 Found 1083 hits with Last Name = 'das' and Initial = 'd'
Found 1083 hits with Last Name = 'das' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM13925
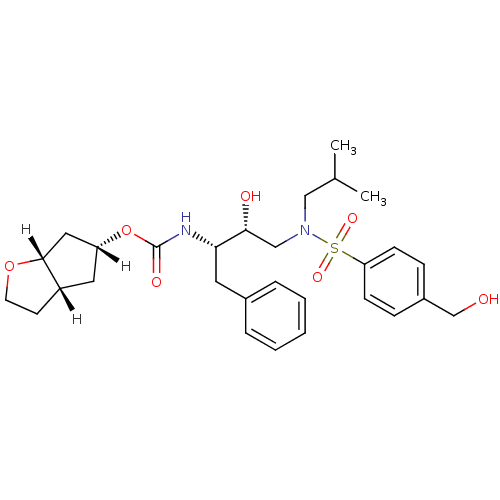
((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(CO)cc1 |r| Show InChI InChI=1S/C29H40N2O7S/c1-20(2)17-31(39(35,36)25-10-8-22(19-32)9-11-25)18-27(33)26(14-21-6-4-3-5-7-21)30-29(34)38-24-15-23-12-13-37-28(23)16-24/h3-11,20,23-24,26-28,32-33H,12-19H2,1-2H3,(H,30,34)/t23-,24+,26-,27+,28+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization in MT2 cells |
J Biol Chem 282: 28709-20 (2007)
Article DOI: 10.1074/jbc.M703938200
BindingDB Entry DOI: 10.7270/Q2JS9T6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM13925

((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(CO)cc1 |r| Show InChI InChI=1S/C29H40N2O7S/c1-20(2)17-31(39(35,36)25-10-8-22(19-32)9-11-25)18-27(33)26(14-21-6-4-3-5-7-21)30-29(34)38-24-15-23-12-13-37-28(23)16-24/h3-11,20,23-24,26-28,32-33H,12-19H2,1-2H3,(H,30,34)/t23-,24+,26-,27+,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00450 | -64.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 49: 5252-61 (2006)
Article DOI: 10.1021/jm060561m
BindingDB Entry DOI: 10.7270/Q23R0R41 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM4685
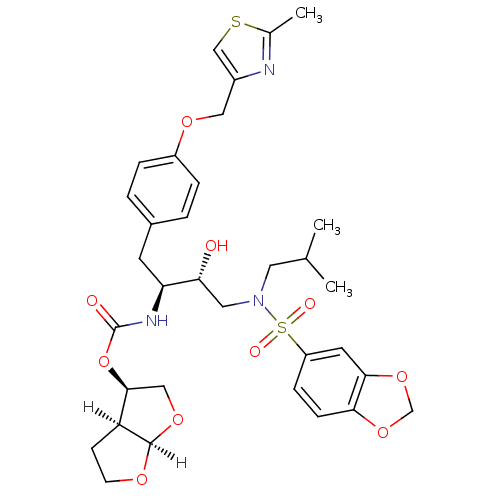
((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@]12OCC[C@@]1([H])[C@H](CO2)OC(=O)N[C@@H](Cc1ccc(OCc2csc(C)n2)cc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C33H41N3O10S2/c1-20(2)14-36(48(39,40)25-8-9-29-30(13-25)45-19-44-29)15-28(37)27(35-33(38)46-31-17-43-32-26(31)10-11-41-32)12-22-4-6-24(7-5-22)42-16-23-18-47-21(3)34-23/h4-9,13,18,20,26-28,31-32,37H,10-12,14-17,19H2,1-3H3,(H,35,38)/t26-,27-,28+,31-,32+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization in MT2 cells |
J Biol Chem 282: 28709-20 (2007)
Article DOI: 10.1074/jbc.M703938200
BindingDB Entry DOI: 10.7270/Q2JS9T6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM9236

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C28H38N2O8S/c1-19(2)16-30(39(33,34)22-11-9-21(35-3)10-12-22)17-25(31)24(15-20-7-5-4-6-8-20)29-28(32)38-26-18-37-27-23(26)13-14-36-27/h4-12,19,23-27,31H,13-18H2,1-3H3,(H,29,32)/t23-,24-,25+,26-,27+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization in MT2 cells |
J Biol Chem 282: 28709-20 (2007)
Article DOI: 10.1074/jbc.M703938200
BindingDB Entry DOI: 10.7270/Q2JS9T6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50379086
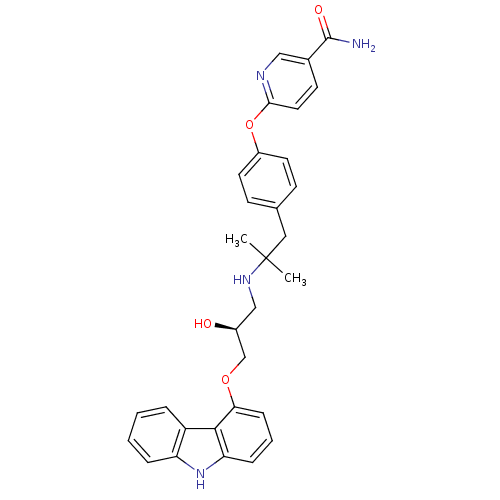
(CHEMBL2012521 | CHEMBL2012522 | LY-377604)Show SMILES CC(C)(Cc1ccc(Oc2ccc(cn2)C(N)=O)cc1)NC[C@H](O)COc1cccc2[nH]c3ccccc3c12 |r| Show InChI InChI=1S/C31H32N4O4/c1-31(2,16-20-10-13-23(14-11-20)39-28-15-12-21(17-33-28)30(32)37)34-18-22(36)19-38-27-9-5-8-26-29(27)24-6-3-4-7-25(24)35-26/h3-15,17,22,34-36H,16,18-19H2,1-2H3,(H2,32,37)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]Iodocyanopindolol from human adrenergic beta2 receptor expressed in insect sf9 cells by scintillation counting |
ACS Med Chem Lett 2: 583-586 (2011)
Article DOI: 10.1021/ml200071k
BindingDB Entry DOI: 10.7270/Q20R9QDP |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0140 | -62.0 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 49: 5252-61 (2006)
Article DOI: 10.1021/jm060561m
BindingDB Entry DOI: 10.7270/Q23R0R41 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50476647
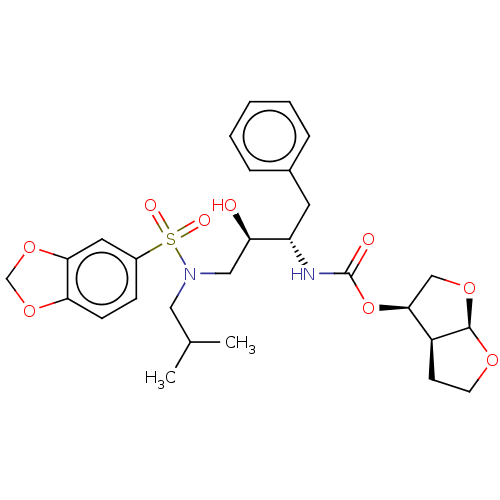
(CHEMBL178593 | GRL-98065)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C28H36N2O9S/c1-18(2)14-30(40(33,34)20-8-9-24-25(13-20)38-17-37-24)15-23(31)22(12-19-6-4-3-5-7-19)29-28(32)39-26-16-36-27-21(26)10-11-35-27/h3-9,13,18,21-23,26-27,31H,10-12,14-17H2,1-2H3,(H,29,32)/t21-,22-,23+,26-,27+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization in MT2 cells |
J Biol Chem 282: 28709-20 (2007)
Article DOI: 10.1074/jbc.M703938200
BindingDB Entry DOI: 10.7270/Q2JS9T6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization in MT2 cells |
J Biol Chem 282: 28709-20 (2007)
Article DOI: 10.1074/jbc.M703938200
BindingDB Entry DOI: 10.7270/Q2JS9T6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50379086

