 Found 732 hits with Last Name = 'uehling' and Initial = 'de'
Found 732 hits with Last Name = 'uehling' and Initial = 'de' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Insulin receptor
(Homo sapiens (Human)) | BDBM50256480
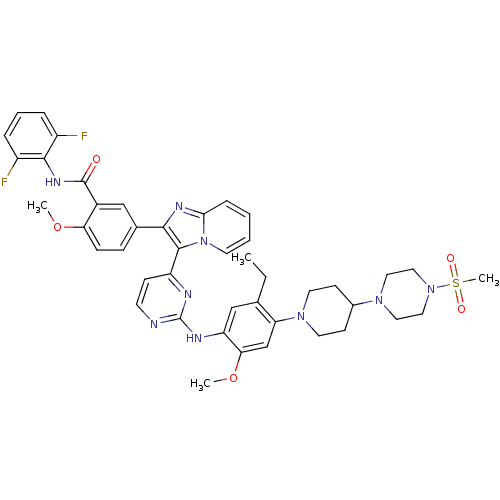
(CHEMBL466397 | N-(2,6-difluorophenyl)-5-(3-(2-(5-e...)Show SMILES CCc1cc(Nc2nccc(n2)-c2c(nc3ccccn23)-c2ccc(OC)c(c2)C(=O)Nc2c(F)cccc2F)c(OC)cc1N1CCC(CC1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C44H47F2N9O5S/c1-5-28-26-35(38(60-3)27-36(28)53-19-15-30(16-20-53)52-21-23-54(24-22-52)61(4,57)58)49-44-47-17-14-34(48-44)42-40(50-39-11-6-7-18-55(39)42)29-12-13-37(59-2)31(25-29)43(56)51-41-32(45)9-8-10-33(41)46/h6-14,17-18,25-27,30H,5,15-16,19-24H2,1-4H3,(H,51,56)(H,47,48,49) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to insulin receptor by liquid scintillation counting |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256480

(CHEMBL466397 | N-(2,6-difluorophenyl)-5-(3-(2-(5-e...)Show SMILES CCc1cc(Nc2nccc(n2)-c2c(nc3ccccn23)-c2ccc(OC)c(c2)C(=O)Nc2c(F)cccc2F)c(OC)cc1N1CCC(CC1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C44H47F2N9O5S/c1-5-28-26-35(38(60-3)27-36(28)53-19-15-30(16-20-53)52-21-23-54(24-22-52)61(4,57)58)49-44-47-17-14-34(48-44)42-40(50-39-11-6-7-18-55(39)42)29-12-13-37(59-2)31(25-29)43(56)51-41-32(45)9-8-10-33(41)46/h6-14,17-18,25-27,30H,5,15-16,19-24H2,1-4H3,(H,51,56)(H,47,48,49) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to IGF1R by liquid scintillation counting |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50256478
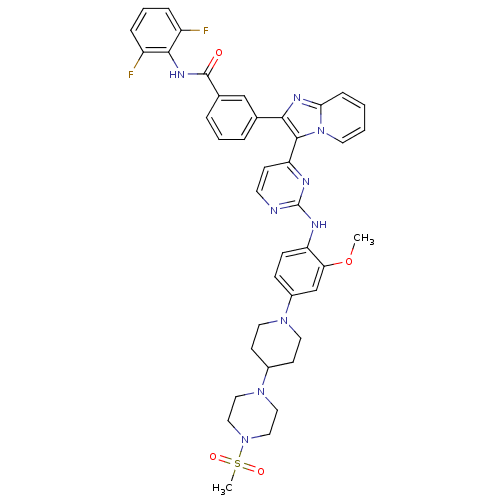
(CHEMBL507714 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c(nc2ccccn12)-c1cccc(c1)C(=O)Nc1c(F)cccc1F)N1CCC(CC1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C41H41F2N9O4S/c1-56-35-26-30(49-19-15-29(16-20-49)50-21-23-51(24-22-50)57(2,54)55)12-13-33(35)45-41-44-17-14-34(46-41)39-37(47-36-11-3-4-18-52(36)39)27-7-5-8-28(25-27)40(53)48-38-31(42)9-6-10-32(38)43/h3-14,17-18,25-26,29H,15-16,19-24H2,1-2H3,(H,48,53)(H,44,45,46) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to insulin receptor by liquid scintillation counting |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256478

(CHEMBL507714 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c(nc2ccccn12)-c1cccc(c1)C(=O)Nc1c(F)cccc1F)N1CCC(CC1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C41H41F2N9O4S/c1-56-35-26-30(49-19-15-29(16-20-49)50-21-23-51(24-22-50)57(2,54)55)12-13-33(35)45-41-44-17-14-34(46-41)39-37(47-36-11-3-4-18-52(36)39)27-7-5-8-28(25-27)40(53)48-38-31(42)9-6-10-32(38)43/h3-14,17-18,25-26,29H,15-16,19-24H2,1-2H3,(H,48,53)(H,44,45,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to IGF1R by liquid scintillation counting |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50002595
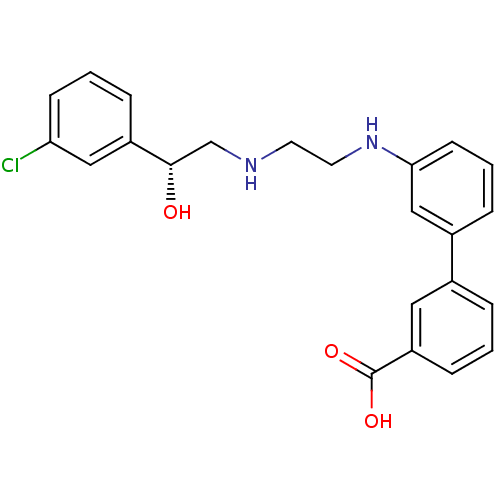
(Solabegron)Show SMILES O[C@@H](CNCCNc1cccc(c1)-c1cccc(c1)C(O)=O)c1cccc(Cl)c1 Show InChI InChI=1S/C23H23ClN2O3/c24-20-8-2-6-18(13-20)22(27)15-25-10-11-26-21-9-3-5-17(14-21)16-4-1-7-19(12-16)23(28)29/h1-9,12-14,22,25-27H,10-11,15H2,(H,28,29)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human cloned beta-1 adrenergic receptor expressed in Sf9 cells |
J Med Chem 49: 2758-71 (2006)
Checked by Author
Article DOI: 10.1021/jm0509445
BindingDB Entry DOI: 10.7270/Q2KD20D5 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50002595

