

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110577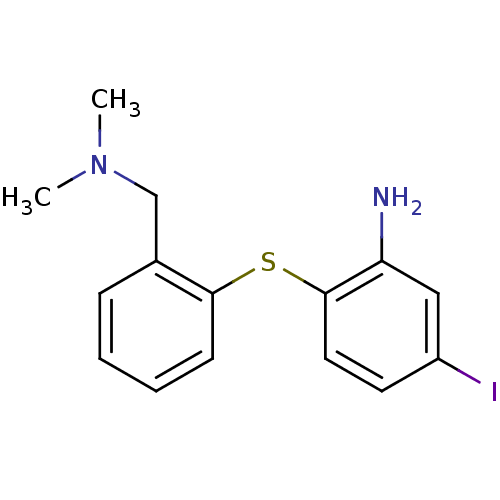 (2-(2-((dimethylamino)methyl)phenylthio)-5-iodoanil...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110578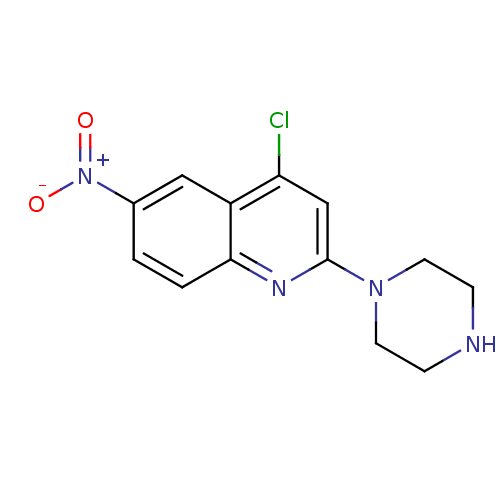 (4-Chloro-6-nitro-2-piperazin-1-yl-quinoline | CHEM...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50063266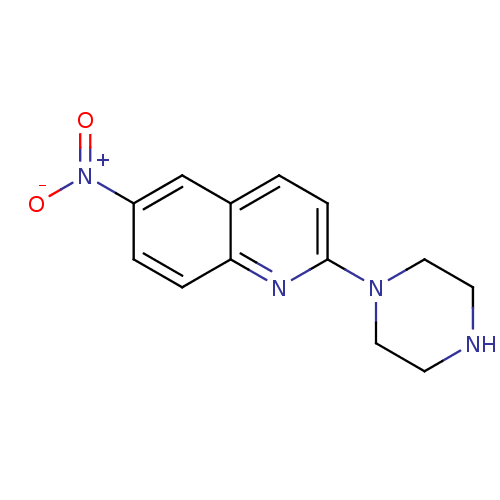 (6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110584 (3-(3-Fluoro-propyl)-6-nitro-2-piperazin-1-yl-quino...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110574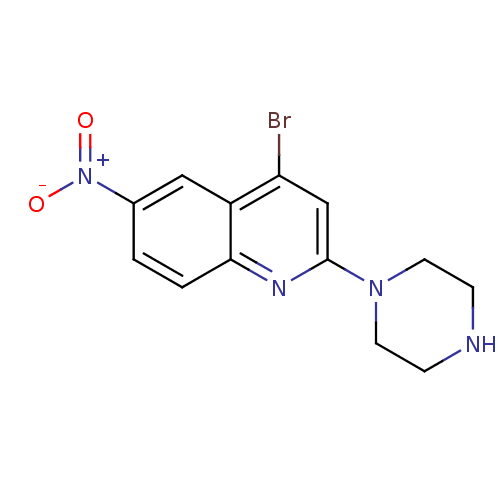 (4-Bromo-6-nitro-2-piperazin-1-yl-quinoline | CHEMB...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM22416 ((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50507371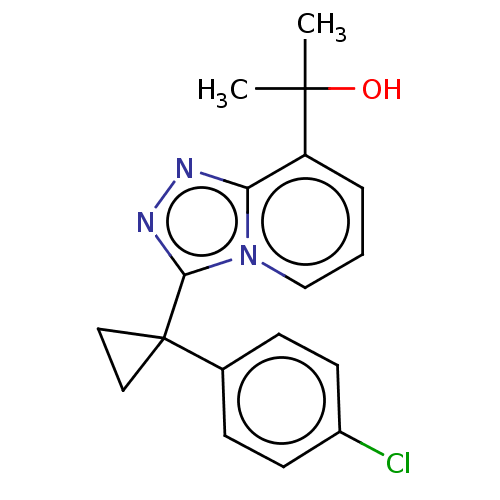 (BMS-823778) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human 11beta-HSD1 expressed in HEK293 cell microsomes using [3H]cortisone as substrate after 4 hrs by homogeneous immuno-ra... | ACS Med Chem Lett 9: 1170-1174 (2018) Article DOI: 10.1021/acsmedchemlett.8b00307 BindingDB Entry DOI: 10.7270/Q20R9SP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110587 (4-Allyl-6-nitro-2-piperazin-1-yl-quinoline | CHEMB...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110585 (4-(4-Bromo-6-nitro-quinolin-2-yl)-piperazine-1-car...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]citalopram binding to the rat cortical Serotonin transporter | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110579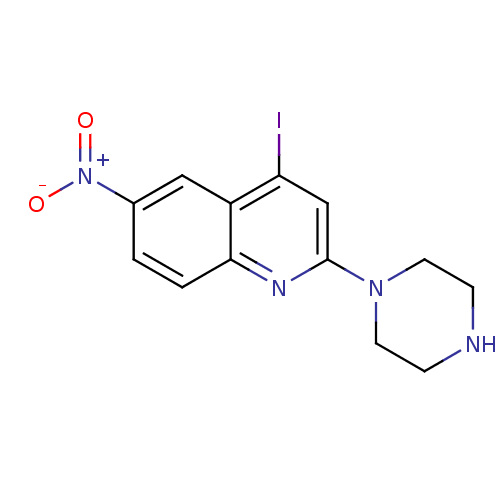 (4-Iodo-6-nitro-2-piperazin-1-yl-quinoline | CHEMBL...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110583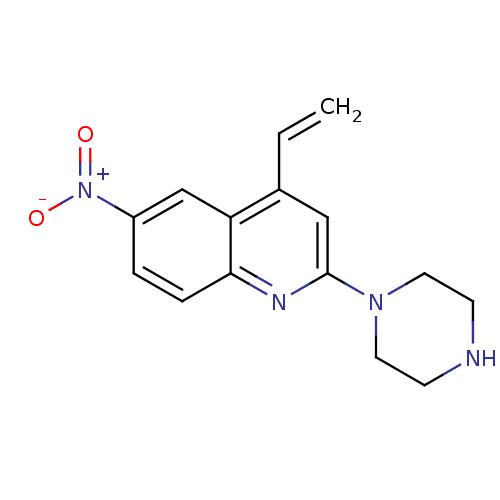 (6-Nitro-2-piperazin-1-yl-4-vinyl-quinoline | CHEMB...