(CHEMBL2012521 | CHEMBL2012522 | LY-377604)Show SMILES CC(C)(Cc1ccc(Oc2ccc(cn2)C(N)=O)cc1)NC[C@H](O)COc1cccc2[nH]c3ccccc3c12 |r| Show InChI InChI=1S/C31H32N4O4/c1-31(2,16-20-10-13-23(14-11-20)39-28-15-12-21(17-33-28)30(32)37)34-18-22(36)19-38-27-9-5-8-26-29(27)24-6-3-4-7-25(24)35-26/h3-15,17,22,34-36H,16,18-19H2,1-2H3,(H2,32,37)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]Iodocyanopindolol from human adrenergic beta1 receptor expressed in insect sf9 cells by scintillation counting |
ACS Med Chem Lett 2: 583-586 (2011)
Article DOI: 10.1021/ml200071k
BindingDB Entry DOI: 10.7270/Q20R9QDP |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50478337

(CHEMBL403306 | GRL-0036A)Show SMILES [H][C@@]12CCO[C@]1([H])C[C@@H](C2)OC(=O)N[C@@H](CSc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(CO)cc1 Show InChI InChI=1S/C29H40N2O7S2/c1-20(2)16-31(40(35,36)25-10-8-21(18-32)9-11-25)17-27(33)26(19-39-24-6-4-3-5-7-24)30-29(34)38-23-14-22-12-13-37-28(22)15-23/h3-11,20,22-23,26-28,32-33H,12-19H2,1-2H3,(H,30,34)/t22-,23+,26-,27+,28+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease dimerization in MT2 cells |
J Biol Chem 282: 28709-20 (2007)
Article DOI: 10.1074/jbc.M703938200
BindingDB Entry DOI: 10.7270/Q2JS9T6J |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM13924
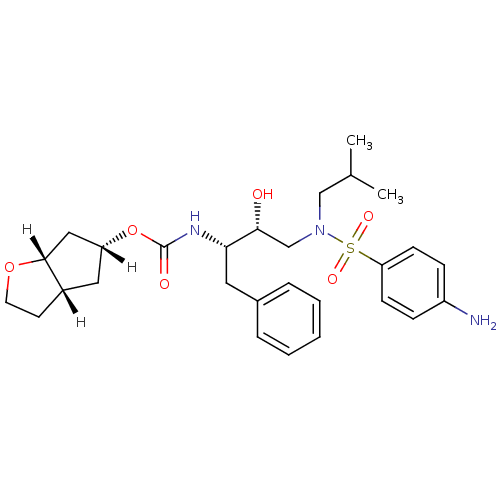
((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C28H39N3O6S/c1-19(2)17-31(38(34,35)24-10-8-22(29)9-11-24)18-26(32)25(14-20-6-4-3-5-7-20)30-28(33)37-23-15-21-12-13-36-27(21)16-23/h3-11,19,21,23,25-27,32H,12-18,29H2,1-2H3,(H,30,33)/t21-,23+,25-,26+,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | -56.2 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 49: 5252-61 (2006)
Article DOI: 10.1021/jm060561m
BindingDB Entry DOI: 10.7270/Q23R0R41 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186291
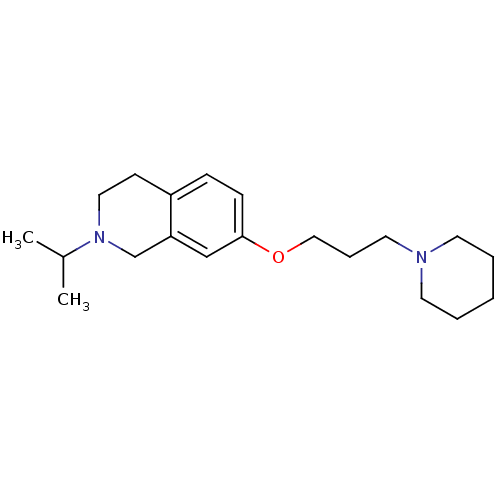
(2-isopropyl-7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-...)Show InChI InChI=1S/C20H32N2O/c1-17(2)22-13-9-18-7-8-20(15-19(18)16-22)23-14-6-12-21-10-4-3-5-11-21/h7-8,15,17H,3-6,9-14,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186270
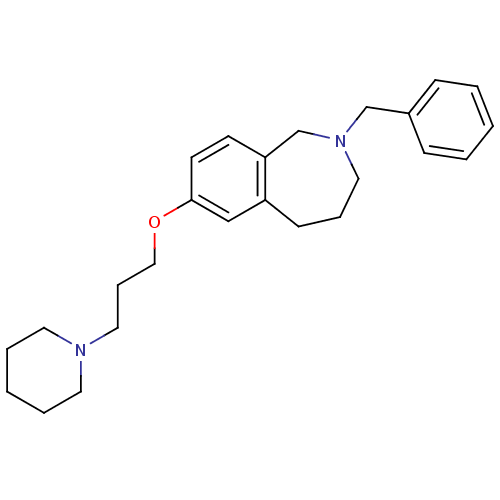
(2-benzyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tet...)Show InChI InChI=1S/C25H34N2O/c1-3-9-22(10-4-1)20-27-16-7-11-23-19-25(13-12-24(23)21-27)28-18-8-17-26-14-5-2-6-15-26/h1,3-4,9-10,12-13,19H,2,5-8,11,14-18,20-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186278
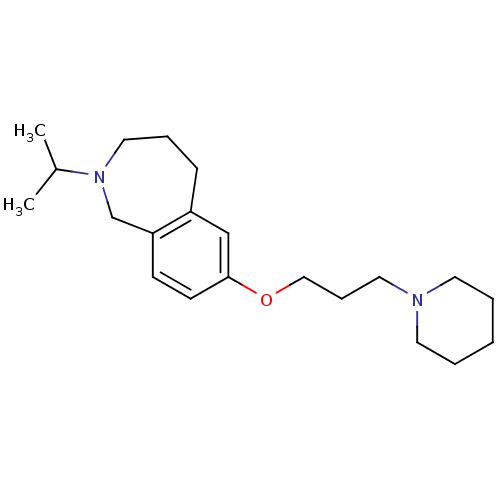
(2-isopropyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-...)Show InChI InChI=1S/C21H34N2O/c1-18(2)23-14-6-8-19-16-21(10-9-20(19)17-23)24-15-7-13-22-11-4-3-5-12-22/h9-10,16,18H,3-8,11-15,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186290
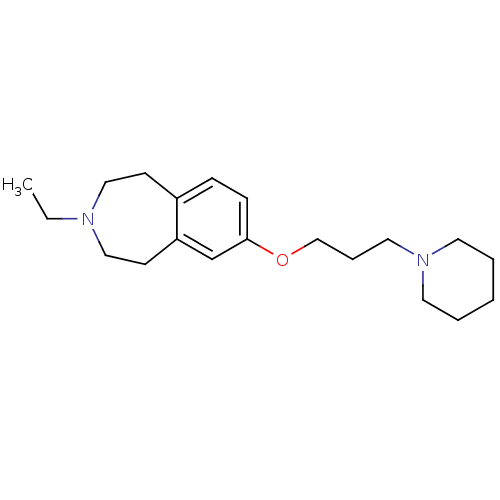
(3-ethyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tetr...)Show InChI InChI=1S/C20H32N2O/c1-2-21-14-9-18-7-8-20(17-19(18)10-15-21)23-16-6-13-22-11-4-3-5-12-22/h7-8,17H,2-6,9-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186309