(Solabegron)Show SMILES O[C@@H](CNCCNc1cccc(c1)-c1cccc(c1)C(O)=O)c1cccc(Cl)c1 Show InChI InChI=1S/C23H23ClN2O3/c24-20-8-2-6-18(13-20)22(27)15-25-10-11-26-21-9-3-5-17(14-21)16-4-1-7-19(12-16)23(28)29/h1-9,12-14,22,25-27H,10-11,15H2,(H,28,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human cloned beta-2 adrenergic receptor expressed in Sf9 cells |
J Med Chem 49: 2758-71 (2006)
Checked by Author
Article DOI: 10.1021/jm0509445
BindingDB Entry DOI: 10.7270/Q2KD20D5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM120095
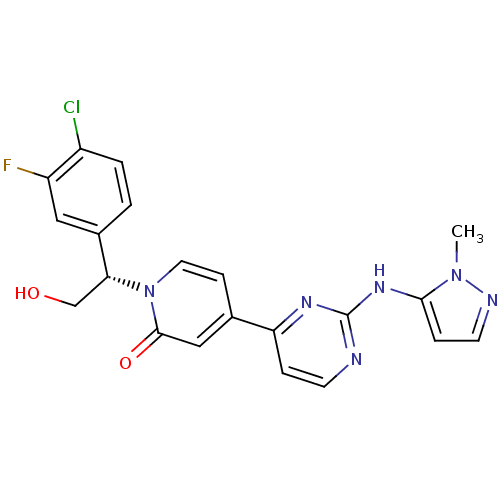
(US10525036, Example GDC-0994 | US10934304, Example...)Show SMILES Cn1nccc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-28-19(5-8-25-28)27-21-24-7-4-17(26-21)13-6-9-29(20(31)11-13)18(12-30)14-2-3-15(22)16(23)10-14/h2-11,18,30H,12H2,1H3,(H,24,26,27)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 4047-56 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.093
BindingDB Entry DOI: 10.7270/Q2VX0JBS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256467
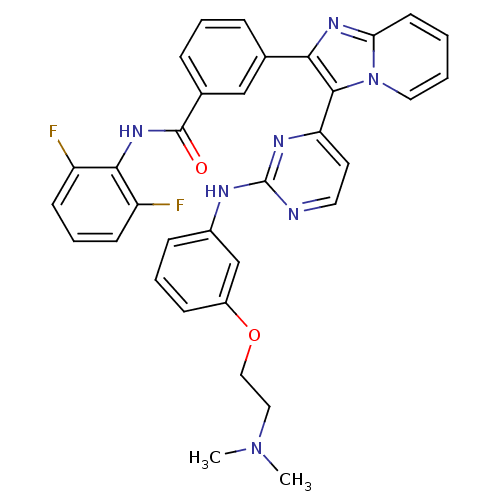
(CHEMBL448668 | N-(2,6-difluorophenyl)-3-(3-(2-(3-(...)Show SMILES CN(C)CCOc1cccc(Nc2nccc(n2)-c2c(nc3ccccn23)-c2cccc(c2)C(=O)Nc2c(F)cccc2F)c1 Show InChI InChI=1S/C34H29F2N7O2/c1-42(2)18-19-45-25-11-6-10-24(21-25)38-34-37-16-15-28(39-34)32-30(40-29-14-3-4-17-43(29)32)22-8-5-9-23(20-22)33(44)41-31-26(35)12-7-13-27(31)36/h3-17,20-21H,18-19H2,1-2H3,(H,41,44)(H,37,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256469
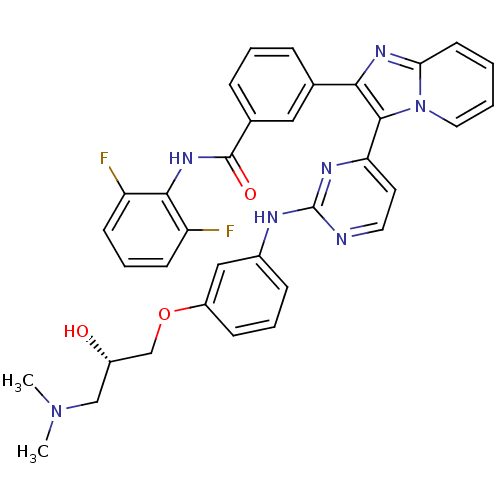
((S)-N-(2,6-difluorophenyl)-3-(3-(2-(3-(3-(dimethyl...)Show SMILES CN(C)C[C@H](O)COc1cccc(Nc2nccc(n2)-c2c(nc3ccccn23)-c2cccc(c2)C(=O)Nc2c(F)cccc2F)c1 |r| Show InChI InChI=1S/C35H31F2N7O3/c1-43(2)20-25(45)21-47-26-11-6-10-24(19-26)39-35-38-16-15-29(40-35)33-31(41-30-14-3-4-17-44(30)33)22-8-5-9-23(18-22)34(46)42-32-27(36)12-7-13-28(32)37/h3-19,25,45H,20-21H2,1-2H3,(H,42,46)(H,38,39,40)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256016
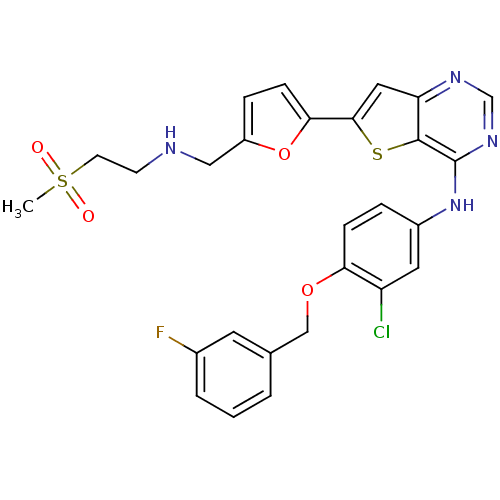
(CHEMBL475768 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C27H24ClFN4O4S2/c1-39(34,35)10-9-30-14-20-6-8-24(37-20)25-13-22-26(38-25)27(32-16-31-22)33-19-5-7-23(21(28)12-19)36-15-17-3-2-4-18(29)11-17/h2-8,11-13,16,30H,9-10,14-15H2,1H3,(H,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50094464

(CHEMBL3590107 | US10525036, Example SCH772984 | US...)Show SMILES O=C(CN1CC[C@H](C1)C(=O)Nc1ccc2[nH]nc(-c3ccncc3)c2c1)N1CCN(CC1)c1ccc(cc1)-c1ncccn1 |r| Show InChI InChI=1S/C22H27NO3/c1-2-15-23-16-13-20(14-17-23)26-21(24)22(25,18-9-5-3-6-10-18)19-11-7-4-8-12-19/h3-12,20,25H,2,13-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) after 45 mins by IMAP assay |
Bioorg Med Chem Lett 25: 4047-56 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.093
BindingDB Entry DOI: 10.7270/Q2VX0JBS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM120095

