 Found 511 hits with Last Name = 'gernert' and Initial = 'dl'
Found 511 hits with Last Name = 'gernert' and Initial = 'dl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50228078
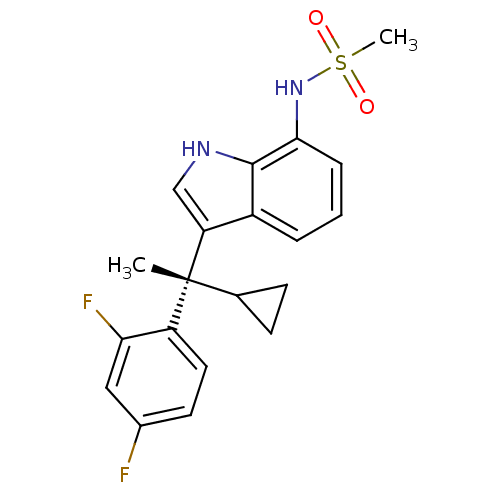
((S)-N-(3-(1-cyclopropyl-1-(2,4-difluorophenyl)ethy...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1F Show InChI InChI=1S/C20H20F2N2O2S/c1-20(12-6-7-12,15-9-8-13(21)10-17(15)22)16-11-23-19-14(16)4-3-5-18(19)24-27(2,25)26/h3-5,8-12,23-24H,6-7H2,1-2H3/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.494 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human mineralocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50228079
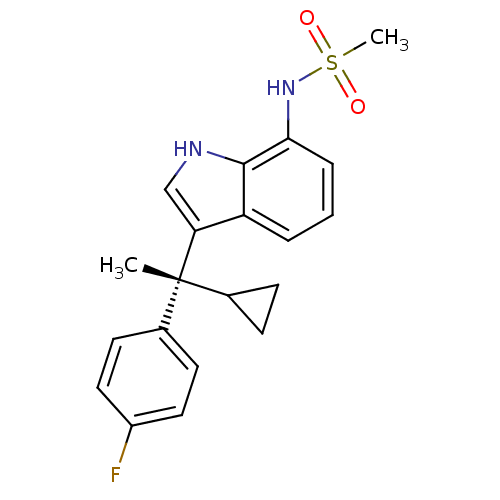
((R)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN2O2S/c1-20(13-6-7-13,14-8-10-15(21)11-9-14)17-12-22-19-16(17)4-3-5-18(19)23-26(2,24)25/h3-5,8-13,22-23H,6-7H2,1-2H3/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human mineralocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50146331
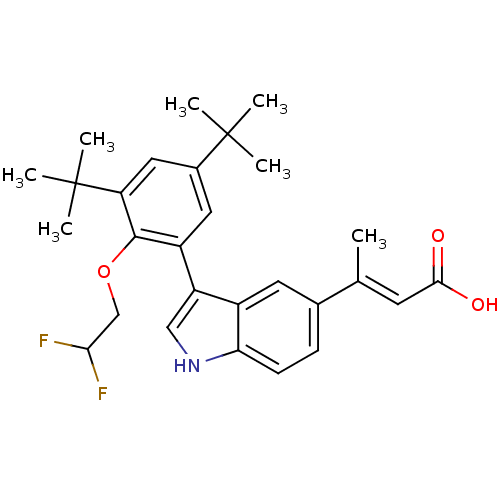
((E)-3-{3-[3,5-Di-tert-butyl-2-(2,2-difluoro-ethoxy...)Show SMILES C\C(=C/C(O)=O)c1ccc2[nH]cc(-c3cc(cc(c3OCC(F)F)C(C)(C)C)C(C)(C)C)c2c1 Show InChI InChI=1S/C28H33F2NO3/c1-16(10-25(32)33)17-8-9-23-19(11-17)21(14-31-23)20-12-18(27(2,3)4)13-22(28(5,6)7)26(20)34-15-24(29)30/h8-14,24,31H,15H2,1-7H3,(H,32,33)/b16-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-9-cis-RA from Retinoid X receptor alpha in CV-1 cells |
Bioorg Med Chem Lett 14: 2759-63 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.073
BindingDB Entry DOI: 10.7270/Q2513XNB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50146327

((E)-3-[3-(3,5-Di-tert-butyl-2-propoxy-phenyl)-1H-i...)Show SMILES CCCOc1c(cc(cc1C(C)(C)C)C(C)(C)C)-c1c[nH]c2ccc(cc12)C(\C)=C\C(O)=O Show InChI InChI=1S/C29H37NO3/c1-9-12-33-27-22(15-20(28(3,4)5)16-24(27)29(6,7)8)23-17-30-25-11-10-19(14-21(23)25)18(2)13-26(31)32/h10-11,13-17,30H,9,12H2,1-8H3,(H,31,32)/b18-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-9-cis-RA from Retinoid X receptor alpha in CV-1 cells |
Bioorg Med Chem Lett 14: 2759-63 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.073
BindingDB Entry DOI: 10.7270/Q2513XNB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50129726
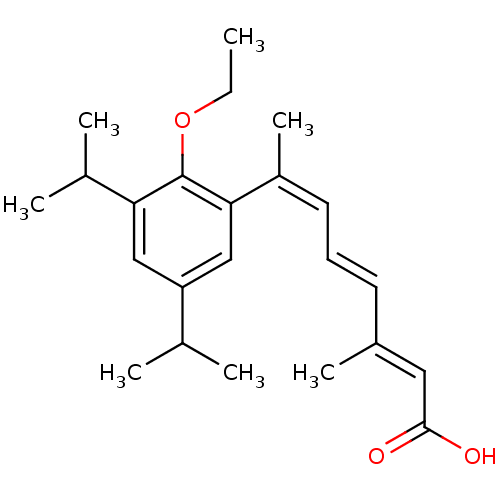
((2E,4E,6Z)-7-(2-Ethoxy-3,5-diisopropyl-phenyl)-3-m...)Show SMILES CCOc1c(cc(cc1\C(C)=C/C=C/C(/C)=C/C(O)=O)C(C)C)C(C)C Show InChI InChI=1S/C23H32O3/c1-8-26-23-20(16(4)5)13-19(15(2)3)14-21(23)18(7)11-9-10-17(6)12-22(24)25/h9-16H,8H2,1-7H3,(H,24,25)/b10-9+,17-12+,18-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-9-cis-RA from Retinoid X receptor alpha in CV-1 cells |
Bioorg Med Chem Lett 14: 2759-63 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.073
BindingDB Entry DOI: 10.7270/Q2513XNB |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50228076
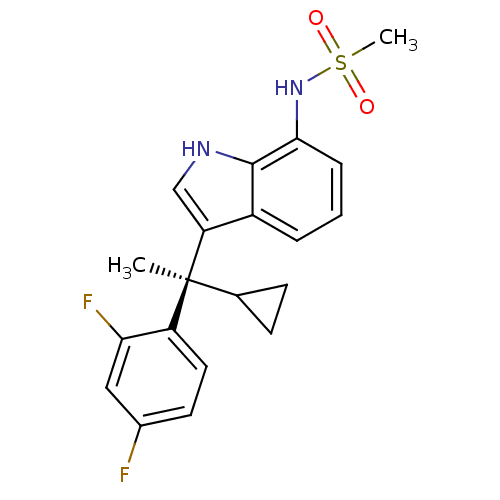
((R)-N-(3-(1-cyclopropyl-1-(2,4-difluorophenyl)ethy...)Show SMILES C[C@@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1F Show InChI InChI=1S/C20H20F2N2O2S/c1-20(12-6-7-12,15-9-8-13(21)10-17(15)22)16-11-23-19-14(16)4-3-5-18(19)24-27(2,25)26/h3-5,8-12,23-24H,6-7H2,1-2H3/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human mineralocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50228080

