

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50048182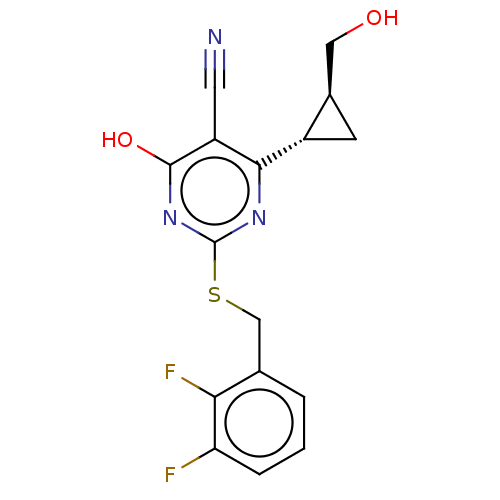 (CHEMBL3310787) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human recombinant CXCR2 receptor expressed in CHO cell membranes by SPA based [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 24: 3285-90 (2014) Article DOI: 10.1016/j.bmcl.2014.06.011 BindingDB Entry DOI: 10.7270/Q2930VTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50048183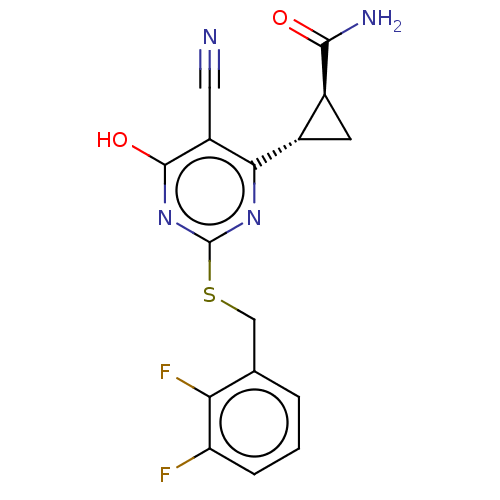 (CHEMBL3310788) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human recombinant CXCR2 receptor expressed in CHO cell membranes by SPA based [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 24: 3285-90 (2014) Article DOI: 10.1016/j.bmcl.2014.06.011 BindingDB Entry DOI: 10.7270/Q2930VTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50048181 (CHEMBL3310786) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human recombinant CXCR2 receptor expressed in CHO cell membranes by SPA based [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 24: 3285-90 (2014) Article DOI: 10.1016/j.bmcl.2014.06.011 BindingDB Entry DOI: 10.7270/Q2930VTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50048228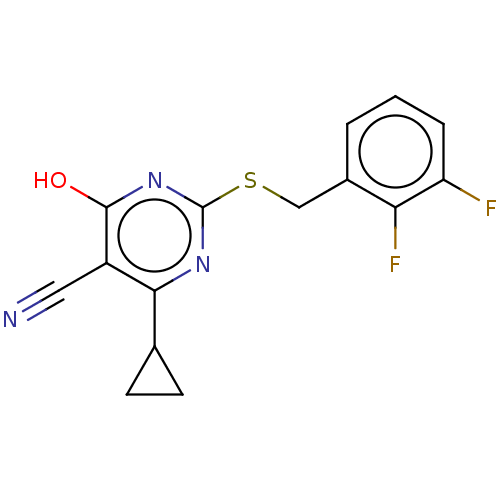 (CHEMBL3310783) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]-GRO-alpha from human recombinant CXCR2 receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 24: 3285-90 (2014) Article DOI: 10.1016/j.bmcl.2014.06.011 BindingDB Entry DOI: 10.7270/Q2930VTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50048228 (CHEMBL3310783) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human recombinant CXCR2 receptor expressed in CHO cell membranes by SPA based [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 24: 3285-90 (2014) Article DOI: 10.1016/j.bmcl.2014.06.011 BindingDB Entry DOI: 10.7270/Q2930VTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50495186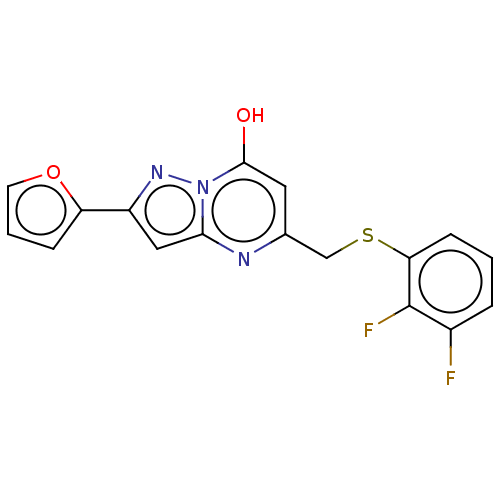 (CHEMBL3105080) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR2 receptor expressed in CHO cells assessed as inhibition