(US10525036, Example GDC-0994 | US10934304, Example...)Show SMILES Cn1nccc1Nc1nccc(n1)-c1ccn([C@H](CO)c2ccc(Cl)c(F)c2)c(=O)c1 |r| Show InChI InChI=1S/C21H18ClFN6O2/c1-28-19(5-8-25-28)27-21-24-7-4-17(26-21)13-6-9-29(20(31)11-13)18(12-30)14-2-3-15(22)16(23)10-14/h2-11,18,30H,12H2,1H3,(H,24,26,27)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) |
Bioorg Med Chem Lett 25: 4047-56 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.093
BindingDB Entry DOI: 10.7270/Q2VX0JBS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256472
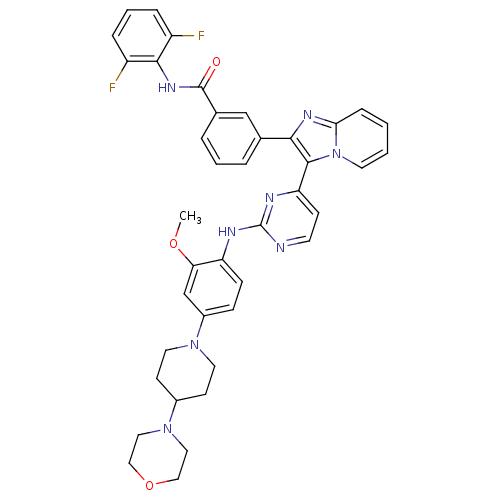
(CHEMBL502652 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c(nc2ccccn12)-c1cccc(c1)C(=O)Nc1c(F)cccc1F)N1CCC(CC1)N1CCOCC1 Show InChI InChI=1S/C40H38F2N8O3/c1-52-34-25-29(48-18-14-28(15-19-48)49-20-22-53-23-21-49)11-12-32(34)44-40-43-16-13-33(45-40)38-36(46-35-10-2-3-17-50(35)38)26-6-4-7-27(24-26)39(51)47-37-30(41)8-5-9-31(37)42/h2-13,16-17,24-25,28H,14-15,18-23H2,1H3,(H,47,51)(H,43,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256473
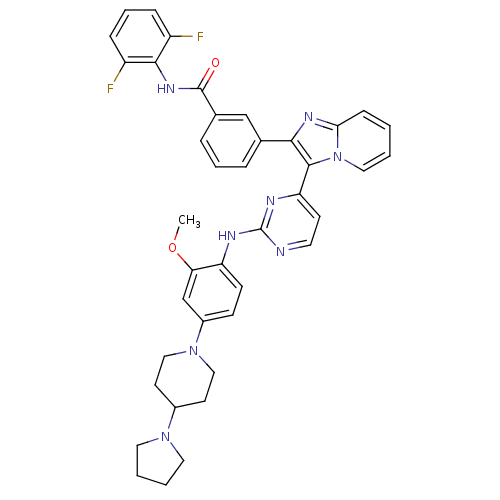
(CHEMBL502198 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c(nc2ccccn12)-c1cccc(c1)C(=O)Nc1c(F)cccc1F)N1CCC(CC1)N1CCCC1 Show InChI InChI=1S/C40H38F2N8O2/c1-52-34-25-29(49-22-16-28(17-23-49)48-19-4-5-20-48)13-14-32(34)44-40-43-18-15-33(45-40)38-36(46-35-12-2-3-21-50(35)38)26-8-6-9-27(24-26)39(51)47-37-30(41)10-7-11-31(37)42/h2-3,6-15,18,21,24-25,28H,4-5,16-17,19-20,22-23H2,1H3,(H,47,51)(H,43,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256474
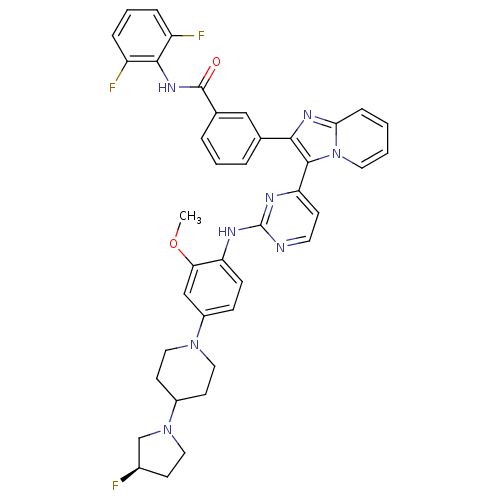
((R)-N-(2,6-difluorophenyl)-3-(3-(2-(4-(4-(3-fluoro...)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c(nc2ccccn12)-c1cccc(c1)C(=O)Nc1c(F)cccc1F)N1CCC(CC1)N1CC[C@@H](F)C1 |r| Show InChI InChI=1S/C40H37F3N8O2/c1-53-34-23-29(49-20-15-28(16-21-49)50-19-14-27(41)24-50)11-12-32(34)45-40-44-17-13-33(46-40)38-36(47-35-10-2-3-18-51(35)38)25-6-4-7-26(22-25)39(52)48-37-30(42)8-5-9-31(37)43/h2-13,17-18,22-23,27-28H,14-16,19-21,24H2,1H3,(H,48,52)(H,44,45,46)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256410
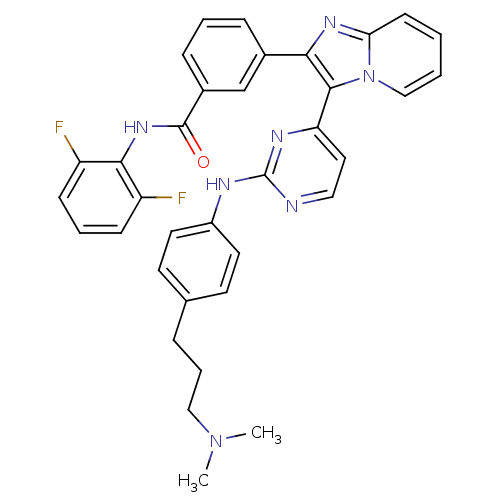
(CHEMBL447668 | N-(2,6-difluorophenyl)-3-(3-(2-(3-(...)Show SMILES CN(C)CCCc1ccc(Nc2nccc(n2)-c2c(nc3ccccn23)-c2cccc(c2)C(=O)Nc2c(F)cccc2F)cc1 Show InChI InChI=1S/C35H31F2N7O/c1-43(2)20-7-8-23-14-16-26(17-15-23)39-35-38-19-18-29(40-35)33-31(41-30-13-3-4-21-44(30)33)24-9-5-10-25(22-24)34(45)42-32-27(36)11-6-12-28(32)37/h3-6,9-19,21-22H,7-8,20H2,1-2H3,(H,42,45)(H,38,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256470
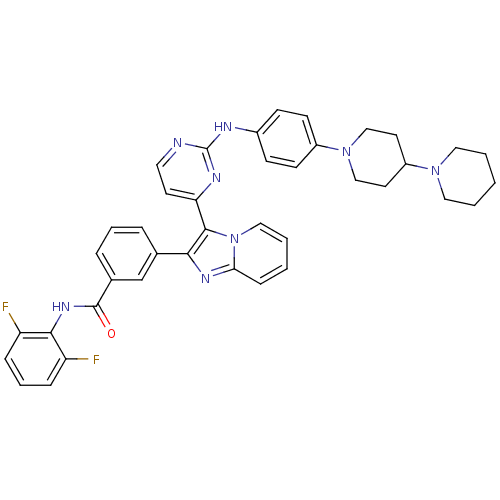
(3-(3-(2-(4-(1,4'-bipiperidin-1'-yl)phenylamino)pyr...)Show SMILES Fc1cccc(F)c1NC(=O)c1cccc(c1)-c1nc2ccccn2c1-c1ccnc(Nc2ccc(cc2)N2CCC(CC2)N2CCCCC2)n1 Show InChI InChI=1S/C40H38F2N8O/c41-32-10-7-11-33(42)37(32)47-39(51)28-9-6-8-27(26-28)36-38(50-23-5-2-12-35(50)46-36)34-17-20-43-40(45-34)44-29-13-15-30(16-14-29)49-24-18-31(19-25-49)48-21-3-1-4-22-48/h2,5-17,20,23,26,31H,1,3-4,18-19,21-22,24-25H2,(H,47,51)(H,43,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256471
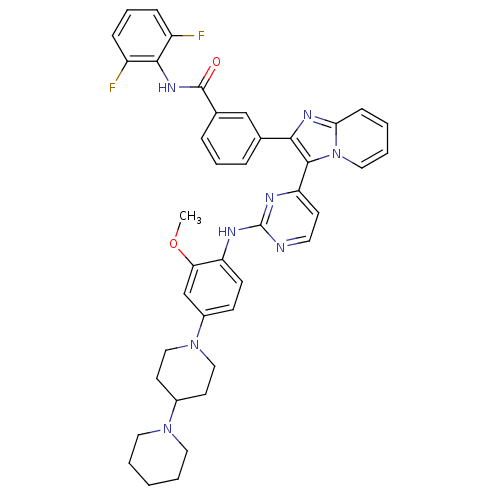
(3-(3-(2-(4-(1,4'-bipiperidin-1'-yl)-2-methoxypheny...)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c(nc2ccccn12)-c1cccc(c1)C(=O)Nc1c(F)cccc1F)N1CCC(CC1)N1CCCCC1 Show InChI InChI=1S/C41H40F2N8O2/c1-53-35-26-30(50-23-17-29(18-24-50)49-20-4-2-5-21-49)14-15-33(35)45-41-44-19-16-34(46-41)39-37(47-36-13-3-6-22-51(36)39)27-9-7-10-28(25-27)40(52)48-38-31(42)11-8-12-32(38)43/h3,6-16,19,22,25-26,29H,2,4-5,17-18,20-21,23-24H2,1H3,(H,48,52)(H,44,45,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256032
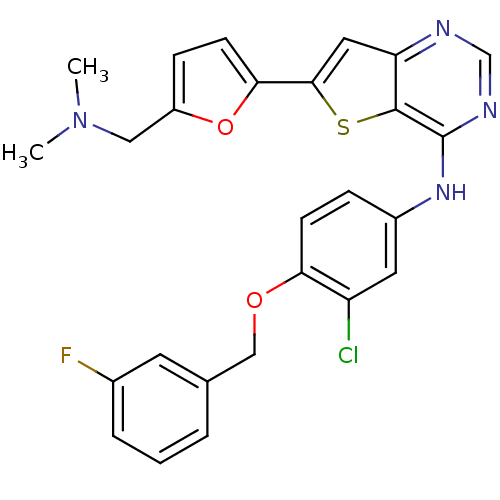
(CHEMBL475594 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CN(C)Cc1ccc(o1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C26H22ClFN4O2S/c1-32(2)13-19-7-9-23(34-19)24-12-21-25(35-24)26(30-15-29-21)31-18-6-8-22(20(27)11-18)33-14-16-4-3-5-17(28)10-16/h3-12,15H,13-14H2,1-2H3,(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256475
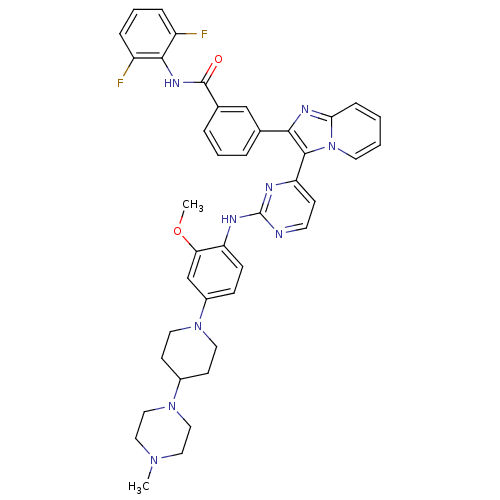
(CHEMBL449110 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c(nc2ccccn12)-c1cccc(c1)C(=O)Nc1c(F)cccc1F)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C41H41F2N9O2/c1-49-21-23-51(24-22-49)29-15-19-50(20-16-29)30-12-13-33(35(26-30)54-2)45-41-44-17-14-34(46-41)39-37(47-36-11-3-4-18-52(36)39)27-7-5-8-28(25-27)40(53)48-38-31(42)9-6-10-32(38)43/h3-14,17-18,25-26,29H,15-16,19-24H2,1-2H3,(H,48,53)(H,44,45,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256477
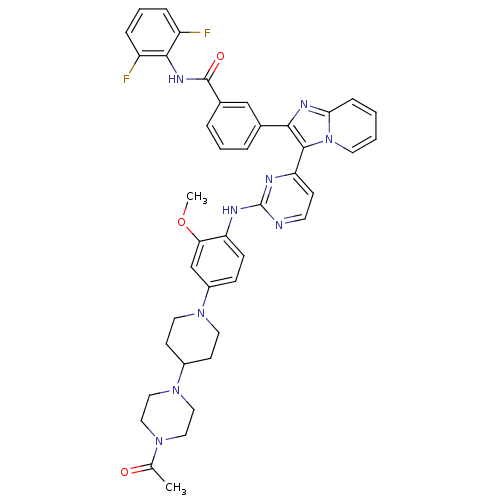
(3-(3-(2-(4-(4-(4-acetylpiperazin-1-yl)piperidin-1-...)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c(nc2ccccn12)-c1cccc(c1)C(=O)Nc1c(F)cccc1F)N1CCC(CC1)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C42H41F2N9O3/c1-27(54)50-21-23-52(24-22-50)30-15-19-51(20-16-30)31-12-13-34(36(26-31)56-2)46-42-45-17-14-35(47-42)40-38(48-37-11-3-4-18-53(37)40)28-7-5-8-29(25-28)41(55)49-39-32(43)9-6-10-33(39)44/h3-14,17-18,25-26,30H,15-16,19-24H2,1-2H3,(H,49,55)(H,45,46,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256036
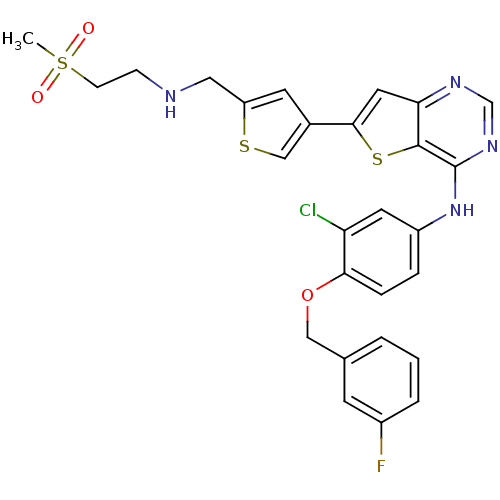
(CHEMBL474431 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CS(=O)(=O)CCNCc1cc(cs1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C27H24ClFN4O3S3/c1-39(34,35)8-7-30-13-21-10-18(15-37-21)25-12-23-26(38-25)27(32-16-31-23)33-20-5-6-24(22(28)11-20)36-14-17-3-2-4-19(29)9-17/h2-6,9-12,15-16,30H,7-8,13-14H2,1H3,(H,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256034
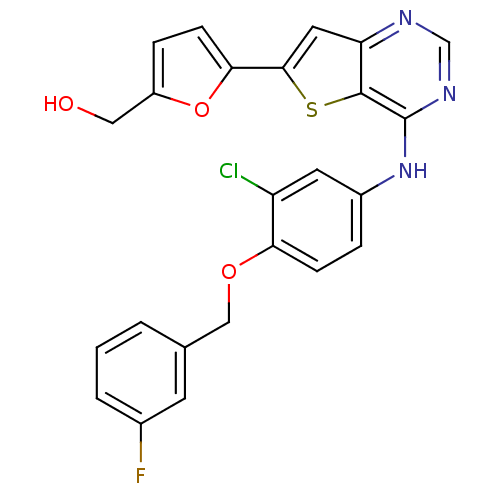
((5-(4-(3-chloro-4-(3-fluorobenzyloxy)phenylamino)t...)Show SMILES OCc1ccc(o1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C24H17ClFN3O3S/c25-18-9-16(4-6-20(18)31-12-14-2-1-3-15(26)8-14)29-24-23-19(27-13-28-24)10-22(33-23)21-7-5-17(11-30)32-21/h1-10,13,30H,11-12H2,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256035
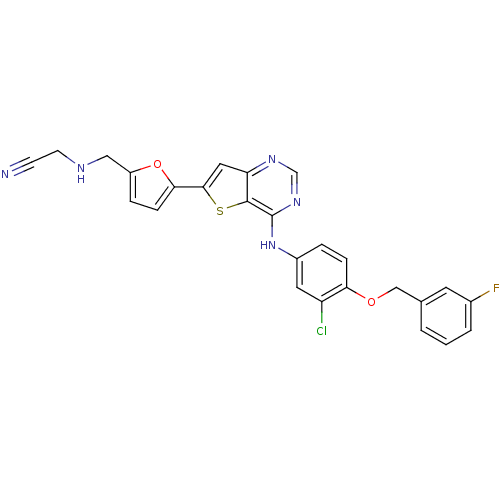
(2-((5-(4-(3-chloro-4-(3-fluorobenzyloxy)phenylamin...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4cc(sc34)-c3ccc(CNCC#N)o3)cc2Cl)c1 Show InChI InChI=1S/C26H19ClFN5O2S/c27-20-11-18(4-6-22(20)34-14-16-2-1-3-17(28)10-16)33-26-25-21(31-15-32-26)12-24(36-25)23-7-5-19(35-23)13-30-9-8-29/h1-7,10-12,15,30H,9,13-14H2,(H,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM50566950
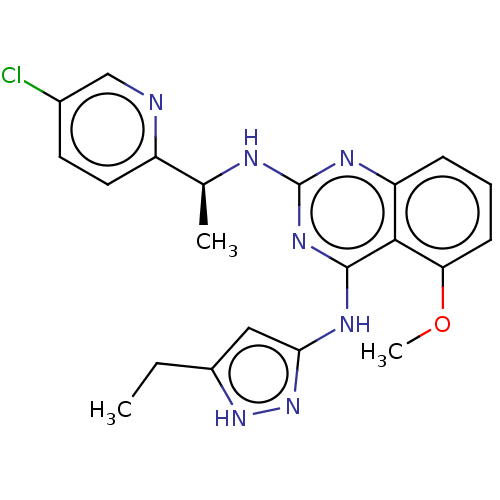
(CHEMBL4852125)Show SMILES CCc1cc(Nc2nc(N[C@@H](C)c3ccc(Cl)cn3)nc3cccc(OC)c23)n[nH]1 |r| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length human GRK6 using casein as substrate measured after 80 mins in presence of [gamma33P]ATP by radiometric scintil... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM50566949
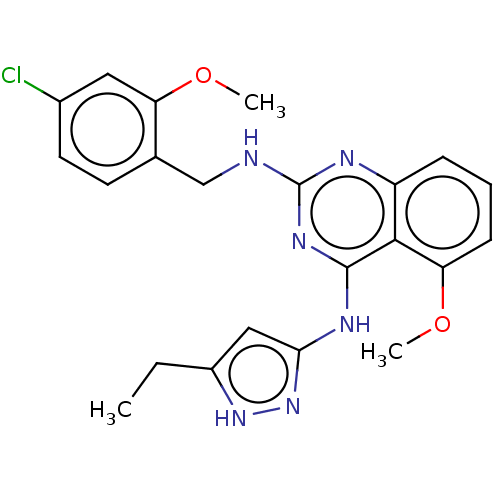
(CHEMBL4877302)Show SMILES CCc1cc(Nc2nc(NCc3ccc(Cl)cc3OC)nc3cccc(OC)c23)n[nH]1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GRK6 using casein as substrate in presence of [gamma33P]ATP by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256468

