 Found 153 hits with Last Name = 'werner' and Initial = 'd'
Found 153 hits with Last Name = 'werner' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339062
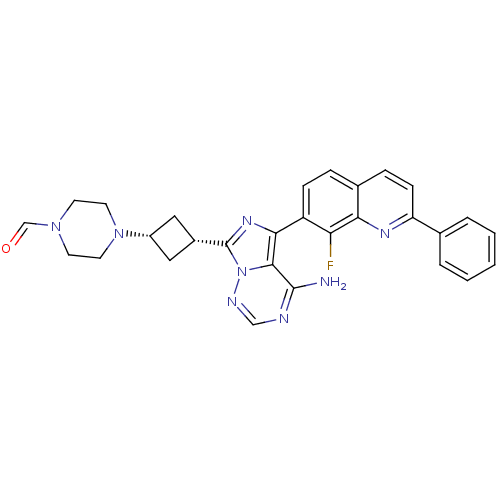
(4-(3-(4-amino-5-(8-fluoro-2-phenylquinolin-7-yl)im...)Show SMILES Nc1ncnn2c(nc(-c3ccc4ccc(nc4c3F)-c3ccccc3)c12)[C@@H]1C[C@@H](C1)N1CCN(CC1)C=O |r,wU:27.31,29.36,(5.56,-13.24,;5.57,-14.78,;4.24,-15.55,;4.24,-17.09,;5.57,-17.86,;6.9,-17.08,;8.37,-17.56,;9.28,-16.31,;8.37,-15.06,;9.12,-13.72,;8.34,-12.4,;9.1,-11.06,;10.64,-11.05,;11.4,-9.71,;12.94,-9.7,;13.72,-11.04,;12.95,-12.37,;11.41,-12.38,;10.67,-13.71,;11.45,-15.04,;15.25,-11.03,;16.03,-12.36,;17.57,-12.35,;18.33,-11.01,;17.55,-9.68,;16.01,-9.69,;6.9,-15.54,;8.84,-19.01,;8.14,-20.39,;9.52,-21.09,;10.21,-19.71,;10,-22.55,;8.96,-23.7,;9.43,-25.15,;10.94,-25.48,;11.97,-24.34,;11.5,-22.87,;11.41,-26.95,;10.37,-28.09,)| Show InChI InChI=1S/C29H27FN8O/c30-24-22(8-6-19-7-9-23(34-25(19)24)18-4-2-1-3-5-18)26-27-28(31)32-16-33-38(27)29(35-26)20-14-21(15-20)37-12-10-36(17-39)11-13-37/h1-9,16-17,20-21H,10-15H2,(H2,31,32,33)/t20-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IGF1R expressed in mouse NIH-3T3 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339064
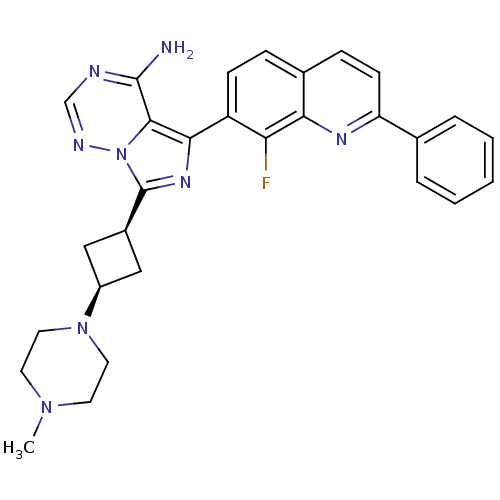
(5-(8-fluoro-2-phenylquinolin-7-yl)-7-(3-(4-methylp...)Show SMILES CN1CCN(CC1)[C@H]1C[C@H](C1)c1nc(-c2ccc3ccc(nc3c2F)-c2ccccc2)c2c(N)ncnn12 |r,wU:9.12,7.7,(40.85,-28.31,;40.38,-26.84,;38.87,-26.51,;38.4,-25.05,;39.44,-23.91,;40.94,-24.23,;41.41,-25.7,;38.96,-22.44,;37.58,-21.74,;38.28,-20.37,;39.66,-21.07,;37.81,-18.92,;38.72,-17.67,;37.81,-16.42,;38.56,-15.08,;37.78,-13.76,;38.54,-12.42,;40.08,-12.4,;40.84,-11.07,;42.38,-11.06,;43.16,-12.39,;42.39,-13.73,;40.85,-13.74,;40.11,-15.07,;40.89,-16.39,;44.69,-12.38,;45.47,-13.72,;47.01,-13.71,;47.77,-12.37,;46.99,-11.04,;45.45,-11.05,;36.34,-16.9,;35.01,-16.13,;35,-14.59,;33.68,-16.9,;33.68,-18.45,;35.01,-19.22,;36.34,-18.44,)| Show InChI InChI=1S/C29H29FN8/c1-36-11-13-37(14-12-36)21-15-20(16-21)29-35-26(27-28(31)32-17-33-38(27)29)22-9-7-19-8-10-23(34-25(19)24(22)30)18-5-3-2-4-6-18/h2-10,17,20-21H,11-16H2,1H3,(H2,31,32,33)/t20-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IGF1R expressed in mouse NIH-3T3 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50336317
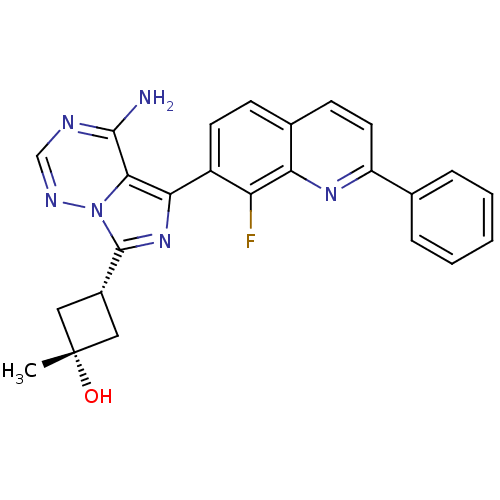
(CHEMBL1667935 | cis-3-[4-Amino-5-(8-fluoro-2-pheny...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2F)-c2ccccc2)c2c(N)ncnn12 |r,wU:4.6,1.1,wD:1.0,(-2.6,-22.44,;-3.93,-21.67,;-4.34,-23.15,;-3.24,-20.29,;-4.61,-19.61,;-5.31,-20.98,;-5.1,-18.14,;-4.2,-16.89,;-5.11,-15.65,;-4.34,-14.32,;-5.11,-12.99,;-4.34,-11.66,;-2.79,-11.65,;-2.03,-10.33,;-.5,-10.33,;.27,-11.66,;-.5,-12.99,;-2.03,-12.99,;-2.8,-14.32,;-2.03,-15.66,;1.81,-11.66,;2.58,-13,;4.12,-13,;4.89,-11.66,;4.11,-10.32,;2.57,-10.33,;-6.58,-16.13,;-7.91,-15.37,;-7.92,-13.83,;-9.24,-16.14,;-9.25,-17.68,;-7.91,-18.46,;-6.57,-17.68,)| Show InChI InChI=1S/C25H21FN6O/c1-25(33)11-16(12-25)24-31-21(22-23(27)28-13-29-32(22)24)17-9-7-15-8-10-18(30-20(15)19(17)26)14-5-3-2-4-6-14/h2-10,13,16,33H,11-12H2,1H3,(H2,27,28,29)/t16-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IGF1R expressed in mouse NIH-3T3 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339063
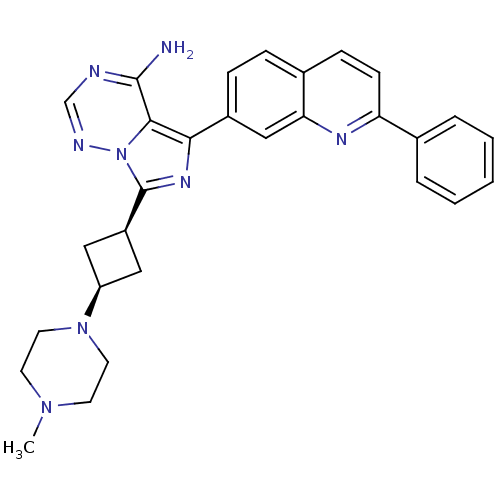
(7-(3-(4-methylpiperazin-1-yl)cyclobutyl)-5-(2-phen...)Show SMILES CN1CCN(CC1)[C@H]1C[C@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)ncnn12 |r,wU:9.12,7.7,(25.86,-27.54,;25.39,-26.08,;23.89,-25.75,;23.42,-24.29,;24.45,-23.15,;25.96,-23.46,;26.43,-24.93,;23.97,-21.68,;22.6,-20.98,;23.3,-19.61,;24.67,-20.31,;22.82,-18.15,;23.73,-16.9,;22.82,-15.66,;23.58,-14.32,;22.79,-13,;23.55,-11.66,;25.09,-11.64,;25.85,-10.31,;27.39,-10.3,;28.17,-11.63,;27.41,-12.97,;25.87,-12.98,;25.12,-14.3,;29.71,-11.62,;30.48,-12.95,;32.02,-12.95,;32.79,-11.61,;32,-10.27,;30.47,-10.29,;21.36,-16.13,;20.02,-15.37,;20.02,-13.83,;18.69,-16.14,;18.69,-17.68,;20.03,-18.45,;21.36,-17.68,)| Show InChI InChI=1S/C29H30N8/c1-35-11-13-36(14-12-35)23-15-22(16-23)29-34-26(27-28(30)31-18-32-37(27)29)21-8-7-20-9-10-24(33-25(20)17-21)19-5-3-2-4-6-19/h2-10,17-18,22-23H,11-16H2,1H3,(H2,30,31,32)/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IGF1R expressed in mouse NIH-3T3 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339061
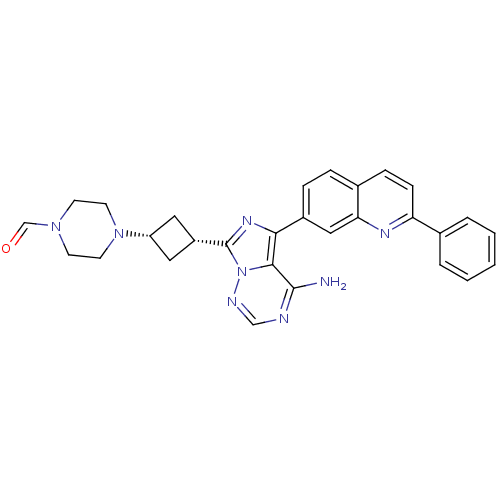
(4-(3-(4-amino-5-(2-phenylquinolin-7-yl)imidazo[1,5...)Show SMILES Nc1ncnn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)[C@@H]1C[C@@H](C1)N1CCN(CC1)C=O |r,wU:26.30,28.35,(-7.24,-13.78,;-7.23,-15.32,;-8.56,-16.09,;-8.56,-17.63,;-7.23,-18.41,;-5.9,-17.63,;-4.43,-18.1,;-3.52,-16.85,;-4.43,-15.61,;-3.68,-14.27,;-4.46,-12.95,;-3.7,-11.61,;-2.16,-11.59,;-1.4,-10.26,;.14,-10.24,;.92,-11.58,;.15,-12.92,;-1.39,-12.92,;-2.13,-14.25,;2.45,-11.57,;3.23,-12.9,;4.77,-12.9,;5.53,-11.56,;4.75,-10.22,;3.21,-10.24,;-5.9,-16.08,;-3.96,-19.56,;-4.66,-20.93,;-3.28,-21.63,;-2.58,-20.26,;-2.8,-23.1,;-3.84,-24.24,;-3.37,-25.7,;-1.86,-26.03,;-.83,-24.88,;-1.3,-23.41,;-1.39,-27.49,;-2.43,-28.63,)| Show InChI InChI=1S/C29H28N8O/c30-28-27-26(21-7-6-20-8-9-24(33-25(20)16-21)19-4-2-1-3-5-19)34-29(37(27)32-17-31-28)22-14-23(15-22)36-12-10-35(18-38)11-13-36/h1-9,16-18,22-23H,10-15H2,(H2,30,31,32)/t22-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IGF1R expressed in mouse NIH-3T3 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50339062