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50239606 (CHEMBL4080667) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... | J Med Chem 60: 4932-4948 (2017) Article DOI: 10.1021/acs.jmedchem.7b00211 BindingDB Entry DOI: 10.7270/Q2DV1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110576 (4-Furan-2-yl-6-nitro-2-piperazin-1-yl-quinoline | ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM30130 (CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110575 (6-Nitro-4-phenyl-2-piperazin-1-yl-quinoline | CHEM...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110586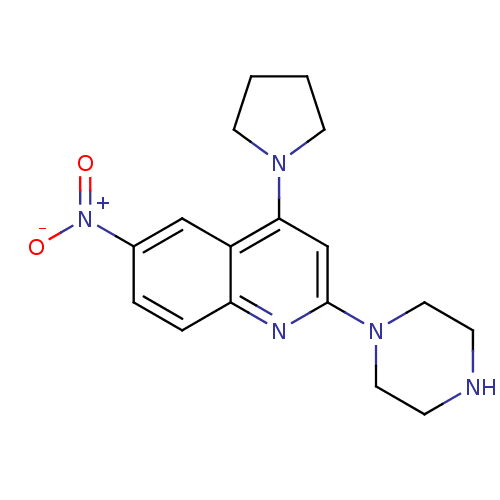 (6-Nitro-2-piperazin-1-yl-4-pyrrolidin-1-yl-quinoli...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110582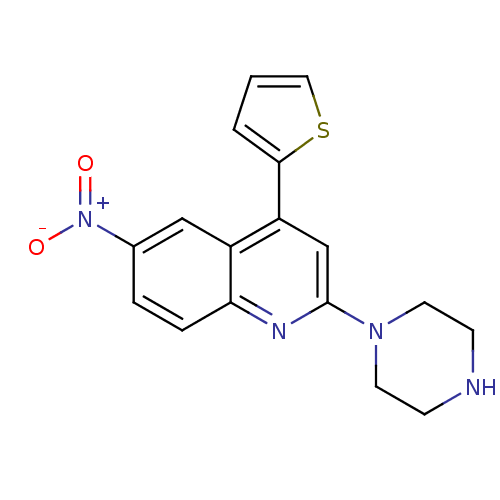 (6-Nitro-2-piperazin-1-yl-4-thiophen-2-yl-quinoline...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110580 (4-Benzyl-6-nitro-2-piperazin-1-yl-quinoline | CHEM...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 260 | -39.1 | 1.24E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Various concentrations of test compound (5, 10, 25, 50 and 100 mM) were dissolved in DMSO (final culture concentration 0.1%) and 4-MU oleate and lipa... | J Enzyme Inhib Med Chem 29: 1-6 (2014) Article DOI: 10.3109/14756366.2012.742517 BindingDB Entry DOI: 10.7270/Q2DJ5DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50507371 (BMS-823778) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant mouse 11beta-HSD1 expressed in HEK293 cell microsomes using [3H]cortisone as substrate after 4 hrs by homogeneous immuno-ra... | ACS Med Chem Lett 9: 1170-1174 (2018) Article DOI: 10.1021/acsmedchemlett.8b00307 BindingDB Entry DOI: 10.7270/Q20R9SP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM246486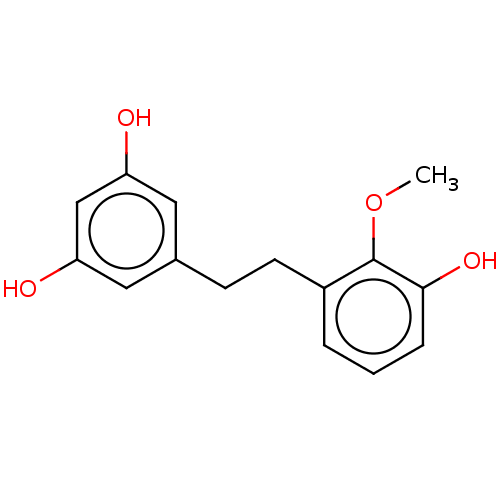 (3,3',5-Trihydroxy-2'-methoxybibenzyl (3)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.82E+3 | -34.1 | 8.77E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Various concentrations of test compound (5, 10, 25, 50 and 100 mM) were dissolved in DMSO (final culture concentration 0.1%) and 4-MU oleate and lipa... | J Enzyme Inhib Med Chem 29: 1-6 (2014) Article DOI: 10.3109/14756366.2012.742517 