)(H,33,42)(H,37,38)(H4,25,26,27)/t11-,12-,13+,14+,15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of canine platelet-rich plasma agregation induced by ADP |
J Med Chem 34: 3114-25 (1991)
BindingDB Entry DOI: 10.7270/Q2445KF0 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50010184
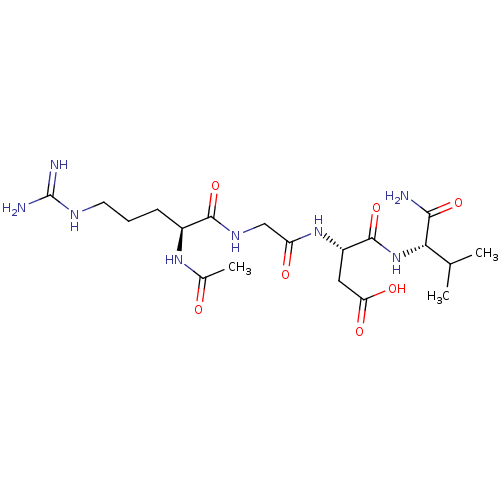
(3-[4-amino(imino)methylamino-1-methylcarboxamido-(...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(N)=O Show InChI InChI=1S/C19H34N8O7/c1-9(2)15(16(20)32)27-18(34)12(7-14(30)31)26-13(29)8-24-17(33)11(25-10(3)28)5-4-6-23-19(21)22/h9,11-12,15H,4-8H2,1-3H3,(H2,20,32)(H,24,33)(H,25,28)(H,26,29)(H,27,34)(H,30,31)(H4,21,22,23)/t11-,12-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of canine platelet-rich plasma agregation induced by ADP |
J Med Chem 34: 3114-25 (1991)
BindingDB Entry DOI: 10.7270/Q2445KF0 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50010167
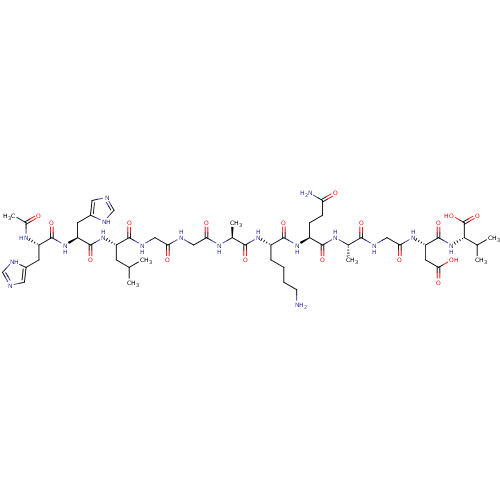
(1N-[1-[5-amino-1-formyl-(1S)-pentylcarbamoyl]-(1S)...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(=O)NCC(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C52H82N18O17/c1-25(2)14-34(68-50(84)36(16-31-19-56-24-61-31)69-49(83)35(64-29(7)71)15-30-18-55-23-60-30)46(80)59-20-39(73)57-21-40(74)62-28(6)45(79)66-32(10-8-9-13-53)48(82)67-33(11-12-38(54)72)47(81)63-27(5)44(78)58-22-41(75)65-37(17-42(76)77)51(85)70-43(26(3)4)52(86)87/h18-19,23-28,32-37,43H,8-17,20-22,53H2,1-7H3,(H2,54,72)(H,55,60)(H,56,61)(H,57,73)(H,58,78)(H,59,80)(H,62,74)(H,63,81)(H,64,71)(H,65,75)(H,66,79)(H,67,82)(H,68,84)(H,69,83)(H,70,85)(H,76,77)(H,86,87)/t27-,28-,32-,33-,34-,35-,36-,37-,43-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of canine platelet-rich plasma agregation induced by ADP |
J Med Chem 34: 3114-25 (1991)
BindingDB Entry DOI: 10.7270/Q2445KF0 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50003326