(4-(3-(4-amino-5-(8-fluoro-2-phenylquinolin-7-yl)im...)Show SMILES Nc1ncnn2c(nc(-c3ccc4ccc(nc4c3F)-c3ccccc3)c12)[C@@H]1C[C@@H](C1)N1CCN(CC1)C=O |r,wU:27.31,29.36,(5.56,-13.24,;5.57,-14.78,;4.24,-15.55,;4.24,-17.09,;5.57,-17.86,;6.9,-17.08,;8.37,-17.56,;9.28,-16.31,;8.37,-15.06,;9.12,-13.72,;8.34,-12.4,;9.1,-11.06,;10.64,-11.05,;11.4,-9.71,;12.94,-9.7,;13.72,-11.04,;12.95,-12.37,;11.41,-12.38,;10.67,-13.71,;11.45,-15.04,;15.25,-11.03,;16.03,-12.36,;17.57,-12.35,;18.33,-11.01,;17.55,-9.68,;16.01,-9.69,;6.9,-15.54,;8.84,-19.01,;8.14,-20.39,;9.52,-21.09,;10.21,-19.71,;10,-22.55,;8.96,-23.7,;9.43,-25.15,;10.94,-25.48,;11.97,-24.34,;11.5,-22.87,;11.41,-26.95,;10.37,-28.09,)| Show InChI InChI=1S/C29H27FN8O/c30-24-22(8-6-19-7-9-23(34-25(19)24)18-4-2-1-3-5-18)26-27-28(31)32-16-33-38(27)29(35-26)20-14-21(15-20)37-12-10-36(17-39)11-13-37/h1-9,16-17,20-21H,10-15H2,(H2,31,32,33)/t20-,21+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IR expressed in human HepG2 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199659
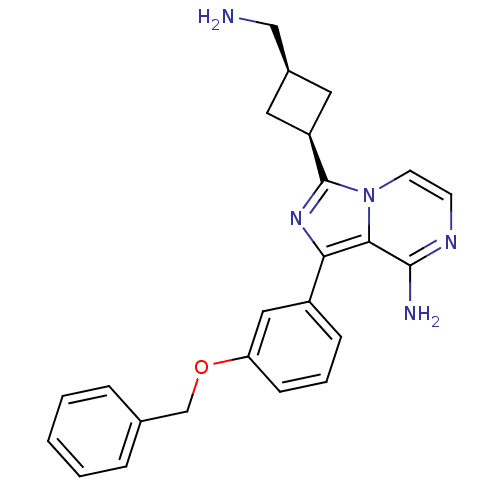
(CHEMBL392788 | US8481733, Comparator 1 | cis-3-(3-...)Show SMILES NC[C@H]1C[C@H](C1)c1nc(-c2cccc(OCc3ccccc3)c2)c2c(N)nccn12 |wU:4.6,2.1,(-3.93,-46.02,;-5.43,-45.7,;-5.91,-44.24,;-7.28,-43.54,;-6.58,-42.18,;-5.21,-42.87,;-7.06,-40.71,;-6.15,-39.45,;-7.06,-38.2,;-6.59,-36.73,;-7.62,-35.6,;-7.15,-34.14,;-5.64,-33.81,;-4.61,-34.96,;-3.1,-34.64,;-2.07,-35.78,;-.56,-35.46,;.46,-36.61,;1.96,-36.29,;2.44,-34.82,;1.4,-33.67,;-.1,-34,;-5.08,-36.42,;-8.54,-38.68,;-9.88,-37.92,;-9.88,-36.38,;-11.2,-38.69,;-11.2,-40.23,;-9.87,-41,;-8.54,-40.23,)| Show InChI InChI=1S/C24H25N5O/c25-14-17-11-19(12-17)24-28-21(22-23(26)27-9-10-29(22)24)18-7-4-8-20(13-18)30-15-16-5-2-1-3-6-16/h1-10,13,17,19H,11-12,14-15,25H2,(H2,26,27)/t17-,19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50339064

(5-(8-fluoro-2-phenylquinolin-7-yl)-7-(3-(4-methylp...)Show SMILES CN1CCN(CC1)[C@H]1C[C@H](C1)c1nc(-c2ccc3ccc(nc3c2F)-c2ccccc2)c2c(N)ncnn12 |r,wU:9.12,7.7,(40.85,-28.31,;40.38,-26.84,;38.87,-26.51,;38.4,-25.05,;39.44,-23.91,;40.94,-24.23,;41.41,-25.7,;38.96,-22.44,;37.58,-21.74,;38.28,-20.37,;39.66,-21.07,;37.81,-18.92,;38.72,-17.67,;37.81,-16.42,;38.56,-15.08,;37.78,-13.76,;38.54,-12.42,;40.08,-12.4,;40.84,-11.07,;42.38,-11.06,;43.16,-12.39,;42.39,-13.73,;40.85,-13.74,;40.11,-15.07,;40.89,-16.39,;44.69,-12.38,;45.47,-13.72,;47.01,-13.71,;47.77,-12.37,;46.99,-11.04,;45.45,-11.05,;36.34,-16.9,;35.01,-16.13,;35,-14.59,;33.68,-16.9,;33.68,-18.45,;35.01,-19.22,;36.34,-18.44,)| Show InChI InChI=1S/C29H29FN8/c1-36-11-13-37(14-12-36)21-15-20(16-21)29-35-26(27-28(31)32-17-33-38(27)29)22-9-7-19-8-10-23(34-25(19)24(22)30)18-5-3-2-4-6-18/h2-10,17,20-21H,11-16H2,1H3,(H2,31,32,33)/t20-,21+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IR expressed in human HepG2 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50336317

(CHEMBL1667935 | cis-3-[4-Amino-5-(8-fluoro-2-pheny...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2F)-c2ccccc2)c2c(N)ncnn12 |r,wU:4.6,1.1,wD:1.0,(-2.6,-22.44,;-3.93,-21.67,;-4.34,-23.15,;-3.24,-20.29,;-4.61,-19.61,;-5.31,-20.98,;-5.1,-18.14,;-4.2,-16.89,;-5.11,-15.65,;-4.34,-14.32,;-5.11,-12.99,;-4.34,-11.66,;-2.79,-11.65,;-2.03,-10.33,;-.5,-10.33,;.27,-11.66,;-.5,-12.99,;-2.03,-12.99,;-2.8,-14.32,;-2.03,-15.66,;1.81,-11.66,;2.58,-13,;4.12,-13,;4.89,-11.66,;4.11,-10.32,;2.57,-10.33,;-6.58,-16.13,;-7.91,-15.37,;-7.92,-13.83,;-9.24,-16.14,;-9.25,-17.68,;-7.91,-18.46,;-6.57,-17.68,)| Show InChI InChI=1S/C25H21FN6O/c1-25(33)11-16(12-25)24-31-21(22-23(27)28-13-29-32(22)24)17-9-7-15-8-10-18(30-20(15)19(17)26)14-5-3-2-4-6-14/h2-10,13,16,33H,11-12H2,1H3,(H2,27,28,29)/t16-,25+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IR expressed in human HepG2 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339059
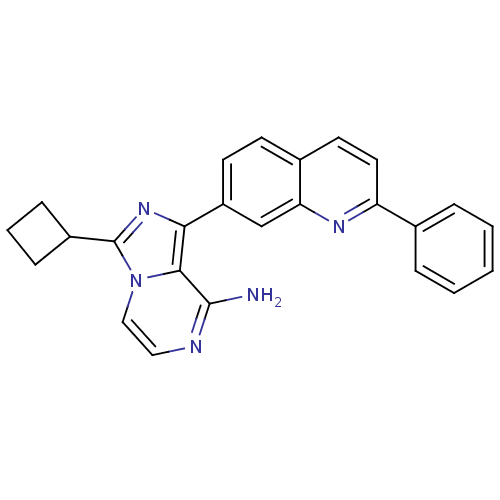
(3-cyclobutyl-1-(2-phenylquinolin-7-yl)-imidazo[1,5...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)C1CCC1 Show InChI InChI=1S/C25H21N5/c26-24-23-22(29-25(18-7-4-8-18)30(23)14-13-27-24)19-10-9-17-11-12-20(28-21(17)15-19)16-5-2-1-3-6-16/h1-3,5-6,9-15,18H,4,7-8H2,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R in presence of 100 uM ATP |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339059