(2-ethyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tetr...)Show InChI InChI=1S/C20H32N2O/c1-2-21-13-6-8-18-16-20(10-9-19(18)17-21)23-15-7-14-22-11-4-3-5-12-22/h9-10,16H,2-8,11-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186269
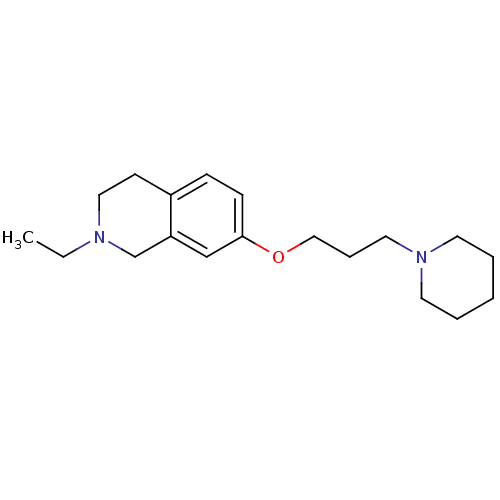
(2-ethyl-7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetr...)Show InChI InChI=1S/C19H30N2O/c1-2-20-13-9-17-7-8-19(15-18(17)16-20)22-14-6-12-21-10-4-3-5-11-21/h7-8,15H,2-6,9-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186292

(2-(cyclohexylmethyl)-7-(3-(piperidin-1-yl)propoxy)...)Show InChI InChI=1S/C24H38N2O/c1-3-8-21(9-4-1)19-26-16-12-22-10-11-24(18-23(22)20-26)27-17-7-15-25-13-5-2-6-14-25/h10-11,18,21H,1-9,12-17,19-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186292