(3-[2-(2-Acetylamino-5-guanidino-pentanoylamino)-ac...)Show SMILES CC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(N)=O Show InChI InChI=1S/C17H30N8O8/c1-8(27)23-9(3-2-4-21-17(19)20)15(32)22-6-12(28)24-10(5-13(29)30)16(33)25-11(7-26)14(18)31/h9-11,26H,2-7H2,1H3,(H2,18,31)(H,22,32)(H,23,27)(H,24,28)(H,25,33)(H,29,30)(H4,19,20,21)/t9-,10-,11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of canine platelet-rich plasma agregation induced by ADP |
J Med Chem 34: 3114-25 (1991)
BindingDB Entry DOI: 10.7270/Q2445KF0 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50055367
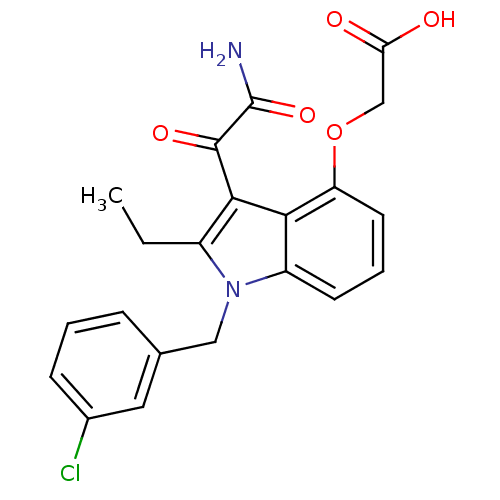
(CHEMBL345986 | [3-Aminooxalyl-1-(3-chloro-benzyl)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1cccc(Cl)c1 Show InChI InChI=1S/C21H19ClN2O5/c1-2-14-19(20(27)21(23)28)18-15(7-4-8-16(18)29-11-17(25)26)24(14)10-12-5-3-6-13(22)9-12/h3-9H,2,10-11H2,1H3,(H2,23,28)(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Compound wastested for inhibition of porcine secreted pancreatic PLA2 |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055371
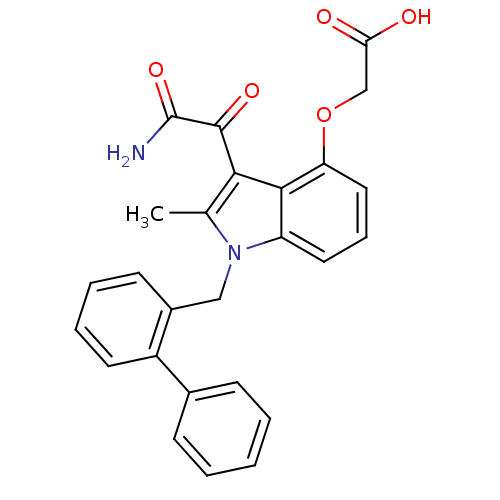
((3-aminooxalyl-1-biphenyl-2-ylmethyl-2-methyl-1H-i...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c1-16-23(25(31)26(27)32)24-20(12-7-13-21(24)33-15-22(29)30)28(16)14-18-10-5-6-11-19(18)17-8-3-2-4-9-17/h2-13H,14-15H2,1H3,(H2,27,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055374
(CHEMBL436456 | [3-Aminooxalyl-1-(2-benzyl-benzyl)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1Cc1ccccc1 Show InChI InChI=1S/C28H26N2O5/c1-2-21-26(27(33)28(29)34)25-22(13-8-14-23(25)35-17-24(31)32)30(21)16-20-12-7-6-11-19(20)15-18-9-4-3-5-10-18/h3-14H,2,15-17H2,1H3,(H2,29,34)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055374

(CHEMBL436456 | [3-Aminooxalyl-1-(2-benzyl-benzyl)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1Cc1ccccc1 Show InChI InChI=1S/C28H26N2O5/c1-2-21-26(27(33)28(29)34)25-22(13-8-14-23(25)35-17-24(31)32)30(21)16-20-12-7-6-11-19(20)15-18-9-4-3-5-10-18/h3-14H,2,15-17H2,1H3,(H2,29,34)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055371