BindingDB Entry DOI: 10.7270/Q2DJ5DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM246500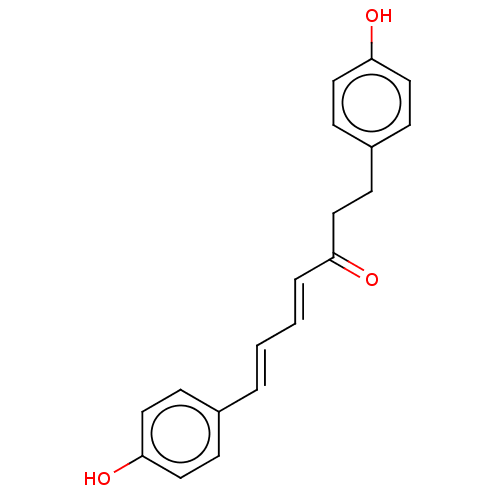 ((4E,6E)-1,7-bis(4-hydroxyphenyl)-4,6-heptadien-3-o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.92E+3 | -33.9 | 9.12E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Various concentrations of test compound (5, 10, 25, 50 and 100 mM) were dissolved in DMSO (final culture concentration 0.1%) and 4-MU oleate and lipa... | J Enzyme Inhib Med Chem 29: 1-6 (2014) Article DOI: 10.3109/14756366.2012.742517 BindingDB Entry DOI: 10.7270/Q2DJ5DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM246501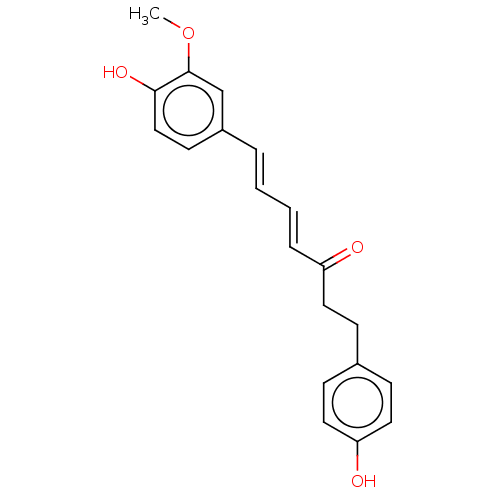 ((4E,6E)-7-(4-hydroxy-3-methoxyphenyl)-1-(4-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.17E+3 | -33.6 | 1.11E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Various concentrations of test compound (5, 10, 25, 50 and 100 mM) were dissolved in DMSO (final culture concentration 0.1%) and 4-MU oleate and lipa... | J Enzyme Inhib Med Chem 29: 1-6 (2014) Article DOI: 10.3109/14756366.2012.742517 BindingDB Entry DOI: 10.7270/Q2DJ5DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM246493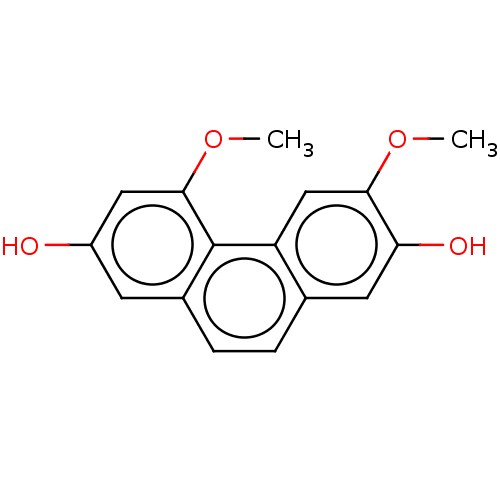 (3,5-Dimethoxy-2,7-phenanthrenediol (11)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 2.67E+3 | -33.1 | 1.27E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Various concentrations of test compound (5, 10, 25, 50 and 100 mM) were dissolved in DMSO (final culture concentration 0.1%) and 4-MU oleate and lipa... | J Enzyme Inhib Med Chem 29: 1-6 (2014) Article DOI: 10.3109/14756366.2012.742517 BindingDB Entry DOI: 10.7270/Q2DJ5DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM246485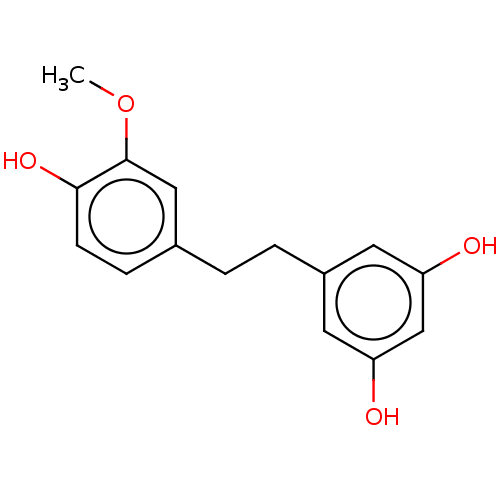 (Tristin (1)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.75E+3 | -33.0 | 1.35E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Various concentrations of test compound (5, 10, 25, 50 and 100 mM) were dissolved in DMSO (final culture concentration 0.1%) and 4-MU oleate and lipa... | J Enzyme Inhib Med Chem 29: 1-6 (2014) Article DOI: 10.3109/14756366.2012.742517 BindingDB Entry DOI: 10.7270/Q2DJ5DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM246499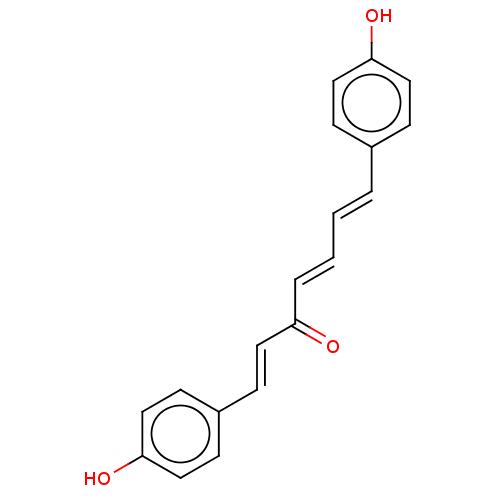 ((1E,4E,6E)-1,7-bis(4-hydroxyphenyl)-1,4,6-heptatri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.34E+3 | -32.5 | 1.64E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Various concentrations of test compound (5, 10, 25, 50 and 100 mM) were dissolved in DMSO (final culture concentration 0.1%) and 4-MU oleate and lipa... | J Enzyme Inhib Med Chem 29: 1-6 (2014) Article DOI: 10.3109/14756366.2012.742517 BindingDB Entry DOI: 10.7270/Q2DJ5DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM246496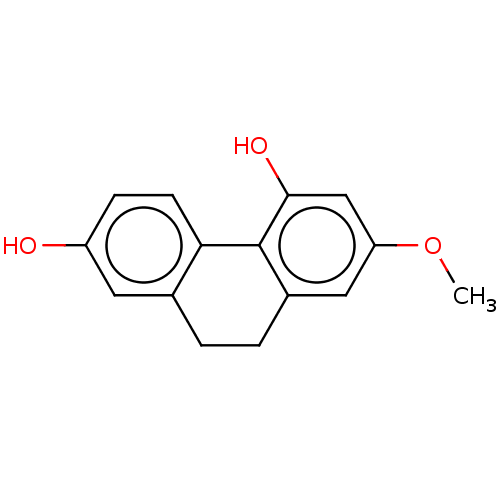 (2,5-Dihydroxy-7-methoxy-9,10-dihydrophenanthrene (...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 4.96E+3 | -31.5 | 2.51E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Various concentrations of test compound (5, 10, 25, 50 and 100 mM) were dissolved in DMSO (final culture concentration 0.1%) and 4-MU oleate and lipa... | J Enzyme Inhib Med Chem 29: 1-6 (2014) Article DOI: 10.3109/14756366.2012.742517 BindingDB Entry DOI: 10.7270/Q2DJ5DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM246488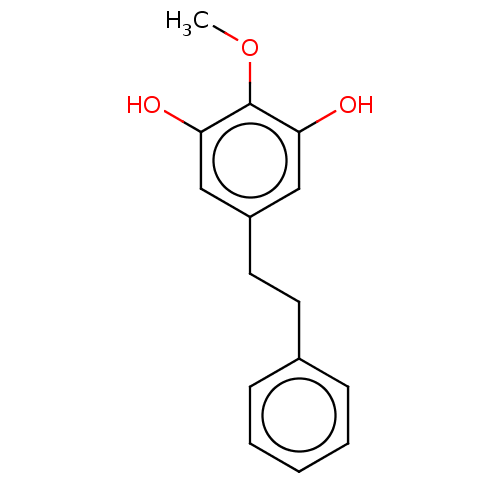 (2',4-Dihydroxy-3,5-dihydroxy-4-methoxybibenzyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.06E+3 | -31.4 | 2.54E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Various concentrations of test compound (5, 10, 25, 50 and 100 mM) were dissolved in DMSO (final culture concentration 0.1%) and 4-MU oleate and lipa... | J Enzyme Inhib Med Chem 29: 1-6 (2014) Article DOI: 10.3109/14756366.2012.742517 BindingDB Entry DOI: 10.7270/Q2DJ5DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50211956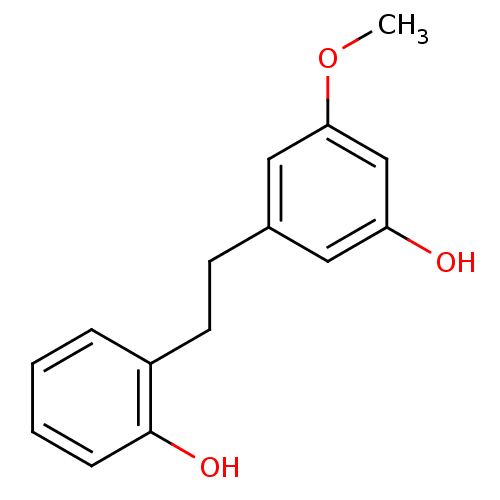 (2-(3-hydroxy-5-methoxyphenethyl)phenol | Batatasin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 