(2-(cyclohexylmethyl)-7-(3-(piperidin-1-yl)propoxy)...)Show InChI InChI=1S/C24H38N2O/c1-3-8-21(9-4-1)19-26-16-12-22-10-11-24(18-23(22)20-26)27-17-7-15-25-13-5-2-6-14-25/h10-11,18,21H,1-9,12-17,19-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186306
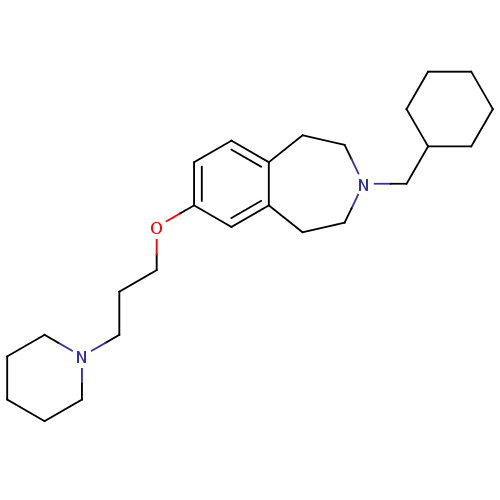
(3-(cyclohexylmethyl)-7-(3-(piperidin-1-yl)propoxy)...)Show InChI InChI=1S/C25H40N2O/c1-3-8-22(9-4-1)21-27-17-12-23-10-11-25(20-24(23)13-18-27)28-19-7-16-26-14-5-2-6-15-26/h10-11,20,22H,1-9,12-19,21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186316
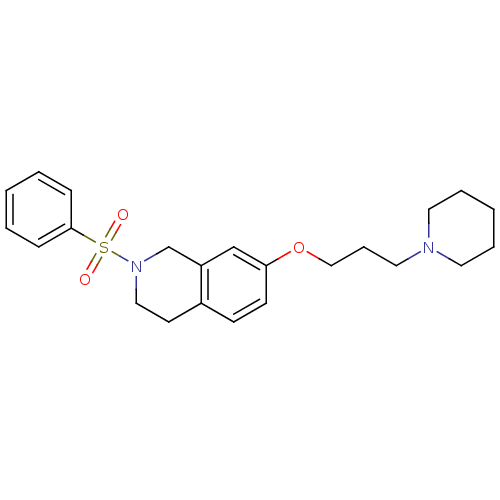
(2-(phenylsulfonyl)-7-(3-(piperidin-1-yl)propoxy)-1...)Show SMILES O=S(=O)(N1CCc2ccc(OCCCN3CCCCC3)cc2C1)c1ccccc1 Show InChI InChI=1S/C23H30N2O3S/c26-29(27,23-8-3-1-4-9-23)25-16-12-20-10-11-22(18-21(20)19-25)28-17-7-15-24-13-5-2-6-14-24/h1,3-4,8-11,18H,2,5-7,12-17,19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186283
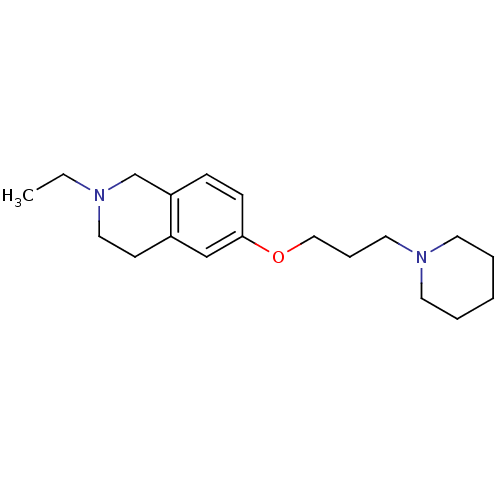
(2-ethyl-6-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetr...)Show InChI InChI=1S/C19H30N2O/c1-2-20-13-9-17-15-19(8-7-18(17)16-20)22-14-6-12-21-10-4-3-5-11-21/h7-8,15H,2-6,9-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186309

(2-ethyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tetr...)Show InChI InChI=1S/C20H32N2O/c1-2-21-13-6-8-18-16-20(10-9-19(18)17-21)23-15-7-14-22-11-4-3-5-12-22/h9-10,16H,2-8,11-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186293
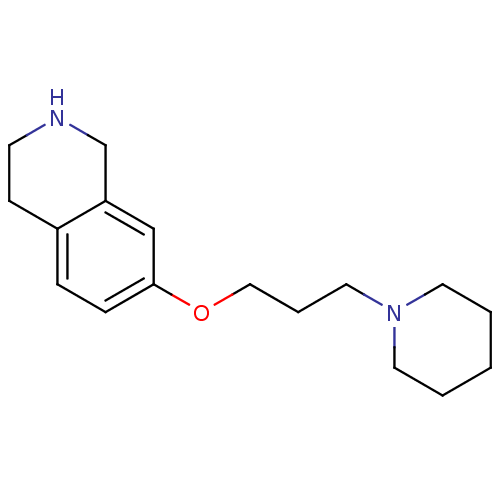
(7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetrahydrois...)Show InChI InChI=1S/C17H26N2O/c1-2-9-19(10-3-1)11-4-12-20-17-6-5-15-7-8-18-14-16(15)13-17/h5-6,13,18H,1-4,7-12,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186269

(2-ethyl-7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetr...)Show InChI InChI=1S/C19H30N2O/c1-2-20-13-9-17-7-8-19(15-18(17)16-20)22-14-6-12-21-10-4-3-5-11-21/h7-8,15H,2-6,9-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186311
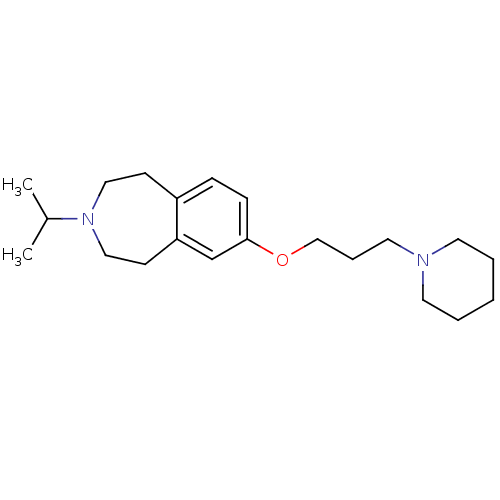
(3-isopropyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-...)Show InChI InChI=1S/C21H34N2O/c1-18(2)23-14-9-19-7-8-21(17-20(19)10-15-23)24-16-6-13-22-11-4-3-5-12-22/h7-8,17-18H,3-6,9-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186270

(2-benzyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tet...)Show InChI InChI=1S/C25H34N2O/c1-3-9-22(10-4-1)20-27-16-7-11-23-19-25(13-12-24(23)21-27)28-18-8-17-26-14-5-2-6-15-26/h1,3-4,9-10,12-13,19H,2,5-8,11,14-18,20-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186291

(2-isopropyl-7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-...)Show InChI InChI=1S/C20H32N2O/c1-17(2)22-13-9-18-7-8-20(15-19(18)16-22)23-14-6-12-21-10-4-3-5-11-21/h7-8,15,17H,3-6,9-14,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186306