(2',15'-dimethyl-5,5'-dioxo-(9'R)-spiro[tetrahydrof...)Show SMILES CC(=O)S[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@H]2CC[C@@]3(C)[C@@H](CC[C@@]33CCC(=O)O3)[C@H]12 |r,t:6| Show InChI InChI=1S/C24H32O4S/c1-14(25)29-19-13-15-12-16(26)4-8-22(15,2)17-5-9-23(3)18(21(17)19)6-10-24(23)11-7-20(27)28-24/h12,17-19,21H,4-11,13H2,1-3H3/t17-,18-,19+,21+,22-,23-,24+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 2.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human mineralocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50129720
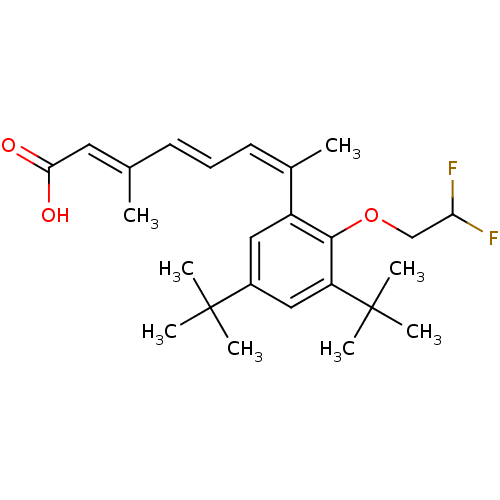
((2E,4E,6Z)-7-[3,5-Di-tert-butyl-2-(2,2-difluoro-et...)Show SMILES C\C(\C=C\C=C(\C)c1cc(cc(c1OCC(F)F)C(C)(C)C)C(C)(C)C)=C/C(O)=O Show InChI InChI=1S/C25H34F2O3/c1-16(12-22(28)29)10-9-11-17(2)19-13-18(24(3,4)5)14-20(25(6,7)8)23(19)30-15-21(26)27/h9-14,21H,15H2,1-8H3,(H,28,29)/b10-9+,16-12+,17-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 3[H]-9-cis-retinoic acid binding to Retinoic acid receptor RXR-alpha expressed in CV-1 cells |
Bioorg Med Chem Lett 13: 3191-5 (2003)
BindingDB Entry DOI: 10.7270/Q2736Q9T |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50146328
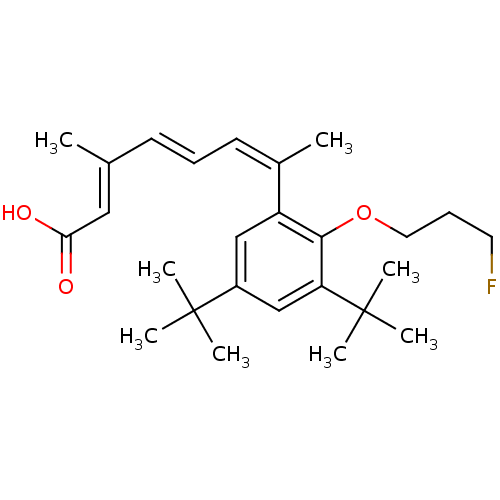
((2E,4E,6Z)-7-[3,5-Di-tert-butyl-2-(3-fluoro-propox...)Show SMILES C\C(\C=C\C=C(\C)c1cc(cc(c1OCCCF)C(C)(C)C)C(C)(C)C)=C/C(O)=O Show InChI InChI=1S/C26H37FO3/c1-18(15-23(28)29)11-9-12-19(2)21-16-20(25(3,4)5)17-22(26(6,7)8)24(21)30-14-10-13-27/h9,11-12,15-17H,10,13-14H2,1-8H3,(H,28,29)/b11-9+,18-15+,19-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration for antagonistic activity against RXR-alpha expressed in CV-1 cells |
Bioorg Med Chem Lett 14: 2759-63 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.073
BindingDB Entry DOI: 10.7270/Q2513XNB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50146337
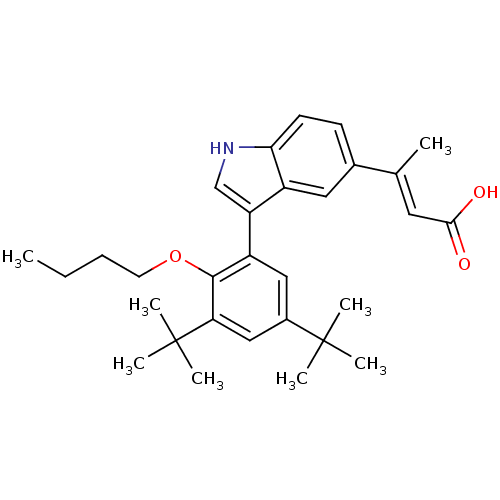
((E)-3-[3-(2-Butoxy-3,5-di-tert-butyl-phenyl)-1H-in...)Show SMILES CCCCOc1c(cc(cc1C(C)(C)C)C(C)(C)C)-c1c[nH]c2ccc(cc12)C(\C)=C\C(O)=O Show InChI InChI=1S/C30H39NO3/c1-9-10-13-34-28-23(16-21(29(3,4)5)17-25(28)30(6,7)8)24-18-31-26-12-11-20(15-22(24)26)19(2)14-27(32)33/h11-12,14-18,31H,9-10,13H2,1-8H3,(H,32,33)/b19-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-9-cis-RA from Retinoid X receptor alpha in CV-1 cells |
Bioorg Med Chem Lett 14: 2759-63 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.073
BindingDB Entry DOI: 10.7270/Q2513XNB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50132579