6.28E+3 | -30.9 | 3.01E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Various concentrations of test compound (5, 10, 25, 50 and 100 mM) were dissolved in DMSO (final culture concentration 0.1%) and 4-MU oleate and lipa... | J Enzyme Inhib Med Chem 29: 1-6 (2014) Article DOI: 10.3109/14756366.2012.742517 BindingDB Entry DOI: 10.7270/Q2DJ5DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM64584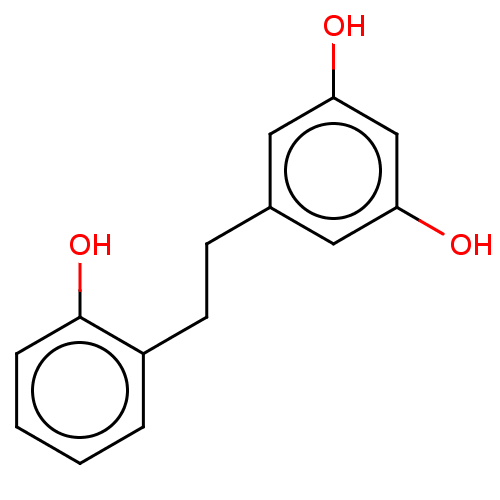 (2',3,5-Trihydroxybibenzyl (2)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.45E+3 | -30.8 | 2.92E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Various concentrations of test compound (5, 10, 25, 50 and 100 mM) were dissolved in DMSO (final culture concentration 0.1%) and 4-MU oleate and lipa... | J Enzyme Inhib Med Chem 29: 1-6 (2014) Article DOI: 10.3109/14756366.2012.742517 BindingDB Entry DOI: 10.7270/Q2DJ5DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM246487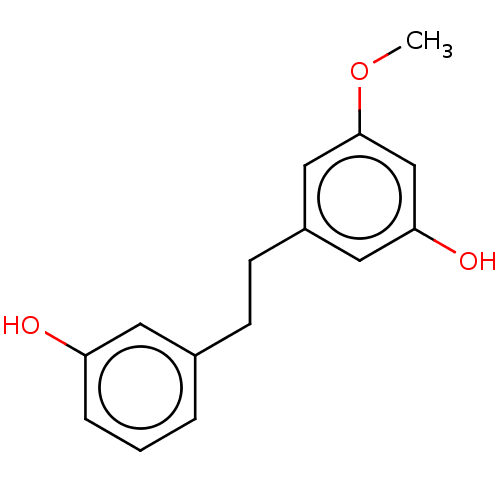 (Batatasin III (4)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 6.79E+3 | -30.7 | 3.23E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Various concentrations of test compound (5, 10, 25, 50 and 100 mM) were dissolved in DMSO (final culture concentration 0.1%) and 4-MU oleate and lipa... | J Enzyme Inhib Med Chem 29: 1-6 (2014) Article DOI: 10.3109/14756366.2012.742517 BindingDB Entry DOI: 10.7270/Q2DJ5DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50131679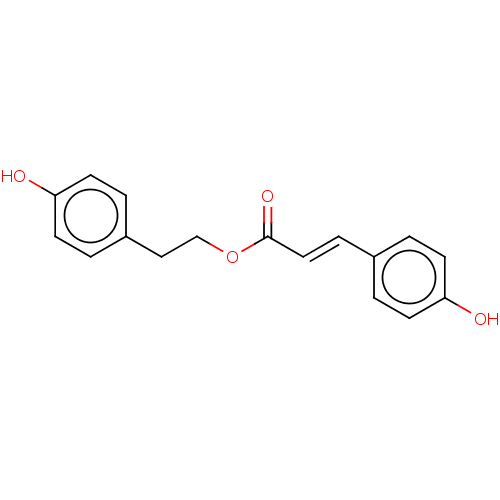 (CHEMBL3628211 | p-Hydroxyphenylethyl p-coumarate (...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.49E+3 | -30.4 | 3.48E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Various concentrations of test compound (5, 10, 25, 50 and 100 mM) were dissolved in DMSO (final culture concentration 0.1%) and 4-MU oleate and lipa... | J Enzyme Inhib Med Chem 29: 1-6 (2014) Article DOI: 10.3109/14756366.2012.742517 BindingDB Entry DOI: 