(3-(cyclohexylmethyl)-7-(3-(piperidin-1-yl)propoxy)...)Show InChI InChI=1S/C25H40N2O/c1-3-8-22(9-4-1)21-27-17-12-23-10-11-25(20-24(23)13-18-27)28-19-7-16-26-14-5-2-6-15-26/h10-11,20,22H,1-9,12-19,21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50396808
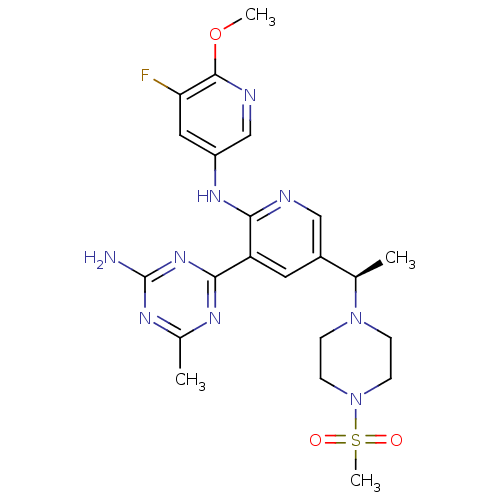
(CHEMBL2170081 | US8772480, 148)Show SMILES COc1ncc(Nc2ncc(cc2-c2nc(C)nc(N)n2)[C@@H](C)N2CCN(CC2)S(C)(=O)=O)cc1F |r| Show InChI InChI=1S/C22H28FN9O3S/c1-13(31-5-7-32(8-6-31)36(4,33)34)15-9-17(20-27-14(2)28-22(24)30-20)19(25-11-15)29-16-10-18(23)21(35-3)26-12-16/h9-13H,5-8H2,1-4H3,(H,25,29)(H2,24,27,28,30)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110gamma expressed in baculovirus infected Hi5 cells using ATP as substrate after 20 mins by spec... |
J Med Chem 55: 7796-816 (2012)
Article DOI: 10.1021/jm300846z
BindingDB Entry DOI: 10.7270/Q2028SPT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50396820
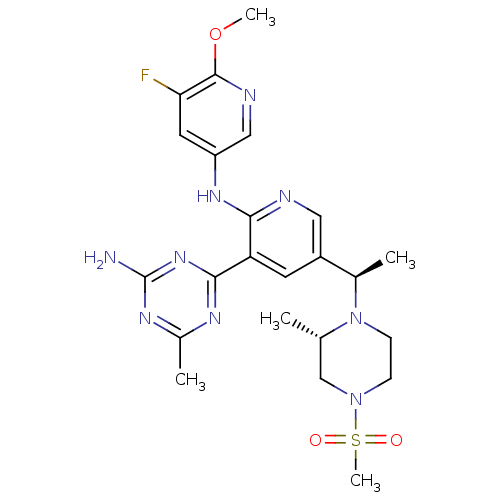
(CHEMBL2170088 | US8772480, 272)Show SMILES COc1ncc(Nc2ncc(cc2-c2nc(C)nc(N)n2)[C@@H](C)N2CCN(C[C@@H]2C)S(C)(=O)=O)cc1F |r| Show InChI InChI=1S/C23H30FN9O3S/c1-13-12-32(37(5,34)35)6-7-33(13)14(2)16-8-18(21-28-15(3)29-23(25)31-21)20(26-10-16)30-17-9-19(24)22(36-4)27-11-17/h8-11,13-14H,6-7,12H2,1-5H3,(H,26,30)(H2,25,28,29,31)/t13-,14+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110alpha/p85alpha expressed in baculovirus infected Sf9 cells using phosphatidylinositol-4,5-bisp... |
J Med Chem 55: 7796-816 (2012)
Article DOI: 10.1021/jm300846z
BindingDB Entry DOI: 10.7270/Q2028SPT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396806
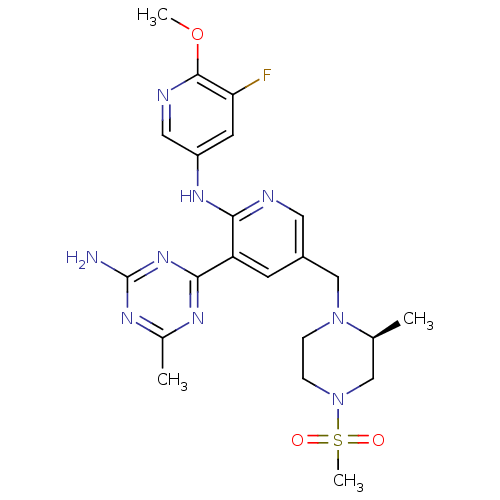
(CHEMBL2170083 | US8772480, 270)Show SMILES COc1ncc(Nc2ncc(CN3CCN(C[C@@H]3C)S(C)(=O)=O)cc2-c2nc(C)nc(N)n2)cc1F |r| Show InChI InChI=1S/C22H28FN9O3S/c1-13-11-32(36(4,33)34)6-5-31(13)12-15-7-17(20-27-14(2)28-22(24)30-20)19(25-9-15)29-16-8-18(23)21(35-3)26-10-16/h7-10,13H,5-6,11-12H2,1-4H3,(H,25,29)(H2,24,27,28,30)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110delta/p85alpha expressed in baculovirus infected Sf9 cells using phosphatidylinositol-4,5-bisp... |
J Med Chem 55: 7796-816 (2012)
Article DOI: 10.1021/jm300846z
BindingDB Entry DOI: 10.7270/Q2028SPT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396807
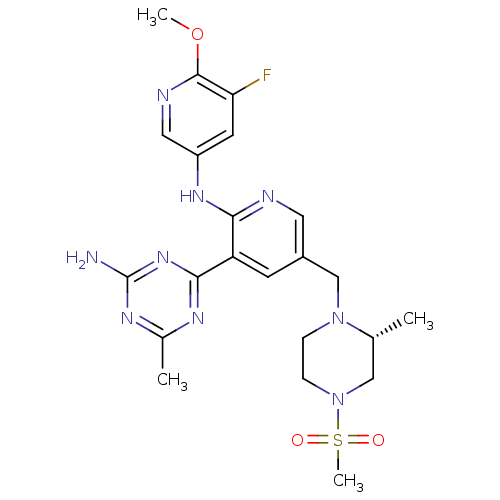
(CHEMBL2170082 | US8772480, 316)Show SMILES COc1ncc(Nc2ncc(CN3CCN(C[C@H]3C)S(C)(=O)=O)cc2-c2nc(C)nc(N)n2)cc1F |r| Show InChI InChI=1S/C22H28FN9O3S/c1-13-11-32(36(4,33)34)6-5-31(13)12-15-7-17(20-27-14(2)28-22(24)30-20)19(25-9-15)29-16-8-18(23)21(35-3)26-10-16/h7-10,13H,5-6,11-12H2,1-4H3,(H,25,29)(H2,24,27,28,30)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110delta/p85alpha expressed in baculovirus infected Sf9 cells using phosphatidylinositol-4,5-bisp... |
J Med Chem 55: 7796-816 (2012)
Article DOI: 10.1021/jm300846z
BindingDB Entry DOI: 10.7270/Q2028SPT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396809
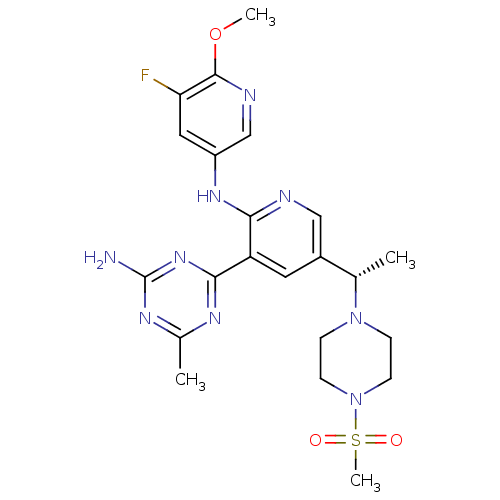
(CHEMBL2170099 | US8772480, 147)Show SMILES COc1ncc(Nc2ncc(cc2-c2nc(C)nc(N)n2)[C@H](C)N2CCN(CC2)S(C)(=O)=O)cc1F |r| Show InChI InChI=1S/C22H28FN9O3S/c1-13(31-5-7-32(8-6-31)36(4,33)34)15-9-17(20-27-14(2)28-22(24)30-20)19(25-11-15)29-16-10-18(23)21(35-3)26-12-16/h9-13H,5-8H2,1-4H3,(H,25,29)(H2,24,27,28,30)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110delta/p85alpha expressed in baculovirus infected Sf9 cells using phosphatidylinositol-4,5-bisp... |
J Med Chem 55: 7796-816 (2012)
Article DOI: 10.1021/jm300846z
BindingDB Entry DOI: 10.7270/Q2028SPT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186296
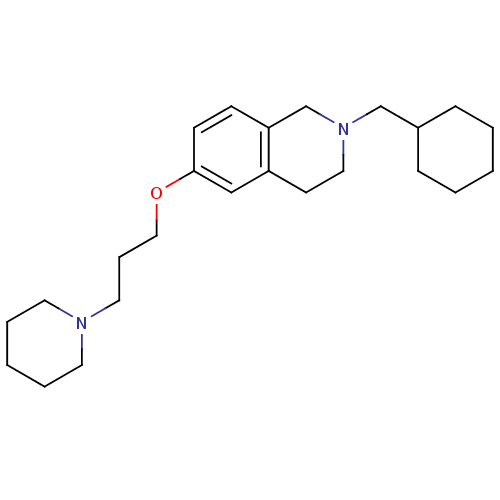
(2-(cyclohexylmethyl)-6-(3-(piperidin-1-yl)propoxy)...)Show InChI InChI=1S/C24H38N2O/c1-3-8-21(9-4-1)19-26-16-12-22-18-24(11-10-23(22)20-26)27-17-7-15-25-13-5-2-6-14-25/h10-11,18,21H,1-9,12-17,19-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186290