(CHEMBL500003 | N-(2,6-difluorophenyl)-3-(3-(2-(4-(...)Show SMILES Fc1cccc(F)c1NC(=O)c1cccc(c1)-c1nc2ccccn2c1-c1ccnc(Nc2ccc(CCN3CCOCC3)cc2)n1 Show InChI InChI=1S/C36H31F2N7O2/c37-28-7-4-8-29(38)33(28)43-35(46)26-6-3-5-25(23-26)32-34(45-17-2-1-9-31(45)42-32)30-14-16-39-36(41-30)40-27-12-10-24(11-13-27)15-18-44-19-21-47-22-20-44/h1-14,16-17,23H,15,18-22H2,(H,43,46)(H,39,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256476
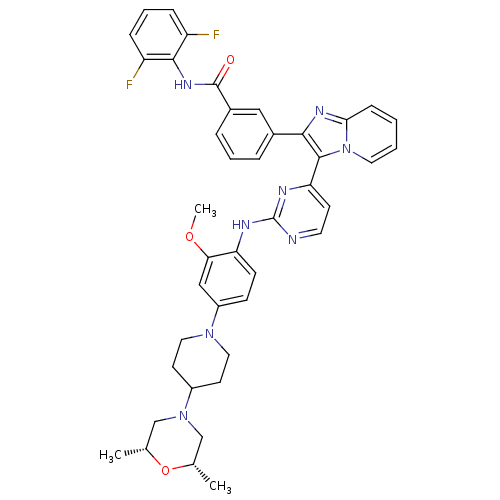
(CHEMBL454796 | N-(2,6-difluorophenyl)-3-(3-(2-(4-(...)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c(nc2ccccn12)-c1cccc(c1)C(=O)Nc1c(F)cccc1F)N1CCC(CC1)N1C[C@H](C)O[C@H](C)C1 |r| Show InChI InChI=1S/C42H42F2N8O3/c1-26-24-51(25-27(2)55-26)30-16-20-50(21-17-30)31-13-14-34(36(23-31)54-3)46-42-45-18-15-35(47-42)40-38(48-37-12-4-5-19-52(37)40)28-8-6-9-29(22-28)41(53)49-39-32(43)10-7-11-33(39)44/h4-15,18-19,22-23,26-27,30H,16-17,20-21,24-25H2,1-3H3,(H,49,53)(H,45,46,47)/t26-,27+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50094464