((2E,4E,6Z)-7-(2-Butoxy-3,5-di-tert-butyl-phenyl)-3...)Show SMILES CCCCOc1c(cc(cc1C(C)(C)C)C(C)(C)C)C(\C)=C/C=C/C(/C)=C/C(O)=O Show InChI InChI=1S/C27H40O3/c1-10-11-15-30-25-22(20(3)14-12-13-19(2)16-24(28)29)17-21(26(4,5)6)18-23(25)27(7,8)9/h12-14,16-18H,10-11,15H2,1-9H3,(H,28,29)/b13-12+,19-16+,20-14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Synergistic activation of BRL49653 mediated PPAR gamma and retinoid X receptor alpha expressed in CV-1 cell transcriptional activation assay |
Bioorg Med Chem Lett 13: 3191-5 (2003)
BindingDB Entry DOI: 10.7270/Q2736Q9T |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50129725
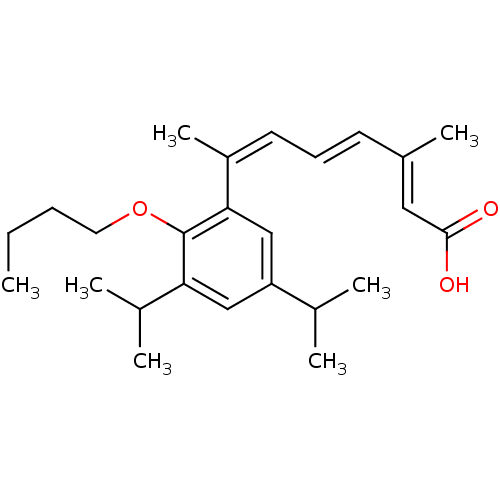
((2E,4E,6Z)-7-(2-Butoxy-3,5-diisopropyl-phenyl)-3-m...)Show SMILES CCCCOc1c(cc(cc1\C(C)=C/C=C/C(/C)=C/C(O)=O)C(C)C)C(C)C Show InChI InChI=1S/C25H36O3/c1-8-9-13-28-25-22(18(4)5)15-21(17(2)3)16-23(25)20(7)12-10-11-19(6)14-24(26)27/h10-12,14-18H,8-9,13H2,1-7H3,(H,26,27)/b11-10+,19-14+,20-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration for antagonistic activity against RXR-alpha expressed in CV-1 cells |
Bioorg Med Chem Lett 14: 2759-63 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.073
BindingDB Entry DOI: 10.7270/Q2513XNB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50132585
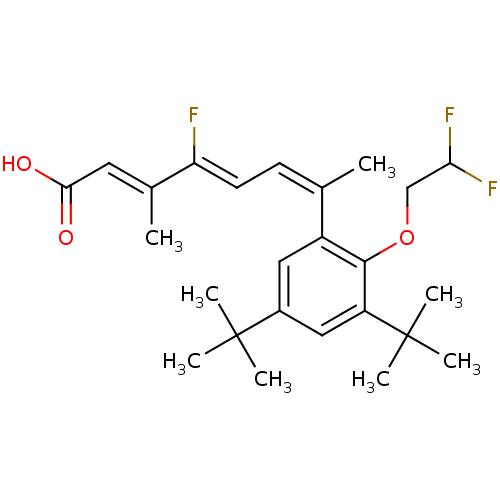
((2E,4Z,6Z)-7-[3,5-Di-tert-butyl-2-(2,2-difluoro-et...)Show SMILES C\C(=C/C(O)=O)\C(\F)=C\C=C(\C)c1cc(cc(c1OCC(F)F)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C25H33F3O3/c1-15(9-10-20(26)16(2)11-22(29)30)18-12-17(24(3,4)5)13-19(25(6,7)8)23(18)31-14-21(27)28/h9-13,21H,14H2,1-8H3,(H,29,30)/b15-9-,16-11+,20-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 3[H]-9-cis-retinoic acid binding to Retinoic acid receptor RXR-alpha expressed in CV-1 cells |
Bioorg Med Chem Lett 13: 3191-5 (2003)
BindingDB Entry DOI: 10.7270/Q2736Q9T |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50146334
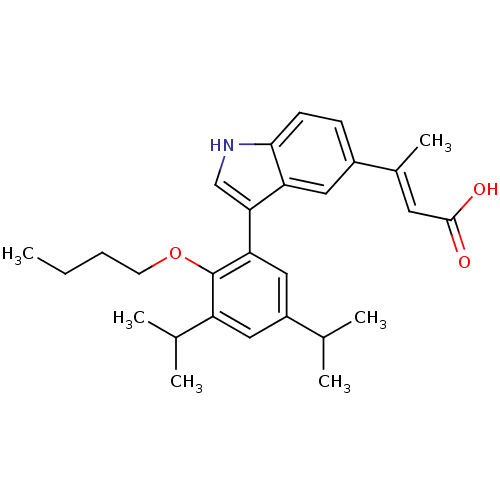
((E)-3-[3-(2-Butoxy-3,5-diisopropyl-phenyl)-1H-indo...)Show SMILES CCCCOc1c(cc(cc1-c1c[nH]c2ccc(cc12)C(\C)=C\C(O)=O)C(C)C)C(C)C Show InChI InChI=1S/C28H35NO3/c1-7-8-11-32-28-22(18(4)5)14-21(17(2)3)15-24(28)25-16-29-26-10-9-20(13-23(25)26)19(6)12-27(30)31/h9-10,12-18,29H,7-8,11H2,1-6H3,(H,30,31)/b19-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-9-cis-RA from Retinoid X receptor alpha in CV-1 cells |
Bioorg Med Chem Lett 14: 2759-63 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.073
BindingDB Entry DOI: 10.7270/Q2513XNB |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50228081