(3-cyclobutyl-1-(2-phenylquinolin-7-yl)-imidazo[1,5...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)C1CCC1 Show InChI InChI=1S/C25H21N5/c26-24-23-22(29-25(18-7-4-8-18)30(23)14-13-27-24)19-10-9-17-11-12-20(28-21(17)15-19)16-5-2-1-3-6-16/h1-3,5-6,9-15,18H,4,7-8H2,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1R by ELISA |
Bioorg Med Chem Lett 21: 2092-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.139
BindingDB Entry DOI: 10.7270/Q23N23QH |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199661
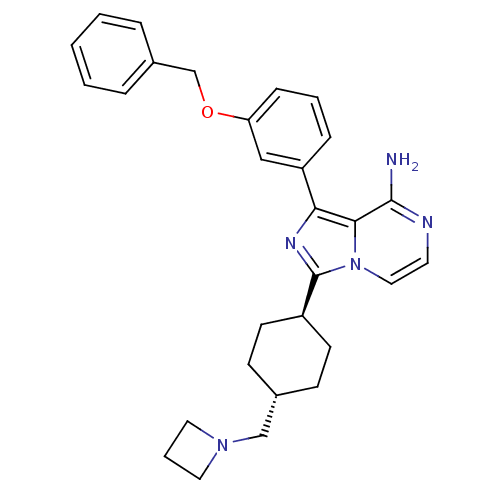
(CHEMBL240035 | trans-3-(-4-(azetidin-1-ylmethyl)cy...)Show SMILES Nc1nccn2c(nc(-c3cccc(OCc4ccccc4)c3)c12)[C@H]1CC[C@H](CN2CCC2)CC1 |wU:27.31,wD:24.27,(7.51,1.57,;7.52,.03,;6.19,-.74,;6.19,-2.29,;7.52,-3.06,;8.86,-2.29,;10.33,-2.77,;11.25,-1.51,;10.33,-.26,;10.81,1.21,;9.77,2.34,;10.24,3.8,;11.76,4.13,;12.79,2.99,;14.29,3.31,;15.32,2.16,;16.83,2.48,;17.85,1.34,;19.36,1.66,;19.83,3.12,;18.8,4.27,;17.29,3.94,;12.31,1.52,;8.86,-.74,;10.81,-4.23,;9.78,-5.38,;10.25,-6.84,;11.75,-7.17,;12.23,-8.63,;13.73,-8.96,;14.56,-10.26,;15.85,-9.42,;15.02,-8.13,;12.79,-6.02,;12.32,-4.55,)| Show InChI InChI=1S/C29H33N5O/c30-28-27-26(24-8-4-9-25(18-24)35-20-22-6-2-1-3-7-22)32-29(34(27)17-14-31-28)23-12-10-21(11-13-23)19-33-15-5-16-33/h1-4,6-9,14,17-18,21,23H,5,10-13,15-16,19-20H2,(H2,30,31)/t21-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339059

(3-cyclobutyl-1-(2-phenylquinolin-7-yl)-imidazo[1,5...)Show SMILES Nc1nccn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)C1CCC1 Show InChI InChI=1S/C25H21N5/c26-24-23-22(29-25(18-7-4-8-18)30(23)14-13-27-24)19-10-9-17-11-12-20(28-21(17)15-19)16-5-2-1-3-6-16/h1-3,5-6,9-15,18H,4,7-8H2,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IGF1R expressed in mouse NIH-3T3 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199632
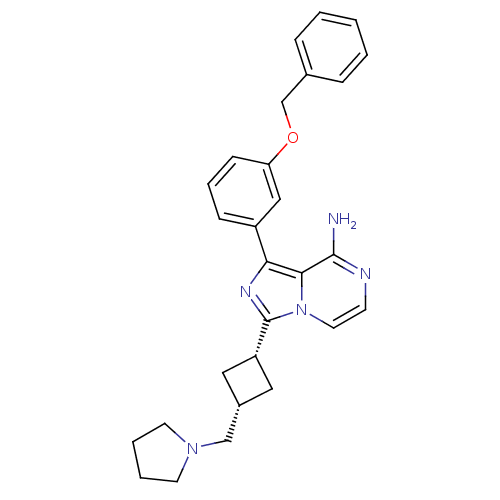
(CHEMBL239985 | cis-1-(3-(benzyloxy)phenyl)-3-(3-(p...)Show SMILES Nc1nccn2c(nc(-c3cccc(OCc4ccccc4)c3)c12)[C@@H]1C[C@H](CN2CCCC2)C1 |wU:24.27,26.30,(25.65,-17.79,;25.65,-19.33,;24.33,-20.1,;24.33,-21.64,;25.66,-22.41,;27,-21.64,;28.47,-22.12,;29.38,-20.86,;28.47,-19.61,;28.95,-18.14,;27.91,-17.01,;28.38,-15.55,;29.89,-15.22,;30.93,-16.37,;32.43,-16.05,;33.46,-17.19,;34.97,-16.87,;35.99,-18.02,;37.5,-17.7,;37.97,-16.23,;36.93,-15.08,;35.43,-15.41,;30.45,-17.83,;26.99,-20.09,;28.95,-23.59,;28.25,-24.95,;29.62,-25.65,;30.1,-27.11,;31.6,-27.43,;32.23,-28.84,;33.77,-28.68,;34.09,-27.17,;32.75,-26.4,;30.32,-24.28,)| Show InChI InChI=1S/C28H31N5O/c29-27-26-25(22-9-6-10-24(17-22)34-19-20-7-2-1-3-8-20)31-28(33(26)14-11-30-27)23-15-21(16-23)18-32-12-4-5-13-32/h1-3,6-11,14,17,21,23H,4-5,12-13,15-16,18-19H2,(H2,29,30)/t21-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199624
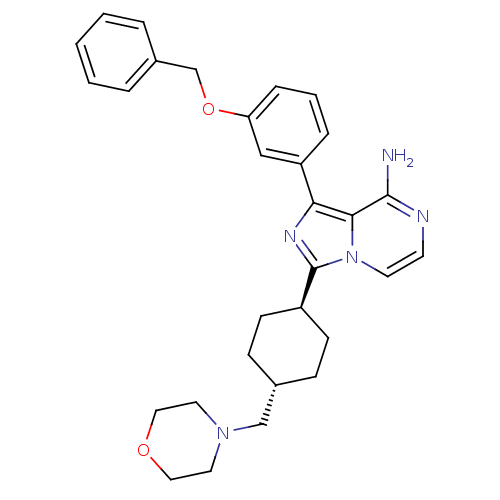
(CHEMBL392082 | trans-1-(3-(benzyloxy)phenyl)-3-(4-...)Show SMILES Nc1nccn2c(nc(-c3cccc(OCc4ccccc4)c3)c12)[C@H]1CC[C@H](CN2CCOCC2)CC1 |wU:27.31,wD:24.27,(-9.11,-17.4,;-9.11,-18.94,;-10.43,-19.71,;-10.45,-21.25,;-9.1,-22.03,;-7.77,-21.26,;-6.29,-21.74,;-5.38,-20.48,;-6.29,-19.22,;-5.82,-17.76,;-6.85,-16.63,;-6.38,-15.16,;-4.87,-14.84,;-3.84,-15.98,;-2.33,-15.66,;-1.3,-16.81,;.21,-16.49,;1.23,-17.63,;2.73,-17.31,;3.21,-15.84,;2.17,-14.7,;.67,-15.02,;-4.31,-17.45,;-7.77,-19.7,;-5.81,-23.2,;-6.85,-24.35,;-6.37,-25.81,;-4.87,-26.13,;-4.4,-27.6,;-2.89,-27.92,;-2.43,-29.39,;-.93,-29.71,;.11,-28.58,;-.36,-27.11,;-1.86,-26.77,;-3.84,-24.99,;-4.31,-23.52,)| Show InChI InChI=1S/C30H35N5O2/c31-29-28-27(25-7-4-8-26(19-25)37-21-23-5-2-1-3-6-23)33-30(35(28)14-13-32-29)24-11-9-22(10-12-24)20-34-15-17-36-18-16-34/h1-8,13-14,19,22,24H,9-12,15-18,20-21H2,(H2,31,32)/t22-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199623
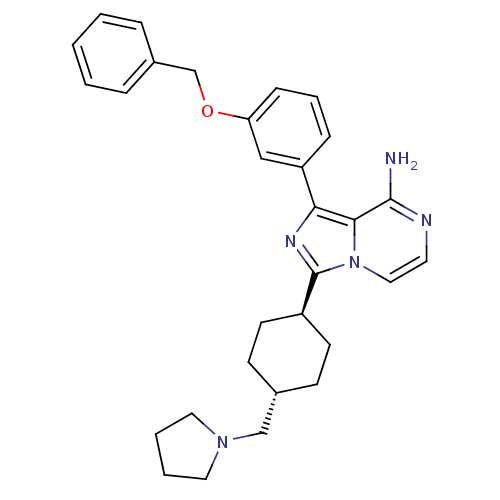
(CHEMBL394155 | trans-1-(3-(benzyloxy)phenyl)-3-(4-...)Show SMILES Nc1nccn2c(nc(-c3cccc(OCc4ccccc4)c3)c12)[C@H]1CC[C@H](CN2CCCC2)CC1 |wU:27.31,wD:24.27,(25.91,1,;25.92,-.54,;24.59,-1.31,;24.59,-2.85,;25.92,-3.62,;27.26,-2.85,;28.74,-3.33,;29.65,-2.08,;28.74,-.82,;29.21,.64,;28.18,1.78,;28.65,3.24,;30.16,3.57,;31.19,2.42,;32.7,2.74,;33.73,1.6,;35.23,1.92,;36.26,.77,;37.76,1.09,;38.24,2.56,;37.2,3.7,;35.7,3.38,;30.71,.96,;27.26,-1.3,;29.21,-4.8,;28.18,-5.94,;28.65,-7.4,;30.16,-7.73,;30.63,-9.2,;32.13,-9.52,;32.76,-10.92,;34.29,-10.76,;34.62,-9.26,;33.29,-8.48,;31.19,-6.59,;30.72,-5.12,)| Show InChI InChI=1S/C30H35N5O/c31-29-28-27(25-9-6-10-26(19-25)36-21-23-7-2-1-3-8-23)33-30(35(28)18-15-32-29)24-13-11-22(12-14-24)20-34-16-4-5-17-34/h1-3,6-10,15,18-19,22,24H,4-5,11-14,16-17,20-21H2,(H2,31,32)/t22-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199668