(CHEMBL3590107 | US10525036, Example SCH772984 | US...)Show SMILES O=C(CN1CC[C@H](C1)C(=O)Nc1ccc2[nH]nc(-c3ccncc3)c2c1)N1CCN(CC1)c1ccc(cc1)-c1ncccn1 |r| Show InChI InChI=1S/C22H27NO3/c1-2-15-23-16-13-20(14-17-23)26-21(24)22(25,18-9-5-3-6-10-18)19-11-7-4-8-12-19/h3-12,20,25H,2,13-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 (unknown origin) after 45 mins by IMAP assay |
Bioorg Med Chem Lett 25: 4047-56 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.093
BindingDB Entry DOI: 10.7270/Q2VX0JBS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256038
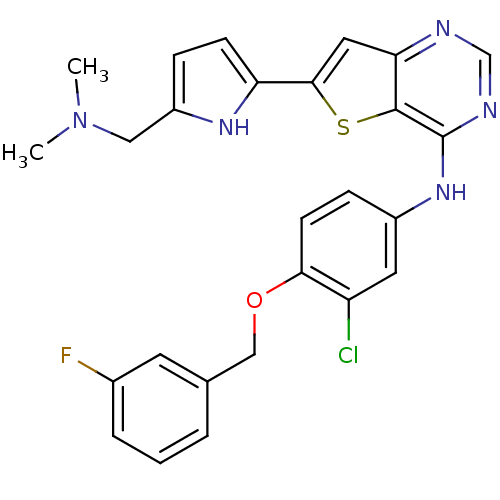
(CHEMBL473437 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CN(C)Cc1ccc([nH]1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C26H23ClFN5OS/c1-33(2)13-19-6-8-21(31-19)24-12-22-25(35-24)26(30-15-29-22)32-18-7-9-23(20(27)11-18)34-14-16-4-3-5-17(28)10-16/h3-12,15,31H,13-14H2,1-2H3,(H,29,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256017
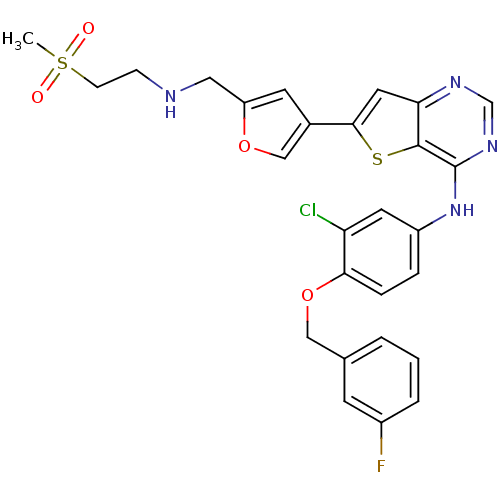
(CHEMBL475769 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CS(=O)(=O)CCNCc1cc(co1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C27H24ClFN4O4S2/c1-39(34,35)8-7-30-13-21-10-18(15-36-21)25-12-23-26(38-25)27(32-16-31-23)33-20-5-6-24(22(28)11-20)37-14-17-3-2-4-19(29)9-17/h2-6,9-12,15-16,30H,7-8,13-14H2,1H3,(H,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50256478