of IL8-induced [35S]GTPgammaS binding by SPA me... | Bioorg Med Chem Lett 24: 72-6 (2014) Article DOI: 10.1016/j.bmcl.2013.11.074 BindingDB Entry DOI: 10.7270/Q2QR513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-phosphate-mannose--protein mannosyltransferase 1 (Candida albicans) | BDBM50149727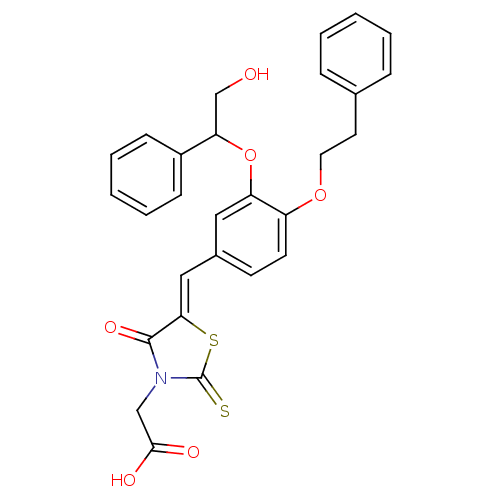 (CHEMBL361420 | {5-[3-(2-Hydroxy-1-phenyl-ethoxy)-4...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxfordshire OX14 4YS Curated by ChEMBL | Assay Description In vitro inhibition of Candida albicans protein mannosyl transferase 1 | Bioorg Med Chem Lett 14: 3975-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.050 BindingDB Entry DOI: 10.7270/Q22J6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-phosphate-mannose--protein mannosyltransferase 1 (Candida albicans) | BDBM50149739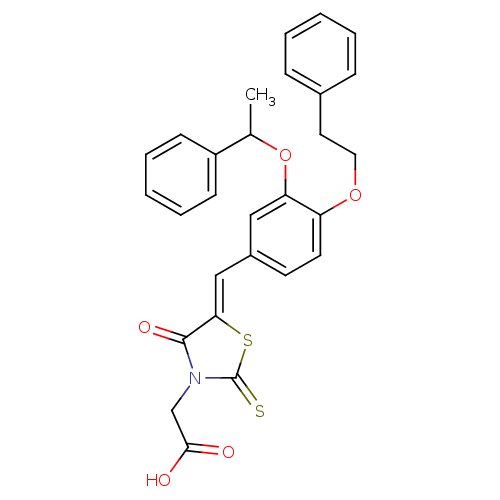 (CHEMBL182293 | {4-Oxo-5-[1-[4-phenethyloxy-3-(1-ph...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxfordshire OX14 4YS Curated by ChEMBL | Assay Description In vitro inhibition of Candida albicans protein mannosyl transferase 1 | Bioorg Med Chem Lett 14: 3975-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.050 BindingDB Entry DOI: 10.7270/Q22J6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50147513 (2-[3-[5-(4-Chloro-phenyl)-benzooxazol-2-yl]-4-(2-m...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd Curated by ChEMBL | Assay Description In vivo inhibitory activity against human Heparanase | Bioorg Med Chem Lett 14: 3269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.03.086 BindingDB Entry DOI: 10.7270/Q2CC1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50147546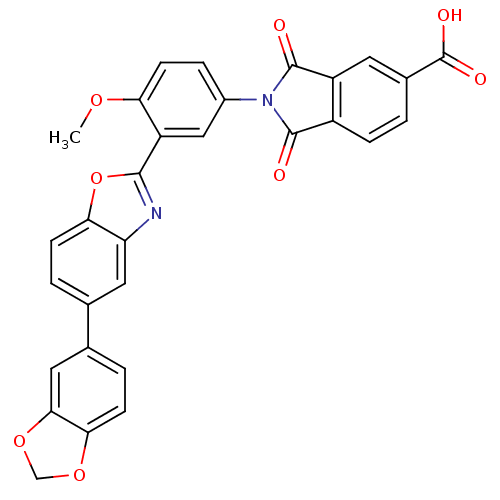 (2-(3-(5-(benzo[d][1,3]dioxol-5-yl)benzo[d]oxazol-2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd Curated by ChEMBL | Assay Description In vivo inhibitory activity against human Heparanase | Bioorg Med Chem Lett 14: 3269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.03.086 BindingDB Entry DOI: 10.7270/Q2CC1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50147534 (1,3-Dioxo-2-[3-(5-phenyl-benzooxazol-2-yl)-4-propy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd Curated by ChEMBL | Assay Description In vivo inhibitory activity against human Heparanase | Bioorg Med Chem Lett 14: 3269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.03.086 BindingDB Entry DOI: 10.7270/Q2CC1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50495172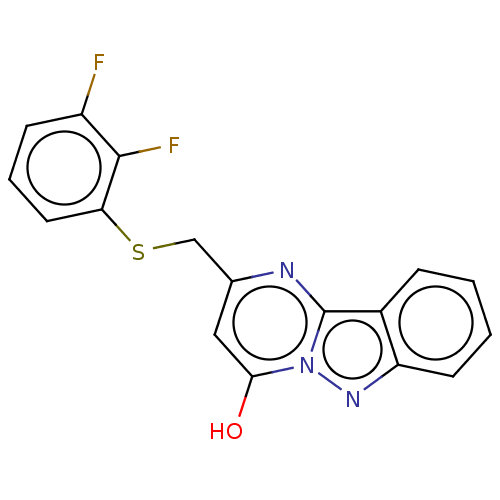 (CHEMBL3105084) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR2 receptor expressed in CHO cells assessed as inhibition of IL8-induced [35S]GTPgammaS binding by SPA me... | Bioorg Med Chem Lett 24: 72-6 (2014) Article DOI: 10.1016/j.bmcl.2013.11.074 BindingDB Entry DOI: 10.7270/Q2QR513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50495184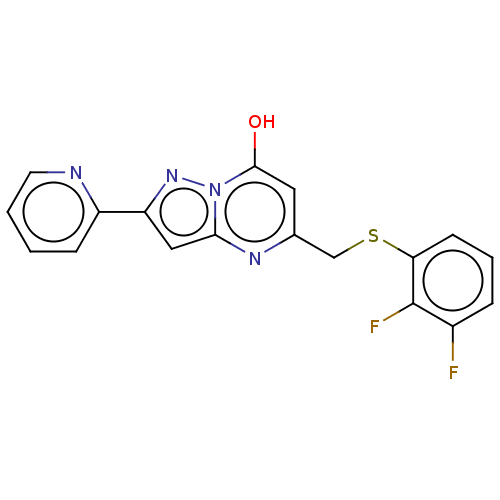 (CHEMBL3105081) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR2 receptor expressed in CHO cells assessed as inhibition of IL8-induced [35S]GTPgammaS binding by SPA me... | Bioorg Med Chem Lett 24: 72-6 (2014) Article DOI: 10.1016/j.bmcl.2013.11.074 BindingDB Entry DOI: 10.7270/Q2QR513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50048224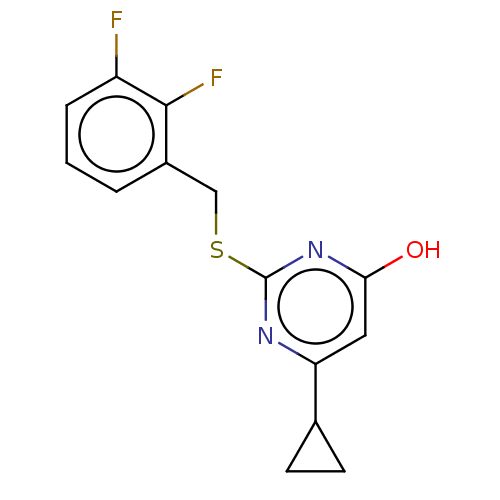 (CHEMBL3310794) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human recombinant CXCR2 receptor expressed in CHO cell membranes by SPA based [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 24: 3285-90 (2014) Article DOI: 10.1016/j.bmcl.2014.06.011 BindingDB Entry DOI: 10.7270/Q2930VTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50048180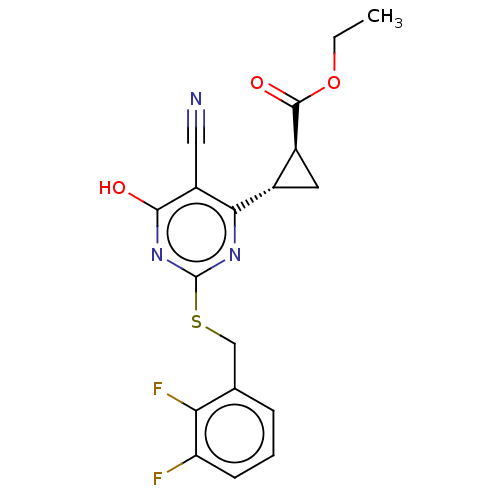 (CHEMBL3310785) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human recombinant CXCR2 receptor expressed in CHO cell membranes by SPA based [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 24: 3285-90 (2014) Article DOI: 10.1016/j.bmcl.2014.06.011 BindingDB Entry DOI: 10.7270/Q2930VTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50495166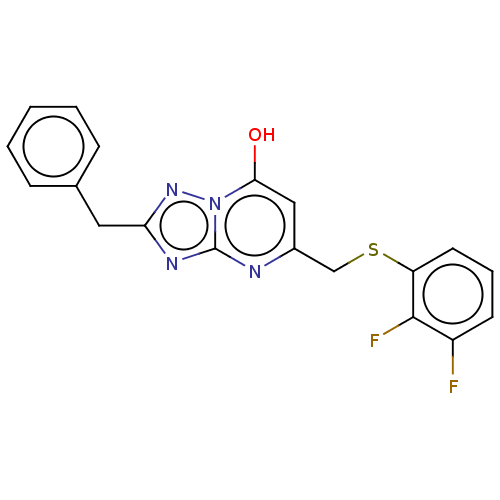 (CHEMBL3104912) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125I]GRO-alpha