(3-ethyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tetr...)Show InChI InChI=1S/C20H32N2O/c1-2-21-14-9-18-7-8-20(17-19(18)10-15-21)23-16-6-13-22-11-4-3-5-12-22/h7-8,17H,2-6,9-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186278

(2-isopropyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-...)Show InChI InChI=1S/C21H34N2O/c1-18(2)23-14-6-8-19-16-21(10-9-20(19)17-23)24-15-7-13-22-11-4-3-5-12-22/h9-10,16,18H,3-8,11-15,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186311

(3-isopropyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-...)Show InChI InChI=1S/C21H34N2O/c1-18(2)23-14-9-19-7-8-21(17-20(19)10-15-23)24-16-6-13-22-11-4-3-5-12-22/h7-8,17-18H,3-6,9-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50056221
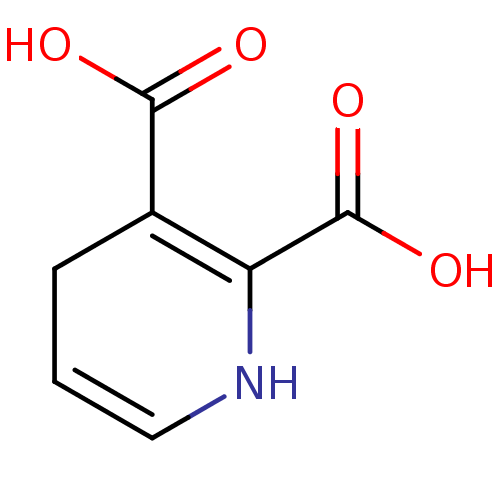
(CHEMBL3322297)Show InChI InChI=1S/C7H7NO4/c9-6(10)4-2-1-3-8-5(4)7(11)12/h1,3,8H,2H2,(H,9,10)(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Thessaly
Curated by ChEMBL
| Assay Description
Inhibition of glycogen phosphorylase b (unknown origin) |
Bioorg Med Chem 22: 4810-25 (2014)
Article DOI: 10.1016/j.bmc.2014.06.058
BindingDB Entry DOI: 10.7270/Q2KS6T58 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396808