((S)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...)Show SMILES C[C@@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN2O2S/c1-20(13-6-7-13,14-8-10-15(21)11-9-14)17-12-22-19-16(17)4-3-5-18(19)23-26(2,24)25/h3-5,8-13,22-23H,6-7H2,1-2H3/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human mineralocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50132580
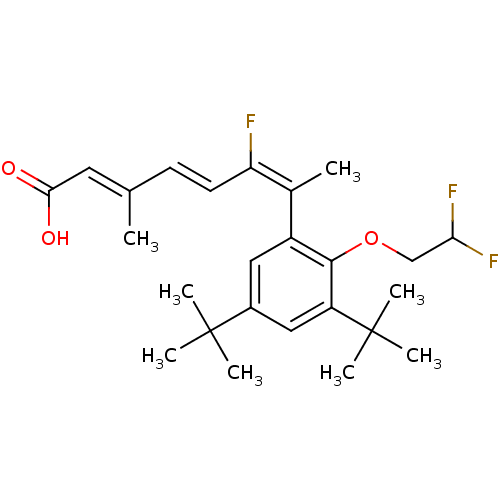
((2E,4E,6E)-7-[3,5-Di-tert-butyl-2-(2,2-difluoro-et...)Show SMILES C\C(\C=C\C(\F)=C(\C)c1cc(cc(c1OCC(F)F)C(C)(C)C)C(C)(C)C)=C/C(O)=O Show InChI InChI=1S/C25H33F3O3/c1-15(11-22(29)30)9-10-20(26)16(2)18-12-17(24(3,4)5)13-19(25(6,7)8)23(18)31-14-21(27)28/h9-13,21H,14H2,1-8H3,(H,29,30)/b10-9+,15-11+,20-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Synergistic activation of BRL49653 mediated PPAR gamma and retinoid X receptor alpha expressed in CV-1 cell transcriptional activation assay |
Bioorg Med Chem Lett 13: 3191-5 (2003)
BindingDB Entry DOI: 10.7270/Q2736Q9T |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50132581
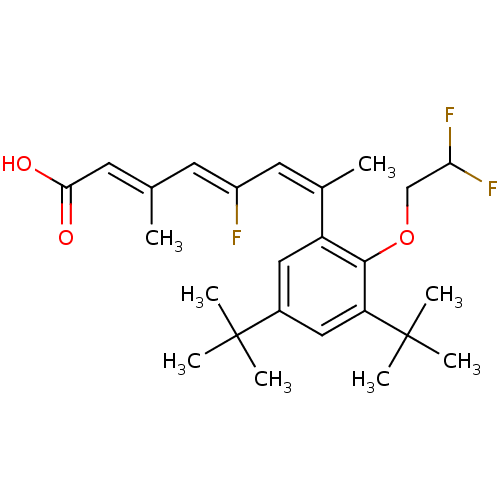
((2E,4Z,6Z)-7-[3,5-Di-tert-butyl-2-(2,2-difluoro-et...)Show SMILES C\C(=C/C(O)=O)\C=C(/F)\C=C(\C)c1cc(cc(c1OCC(F)F)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C25H33F3O3/c1-15(10-22(29)30)9-18(26)11-16(2)19-12-17(24(3,4)5)13-20(25(6,7)8)23(19)31-14-21(27)28/h9-13,21H,14H2,1-8H3,(H,29,30)/b15-10+,16-11-,18-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Synergistic activation of BRL49653 mediated PPAR gamma and retinoid X receptor alpha expressed in CV-1 cell transcriptional activation assay |
Bioorg Med Chem Lett 13: 3191-5 (2003)
BindingDB Entry DOI: 10.7270/Q2736Q9T |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50228078

((S)-N-(3-(1-cyclopropyl-1-(2,4-difluorophenyl)ethy...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1F Show InChI InChI=1S/C20H20F2N2O2S/c1-20(12-6-7-12,15-9-8-13(21)10-17(15)22)16-11-23-19-14(16)4-3-5-18(19)24-27(2,25)26/h3-5,8-12,23-24H,6-7H2,1-2H3/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50132584
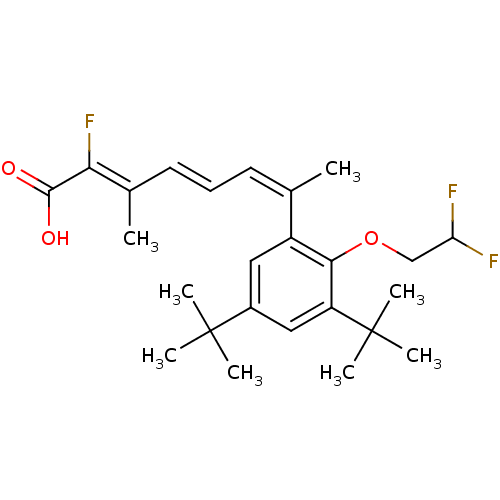
((2Z,4E,6Z)-7-[3,5-Di-tert-butyl-2-(2,2-difluoro-et...)Show SMILES C\C(\C=C\C=C(\C)c1cc(cc(c1OCC(F)F)C(C)(C)C)C(C)(C)C)=C(\F)C(O)=O Show InChI InChI=1S/C25H33F3O3/c1-15(10-9-11-16(2)21(28)23(29)30)18-12-17(24(3,4)5)13-19(25(6,7)8)22(18)31-14-20(26)27/h9-13,20H,14H2,1-8H3,(H,29,30)/b11-9+,15-10-,21-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity against Retinoic acid receptor RXR-alpha expressed in CV-1 cell transcriptional activation assay |
Bioorg Med Chem Lett 13: 3191-5 (2003)
BindingDB Entry DOI: 10.7270/Q2736Q9T |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50129728