(CHEMBL240034 | trans-1-(3-(benzyloxy)phenyl)-3-(4-...)Show SMILES CCN(CC)C[C@H]1CC[C@@H](CC1)c1nc(-c2cccc(OCc3ccccc3)c2)c2c(N)nccn12 |wU:6.5,wD:9.12,(.11,-7.88,;-1.4,-7.56,;-2.43,-8.7,;-1.96,-10.17,;-.45,-10.49,;-3.94,-8.38,;-4.41,-6.91,;-5.91,-6.58,;-6.38,-5.12,;-5.35,-3.98,;-3.84,-4.29,;-3.37,-5.77,;-5.83,-2.51,;-4.92,-1.25,;-5.83,0,;-5.35,1.47,;-6.39,2.6,;-5.92,4.06,;-4.41,4.39,;-3.37,3.24,;-1.87,3.56,;-.84,2.42,;.67,2.74,;1.69,1.59,;3.2,1.91,;3.67,3.38,;2.63,4.53,;1.13,4.2,;-3.85,1.78,;-7.3,-.48,;-8.65,.28,;-8.65,1.82,;-9.97,-.49,;-9.97,-2.03,;-8.64,-2.8,;-7.3,-2.03,)| Show InChI InChI=1S/C30H37N5O/c1-3-34(4-2)20-22-13-15-24(16-14-22)30-33-27(28-29(31)32-17-18-35(28)30)25-11-8-12-26(19-25)36-21-23-9-6-5-7-10-23/h5-12,17-19,22,24H,3-4,13-16,20-21H2,1-2H3,(H2,31,32)/t22-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199666
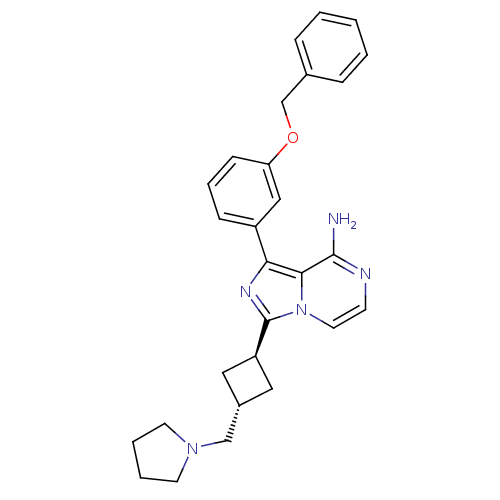
(CHEMBL437558 | trans-1-(3-(benzyloxy)phenyl)-3-(3-...)Show SMILES Nc1nccn2c(nc(-c3cccc(OCc4ccccc4)c3)c12)[C@H]1C[C@H](CN2CCCC2)C1 |wU:24.27,wD:26.30,(8.59,-17.92,;8.59,-19.46,;7.27,-20.23,;7.27,-21.77,;8.6,-22.54,;9.94,-21.77,;11.41,-22.25,;12.32,-20.99,;11.41,-19.74,;11.89,-18.27,;10.85,-17.14,;11.32,-15.68,;12.83,-15.35,;13.87,-16.5,;15.37,-16.18,;16.4,-17.32,;17.91,-17,;18.93,-18.14,;20.44,-17.83,;20.91,-16.36,;19.87,-15.21,;18.37,-15.54,;13.39,-17.96,;9.94,-20.22,;11.89,-23.71,;13.26,-24.41,;12.56,-25.78,;13.04,-27.24,;14.55,-27.56,;15.17,-28.97,;16.71,-28.81,;17.03,-27.3,;15.69,-26.53,;11.19,-25.08,)| Show InChI InChI=1S/C28H31N5O/c29-27-26-25(22-9-6-10-24(17-22)34-19-20-7-2-1-3-8-20)31-28(33(26)14-11-30-27)23-15-21(16-23)18-32-12-4-5-13-32/h1-3,6-11,14,17,21,23H,4-5,12-13,15-16,18-19H2,(H2,29,30)/t21-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199640
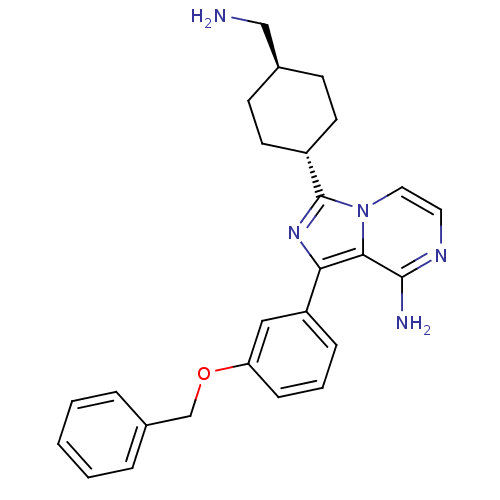
(CHEMBL392284 | trans3-(-4-(aminomethyl)cyclohexyl)...)Show SMILES NC[C@H]1CC[C@@H](CC1)c1nc(-c2cccc(OCc3ccccc3)c2)c2c(N)nccn12 |wU:2.1,wD:5.8,(13.4,-48.47,;11.89,-48.15,;11.42,-46.68,;9.92,-46.36,;9.44,-44.9,;10.48,-43.75,;11.98,-44.07,;12.45,-45.54,;10,-42.29,;10.91,-41.03,;10,-39.77,;10.47,-38.31,;9.44,-37.18,;9.91,-35.71,;11.42,-35.39,;12.45,-36.53,;13.96,-36.21,;14.99,-37.36,;16.5,-37.04,;17.52,-38.18,;19.02,-37.86,;19.5,-36.39,;18.46,-35.25,;16.96,-35.57,;11.98,-38,;8.52,-40.25,;7.18,-39.49,;7.18,-37.95,;5.86,-40.26,;5.86,-41.81,;7.19,-42.58,;8.52,-41.81,)| Show InChI InChI=1S/C26H29N5O/c27-16-18-9-11-20(12-10-18)26-30-23(24-25(28)29-13-14-31(24)26)21-7-4-8-22(15-21)32-17-19-5-2-1-3-6-19/h1-8,13-15,18,20H,9-12,16-17,27H2,(H2,28,29)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199686
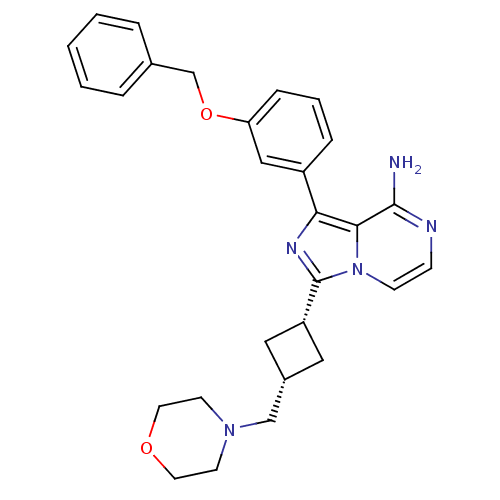
(CHEMBL393314 | cis-1-(3-(benzyloxy)phenyl)-3-(3-(m...)Show SMILES Nc1nccn2c(nc(-c3cccc(OCc4ccccc4)c3)c12)[C@@H]1C[C@H](CN2CCOCC2)C1 |wU:24.27,26.30,(-9.93,2.7,;-9.92,1.16,;-11.25,.39,;-11.25,-1.15,;-9.92,-1.92,;-8.58,-1.15,;-7.1,-1.63,;-6.19,-.37,;-7.1,.88,;-6.63,2.35,;-7.66,3.48,;-7.19,4.94,;-5.68,5.27,;-4.65,4.12,;-3.14,4.44,;-2.11,3.3,;-.61,3.62,;.42,2.48,;1.92,2.79,;2.4,4.26,;1.36,5.41,;-.14,5.08,;-5.12,2.66,;-8.58,.4,;-6.63,-3.09,;-7.33,-4.46,;-5.95,-5.16,;-5.48,-6.62,;-3.97,-6.94,;-3.51,-8.41,;-2.01,-8.73,;-.97,-7.59,;-1.44,-6.12,;-2.95,-5.8,;-5.26,-3.79,)| Show InChI InChI=1S/C28H31N5O2/c29-27-26-25(22-7-4-8-24(17-22)35-19-20-5-2-1-3-6-20)31-28(33(26)10-9-30-27)23-15-21(16-23)18-32-11-13-34-14-12-32/h1-10,17,21,23H,11-16,18-19H2,(H2,29,30)/t21-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339060
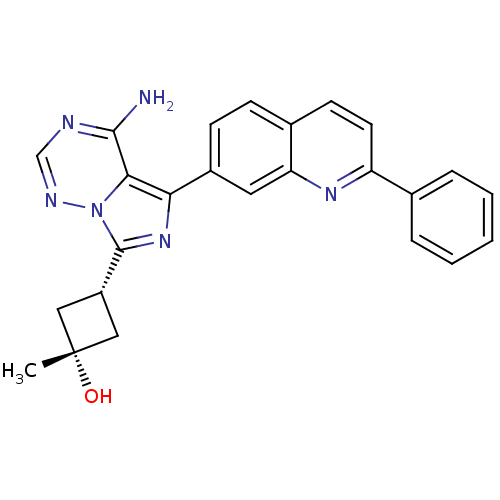
(CHEMBL1688359 | cis-3-(4-amino-5-(2-phenylquinolin...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)ncnn12 |r,wU:4.6,1.1,wD:1.0,(26.46,-7.08,;25.39,-6.01,;24.98,-7.48,;26.09,-4.63,;24.71,-3.93,;24.01,-5.31,;24.24,-2.48,;25.15,-1.23,;24.24,.02,;24.99,1.36,;24.21,2.68,;24.97,4.02,;26.51,4.03,;27.27,5.37,;28.81,5.38,;29.59,4.04,;28.82,2.71,;27.29,2.7,;26.54,1.37,;31.12,4.05,;31.9,2.72,;33.44,2.73,;34.2,4.07,;33.42,5.4,;31.88,5.39,;22.77,-.46,;21.44,.31,;21.44,1.85,;20.11,-.46,;20.11,-2.01,;21.44,-2.78,;22.77,-2,)| Show InChI InChI=1S/C25H22N6O/c1-25(32)12-18(13-25)24-30-21(22-23(26)27-14-28-31(22)24)17-8-7-16-9-10-19(29-20(16)11-17)15-5-3-2-4-6-15/h2-11,14,18,32H,12-13H2,1H3,(H2,26,27,28)/t18-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IGF1R expressed in mouse NIH-3T3 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199626