(CHEMBL507714 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c(nc2ccccn12)-c1cccc(c1)C(=O)Nc1c(F)cccc1F)N1CCC(CC1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C41H41F2N9O4S/c1-56-35-26-30(49-19-15-29(16-20-49)50-21-23-51(24-22-50)57(2,54)55)12-13-33(35)45-41-44-17-14-34(46-41)39-37(47-36-11-3-4-18-52(36)39)27-7-5-8-28(25-27)40(53)48-38-31(42)9-6-10-32(38)43/h3-14,17-18,25-26,29H,15-16,19-24H2,1-2H3,(H,48,53)(H,44,45,46) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged insulin receptor expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256033
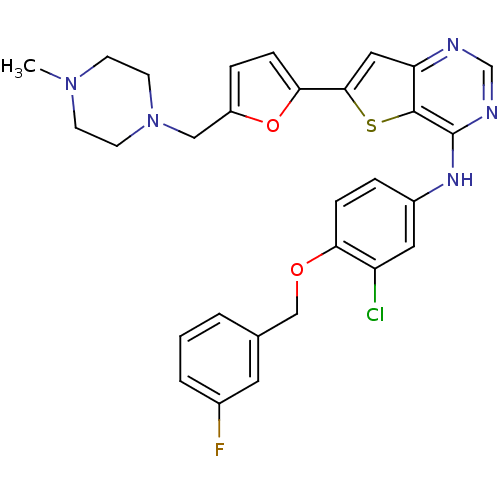
(CHEMBL475761 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CN1CCN(Cc2ccc(o2)-c2cc3ncnc(Nc4ccc(OCc5cccc(F)c5)c(Cl)c4)c3s2)CC1 Show InChI InChI=1S/C29H27ClFN5O2S/c1-35-9-11-36(12-10-35)16-22-6-8-26(38-22)27-15-24-28(39-27)29(33-18-32-24)34-21-5-7-25(23(30)14-21)37-17-19-3-2-4-20(31)13-19/h2-8,13-15,18H,9-12,16-17H2,1H3,(H,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256478