(CHEMBL2170081 | US8772480, 148)Show SMILES COc1ncc(Nc2ncc(cc2-c2nc(C)nc(N)n2)[C@@H](C)N2CCN(CC2)S(C)(=O)=O)cc1F |r| Show InChI InChI=1S/C22H28FN9O3S/c1-13(31-5-7-32(8-6-31)36(4,33)34)15-9-17(20-27-14(2)28-22(24)30-20)19(25-11-15)29-16-10-18(23)21(35-3)26-12-16/h9-13H,5-8H2,1-4H3,(H,25,29)(H2,24,27,28,30)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110delta/p85alpha expressed in baculovirus infected Sf9 cells using phosphatidylinositol-4,5-bisp... |
J Med Chem 55: 7796-816 (2012)
Article DOI: 10.1021/jm300846z
BindingDB Entry DOI: 10.7270/Q2028SPT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50396821
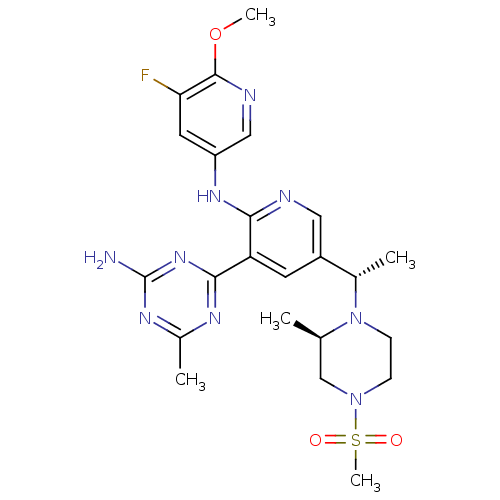
(CHEMBL2170087 | US8772480, 361)Show SMILES COc1ncc(Nc2ncc(cc2-c2nc(C)nc(N)n2)[C@H](C)N2CCN(C[C@H]2C)S(C)(=O)=O)cc1F |r| Show InChI InChI=1S/C23H30FN9O3S/c1-13-12-32(37(5,34)35)6-7-33(13)14(2)16-8-18(21-28-15(3)29-23(25)31-21)20(26-10-16)30-17-9-19(24)22(36-4)27-11-17/h8-11,13-14H,6-7,12H2,1-5H3,(H,26,30)(H2,25,28,29,31)/t13-,14+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110alpha/p85alpha expressed in baculovirus infected Sf9 cells using phosphatidylinositol-4,5-bisp... |
J Med Chem 55: 7796-816 (2012)
Article DOI: 10.1021/jm300846z
BindingDB Entry DOI: 10.7270/Q2028SPT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396822
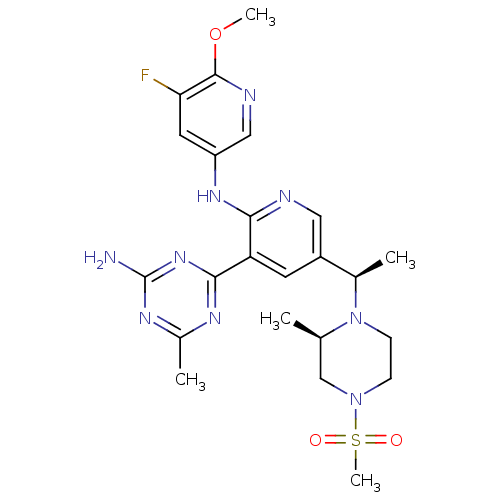
(CHEMBL2170086 | US8772480, 360)Show SMILES COc1ncc(Nc2ncc(cc2-c2nc(C)nc(N)n2)[C@@H](C)N2CCN(C[C@H]2C)S(C)(=O)=O)cc1F |r| Show InChI InChI=1S/C23H30FN9O3S/c1-13-12-32(37(5,34)35)6-7-33(13)14(2)16-8-18(21-28-15(3)29-23(25)31-21)20(26-10-16)30-17-9-19(24)22(36-4)27-11-17/h8-11,13-14H,6-7,12H2,1-5H3,(H,26,30)(H2,25,28,29,31)/t13-,14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110delta/p85alpha expressed in baculovirus infected Sf9 cells using phosphatidylinositol-4,5-bisp... |
J Med Chem 55: 7796-816 (2012)
Article DOI: 10.1021/jm300846z
BindingDB Entry DOI: 10.7270/Q2028SPT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50396806

(CHEMBL2170083 | US8772480, 270)Show SMILES COc1ncc(Nc2ncc(CN3CCN(C[C@@H]3C)S(C)(=O)=O)cc2-c2nc(C)nc(N)n2)cc1F |r| Show InChI InChI=1S/C22H28FN9O3S/c1-13-11-32(36(4,33)34)6-5-31(13)12-15-7-17(20-27-14(2)28-22(24)30-20)19(25-9-15)29-16-8-18(23)21(35-3)26-10-16/h7-10,13H,5-6,11-12H2,1-4H3,(H,25,29)(H2,24,27,28,30)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110alpha/p85alpha expressed in baculovirus infected Sf9 cells using phosphatidylinositol-4,5-bisp... |
J Med Chem 55: 7796-816 (2012)
Article DOI: 10.1021/jm300846z
BindingDB Entry DOI: 10.7270/Q2028SPT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396821

(CHEMBL2170087 | US8772480, 361)Show SMILES COc1ncc(Nc2ncc(cc2-c2nc(C)nc(N)n2)[C@H](C)N2CCN(C[C@H]2C)S(C)(=O)=O)cc1F |r| Show InChI InChI=1S/C23H30FN9O3S/c1-13-12-32(37(5,34)35)6-7-33(13)14(2)16-8-18(21-28-15(3)29-23(25)31-21)20(26-10-16)30-17-9-19(24)22(36-4)27-11-17/h8-11,13-14H,6-7,12H2,1-5H3,(H,26,30)(H2,25,28,29,31)/t13-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110delta/p85alpha expressed in baculovirus infected Sf9 cells using phosphatidylinositol-4,5-bisp... |
J Med Chem 55: 7796-816 (2012)
Article DOI: 10.1021/jm300846z
BindingDB Entry DOI: 10.7270/Q2028SPT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186281
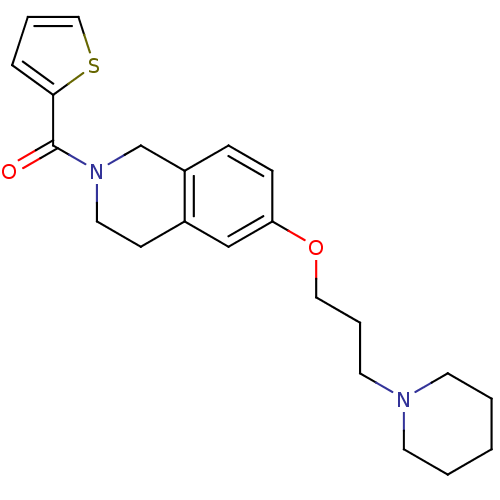
((6-(3-(piperidin-1-yl)propoxy)-3,4-dihydroisoquino...)Show InChI InChI=1S/C22H28N2O2S/c25-22(21-6-4-15-27-21)24-13-9-18-16-20(8-7-19(18)17-24)26-14-5-12-23-10-2-1-3-11-23/h4,6-8,15-16H,1-3,5,9-14,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186295
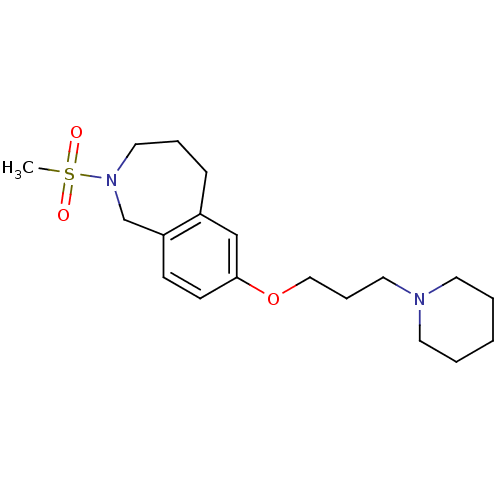
(2-(methylsulfonyl)-7-(3-(piperidin-1-yl)propoxy)-2...)Show InChI InChI=1S/C19H30N2O3S/c1-25(22,23)21-13-5-7-17-15-19(9-8-18(17)16-21)24-14-6-12-20-10-3-2-4-11-20/h8-9,15H,2-7,10-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50396807