10.7270/Q2DJ5DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM246495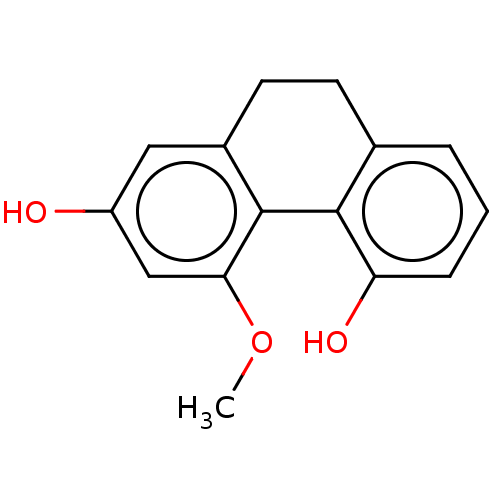 (Hircinol (13)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 8.57E+3 | -30.1 | 4.06E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Various concentrations of test compound (5, 10, 25, 50 and 100 mM) were dissolved in DMSO (final culture concentration 0.1%) and 4-MU oleate and lipa... | J Enzyme Inhib Med Chem 29: 1-6 (2014) Article DOI: 10.3109/14756366.2012.742517 BindingDB Entry DOI: 10.7270/Q2DJ5DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM246489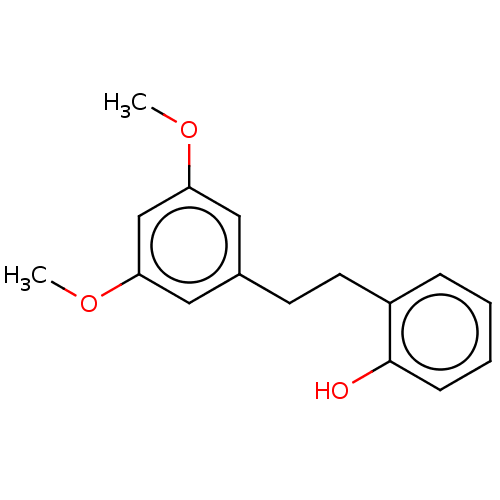 (3,5-Dimethoxy-2'hydroxybibenzyl (7)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.28E+3 | -29.9 | 4.41E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Various concentrations of test compound (5, 10, 25, 50 and 100 mM) were dissolved in DMSO (final culture concentration 0.1%) and 4-MU oleate and lipa... | J Enzyme Inhib Med Chem 29: 1-6 (2014) Article DOI: 10.3109/14756366.2012.742517 BindingDB Entry DOI: 10.7270/Q2DJ5DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM246492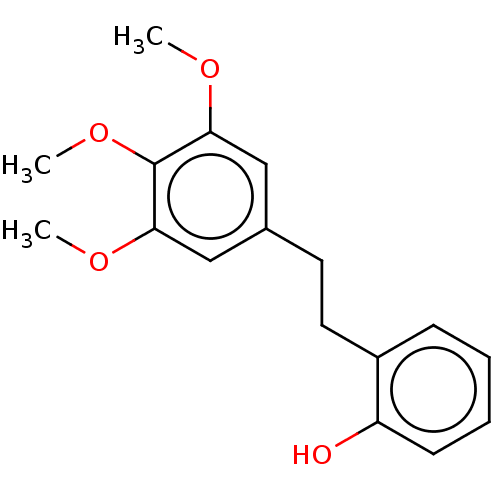 (Batatasin V (10)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 9.66E+3 | -29.8 | 4.57E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Seoul National University | Assay Description Various concentrations of test compound (5, 10, 25, 50 and 100 mM) were dissolved in DMSO (final culture concentration 0.1%) and 4-MU oleate and lipa... | J Enzyme Inhib Med Chem 29: 1-6 (2014) Article DOI: 10.3109/14756366.2012.742517 BindingDB Entry DOI: 10.7270/Q2DJ5DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50239610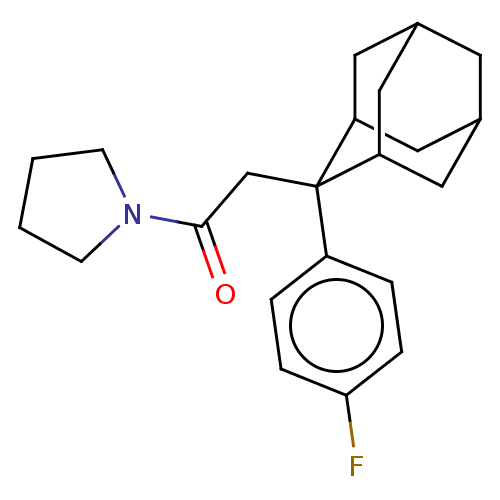 (CHEMBL4073961) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... | J Med Chem 60: 4932-4948 (2017) Article DOI: 10.1021/acs.jmedchem.7b00211 BindingDB Entry DOI: 10.7270/Q2DV1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50507376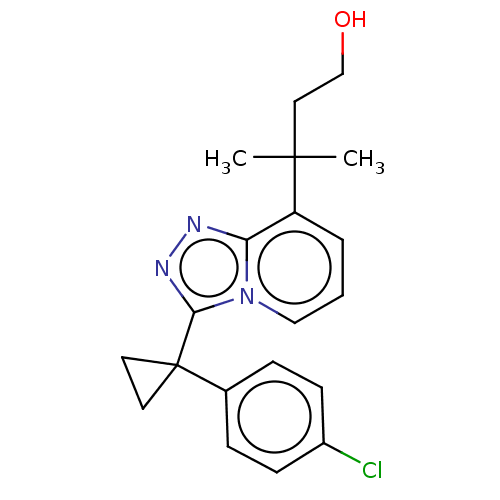 (CHEMBL4453347) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human 