((2E,4E,6Z)-7-(3,5-Di-tert-butyl-2-ethoxy-phenyl)-3...)Show SMILES CCOc1c(cc(cc1C(C)(C)C)C(C)(C)C)C(\C)=C/C=C/C(/C)=C/C(O)=O Show InChI InChI=1S/C25H36O3/c1-10-28-23-20(18(3)13-11-12-17(2)14-22(26)27)15-19(24(4,5)6)16-21(23)25(7,8)9/h11-16H,10H2,1-9H3,(H,26,27)/b12-11+,17-14+,18-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 3[H]-9-cis-retinoic acid binding to Retinoic acid receptor RXR-alpha expressed in CV-1 cells |
Bioorg Med Chem Lett 13: 3191-5 (2003)
BindingDB Entry DOI: 10.7270/Q2736Q9T |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50146330
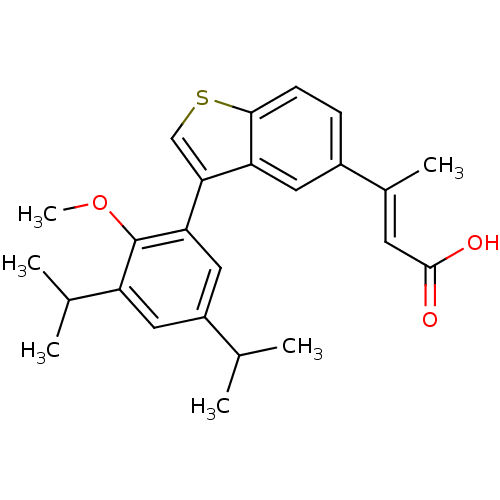
((E)-3-[3-(3,5-Diisopropyl-2-methoxy-phenyl)-benzo[...)Show SMILES COc1c(cc(cc1-c1csc2ccc(cc12)C(\C)=C\C(O)=O)C(C)C)C(C)C Show InChI InChI=1S/C25H28O3S/c1-14(2)18-11-19(15(3)4)25(28-6)21(12-18)22-13-29-23-8-7-17(10-20(22)23)16(5)9-24(26)27/h7-15H,1-6H3,(H,26,27)/b16-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-9-cis-RA from Retinoid X receptor alpha in CV-1 cells |
Bioorg Med Chem Lett 14: 2759-63 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.073
BindingDB Entry DOI: 10.7270/Q2513XNB |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50228080

(2',15'-dimethyl-5,5'-dioxo-(9'R)-spiro[tetrahydrof...)Show SMILES CC(=O)S[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@H]2CC[C@@]3(C)[C@@H](CC[C@@]33CCC(=O)O3)[C@H]12 |r,t:6| Show InChI InChI=1S/C24H32O4S/c1-14(25)29-19-13-15-12-16(26)4-8-22(15,2)17-5-9-23(3)18(21(17)19)6-10-24(23)11-7-20(27)28-24/h12,17-19,21H,4-11,13H2,1-3H3/t17-,18-,19+,21+,22-,23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 32.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50228080

(2',15'-dimethyl-5,5'-dioxo-(9'R)-spiro[tetrahydrof...)Show SMILES CC(=O)S[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@H]2CC[C@@]3(C)[C@@H](CC[C@@]33CCC(=O)O3)[C@H]12 |r,t:6| Show InChI InChI=1S/C24H32O4S/c1-14(25)29-19-13-15-12-16(26)4-8-22(15,2)17-5-9-23(3)18(21(17)19)6-10-24(23)11-7-20(27)28-24/h12,17-19,21H,4-11,13H2,1-3H3/t17-,18-,19+,21+,22-,23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 39.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human androgen receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50132583
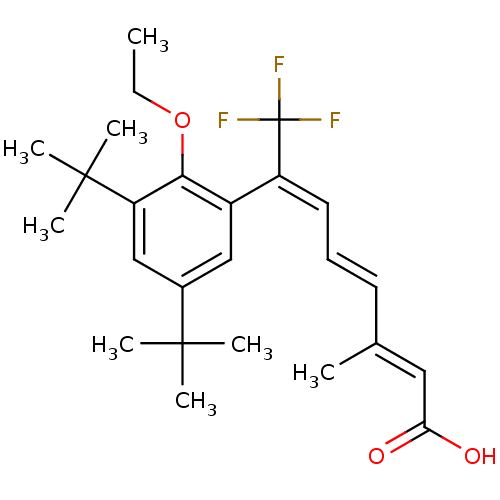
((2E,4E,6E)-7-(3,5-Di-tert-butyl-2-ethoxy-phenyl)-8...)Show SMILES CCOc1c(cc(cc1C(C)(C)C)C(C)(C)C)C(=C/C=C/C(/C)=C/C(O)=O)\C(F)(F)F Show InChI InChI=1S/C25H33F3O3/c1-9-31-22-18(14-17(23(3,4)5)15-20(22)24(6,7)8)19(25(26,27)28)12-10-11-16(2)13-21(29)30/h10-15H,9H2,1-8H3,(H,29,30)/b11-10+,16-13+,19-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 3[H]-9-cis-retinoic acid binding to Retinoic acid receptor RXR-alpha expressed in CV-1 cells |
Bioorg Med Chem Lett 13: 3191-5 (2003)
BindingDB Entry DOI: 10.7270/Q2736Q9T |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50146326