((3-aminooxalyl-1-biphenyl-2-ylmethyl-2-methyl-1H-i...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c1-16-23(25(31)26(27)32)24-20(12-7-13-21(24)33-15-22(29)30)28(16)14-18-10-5-6-11-19(18)17-8-3-2-4-9-17/h2-13H,14-15H2,1H3,(H2,27,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055378

(CHEMBL345488 | [3-Aminooxalyl-1-(2,6-dichloro-benz...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1c(Cl)cccc1Cl Show InChI InChI=1S/C20H16Cl2N2O5/c1-10-17(19(27)20(23)28)18-14(6-3-7-15(18)29-9-16(25)26)24(10)8-11-12(21)4-2-5-13(11)22/h2-7H,8-9H2,1H3,(H2,23,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055387

((R)-2-(3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-...)Show SMILES C[C@@H](Oc1cccc2n(Cc3ccccc3)c(C)c(C(=O)C(N)=O)c12)C(O)=O Show InChI InChI=1S/C21H20N2O5/c1-12-17(19(24)20(22)25)18-15(23(12)11-14-7-4-3-5-8-14)9-6-10-16(18)28-13(2)21(26)27/h3-10,13H,11H2,1-2H3,(H2,22,25)(H,26,27)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055401

((3-Aminooxalyl-1-biphenyl-2-ylmethyl-2-ethyl-1H-in...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C27H24N2O5/c1-2-20-25(26(32)27(28)33)24-21(13-8-14-22(24)34-16-23(30)31)29(20)15-18-11-6-7-12-19(18)17-9-4-3-5-10-17/h3-14H,2,15-16H2,1H3,(H2,28,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055401

((3-Aminooxalyl-1-biphenyl-2-ylmethyl-2-ethyl-1H-in...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C27H24N2O5/c1-2-20-25(26(32)27(28)33)24-21(13-8-14-22(24)34-16-23(30)31)29(20)15-18-11-6-7-12-19(18)17-9-4-3-5-10-17/h3-14H,2,15-16H2,1H3,(H2,28,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055379

((3-Aminooxalyl-1-biphenyl-2-ylmethyl-2-cyclopropyl...)Show SMILES NC(=O)C(=O)c1c(C2CC2)n(Cc2ccccc2-c2ccccc2)c2cccc(OCC(O)=O)c12 Show InChI InChI=1S/C28H24N2O5/c29-28(34)27(33)25-24-21(11-6-12-22(24)35-16-23(31)32)30(26(25)18-13-14-18)15-19-9-4-5-10-20(19)17-7-2-1-3-8-17/h1-12,18H,13-16H2,(H2,29,34)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50055384
((3-Aminooxalyl-1-biphenyl-3-ylmethyl-2-methyl-1H-i...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c1-16-23(25(31)26(27)32)24-20(11-6-12-21(24)33-15-22(29)30)28(16)14-17-7-5-10-19(13-17)18-8-3-2-4-9-18/h2-13H,14-15H2,1H3,(H2,27,32)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of porcine secretory pancreatic Phospholipase A2 |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055367

(CHEMBL345986 | [3-Aminooxalyl-1-(3-chloro-benzyl)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1cccc(Cl)c1 Show InChI InChI=1S/C21H19ClN2O5/c1-2-14-19(20(27)21(23)28)18-15(7-4-8-16(18)29-11-17(25)26)24(14)10-12-5-3-6-13(22)9-12/h3-9H,2,10-11H2,1H3,(H2,23,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM185362