11beta-HSD1 expressed in HEK293 cell microsomes using [3H]cortisone as substrate after 4 hrs by homogeneous immuno-ra... | ACS Med Chem Lett 9: 1170-1174 (2018) Article DOI: 10.1021/acsmedchemlett.8b00307 BindingDB Entry DOI: 10.7270/Q20R9SP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50239632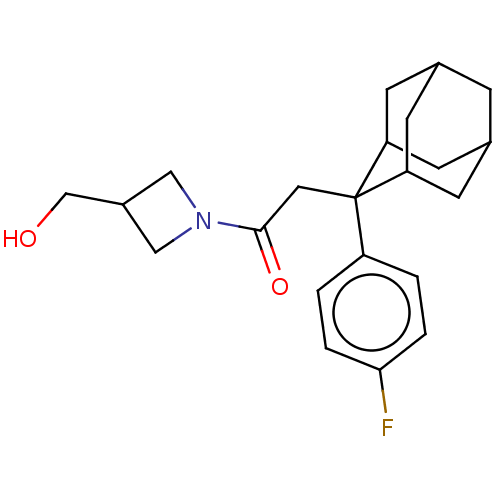 (CHEMBL4071232) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... | J Med Chem 60: 4932-4948 (2017) Article DOI: 10.1021/acs.jmedchem.7b00211 BindingDB Entry DOI: 10.7270/Q2DV1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50239628 (CHEMBL4102283) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibitory activity against human plasma renin | J Med Chem 60: 4932-4948 (2017) Article DOI: 10.1021/acs.jmedchem.7b00211 BindingDB Entry DOI: 10.7270/Q2DV1N23 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50239619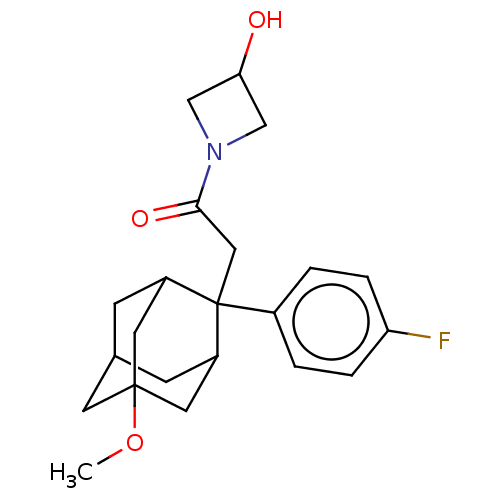 (CHEMBL4087497) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... | J Med Chem 60: 4932-4948 (2017) Article DOI: 10.1021/acs.jmedchem.7b00211 BindingDB Entry DOI: 10.7270/Q2DV1N23 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50239603 (CHEMBL4101370) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [3H]PIA from adenosine A1 receptor of rat brain membranes | J Med Chem 60: 4932-4948 (2017) Article DOI: 10.1021/acs.jmedchem.7b00211 BindingDB Entry DOI: 10.7270/Q2DV1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50239616 (CHEMBL4078671) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... | J Med Chem 60: 4932-4948 (2017) Article DOI: 10.1021/acs.jmedchem.7b00211 BindingDB Entry DOI: 10.7270/Q2DV1N23 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50239614 (CHEMBL4067777) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... | J Med Chem 60: 4932-4948 (2017) Article DOI: 10.1021/acs.jmedchem.7b00211 BindingDB Entry DOI: 10.7270/Q2DV1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50239609 (CHEMBL4093016) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... | J Med Chem 60: 4932-4948 (2017) Article DOI: 10.1021/acs.jmedchem.7b00211 BindingDB Entry DOI: 10.7270/Q2DV1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50356439 (CHEMBL1911707) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation plate reader | Bioorg Med Chem Lett 21: 6693-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.058 BindingDB Entry DOI: 10.7270/Q2FN16M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50239633 (CHEMBL4102950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... | J Med Chem 60: 4932-4948 (2017) Article DOI: 10.1021/acs.jmedchem.7b00211 BindingDB Entry DOI: 10.7270/Q2DV1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50239615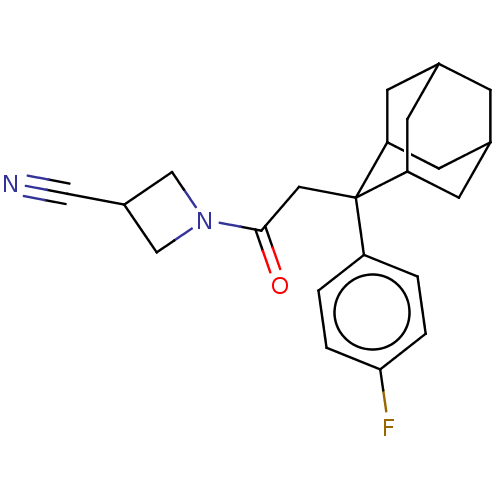 (CHEMBL4095204) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... | J Med Chem 60: 4932-4948 (2017) Article DOI: 10.1021/acs.jmedchem.7b00211 BindingDB Entry DOI: 10.7270/Q2DV1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50445575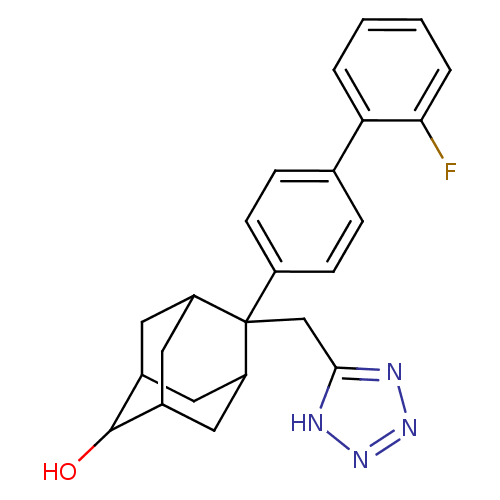 (CHEMBL3103523) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 | Bioorg Med Chem Lett 24: 654-60 (2014) Article DOI: 10.1016/j.bmcl.2013.11.066 BindingDB Entry DOI: 10.7270/Q20Z74RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50445576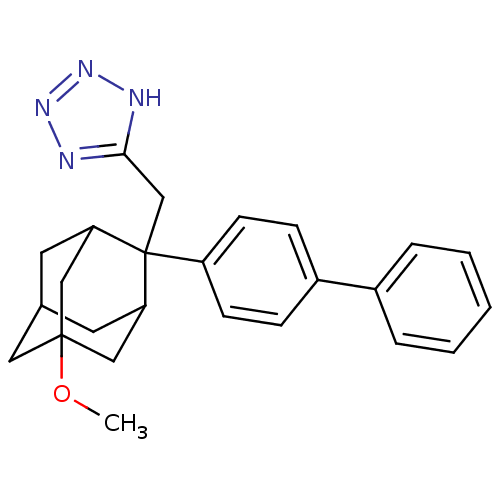 (CHEMBL3103439) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 | Bioorg Med Chem Lett 24: 654-60 (2014) Article DOI: 10.1016/j.bmcl.2013.11.066 BindingDB Entry DOI: 10.7270/Q20Z74RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50239621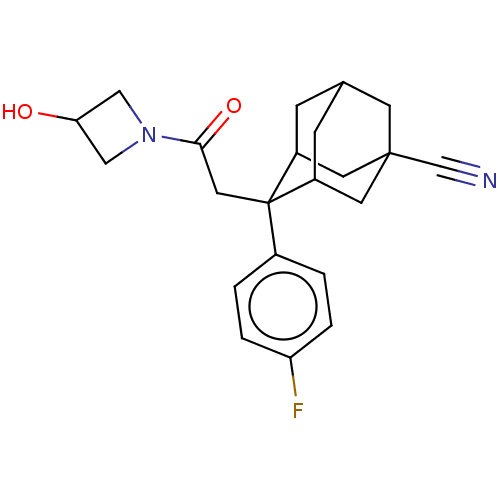 (CHEMBL4060170) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human microsomes using [3H]cortisone as substrate preincubated for 10 mins followed by substrate addition measured after... | J Med Chem 60: 4932-4948 (2017) Article DOI: 10.1021/acs.jmedchem.7b00211 BindingDB Entry DOI: 10.7270/Q2DV1N23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1275 total ) | Next | Last >> |