((E)-3-[3-(2-Ethoxy-3,5-diisopropyl-phenyl)-1H-inda...)Show SMILES CCOc1c(cc(cc1-c1[nH]nc2ccc(cc12)C(C)=CC(O)=O)C(C)C)C(C)C |w:20.23| Show InChI InChI=1S/C25H30N2O3/c1-7-30-25-19(15(4)5)12-18(14(2)3)13-21(25)24-20-11-17(16(6)10-23(28)29)8-9-22(20)26-27-24/h8-15H,7H2,1-6H3,(H,26,27)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration for antagonistic activity against RXR-alpha expressed in CV-1 cells |
Bioorg Med Chem Lett 14: 2759-63 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.073
BindingDB Entry DOI: 10.7270/Q2513XNB |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50228077
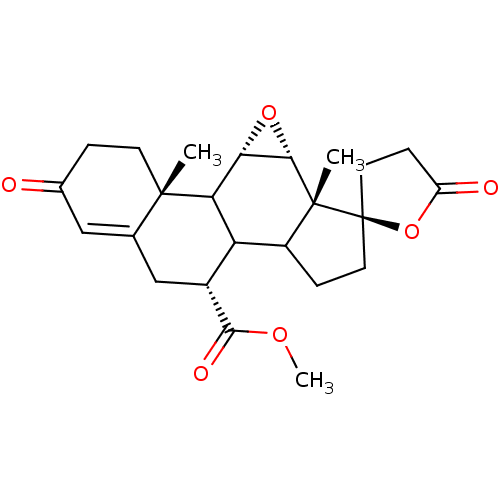
(CHEMBL237122 | epierenone)Show SMILES COC(=O)[C@@H]1CC2=CC(=O)CC[C@]2(C)C2[C@@H]3O[C@@H]3[C@@]3(C)C(CC[C@@]33CCC(=O)O3)C12 |t:6| Show InChI InChI=1S/C24H30O6/c1-22-7-4-13(25)10-12(22)11-14(21(27)28-3)17-15-5-8-24(9-6-16(26)30-24)23(15,2)20-19(29-20)18(17)22/h10,14-15,17-20H,4-9,11H2,1-3H3/t14-,15?,17?,18?,19+,20+,22+,23-,24-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human mineralocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50146325
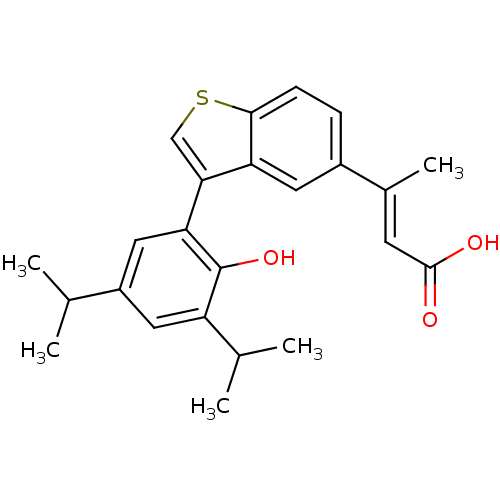
((E)-3-[3-(2-Hydroxy-3,5-diisopropyl-phenyl)-benzo[...)Show SMILES CC(C)c1cc(C(C)C)c(O)c(c1)-c1csc2ccc(cc12)C(\C)=C\C(O)=O Show InChI InChI=1S/C24H26O3S/c1-13(2)17-10-18(14(3)4)24(27)20(11-17)21-12-28-22-7-6-16(9-19(21)22)15(5)8-23(25)26/h6-14,27H,1-5H3,(H,25,26)/b15-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-9-cis-RA from Retinoid X receptor alpha in CV-1 cells |
Bioorg Med Chem Lett 14: 2759-63 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.073
BindingDB Entry DOI: 10.7270/Q2513XNB |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50228079

((R)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN2O2S/c1-20(13-6-7-13,14-8-10-15(21)11-9-14)17-12-22-19-16(17)4-3-5-18(19)23-26(2,24)25/h3-5,8-13,22-23H,6-7H2,1-2H3/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human progesterone receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50228078

((S)-N-(3-(1-cyclopropyl-1-(2,4-difluorophenyl)ethy...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1F Show InChI InChI=1S/C20H20F2N2O2S/c1-20(12-6-7-12,15-9-8-13(21)10-17(15)22)16-11-23-19-14(16)4-3-5-18(19)24-27(2,25)26/h3-5,8-12,23-24H,6-7H2,1-2H3/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human progesterone receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50228079

((R)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN2O2S/c1-20(13-6-7-13,14-8-10-15(21)11-9-14)17-12-22-19-16(17)4-3-5-18(19)23-26(2,24)25/h3-5,8-13,22-23H,6-7H2,1-2H3/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50228081

((S)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...)Show SMILES C[C@@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN2O2S/c1-20(13-6-7-13,14-8-10-15(21)11-9-14)17-12-22-19-16(17)4-3-5-18(19)23-26(2,24)25/h3-5,8-13,22-23H,6-7H2,1-2H3/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human progesterone receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50228081

((S)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...)Show SMILES C[C@@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN2O2S/c1-20(13-6-7-13,14-8-10-15(21)11-9-14)17-12-22-19-16(17)4-3-5-18(19)23-26(2,24)25/h3-5,8-13,22-23H,6-7H2,1-2H3/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 387 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50146335
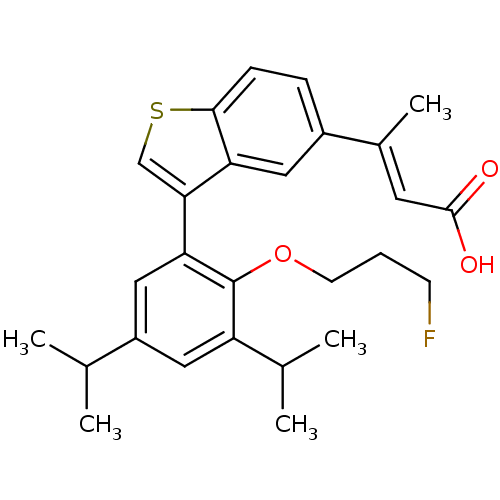
((E)-3-{3-[2-(3-Fluoro-propoxy)-3,5-diisopropyl-phe...)Show SMILES CC(C)c1cc(C(C)C)c(OCCCF)c(c1)-c1csc2ccc(cc12)C(\C)=C\C(O)=O Show InChI InChI=1S/C27H31FO3S/c1-16(2)20-13-21(17(3)4)27(31-10-6-9-28)23(14-20)24-15-32-25-8-7-19(12-22(24)25)18(5)11-26(29)30/h7-8,11-17H,6,9-10H2,1-5H3,(H,29,30)/b18-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 395 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-9-cis-RA from Retinoid X receptor alpha in CV-1 cells |
Bioorg Med Chem Lett 14: 2759-63 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.073
BindingDB Entry DOI: 10.7270/Q2513XNB |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50228080

(2',15'-dimethyl-5,5'-dioxo-(9'R)-spiro[tetrahydrof...)Show SMILES CC(=O)S[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@H]2CC[C@@]3(C)[C@@H](CC[C@@]33CCC(=O)O3)[C@H]12 |r,t:6| Show InChI InChI=1S/C24H32O4S/c1-14(25)29-19-13-15-12-16(26)4-8-22(15,2)17-5-9-23(3)18(21(17)19)6-10-24(23)11-7-20(27)28-24/h12,17-19,21H,4-11,13H2,1-3H3/t17-,18-,19+,21+,22-,23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human progesterone receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50228081