(US9156831, 3)Show SMILES CCn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CCO Show InChI InChI=1S/C22H31N9O3/c1-5-31-19(26-18(29-31)13-6-9-30(10-7-13)15(33)8-11-32)14-12-24-17(23)16(25-14)20-27-28-21(34-20)22(2,3)4/h12-13,32H,5-11H2,1-4H3,(H2,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055370
((3-Aminooxalyl-2-methyl-1-octyl-1H-indol-4-yloxy)-...)Show SMILES CCCCCCCCn1c(C)c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc12 Show InChI InChI=1S/C21H28N2O5/c1-3-4-5-6-7-8-12-23-14(2)18(20(26)21(22)27)19-15(23)10-9-11-16(19)28-13-17(24)25/h9-11H,3-8,12-13H2,1-2H3,(H2,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055380
((3-Aminooxalyl-1-benzyl-2-ethyl-6-methyl-1H-indol-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(CC(O)=O)cc(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O4/c1-3-16-20(21(27)22(23)28)19-15(11-18(25)26)9-13(2)10-17(19)24(16)12-14-7-5-4-6-8-14/h4-10H,3,11-12H2,1-2H3,(H2,23,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055380

((3-Aminooxalyl-1-benzyl-2-ethyl-6-methyl-1H-indol-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(CC(O)=O)cc(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O4/c1-3-16-20(21(27)22(23)28)19-15(11-18(25)26)9-13(2)10-17(19)24(16)12-14-7-5-4-6-8-14/h4-10H,3,11-12H2,1-2H3,(H2,23,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055384

((3-Aminooxalyl-1-biphenyl-3-ylmethyl-2-methyl-1H-i...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c1-16-23(25(31)26(27)32)24-20(11-6-12-21(24)33-15-22(29)30)28(16)14-17-7-5-10-19(13-17)18-8-3-2-4-9-18/h2-13H,14-15H2,1H3,(H2,27,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055368

((3-Aminooxalyl-2-methyl-1-naphthalen-1-ylmethyl-1H...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1cccc2ccccc12 Show InChI InChI=1S/C24H20N2O5/c1-14-21(23(29)24(25)30)22-18(10-5-11-19(22)31-13-20(27)28)26(14)12-16-8-4-7-15-6-2-3-9-17(15)16/h2-11H,12-13H2,1H3,(H2,25,30)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055384

((3-Aminooxalyl-1-biphenyl-3-ylmethyl-2-methyl-1H-i...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c1-16-23(25(31)26(27)32)24-20(11-6-12-21(24)33-15-22(29)30)28(16)14-17-7-5-10-19(13-17)18-8-3-2-4-9-18/h2-13H,14-15H2,1H3,(H2,27,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055394
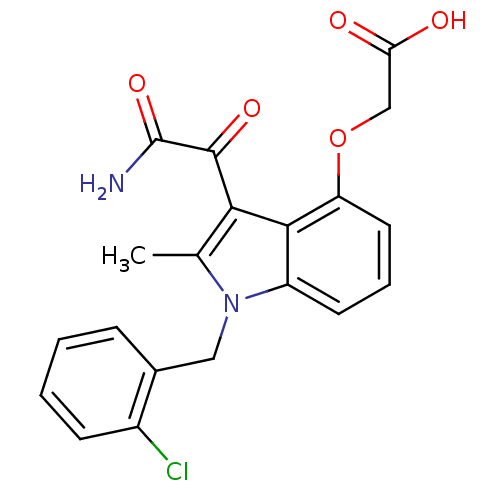
(CHEMBL148795 | [3-Aminooxalyl-1-(2-chloro-benzyl)-...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1Cl Show InChI InChI=1S/C20H17ClN2O5/c1-11-17(19(26)20(22)27)18-14(7-4-8-15(18)28-10-16(24)25)23(11)9-12-5-2-3-6-13(12)21/h2-8H,9-10H2,1H3,(H2,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055385
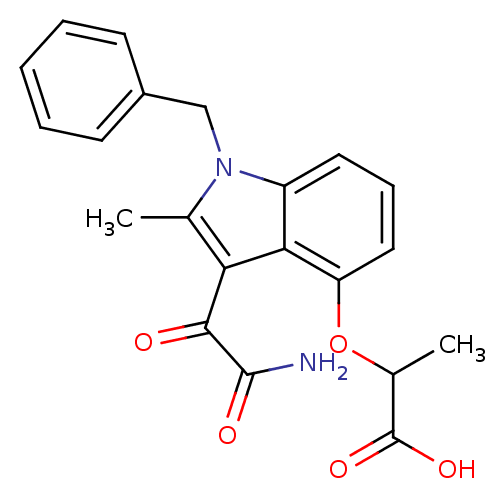
(2-(3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-ylox...)Show SMILES CC(Oc1cccc2n(Cc3ccccc3)c(C)c(C(=O)C(N)=O)c12)C(O)=O Show InChI InChI=1S/C21H20N2O5/c1-12-17(19(24)20(22)25)18-15(23(12)11-14-7-4-3-5-8-14)9-6-10-16(18)28-13(2)21(26)27/h3-10,13H,11H2,1-2H3,(H2,22,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM185358