(CHEMBL401429 | cis-1-(3-(benzyloxy)phenyl)-3-(3-((...)Show SMILES CN(C)C[C@H]1C[C@H](C1)c1nc(-c2cccc(OCc3ccccc3)c2)c2c(N)nccn12 |wU:6.8,4.3,(13.74,-47.62,;13.26,-46.15,;14.29,-45.01,;11.76,-45.84,;11.28,-44.37,;9.91,-43.67,;10.61,-42.31,;11.98,-43,;10.13,-40.84,;11.04,-39.59,;10.13,-38.33,;10.6,-36.87,;9.57,-35.73,;10.04,-34.27,;11.55,-33.94,;12.58,-35.09,;14.09,-34.77,;15.12,-35.91,;16.63,-35.59,;17.65,-36.74,;19.15,-36.42,;19.63,-34.95,;18.59,-33.8,;17.09,-34.13,;12.11,-36.55,;8.65,-38.81,;7.31,-38.05,;7.3,-36.51,;5.99,-38.82,;5.98,-40.36,;7.31,-41.13,;8.65,-40.36,)| Show InChI InChI=1S/C26H29N5O/c1-30(2)16-19-13-21(14-19)26-29-23(24-25(27)28-11-12-31(24)26)20-9-6-10-22(15-20)32-17-18-7-4-3-5-8-18/h3-12,15,19,21H,13-14,16-17H2,1-2H3,(H2,27,28)/t19-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50340954
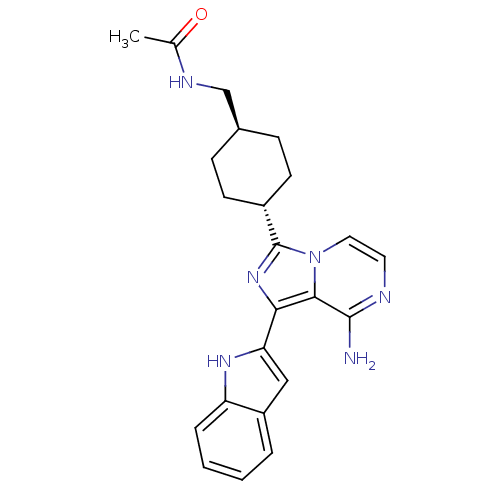
(CHEMBL1762110 | Trans-N-((-4-(8-amino-1-(1H-indol-...)Show SMILES CC(=O)NC[C@H]1CC[C@@H](CC1)c1nc(-c2cc3ccccc3[nH]2)c2c(N)nccn12 |r,wU:8.11,wD:5.4,(30.4,-52.78,;31.44,-51.63,;32.94,-51.96,;30.97,-50.17,;32,-49.03,;31.53,-47.56,;32.56,-46.41,;32.09,-44.94,;30.58,-44.63,;29.54,-45.77,;30.01,-47.24,;30.1,-43.16,;31.01,-41.9,;30.1,-40.65,;30.58,-39.19,;29.67,-37.94,;30.58,-36.7,;30.27,-35.2,;31.41,-34.17,;32.87,-34.65,;33.19,-36.15,;32.04,-37.18,;32.04,-38.72,;28.62,-41.13,;27.29,-40.37,;27.29,-38.83,;25.96,-41.14,;25.96,-42.68,;27.29,-43.45,;28.63,-42.68,)| Show InChI InChI=1S/C23H26N6O/c1-14(30)26-13-15-6-8-16(9-7-15)23-28-20(21-22(24)25-10-11-29(21)23)19-12-17-4-2-3-5-18(17)27-19/h2-5,10-12,15-16,27H,6-9,13H2,1H3,(H2,24,25)(H,26,30)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of cRaf |
Bioorg Med Chem Lett 21: 2092-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.139
BindingDB Entry DOI: 10.7270/Q23N23QH |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199626

(CHEMBL401429 | cis-1-(3-(benzyloxy)phenyl)-3-(3-((...)Show SMILES CN(C)C[C@H]1C[C@H](C1)c1nc(-c2cccc(OCc3ccccc3)c2)c2c(N)nccn12 |wU:6.8,4.3,(13.74,-47.62,;13.26,-46.15,;14.29,-45.01,;11.76,-45.84,;11.28,-44.37,;9.91,-43.67,;10.61,-42.31,;11.98,-43,;10.13,-40.84,;11.04,-39.59,;10.13,-38.33,;10.6,-36.87,;9.57,-35.73,;10.04,-34.27,;11.55,-33.94,;12.58,-35.09,;14.09,-34.77,;15.12,-35.91,;16.63,-35.59,;17.65,-36.74,;19.15,-36.42,;19.63,-34.95,;18.59,-33.8,;17.09,-34.13,;12.11,-36.55,;8.65,-38.81,;7.31,-38.05,;7.3,-36.51,;5.99,-38.82,;5.98,-40.36,;7.31,-41.13,;8.65,-40.36,)| Show InChI InChI=1S/C26H29N5O/c1-30(2)16-19-13-21(14-19)26-29-23(24-25(27)28-11-12-31(24)26)20-9-6-10-22(15-20)32-17-18-7-4-3-5-8-18/h3-12,15,19,21H,13-14,16-17H2,1-2H3,(H2,27,28)/t19-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 191 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-stimulated phosphorylation of human recombinant IGF1R expressed in NIH3T3 cells |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199641
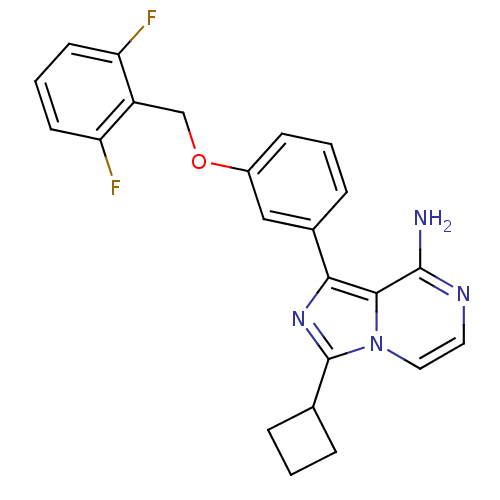
(1-(3-(2,6-difluorobenzyloxy)phenyl)-3-cyclobutylim...)Show SMILES Nc1nccn2c(nc(-c3cccc(OCc4c(F)cccc4F)c3)c12)C1CCC1 Show InChI InChI=1S/C23H20F2N4O/c24-18-8-3-9-19(25)17(18)13-30-16-7-2-6-15(12-16)20-21-22(26)27-10-11-29(21)23(28-20)14-4-1-5-14/h2-3,6-12,14H,1,4-5,13H2,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199650
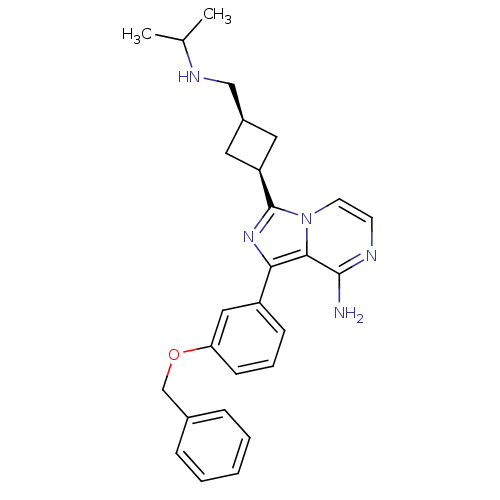
(CHEMBL240613 | cis-1-(3-(benzyloxy)phenyl)-3-(3-((...)Show SMILES CC(C)NC[C@H]1C[C@H](C1)c1nc(-c2cccc(OCc3ccccc3)c2)c2c(N)nccn12 |wU:7.9,5.4,(15.38,-7.53,;13.87,-7.21,;13.4,-5.74,;12.84,-8.35,;11.34,-8.03,;10.86,-6.57,;9.49,-5.87,;10.19,-4.51,;11.56,-5.2,;9.71,-3.04,;10.62,-1.79,;9.71,-.53,;10.18,.93,;9.15,2.07,;9.62,3.53,;11.13,3.86,;12.16,2.71,;13.67,3.03,;14.7,1.89,;16.21,2.21,;17.23,1.06,;18.73,1.38,;19.21,2.85,;18.17,4,;16.67,3.67,;11.69,1.25,;8.23,-1.01,;6.89,-.25,;6.88,1.29,;5.57,-1.02,;5.56,-2.56,;6.9,-3.33,;8.23,-2.56,)| Show InChI InChI=1S/C27H31N5O/c1-18(2)30-16-20-13-22(14-20)27-31-24(25-26(28)29-11-12-32(25)27)21-9-6-10-23(15-21)33-17-19-7-4-3-5-8-19/h3-12,15,18,20,22,30H,13-14,16-17H2,1-2H3,(H2,28,29)/t20-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199630
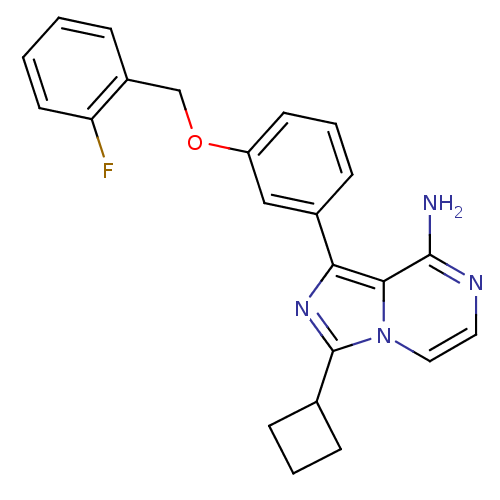
(1-(3-(2-fluorobenzyloxy)phenyl)-3-cyclobutylimidaz...)Show SMILES Nc1nccn2c(nc(-c3cccc(OCc4ccccc4F)c3)c12)C1CCC1 Show InChI InChI=1S/C23H21FN4O/c24-19-10-2-1-5-17(19)14-29-18-9-4-8-16(13-18)20-21-22(25)26-11-12-28(21)23(27-20)15-6-3-7-15/h1-2,4-5,8-13,15H,3,6-7,14H2,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199631
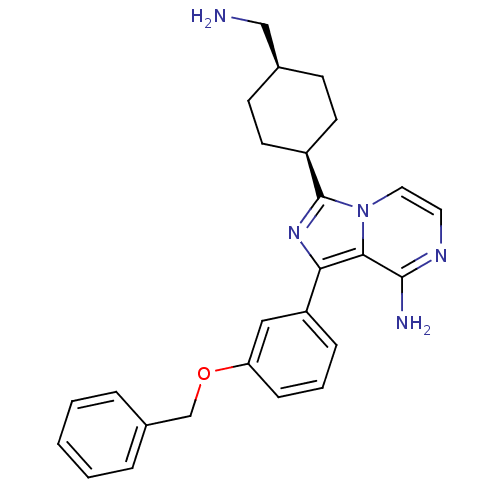
(CHEMBL394154 | cis-3-(4-(aminomethyl)cyclohexyl)-1...)Show SMILES NC[C@H]1CC[C@H](CC1)c1nc(-c2cccc(OCc3ccccc3)c2)c2c(N)nccn12 |wU:5.8,2.1,(30.07,-50.41,;28.57,-50.08,;28.1,-48.62,;26.59,-48.29,;26.12,-46.83,;27.15,-45.68,;28.66,-46,;29.13,-47.47,;26.68,-44.22,;27.59,-42.96,;26.67,-41.71,;27.15,-40.24,;26.12,-39.11,;26.58,-37.65,;28.1,-37.32,;29.13,-38.47,;30.63,-38.15,;31.66,-39.29,;33.17,-38.97,;34.19,-40.11,;35.7,-39.79,;36.18,-38.33,;35.14,-37.18,;33.63,-37.51,;28.65,-39.93,;25.2,-42.19,;23.86,-41.43,;23.85,-39.89,;22.53,-42.2,;22.53,-43.74,;23.86,-44.51,;25.2,-43.74,)| Show InChI InChI=1S/C26H29N5O/c27-16-18-9-11-20(12-10-18)26-30-23(24-25(28)29-13-14-31(24)26)21-7-4-8-22(15-21)32-17-19-5-2-1-3-6-19/h1-8,13-15,18,20H,9-12,16-17,27H2,(H2,28,29)/t18-,20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 228 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199638