((S)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...)Show SMILES C[C@@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN2O2S/c1-20(13-6-7-13,14-8-10-15(21)11-9-14)17-12-22-19-16(17)4-3-5-18(19)23-26(2,24)25/h3-5,8-13,22-23H,6-7H2,1-2H3/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human androgen receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50146329
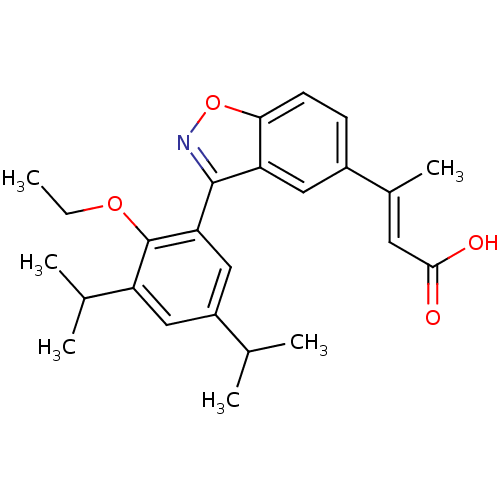
((E)-3-[3-(2-Ethoxy-3,5-diisopropyl-phenyl)-benzo[d...)Show SMILES CCOc1c(cc(cc1-c1noc2ccc(cc12)C(\C)=C\C(O)=O)C(C)C)C(C)C Show InChI InChI=1S/C25H29NO4/c1-7-29-25-19(15(4)5)12-18(14(2)3)13-21(25)24-20-11-17(16(6)10-23(27)28)8-9-22(20)30-26-24/h8-15H,7H2,1-6H3,(H,27,28)/b16-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 648 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration for antagonistic activity against RXR-alpha expressed in CV-1 cells |
Bioorg Med Chem Lett 14: 2759-63 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.073
BindingDB Entry DOI: 10.7270/Q2513XNB |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50228078

((S)-N-(3-(1-cyclopropyl-1-(2,4-difluorophenyl)ethy...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1F Show InChI InChI=1S/C20H20F2N2O2S/c1-20(12-6-7-12,15-9-8-13(21)10-17(15)22)16-11-23-19-14(16)4-3-5-18(19)24-27(2,25)26/h3-5,8-12,23-24H,6-7H2,1-2H3/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human androgen receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50146332
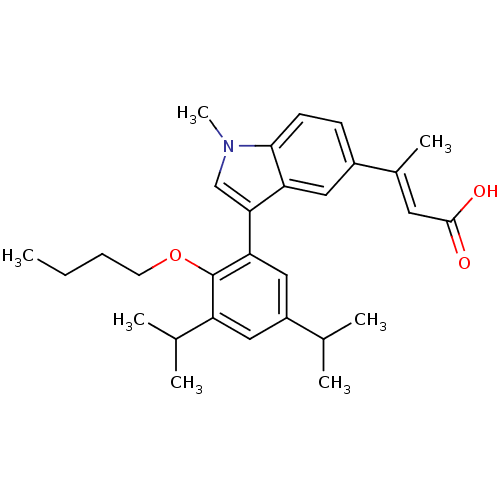
((E)-3-[3-(2-Butoxy-3,5-diisopropyl-phenyl)-1-methy...)Show SMILES CCCCOc1c(cc(cc1-c1cn(C)c2ccc(cc12)C(\C)=C\C(O)=O)C(C)C)C(C)C Show InChI InChI=1S/C29H37NO3/c1-8-9-12-33-29-23(19(4)5)15-22(18(2)3)16-25(29)26-17-30(7)27-11-10-21(14-24(26)27)20(6)13-28(31)32/h10-11,13-19H,8-9,12H2,1-7H3,(H,31,32)/b20-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-9-cis-RA from Retinoid X receptor alpha in CV-1 cells |
Bioorg Med Chem Lett 14: 2759-63 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.073
BindingDB Entry DOI: 10.7270/Q2513XNB |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50228079

((R)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN2O2S/c1-20(13-6-7-13,14-8-10-15(21)11-9-14)17-12-22-19-16(17)4-3-5-18(19)23-26(2,24)25/h3-5,8-13,22-23H,6-7H2,1-2H3/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 849 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human androgen receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50228081

((S)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...)Show SMILES C[C@@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN2O2S/c1-20(13-6-7-13,14-8-10-15(21)11-9-14)17-12-22-19-16(17)4-3-5-18(19)23-26(2,24)25/h3-5,8-13,22-23H,6-7H2,1-2H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human estrogen receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50228077

(CHEMBL237122 | epierenone)Show SMILES COC(=O)[C@@H]1CC2=CC(=O)CC[C@]2(C)C2[C@@H]3O[C@@H]3[C@@]3(C)C(CC[C@@]33CCC(=O)O3)C12 |t:6| Show InChI InChI=1S/C24H30O6/c1-22-7-4-13(25)10-12(22)11-14(21(27)28-3)17-15-5-8-24(9-6-16(26)30-24)23(15,2)20-19(29-20)18(17)22/h10,14-15,17-20H,4-9,11H2,1-3H3/t14-,15?,17?,18?,19+,20+,22+,23-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human estrogen receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50228079

((R)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1 Show InChI InChI=1S/C20H21FN2O2S/c1-20(13-6-7-13,14-8-10-15(21)11-9-14)17-12-22-19-16(17)4-3-5-18(19)23-26(2,24)25/h3-5,8-13,22-23H,6-7H2,1-2H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human estrogen receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50228080

(2',15'-dimethyl-5,5'-dioxo-(9'R)-spiro[tetrahydrof...)Show SMILES CC(=O)S[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@H]2CC[C@@]3(C)[C@@H](CC[C@@]33CCC(=O)O3)[C@H]12 |r,t:6| Show InChI InChI=1S/C24H32O4S/c1-14(25)29-19-13-15-12-16(26)4-8-22(15,2)17-5-9-23(3)18(21(17)19)6-10-24(23)11-7-20(27)28-24/h12,17-19,21H,4-11,13H2,1-3H3/t17-,18-,19+,21+,22-,23-,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human estrogen receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50228078