(US9156831, 9)Show SMILES CCn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CC(O)=O Show InChI InChI=1S/C22H29N9O4/c1-5-31-19(26-18(29-31)12-6-8-30(9-7-12)14(32)10-15(33)34)13-11-24-17(23)16(25-13)20-27-28-21(35-20)22(2,3)4/h11-12H,5-10H2,1-4H3,(H2,23,24)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM185362

(US9156831, 3)Show SMILES CCn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CCO Show InChI InChI=1S/C22H31N9O3/c1-5-31-19(26-18(29-31)13-6-9-30(10-7-13)15(33)8-11-32)14-12-24-17(23)16(25-14)20-27-28-21(34-20)22(2,3)4/h12-13,32H,5-11H2,1-4H3,(H2,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055385

(2-(3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-ylox...)Show SMILES CC(Oc1cccc2n(Cc3ccccc3)c(C)c(C(=O)C(N)=O)c12)C(O)=O Show InChI InChI=1S/C21H20N2O5/c1-12-17(19(24)20(22)25)18-15(23(12)11-14-7-4-3-5-8-14)9-6-10-16(18)28-13(2)21(26)27/h3-10,13H,11H2,1-2H3,(H2,22,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055305
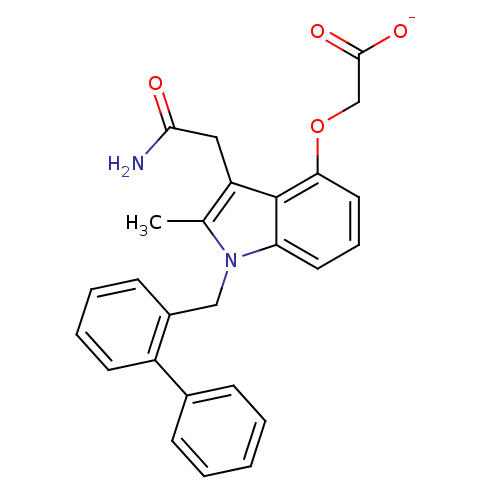
(CHEMBL423762 | Sodium; (1-biphenyl-2-ylmethyl-3-ca...)Show SMILES Cc1c(CC(N)=O)c2c(OCC([O-])=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C26H24N2O4/c1-17-21(14-24(27)29)26-22(12-7-13-23(26)32-16-25(30)31)28(17)15-19-10-5-6-11-20(19)18-8-3-2-4-9-18/h2-13H,14-16H2,1H3,(H2,27,29)(H,30,31)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human non-pancreatic secretory phospholipase A2 (PLA2) in a chromogenic assay |
J Med Chem 39: 5137-58 (1997)
Article DOI: 10.1021/jm960486n
BindingDB Entry DOI: 10.7270/Q2639NV0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM185358

(US9156831, 9)Show SMILES CCn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CC(O)=O Show InChI InChI=1S/C22H29N9O4/c1-5-31-19(26-18(29-31)12-6-8-30(9-7-12)14(32)10-15(33)34)13-11-24-17(23)16(25-13)20-27-28-21(35-20)22(2,3)4/h11-12H,5-10H2,1-4H3,(H2,23,24)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055383