(CHEMBL393313 | cis-1-(3-(benzyloxy)phenyl)-3-(3-(p...)Show SMILES Nc1nccn2c(nc(-c3cccc(OCc4ccccc4)c3)c12)[C@@H]1C[C@H](CN2CCCCC2)C1 |wU:24.27,26.30,(24.76,-37.15,;24.77,-38.69,;23.45,-39.46,;23.44,-41,;24.78,-41.77,;26.11,-41,;27.59,-41.48,;28.5,-40.22,;27.59,-38.97,;28.06,-37.5,;27.03,-36.37,;27.5,-34.91,;29.01,-34.58,;30.04,-35.73,;31.55,-35.41,;32.58,-36.55,;34.09,-36.23,;35.11,-37.38,;36.61,-37.06,;37.09,-35.59,;36.05,-34.44,;34.55,-34.77,;29.57,-37.19,;26.11,-39.45,;28.07,-42.94,;27.37,-44.31,;28.74,-45.01,;29.22,-46.47,;30.72,-46.79,;31.19,-48.26,;32.69,-48.58,;33.72,-47.44,;33.25,-45.97,;31.74,-45.65,;29.44,-43.64,)| Show InChI InChI=1S/C29H33N5O/c30-28-27-26(23-10-7-11-25(18-23)35-20-21-8-3-1-4-9-21)32-29(34(27)15-12-31-28)24-16-22(17-24)19-33-13-5-2-6-14-33/h1,3-4,7-12,15,18,22,24H,2,5-6,13-14,16-17,19-20H2,(H2,30,31)/t22-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 237 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199676

(1-(3-(2-chloro-6-fluorobenzyloxy)phenyl)-3-cyclobu...)Show SMILES Nc1nccn2c(nc(-c3cccc(OCc4c(F)cccc4Cl)c3)c12)C1CCC1 Show InChI InChI=1S/C23H20ClFN4O/c24-18-8-3-9-19(25)17(18)13-30-16-7-2-6-15(12-16)20-21-22(26)27-10-11-29(21)23(28-20)14-4-1-5-14/h2-3,6-12,14H,1,4-5,13H2,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 248 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50339061

(4-(3-(4-amino-5-(2-phenylquinolin-7-yl)imidazo[1,5...)Show SMILES Nc1ncnn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)[C@@H]1C[C@@H](C1)N1CCN(CC1)C=O |r,wU:26.30,28.35,(-7.24,-13.78,;-7.23,-15.32,;-8.56,-16.09,;-8.56,-17.63,;-7.23,-18.41,;-5.9,-17.63,;-4.43,-18.1,;-3.52,-16.85,;-4.43,-15.61,;-3.68,-14.27,;-4.46,-12.95,;-3.7,-11.61,;-2.16,-11.59,;-1.4,-10.26,;.14,-10.24,;.92,-11.58,;.15,-12.92,;-1.39,-12.92,;-2.13,-14.25,;2.45,-11.57,;3.23,-12.9,;4.77,-12.9,;5.53,-11.56,;4.75,-10.22,;3.21,-10.24,;-5.9,-16.08,;-3.96,-19.56,;-4.66,-20.93,;-3.28,-21.63,;-2.58,-20.26,;-2.8,-23.1,;-3.84,-24.24,;-3.37,-25.7,;-1.86,-26.03,;-.83,-24.88,;-1.3,-23.41,;-1.39,-27.49,;-2.43,-28.63,)| Show InChI InChI=1S/C29H28N8O/c30-28-27-26(21-7-6-20-8-9-24(33-25(20)16-21)19-4-2-1-3-5-19)34-29(37(27)32-17-31-28)22-14-23(15-22)36-12-10-35(18-38)11-13-36/h1-9,16-18,22-23H,10-15H2,(H2,30,31,32)/t22-,23+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IR expressed in human HepG2 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339058
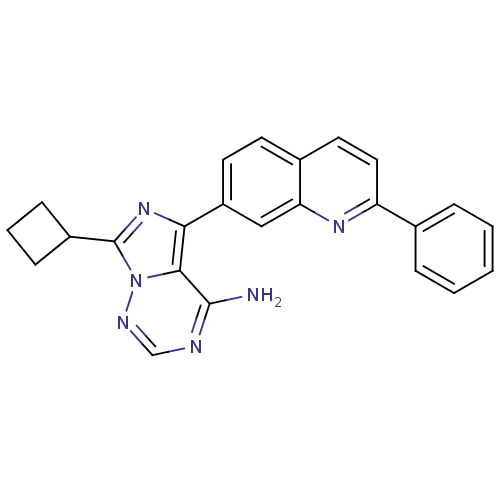
(7-cyclobutyl-5-(2-phenylquinolin-7-yl)imidazo[1,5-...)Show SMILES Nc1ncnn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)C1CCC1 Show InChI InChI=1S/C24H20N6/c25-23-22-21(29-24(17-7-4-8-17)30(22)27-14-26-23)18-10-9-16-11-12-19(28-20(16)13-18)15-5-2-1-3-6-15/h1-3,5-6,9-14,17H,4,7-8H2,(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human IGF1R in presence of 100 uM ATP |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50339063

(7-(3-(4-methylpiperazin-1-yl)cyclobutyl)-5-(2-phen...)Show SMILES CN1CCN(CC1)[C@H]1C[C@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)ncnn12 |r,wU:9.12,7.7,(25.86,-27.54,;25.39,-26.08,;23.89,-25.75,;23.42,-24.29,;24.45,-23.15,;25.96,-23.46,;26.43,-24.93,;23.97,-21.68,;22.6,-20.98,;23.3,-19.61,;24.67,-20.31,;22.82,-18.15,;23.73,-16.9,;22.82,-15.66,;23.58,-14.32,;22.79,-13,;23.55,-11.66,;25.09,-11.64,;25.85,-10.31,;27.39,-10.3,;28.17,-11.63,;27.41,-12.97,;25.87,-12.98,;25.12,-14.3,;29.71,-11.62,;30.48,-12.95,;32.02,-12.95,;32.79,-11.61,;32,-10.27,;30.47,-10.29,;21.36,-16.13,;20.02,-15.37,;20.02,-13.83,;18.69,-16.14,;18.69,-17.68,;20.03,-18.45,;21.36,-17.68,)| Show InChI InChI=1S/C29H30N8/c1-35-11-13-36(14-12-35)23-15-22(16-23)29-34-26(27-28(30)31-18-32-37(27)29)21-8-7-20-9-10-24(33-25(20)17-21)19-5-3-2-4-6-19/h2-10,17-18,22-23H,11-16H2,1H3,(H2,30,31,32)/t22-,23+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IR expressed in human HepG2 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199665

(1-(3-(2,5-difluorobenzyloxy)phenyl)-3-cyclobutylim...)Show SMILES Nc1nccn2c(nc(-c3cccc(OCc4cc(F)ccc4F)c3)c12)C1CCC1 Show InChI InChI=1S/C23H20F2N4O/c24-17-7-8-19(25)16(11-17)13-30-18-6-2-5-15(12-18)20-21-22(26)27-9-10-29(21)23(28-20)14-3-1-4-14/h2,5-12,14H,1,3-4,13H2,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 329 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199665

(1-(3-(2,5-difluorobenzyloxy)phenyl)-3-cyclobutylim...)Show SMILES Nc1nccn2c(nc(-c3cccc(OCc4cc(F)ccc4F)c3)c12)C1CCC1 Show InChI InChI=1S/C23H20F2N4O/c24-17-7-8-19(25)16(11-17)13-30-18-6-2-5-15(12-18)20-21-22(26)27-9-10-29(21)23(28-20)14-3-1-4-14/h2,5-12,14H,1,3-4,13H2,(H2,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 329 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50339058