((S)-N-(3-(1-cyclopropyl-1-(2,4-difluorophenyl)ethy...)Show SMILES C[C@](C1CC1)(c1c[nH]c2c(NS(C)(=O)=O)cccc12)c1ccc(F)cc1F Show InChI InChI=1S/C20H20F2N2O2S/c1-20(12-6-7-12,15-9-8-13(21)10-17(15)22)16-11-23-19-14(16)4-3-5-18(19)24-27(2,25)26/h3-5,8-12,23-24H,6-7H2,1-2H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human estrogen receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50228077

(CHEMBL237122 | epierenone)Show SMILES COC(=O)[C@@H]1CC2=CC(=O)CC[C@]2(C)C2[C@@H]3O[C@@H]3[C@@]3(C)C(CC[C@@]33CCC(=O)O3)C12 |t:6| Show InChI InChI=1S/C24H30O6/c1-22-7-4-13(25)10-12(22)11-14(21(27)28-3)17-15-5-8-24(9-6-16(26)30-24)23(15,2)20-19(29-20)18(17)22/h10,14-15,17-20H,4-9,11H2,1-3H3/t14-,15?,17?,18?,19+,20+,22+,23-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50146336

((E)-3-[3-(2-Ethoxy-3,5-diisopropyl-phenyl)-imidazo...)Show SMILES CCOc1c(cc(cc1-c1cnc2ccc(cn12)C(\C)=C\C(O)=O)C(C)C)C(C)C Show InChI InChI=1S/C25H30N2O3/c1-7-30-25-20(16(4)5)11-19(15(2)3)12-21(25)22-13-26-23-9-8-18(14-27(22)23)17(6)10-24(28)29/h8-16H,7H2,1-6H3,(H,28,29)/b17-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-9-cis-RA from Retinoid X receptor alpha in CV-1 cells |
Bioorg Med Chem Lett 14: 2759-63 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.073
BindingDB Entry DOI: 10.7270/Q2513XNB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50132582
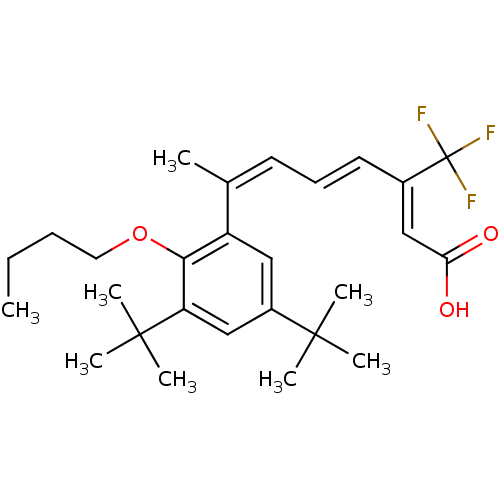
((2Z,4E,6Z)-7-(2-Butoxy-3,5-di-tert-butyl-phenyl)-3...)Show SMILES CCCCOc1c(cc(cc1C(C)(C)C)C(C)(C)C)C(\C)=C/C=C/C(=C/C(O)=O)/C(F)(F)F Show InChI InChI=1S/C27H37F3O3/c1-9-10-14-33-24-21(15-20(25(3,4)5)16-22(24)26(6,7)8)18(2)12-11-13-19(17-23(31)32)27(28,29)30/h11-13,15-17H,9-10,14H2,1-8H3,(H,31,32)/b13-11+,18-12-,19-17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 3[H]-9-cis-retinoic acid binding to Retinoic acid receptor RXR-alpha expressed in CV-1 cells |
Bioorg Med Chem Lett 13: 3191-5 (2003)
BindingDB Entry DOI: 10.7270/Q2736Q9T |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50129728

((2E,4E,6Z)-7-(3,5-Di-tert-butyl-2-ethoxy-phenyl)-3...)Show SMILES CCOc1c(cc(cc1C(C)(C)C)C(C)(C)C)C(\C)=C/C=C/C(/C)=C/C(O)=O Show InChI InChI=1S/C25H36O3/c1-10-28-23-20(18(3)13-11-12-17(2)14-22(26)27)15-19(24(4,5)6)16-21(23)25(7,8)9/h11-16H,10H2,1-9H3,(H,26,27)/b12-11+,17-14+,18-13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 3[H]ATRA binding to retinoid acid receptor gamma expressed in CV-1 cells |
Bioorg Med Chem Lett 13: 3191-5 (2003)
BindingDB Entry DOI: 10.7270/Q2736Q9T |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50228077

(CHEMBL237122 | epierenone)Show SMILES COC(=O)[C@@H]1CC2=CC(=O)CC[C@]2(C)C2[C@@H]3O[C@@H]3[C@@]3(C)C(CC[C@@]33CCC(=O)O3)C12 |t:6| Show InChI InChI=1S/C24H30O6/c1-22-7-4-13(25)10-12(22)11-14(21(27)28-3)17-15-5-8-24(9-6-16(26)30-24)23(15,2)20-19(29-20)18(17)22/h10,14-15,17-20H,4-9,11H2,1-3H3/t14-,15?,17?,18?,19+,20+,22+,23-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human androgen receptor |
J Med Chem 50: 6443-5 (2007)
Article DOI: 10.1021/jm701186z
BindingDB Entry DOI: 10.7270/Q23B5ZVP |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50132585

((2E,4Z,6Z)-7-[3,5-Di-tert-butyl-2-(2,2-difluoro-et...)Show SMILES C\C(=C/C(O)=O)\C(\F)=C\C=C(\C)c1cc(cc(c1OCC(F)F)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C25H33F3O3/c1-15(9-10-20(26)16(2)11-22(29)30)18-12-17(24(3,4)5)13-19(25(6,7)8)23(18)31-14-21(27)28/h9-13,21H,14H2,1-8H3,(H,29,30)/b15-9-,16-11+,20-10- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of 3[H]ATRA binding to retinoid acid receptor gamma expressed in CV-1 cells |
Bioorg Med Chem Lett 13: 3191-5 (2003)
BindingDB Entry DOI: 10.7270/Q2736Q9T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data