(CHEMBL507714 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c(nc2ccccn12)-c1cccc(c1)C(=O)Nc1c(F)cccc1F)N1CCC(CC1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C41H41F2N9O4S/c1-56-35-26-30(49-19-15-29(16-20-49)50-21-23-51(24-22-50)57(2,54)55)12-13-33(35)45-41-44-17-14-34(46-41)39-37(47-36-11-3-4-18-52(36)39)27-7-5-8-28(25-27)40(53)48-38-31(42)9-6-10-32(38)43/h3-14,17-18,25-26,29H,15-16,19-24H2,1-2H3,(H,48,53)(H,44,45,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM50566953
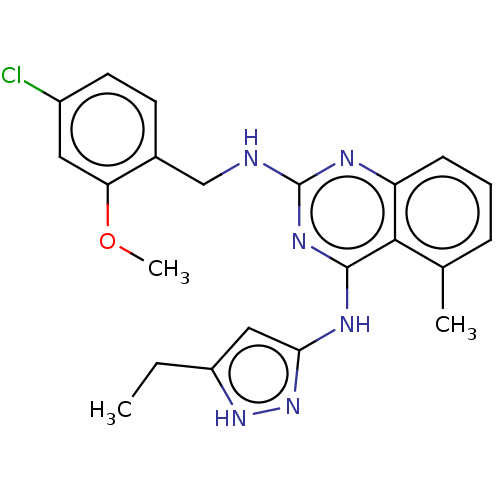
(CHEMBL4860332)Show SMILES CCc1cc(Nc2nc(NCc3ccc(Cl)cc3OC)nc3cccc(C)c23)n[nH]1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length human GRK6 using casein as substrate measured after 80 mins in presence of [gamma33P]ATP by radiometric scintil... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50256040
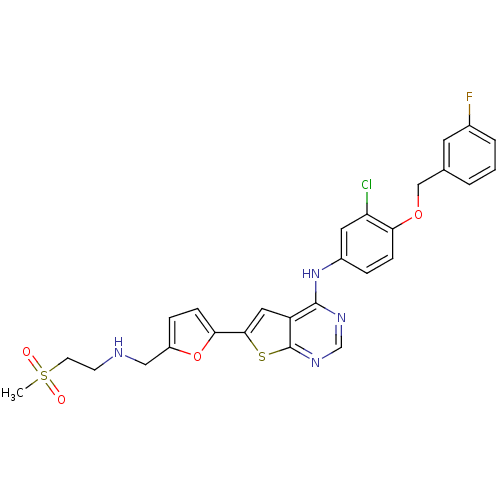
(CHEMBL480349 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1cc2c(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)ncnc2s1 Show InChI InChI=1S/C27H24ClFN4O4S2/c1-39(34,35)10-9-30-14-20-6-8-24(37-20)25-13-21-26(31-16-32-27(21)38-25)33-19-5-7-23(22(28)12-19)36-15-17-3-2-4-18(29)11-17/h2-8,11-13,16,30H,9-10,14-15H2,1H3,(H,31,32,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256039
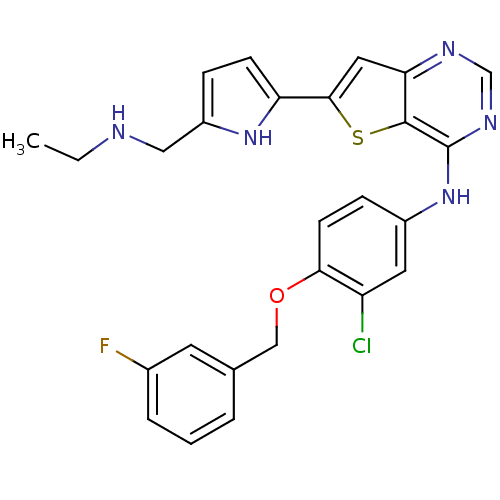
(CHEMBL473438 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CCNCc1ccc([nH]1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C26H23ClFN5OS/c1-2-29-13-19-6-8-21(32-19)24-12-22-25(35-24)26(31-15-30-22)33-18-7-9-23(20(27)11-18)34-14-16-4-3-5-17(28)10-16/h3-12,15,29,32H,2,13-14H2,1H3,(H,30,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
Rhodopsin kinase GRK7
(Homo sapiens (Human)) | BDBM50566949

(CHEMBL4877302)Show SMILES CCc1cc(Nc2nc(NCc3ccc(Cl)cc3OC)nc3cccc(OC)c23)n[nH]1 | NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full length human GRK7 using casein as substrate incubated for 40 mins in presence of [gamma-33ATP] by scintillation counti... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
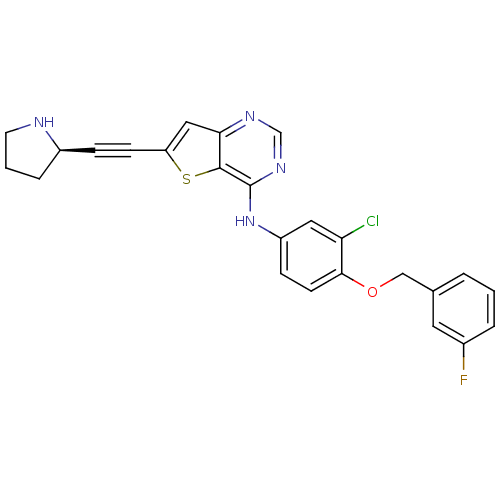
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM27973
(6-Ethynylthieno[3,2-d]pyrimidine, 8 | N-{3-chloro-...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4cc(sc34)C#C[C@H]3CCCN3)cc2Cl)c1 |r| Show InChI InChI=1S/C25H20ClFN4OS/c26-21-12-19(7-9-23(21)32-14-16-3-1-4-17(27)11-16)31-25-24-22(29-15-30-25)13-20(33-24)8-6-18-5-2-10-28-18/h1,3-4,7,9,11-13,15,18,28H,2,5,10,14H2,(H,29,30,31)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GSK
| Assay Description
Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP/[gamma-33P]ATP, and purified kinase in t... |
Proc Natl Acad Sci U S A 105: 2773-8 (2008)
Article DOI: 10.1073/pnas.0708281105
BindingDB Entry DOI: 10.7270/Q27S7M35 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM27973

(6-Ethynylthieno[3,2-d]pyrimidine, 8 | N-{3-chloro-...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4cc(sc34)C#C[C@H]3CCCN3)cc2Cl)c1 |r| Show InChI InChI=1S/C25H20ClFN4OS/c26-21-12-19(7-9-23(21)32-14-16-3-1-4-17(27)11-16)31-25-24-22(29-15-30-25)13-20(33-24)8-6-18-5-2-10-28-18/h1,3-4,7,9,11-13,15,18,28H,2,5,10,14H2,(H,29,30,31)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR kinase |
Bioorg Med Chem Lett 18: 5738-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.090
BindingDB Entry DOI: 10.7270/Q2H70FTT |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50256479
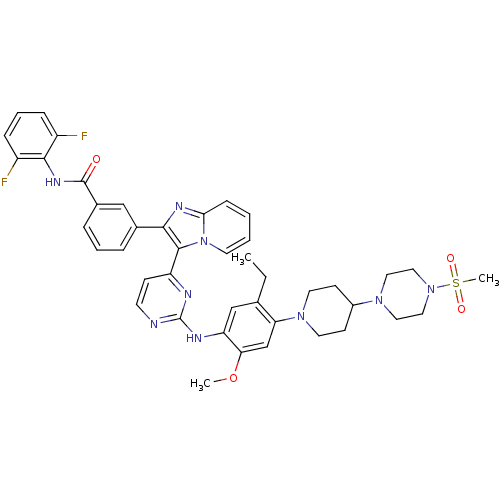
(CHEMBL448929 | N-(2,6-difluorophenyl)-3-(3-(2-(5-e...)Show SMILES CCc1cc(Nc2nccc(n2)-c2c(nc3ccccn23)-c2cccc(c2)C(=O)Nc2c(F)cccc2F)c(OC)cc1N1CCC(CC1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C43H45F2N9O4S/c1-4-28-26-35(37(58-2)27-36(28)52-19-15-31(16-20-52)51-21-23-53(24-22-51)59(3,56)57)48-43-46-17-14-34(47-43)41-39(49-38-13-5-6-18-54(38)41)29-9-7-10-30(25-29)42(55)50-40-32(44)11-8-12-33(40)45/h5-14,17-18,25-27,31H,4,15-16,19-24H2,1-3H3,(H,50,55)(H,46,47,48) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged insulin receptor expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256479