(7-cyclobutyl-5-(2-phenylquinolin-7-yl)imidazo[1,5-...)Show SMILES Nc1ncnn2c(nc(-c3ccc4ccc(nc4c3)-c3ccccc3)c12)C1CCC1 Show InChI InChI=1S/C24H20N6/c25-23-22-21(29-24(17-7-4-8-17)30(22)27-14-26-23)18-10-9-16-11-12-19(28-20(16)13-18)15-5-2-1-3-6-15/h1-3,5-6,9-14,17H,4,7-8H2,(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of auto-phosphorylation of human IGF1R expressed in mouse NIH-3T3 cells after 2 hrs by luminometry |
ACS Med Chem Lett 1: 510-515 (2010)
Article DOI: 10.1021/ml100178g
BindingDB Entry DOI: 10.7270/Q2J966NZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199689
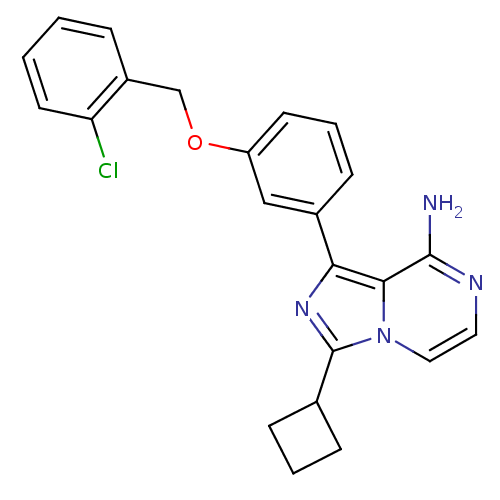
(1-(3-(2-chlorobenzyloxy)phenyl)-3-cyclobutylimidaz...)Show SMILES Nc1nccn2c(nc(-c3cccc(OCc4ccccc4Cl)c3)c12)C1CCC1 Show InChI InChI=1S/C23H21ClN4O/c24-19-10-2-1-5-17(19)14-29-18-9-4-8-16(13-18)20-21-22(25)26-11-12-28(21)23(27-20)15-6-3-7-15/h1-2,4-5,8-13,15H,3,6-7,14H2,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 343 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199638

(CHEMBL393313 | cis-1-(3-(benzyloxy)phenyl)-3-(3-(p...)Show SMILES Nc1nccn2c(nc(-c3cccc(OCc4ccccc4)c3)c12)[C@@H]1C[C@H](CN2CCCCC2)C1 |wU:24.27,26.30,(24.76,-37.15,;24.77,-38.69,;23.45,-39.46,;23.44,-41,;24.78,-41.77,;26.11,-41,;27.59,-41.48,;28.5,-40.22,;27.59,-38.97,;28.06,-37.5,;27.03,-36.37,;27.5,-34.91,;29.01,-34.58,;30.04,-35.73,;31.55,-35.41,;32.58,-36.55,;34.09,-36.23,;35.11,-37.38,;36.61,-37.06,;37.09,-35.59,;36.05,-34.44,;34.55,-34.77,;29.57,-37.19,;26.11,-39.45,;28.07,-42.94,;27.37,-44.31,;28.74,-45.01,;29.22,-46.47,;30.72,-46.79,;31.19,-48.26,;32.69,-48.58,;33.72,-47.44,;33.25,-45.97,;31.74,-45.65,;29.44,-43.64,)| Show InChI InChI=1S/C29H33N5O/c30-28-27-26(23-10-7-11-25(18-23)35-20-21-8-3-1-4-9-21)32-29(34(27)15-12-31-28)24-16-22(17-24)19-33-13-5-2-6-14-33/h1,3-4,7-12,15,18,22,24H,2,5-6,13-14,16-17,19-20H2,(H2,30,31)/t22-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 346 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-stimulated phosphorylation of human recombinant IGF1R expressed in NIH3T3 cells |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199650

(CHEMBL240613 | cis-1-(3-(benzyloxy)phenyl)-3-(3-((...)Show SMILES CC(C)NC[C@H]1C[C@H](C1)c1nc(-c2cccc(OCc3ccccc3)c2)c2c(N)nccn12 |wU:7.9,5.4,(15.38,-7.53,;13.87,-7.21,;13.4,-5.74,;12.84,-8.35,;11.34,-8.03,;10.86,-6.57,;9.49,-5.87,;10.19,-4.51,;11.56,-5.2,;9.71,-3.04,;10.62,-1.79,;9.71,-.53,;10.18,.93,;9.15,2.07,;9.62,3.53,;11.13,3.86,;12.16,2.71,;13.67,3.03,;14.7,1.89,;16.21,2.21,;17.23,1.06,;18.73,1.38,;19.21,2.85,;18.17,4,;16.67,3.67,;11.69,1.25,;8.23,-1.01,;6.89,-.25,;6.88,1.29,;5.57,-1.02,;5.56,-2.56,;6.9,-3.33,;8.23,-2.56,)| Show InChI InChI=1S/C27H31N5O/c1-18(2)30-16-20-13-22(14-20)27-31-24(25-26(28)29-11-12-32(25)27)21-9-6-10-23(15-21)33-17-19-7-4-3-5-8-19/h3-12,15,18,20,22,30H,13-14,16-17H2,1-2H3,(H2,28,29)/t20-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 376 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-stimulated phosphorylation of human recombinant IGF1R expressed in NIH3T3 cells |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199661

(CHEMBL240035 | trans-3-(-4-(azetidin-1-ylmethyl)cy...)Show SMILES Nc1nccn2c(nc(-c3cccc(OCc4ccccc4)c3)c12)[C@H]1CC[C@H](CN2CCC2)CC1 |wU:27.31,wD:24.27,(7.51,1.57,;7.52,.03,;6.19,-.74,;6.19,-2.29,;7.52,-3.06,;8.86,-2.29,;10.33,-2.77,;11.25,-1.51,;10.33,-.26,;10.81,1.21,;9.77,2.34,;10.24,3.8,;11.76,4.13,;12.79,2.99,;14.29,3.31,;15.32,2.16,;16.83,2.48,;17.85,1.34,;19.36,1.66,;19.83,3.12,;18.8,4.27,;17.29,3.94,;12.31,1.52,;8.86,-.74,;10.81,-4.23,;9.78,-5.38,;10.25,-6.84,;11.75,-7.17,;12.23,-8.63,;13.73,-8.96,;14.56,-10.26,;15.85,-9.42,;15.02,-8.13,;12.79,-6.02,;12.32,-4.55,)| Show InChI InChI=1S/C29H33N5O/c30-28-27-26(24-8-4-9-25(18-24)35-20-22-6-2-1-3-7-22)32-29(34(27)17-14-31-28)23-12-10-21(11-13-23)19-33-15-5-16-33/h1-4,6-9,14,17-18,21,23H,5,10-13,15-16,19-20H2,(H2,30,31)/t21-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-stimulated phosphorylation of human recombinant IGF1R expressed in NIH3T3 cells |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199632

(CHEMBL239985 | cis-1-(3-(benzyloxy)phenyl)-3-(3-(p...)Show SMILES Nc1nccn2c(nc(-c3cccc(OCc4ccccc4)c3)c12)[C@@H]1C[C@H](CN2CCCC2)C1 |wU:24.27,26.30,(25.65,-17.79,;25.65,-19.33,;24.33,-20.1,;24.33,-21.64,;25.66,-22.41,;27,-21.64,;28.47,-22.12,;29.38,-20.86,;28.47,-19.61,;28.95,-18.14,;27.91,-17.01,;28.38,-15.55,;29.89,-15.22,;30.93,-16.37,;32.43,-16.05,;33.46,-17.19,;34.97,-16.87,;35.99,-18.02,;37.5,-17.7,;37.97,-16.23,;36.93,-15.08,;35.43,-15.41,;30.45,-17.83,;26.99,-20.09,;28.95,-23.59,;28.25,-24.95,;29.62,-25.65,;30.1,-27.11,;31.6,-27.43,;32.23,-28.84,;33.77,-28.68,;34.09,-27.17,;32.75,-26.4,;30.32,-24.28,)| Show InChI InChI=1S/C28H31N5O/c29-27-26-25(22-9-6-10-24(17-22)34-19-20-7-2-1-3-8-20)31-28(33(26)14-11-30-27)23-15-21(16-23)18-32-12-4-5-13-32/h1-3,6-11,14,17,21,23H,4-5,12-13,15-16,18-19H2,(H2,29,30)/t21-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 401 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-stimulated phosphorylation of human recombinant IGF1R expressed in NIH3T3 cells |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199668