(CHEMBL2170082 | US8772480, 316)Show SMILES COc1ncc(Nc2ncc(CN3CCN(C[C@H]3C)S(C)(=O)=O)cc2-c2nc(C)nc(N)n2)cc1F |r| Show InChI InChI=1S/C22H28FN9O3S/c1-13-11-32(36(4,33)34)6-5-31(13)12-15-7-17(20-27-14(2)28-22(24)30-20)19(25-9-15)29-16-8-18(23)21(35-3)26-10-16/h7-10,13H,5-6,11-12H2,1-4H3,(H,25,29)(H2,24,27,28,30)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110alpha/p85alpha expressed in baculovirus infected Sf9 cells using phosphatidylinositol-4,5-bisp... |
J Med Chem 55: 7796-816 (2012)
Article DOI: 10.1021/jm300846z
BindingDB Entry DOI: 10.7270/Q2028SPT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396805
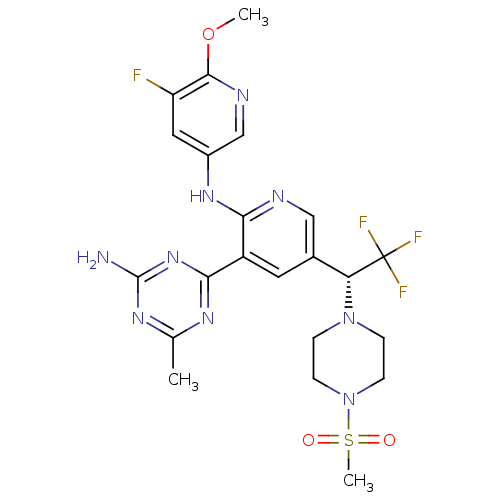
(CHEMBL2170084)Show SMILES COc1ncc(Nc2ncc(cc2-c2nc(C)nc(N)n2)[C@@H](N2CCN(CC2)S(C)(=O)=O)C(F)(F)F)cc1F |r| Show InChI InChI=1S/C22H25F4N9O3S/c1-12-30-19(33-21(27)31-12)15-8-13(10-28-18(15)32-14-9-16(23)20(38-2)29-11-14)17(22(24,25)26)34-4-6-35(7-5-34)39(3,36)37/h8-11,17H,4-7H2,1-3H3,(H,28,32)(H2,27,30,31,33)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110delta/p85alpha expressed in baculovirus infected Sf9 cells using phosphatidylinositol-4,5-bisp... |
J Med Chem 55: 7796-816 (2012)
Article DOI: 10.1021/jm300846z
BindingDB Entry DOI: 10.7270/Q2028SPT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50396808

(CHEMBL2170081 | US8772480, 148)Show SMILES COc1ncc(Nc2ncc(cc2-c2nc(C)nc(N)n2)[C@@H](C)N2CCN(CC2)S(C)(=O)=O)cc1F |r| Show InChI InChI=1S/C22H28FN9O3S/c1-13(31-5-7-32(8-6-31)36(4,33)34)15-9-17(20-27-14(2)28-22(24)30-20)19(25-11-15)29-16-10-18(23)21(35-3)26-12-16/h9-13H,5-8H2,1-4H3,(H,25,29)(H2,24,27,28,30)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110alpha/p85alpha expressed in baculovirus infected Sf9 cells using phosphatidylinositol-4,5-bisp... |
J Med Chem 55: 7796-816 (2012)
Article DOI: 10.1021/jm300846z
BindingDB Entry DOI: 10.7270/Q2028SPT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394846
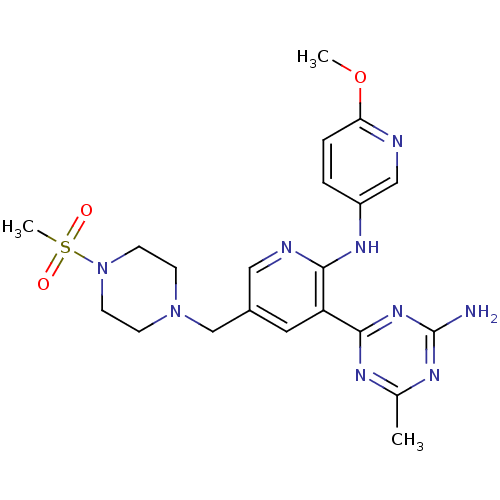
(CHEMBL2165017 | US8772480, 35)Show SMILES COc1ccc(Nc2ncc(CN3CCN(CC3)S(C)(=O)=O)cc2-c2nc(C)nc(N)n2)cn1 Show InChI InChI=1S/C21H27N9O3S/c1-14-25-20(28-21(22)26-14)17-10-15(13-29-6-8-30(9-7-29)34(3,31)32)11-24-19(17)27-16-4-5-18(33-2)23-12-16/h4-5,10-12H,6-9,13H2,1-3H3,(H,24,27)(H2,22,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal polyHis-tagged PI3K p110delta/p85alpha expressed in baculovirus infected Sf9 cells using phosphatidylinositol-4,5-bisp... |
J Med Chem 55: 7796-816 (2012)
Article DOI: 10.1021/jm300846z
BindingDB Entry DOI: 10.7270/Q2028SPT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data