(CHEMBL448929 | N-(2,6-difluorophenyl)-3-(3-(2-(5-e...)Show SMILES CCc1cc(Nc2nccc(n2)-c2c(nc3ccccn23)-c2cccc(c2)C(=O)Nc2c(F)cccc2F)c(OC)cc1N1CCC(CC1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C43H45F2N9O4S/c1-4-28-26-35(37(58-2)27-36(28)52-19-15-31(16-20-52)51-21-23-53(24-22-51)59(3,56)57)48-43-46-17-14-34(47-43)41-39(49-38-13-5-6-18-54(38)41)29-9-7-10-30(25-29)42(55)50-40-32(44)11-8-12-33(40)45/h5-14,17-18,25-27,31H,4,15-16,19-24H2,1-3H3,(H,50,55)(H,46,47,48) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50256466
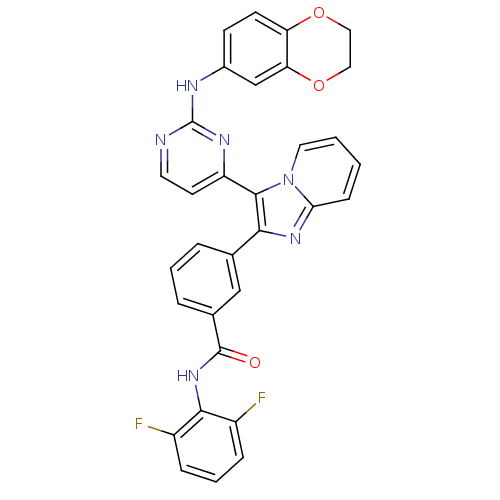
(CHEMBL444031 | N-(2,6-difluorophenyl)-3-(3-(2-(2,3...)Show SMILES Fc1cccc(F)c1NC(=O)c1cccc(c1)-c1nc2ccccn2c1-c1ccnc(Nc2ccc3OCCOc3c2)n1 Show InChI InChI=1S/C32H22F2N6O3/c33-22-7-4-8-23(34)29(22)39-31(41)20-6-3-5-19(17-20)28-30(40-14-2-1-9-27(40)38-28)24-12-13-35-32(37-24)36-21-10-11-25-26(18-21)43-16-15-42-25/h1-14,17-18H,15-16H2,(H,39,41)(H,35,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256020
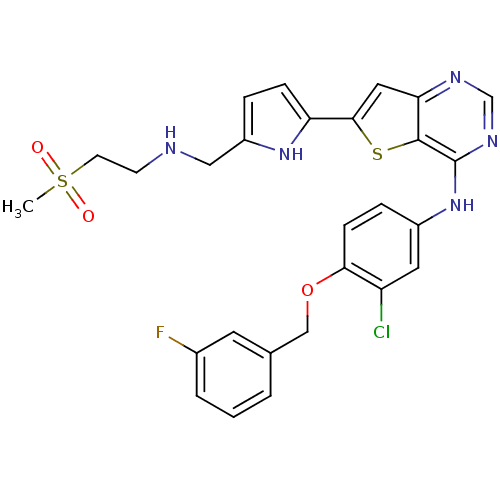
(CHEMBL475446 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES CS(=O)(=O)CCNCc1ccc([nH]1)-c1cc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2s1 Show InChI InChI=1S/C27H25ClFN5O3S2/c1-39(35,36)10-9-30-14-20-5-7-22(33-20)25-13-23-26(38-25)27(32-16-31-23)34-19-6-8-24(21(28)12-19)37-15-17-3-2-4-18(29)11-17/h2-8,11-13,16,30,33H,9-10,14-15H2,1H3,(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM50566946
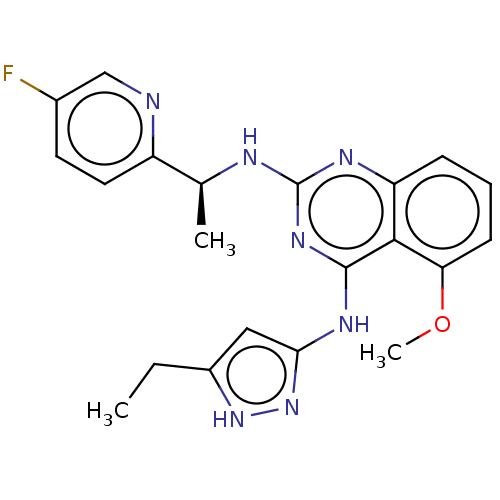
(CHEMBL4854871)Show SMILES CCc1cc(Nc2nc(N[C@@H](C)c3ccc(F)cn3)nc3cccc(OC)c23)n[nH]1 |r| | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length human GRK6 using casein as substrate measured after 80 mins in presence of [gamma33P]ATP by radiometric scintil... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50256018

(CHEMBL473427 | N-(3-chloro-4-(3-fluorobenzyloxy)ph...)Show SMILES Fc1cccc(COc2ccc(Nc3ncnc4cc(sc34)-c3cccs3)cc2Cl)c1 Show InChI InChI=1S/C23H15ClFN3OS2/c24-17-10-16(6-7-19(17)29-12-14-3-1-4-15(25)9-14)28-23-22-18(26-13-27-23)11-21(31-22)20-5-2-8-30-20/h1-11,13H,12H2,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 19: 817-20 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.011
BindingDB Entry DOI: 10.7270/Q2TH8NNN |
More data for this
Ligand-Target Pair | |
G protein-coupled receptor kinase 6
(Homo sapiens (Human)) | BDBM50566949

(CHEMBL4877302)Show SMILES CCc1cc(Nc2nc(NCc3ccc(Cl)cc3OC)nc3cccc(OC)c23)n[nH]1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length human GRK6 using casein as substrate measured after 80 mins in presence of [gamma33P]ATP by radiometric scintil... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00506
BindingDB Entry DOI: 10.7270/Q20K2D97 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50256470

(3-(3-(2-(4-(1,4'-bipiperidin-1'-yl)phenylamino)pyr...)Show SMILES Fc1cccc(F)c1NC(=O)c1cccc(c1)-c1nc2ccccn2c1-c1ccnc(Nc2ccc(cc2)N2CCC(CC2)N2CCCCC2)n1 Show InChI InChI=1S/C40H38F2N8O/c41-32-10-7-11-33(42)37(32)47-39(51)28-9-6-8-27(26-28)36-38(50-23-5-2-12-35(50)46-36)34-17-20-43-40(45-34)44-29-13-15-30(16-14-29)49-24-18-31(19-25-49)48-21-3-1-4-22-48/h2,5-17,20,23,26,31H,1,3-4,18-19,21-22,24-25H2,(H,47,51)(H,43,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 19: 1004-8 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.058
BindingDB Entry DOI: 10.7270/Q24T6J8M |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 23 |
GSK
| Assay Description
Assays were performed in 96-well microtiter plates in reaction buffer containing biotinylated substrate, ATP/[gamma-33P]ATP, and purified kinase in t... |
Proc Natl Acad Sci U S A 105: 2773-8 (2008)
Article DOI: 10.1073/pnas.0708281105
BindingDB Entry DOI: 10.7270/Q27S7M35 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 16: 2419-22 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.111
BindingDB Entry DOI: 10.7270/Q2JW8DH8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data