(CHEMBL240034 | trans-1-(3-(benzyloxy)phenyl)-3-(4-...)Show SMILES CCN(CC)C[C@H]1CC[C@@H](CC1)c1nc(-c2cccc(OCc3ccccc3)c2)c2c(N)nccn12 |wU:6.5,wD:9.12,(.11,-7.88,;-1.4,-7.56,;-2.43,-8.7,;-1.96,-10.17,;-.45,-10.49,;-3.94,-8.38,;-4.41,-6.91,;-5.91,-6.58,;-6.38,-5.12,;-5.35,-3.98,;-3.84,-4.29,;-3.37,-5.77,;-5.83,-2.51,;-4.92,-1.25,;-5.83,0,;-5.35,1.47,;-6.39,2.6,;-5.92,4.06,;-4.41,4.39,;-3.37,3.24,;-1.87,3.56,;-.84,2.42,;.67,2.74,;1.69,1.59,;3.2,1.91,;3.67,3.38,;2.63,4.53,;1.13,4.2,;-3.85,1.78,;-7.3,-.48,;-8.65,.28,;-8.65,1.82,;-9.97,-.49,;-9.97,-2.03,;-8.64,-2.8,;-7.3,-2.03,)| Show InChI InChI=1S/C30H37N5O/c1-3-34(4-2)20-22-13-15-24(16-14-22)30-33-27(28-29(31)32-17-18-35(28)30)25-11-8-12-26(19-25)36-21-23-9-6-5-7-10-23/h5-12,17-19,22,24H,3-4,13-16,20-21H2,1-2H3,(H2,31,32)/t22-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-stimulated phosphorylation of human recombinant IGF1R expressed in NIH3T3 cells |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Mus musculus) | BDBM50511189
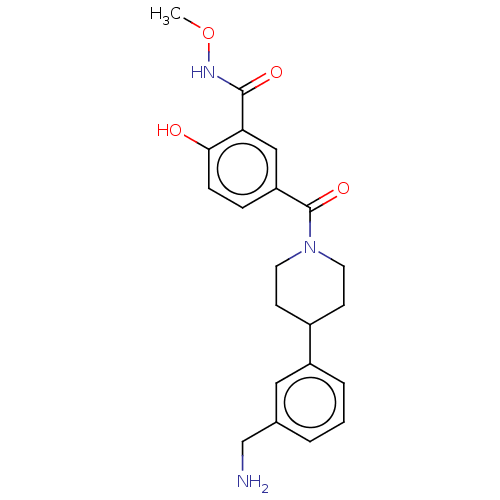
(CHEMBL4571556)Show SMILES CONC(=O)c1cc(ccc1O)C(=O)N1CCC(CC1)c1cccc(CN)c1 Show InChI InChI=1S/C21H25N3O4/c1-28-23-20(26)18-12-17(5-6-19(18)25)21(27)24-9-7-15(8-10-24)16-4-2-3-14(11-16)13-22/h2-6,11-12,15,25H,7-10,13,22H2,1H3,(H,23,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 455 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medicine
Curated by ChEMBL
| Assay Description
Inhibition of mouse MCP6 pre-incubated for 3 hrs before N-tert-butoxycarbonyl-Gln-Ala-Arg-AMC substrate addition and measured every 30 secs for 15 mi... |
J Med Chem 63: 3004-3027 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01689
BindingDB Entry DOI: 10.7270/Q2N3018J |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199625
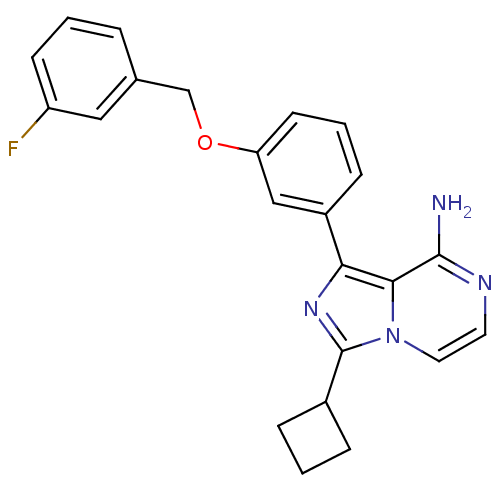
(1-(3-(3-fluorobenzyloxy)phenyl)-3-cyclobutylimidaz...)Show SMILES Nc1nccn2c(nc(-c3cccc(OCc4cccc(F)c4)c3)c12)C1CCC1 Show InChI InChI=1S/C23H21FN4O/c24-18-8-1-4-15(12-18)14-29-19-9-3-7-17(13-19)20-21-22(25)26-10-11-28(21)23(27-20)16-5-2-6-16/h1,3-4,7-13,16H,2,5-6,14H2,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199683

(3-(8-amino-3-cyclobutylimidazo[1,5-a]pyrazin-1-yl)...)Show InChI InChI=1S/C16H16N4O/c17-15-14-13(11-5-2-6-12(21)9-11)19-16(10-3-1-4-10)20(14)8-7-18-15/h2,5-10,21H,1,3-4H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 518 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199683

(3-(8-amino-3-cyclobutylimidazo[1,5-a]pyrazin-1-yl)...)Show InChI InChI=1S/C16H16N4O/c17-15-14-13(11-5-2-6-12(21)9-11)19-16(10-3-1-4-10)20(14)8-7-18-15/h2,5-10,21H,1,3-4H2,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1R by ELISA |
Bioorg Med Chem Lett 21: 2092-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.139
BindingDB Entry DOI: 10.7270/Q23N23QH |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199629
(CHEMBL393517 | trans-3-(8-amino-1-(3-(benzyloxy)ph...)Show SMILES NC(=O)[C@H]1C[C@@H](C1)c1nc(-c2cccc(OCc3ccccc3)c2)c2c(N)nccn12 |wU:5.7,wD:3.2,(-3.43,-24.86,;-4.94,-24.54,;-5.97,-25.68,;-5.41,-23.07,;-4.72,-21.7,;-6.09,-21.01,;-6.79,-22.38,;-6.56,-19.55,;-5.65,-18.29,;-6.56,-17.03,;-6.09,-15.57,;-7.12,-14.44,;-6.66,-12.97,;-5.14,-12.65,;-4.11,-13.79,;-2.6,-13.47,;-1.57,-14.62,;-.07,-14.3,;.96,-15.44,;2.46,-15.12,;2.94,-13.66,;1.9,-12.51,;.4,-12.83,;-4.59,-15.26,;-8.04,-17.52,;-9.38,-16.75,;-9.39,-15.21,;-10.71,-17.52,;-10.71,-19.07,;-9.38,-19.84,;-8.04,-19.07,)| Show InChI InChI=1S/C24H23N5O2/c25-22-21-20(16-7-4-8-19(13-16)31-14-15-5-2-1-3-6-15)28-24(29(21)10-9-27-22)18-11-17(12-18)23(26)30/h1-10,13,17-18H,11-12,14H2,(H2,25,27)(H2,26,30)/t17-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 526 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-IGF1R activity |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199640

(CHEMBL392284 | trans3-(-4-(aminomethyl)cyclohexyl)...)Show SMILES NC[C@H]1CC[C@@H](CC1)c1nc(-c2cccc(OCc3ccccc3)c2)c2c(N)nccn12 |wU:2.1,wD:5.8,(13.4,-48.47,;11.89,-48.15,;11.42,-46.68,;9.92,-46.36,;9.44,-44.9,;10.48,-43.75,;11.98,-44.07,;12.45,-45.54,;10,-42.29,;10.91,-41.03,;10,-39.77,;10.47,-38.31,;9.44,-37.18,;9.91,-35.71,;11.42,-35.39,;12.45,-36.53,;13.96,-36.21,;14.99,-37.36,;16.5,-37.04,;17.52,-38.18,;19.02,-37.86,;19.5,-36.39,;18.46,-35.25,;16.96,-35.57,;11.98,-38,;8.52,-40.25,;7.18,-39.49,;7.18,-37.95,;5.86,-40.26,;5.86,-41.81,;7.19,-42.58,;8.52,-41.81,)| Show InChI InChI=1S/C26H29N5O/c27-16-18-9-11-20(12-10-18)26-30-23(24-25(28)29-13-14-31(24)26)21-7-4-8-22(15-21)32-17-19-5-2-1-3-6-19/h1-8,13-15,18,20H,9-12,16-17,27H2,(H2,28,29)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 534 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-stimulated phosphorylation of human recombinant IGF1R expressed in NIH3T3 cells |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199623

(CHEMBL394155 | trans-1-(3-(benzyloxy)phenyl)-3-(4-...)Show SMILES Nc1nccn2c(nc(-c3cccc(OCc4ccccc4)c3)c12)[C@H]1CC[C@H](CN2CCCC2)CC1 |wU:27.31,wD:24.27,(25.91,1,;25.92,-.54,;24.59,-1.31,;24.59,-2.85,;25.92,-3.62,;27.26,-2.85,;28.74,-3.33,;29.65,-2.08,;28.74,-.82,;29.21,.64,;28.18,1.78,;28.65,3.24,;30.16,3.57,;31.19,2.42,;32.7,2.74,;33.73,1.6,;35.23,1.92,;36.26,.77,;37.76,1.09,;38.24,2.56,;37.2,3.7,;35.7,3.38,;30.71,.96,;27.26,-1.3,;29.21,-4.8,;28.18,-5.94,;28.65,-7.4,;30.16,-7.73,;30.63,-9.2,;32.13,-9.52,;32.76,-10.92,;34.29,-10.76,;34.62,-9.26,;33.29,-8.48,;31.19,-6.59,;30.72,-5.12,)| Show InChI InChI=1S/C30H35N5O/c31-29-28-27(25-9-6-10-26(19-25)36-21-23-7-2-1-3-8-23)33-30(35(28)18-15-32-29)24-13-11-22(12-14-24)20-34-16-4-5-17-34/h1-3,6-10,15,18-19,22,24H,4-5,11-14,16-17,20-21H2,(H2,31,32)/t22-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 547 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-stimulated phosphorylation of human recombinant IGF1R expressed in NIH3T3 cells |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50199631

(CHEMBL394154 | cis-3-(4-(aminomethyl)cyclohexyl)-1...)Show SMILES NC[C@H]1CC[C@H](CC1)c1nc(-c2cccc(OCc3ccccc3)c2)c2c(N)nccn12 |wU:5.8,2.1,(30.07,-50.41,;28.57,-50.08,;28.1,-48.62,;26.59,-48.29,;26.12,-46.83,;27.15,-45.68,;28.66,-46,;29.13,-47.47,;26.68,-44.22,;27.59,-42.96,;26.67,-41.71,;27.15,-40.24,;26.12,-39.11,;26.58,-37.65,;28.1,-37.32,;29.13,-38.47,;30.63,-38.15,;31.66,-39.29,;33.17,-38.97,;34.19,-40.11,;35.7,-39.79,;36.18,-38.33,;35.14,-37.18,;33.63,-37.51,;28.65,-39.93,;25.2,-42.19,;23.86,-41.43,;23.85,-39.89,;22.53,-42.2,;22.53,-43.74,;23.86,-44.51,;25.2,-43.74,)| Show InChI InChI=1S/C26H29N5O/c27-16-18-9-11-20(12-10-18)26-30-23(24-25(28)29-13-14-31(24)26)21-7-4-8-22(15-21)32-17-19-5-2-1-3-6-19/h1-8,13-15,18,20H,9-12,16-17,27H2,(H2,28,29)/t18-,20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IGF1-stimulated phosphorylation of human recombinant IGF1R expressed in NIH3T3 cells |
Bioorg Med Chem Lett 17: 1091-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.016
BindingDB Entry DOI: 10.7270/Q2QZ29MB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data