((3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-yloxy)...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C20H18N2O5/c1-12-17(19(25)20(21)26)18-14(8-5-9-15(18)27-11-16(23)24)22(12)10-13-6-3-2-4-7-13/h2-9H,10-11H2,1H3,(H2,21,26)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055387

((R)-2-(3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-...)Show SMILES C[C@@H](Oc1cccc2n(Cc3ccccc3)c(C)c(C(=O)C(N)=O)c12)C(O)=O Show InChI InChI=1S/C21H20N2O5/c1-12-17(19(24)20(22)25)18-15(23(12)11-14-7-4-3-5-8-14)9-6-10-16(18)28-13(2)21(26)27/h3-10,13H,11H2,1-2H3,(H2,22,25)(H,26,27)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055391

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-ind...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OC(C)C(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-19(20(25)21(23)26)18-16(24(15)12-14-8-5-4-6-9-14)10-7-11-17(18)29-13(2)22(27)28/h4-11,13H,3,12H2,1-2H3,(H2,23,26)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM185354

(US9156831, 5)Show SMILES C[C@H](O)CC(=O)N1CCC(CC1)c1nc(-c2cnc(N)c(n2)-c2nnc(o2)C(C)(C)C)n(C)n1 |r| Show InChI InChI=1S/C22H31N9O3/c1-12(32)10-15(33)31-8-6-13(7-9-31)18-26-19(30(5)29-18)14-11-24-17(23)16(25-14)20-27-28-21(34-20)22(2,3)4/h11-13,32H,6-10H2,1-5H3,(H2,23,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM185355
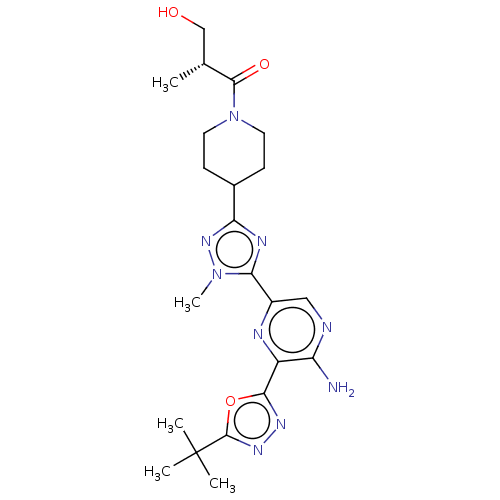
(US9156831, 6)Show SMILES C[C@H](CO)C(=O)N1CCC(CC1)c1nc(-c2cnc(N)c(n2)-c2nnc(o2)C(C)(C)C)n(C)n1 |r| Show InChI InChI=1S/C22H31N9O3/c1-12(11-32)20(33)31-8-6-13(7-9-31)17-26-18(30(5)29-17)14-10-24-16(23)15(25-14)19-27-28-21(34-19)22(2,3)4/h10,12-13,32H,6-9,11H2,1-5H3,(H2,23,24)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <12 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM185357

(US9156831, 8)Show SMILES Cn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CC(O)=O Show InChI InChI=1S/C21H27N9O4/c1-21(2,3)20-27-26-19(34-20)15-16(22)23-10-12(24-15)18-25-17(28-29(18)4)11-5-7-30(8-6-11)13(31)9-14(32)33/h10-11H,5-9H2,1-4H3,(H2,22,23)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM185353

(US9156831, 4)Show SMILES C[C@@H](O)CC(=O)N1CCC(CC1)c1nc(-c2cnc(N)c(n2)-c2nnc(o2)C(C)(C)C)n(C)n1 |r| Show InChI InChI=1S/C22H31N9O3/c1-12(32)10-15(33)31-8-6-13(7-9-31)18-26-19(30(5)29-18)14-11-24-17(23)16(25-14)20-27-28-21(34-20)22(2,3)4/h11-13,32H,6-10H2,1-5H3,(H2,23,24)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <12 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055391

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-ind...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OC(C)C(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-19(20(25)21(23)26)18-16(24(15)12-14-8-5-4-6-9-14)10-7-11-17(18)29-13(2)22(27)28/h4-11,13H,3,12H2,1-2H3,(H2,23,26)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM185361

(US9156831, 1)Show SMILES Cn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)CCO Show InChI InChI=1S/C21H29N9O3/c1-21(2,3)20-27-26-19(33-20)15-16(22)23-11-13(24-15)18-25-17(28-29(18)4)12-5-8-30(9-6-12)14(32)7-10-31/h11-12,31H,5-10H2,1-4H3,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <14 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055305

(CHEMBL423762 | Sodium; (1-biphenyl-2-ylmethyl-3-ca...)Show SMILES Cc1c(CC(N)=O)c2c(OCC([O-])=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C26H24N2O4/c1-17-21(14-24(27)29)26-22(12-7-13-23(26)32-16-25(30)31)28(17)15-19-10-5-6-11-20(19)18-8-3-2-4-9-18/h2-13H,14-16H2,1H3,(H2,27,29)(H,30,31)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against porcine pancreatic Phospholipase A2 |
J Med Chem 39: 5137-58 (1997)
Article DOI: 10.1021/jm960486n
BindingDB Entry DOI: 10.7270/Q2639NV0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM185356
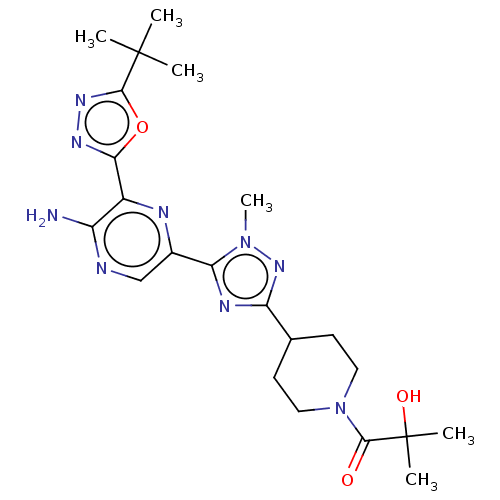
(US9156831, 7)Show SMILES Cn1nc(nc1-c1cnc(N)c(n1)-c1nnc(o1)C(C)(C)C)C1CCN(CC1)C(=O)C(C)(C)O Show InChI InChI=1S/C22H31N9O3/c1-21(2,3)19-28-27-18(34-19)14-15(23)24-11-13(25-14)17-26-16(29-30(17)6)12-7-9-31(10-8-12)20(32)22(4,5)33/h11-12,33H,7-10H2,1-6H3,(H2,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | <14 | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca AB
US Patent
| Assay Description
The inhibition of PI3K-beta, PI3K-alpha, PI3K-gamma and PI3K-delta was evaluated in a Kinase Glo based enzyme activity assay using human recombinant ... |
US Patent US9156831 (2015)
BindingDB Entry DOI: 10.7270/Q2NV9H2N |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055315

(CHEMBL359304 | [3-(1-Benzyl-3-carbamoylmethyl-2-et...)Show SMILES CCc1c(CC(N)=O)c2cc(OCCCP(O)(O)=O)c(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C23H29N2O5P/c1-3-20-19(14-23(24)26)18-13-22(30-10-7-11-31(27,28)29)16(2)12-21(18)25(20)15-17-8-5-4-6-9-17/h4-6,8-9,12-13H,3,7,10-11,14-15H2,1-2H3,(H2,24,26)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human non-pancreatic secretory phospholipase A2 (PLA2) in a chromogenic assay |
J Med Chem 39: 5137-58 (1997)
Article DOI: 10.1021/jm960486n
BindingDB Entry DOI: 10.7270/Q2639NV0 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50055383

((3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-yloxy)...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C20H18N2O5/c1-12-17(19(25)20(21)26)18-14(8-5-9-15(18)27-11-16(23)24)22(12)10-13-6-3-2-4-7-13/h2-9H,10-11H2,1H3,(H2,21,26)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of porcine secretory pancreatic Phospholipase A2 |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data