from human recombinant CXCR2 receptor expressed in CHO cells | Bioorg Med Chem Lett 24: 72-6 (2014) Article DOI: 10.1016/j.bmcl.2013.11.074 BindingDB Entry DOI: 10.7270/Q2QR513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-phosphate-mannose--protein mannosyltransferase 1 (Candida albicans) | BDBM50149752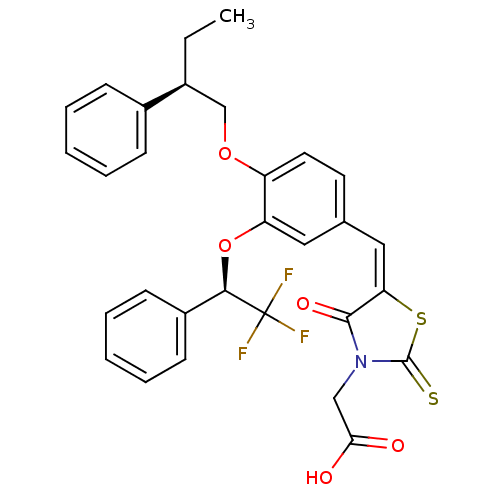 (CHEMBL361718 | {4-Oxo-5-[4-((R)-2-phenyl-butoxy)-3...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxfordshire OX14 4YS Curated by ChEMBL | Assay Description In vitro inhibition of Candida albicans protein mannosyl transferase 1 | Bioorg Med Chem Lett 14: 3975-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.050 BindingDB Entry DOI: 10.7270/Q22J6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-phosphate-mannose--protein mannosyltransferase 1 (Candida albicans) | BDBM50149777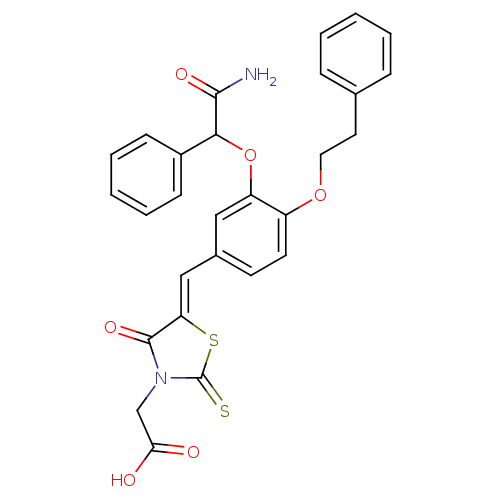 (CHEMBL366064 | {5-[1-[3-(Carbamoyl-phenyl-methoxy)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxfordshire OX14 4YS Curated by ChEMBL | Assay Description In vitro inhibition of Candida albicans protein mannosyl transferase 1 | Bioorg Med Chem Lett 14: 3975-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.050 BindingDB Entry DOI: 10.7270/Q22J6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50495166 (CHEMBL3104912) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR2 receptor expressed in CHO cells assessed as inhibition of IL8-induced [35S]GTPgammaS binding by SPA me... | Bioorg Med Chem Lett 24: 72-6 (2014) Article DOI: 10.1016/j.bmcl.2013.11.074 BindingDB Entry DOI: 10.7270/Q2QR513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50147544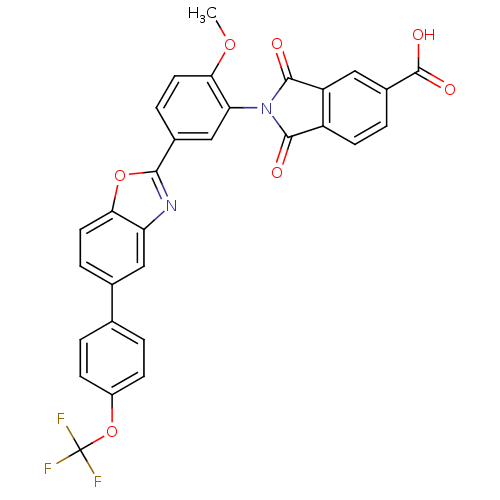 (2-(2-methoxy-5-(5-(4-(trifluoromethoxy)phenyl)benz...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd Curated by ChEMBL | Assay Description In vivo inhibitory activity against human Heparanase | Bioorg Med Chem Lett 14: 3269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.03.086 BindingDB Entry DOI: 10.7270/Q2CC1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50147533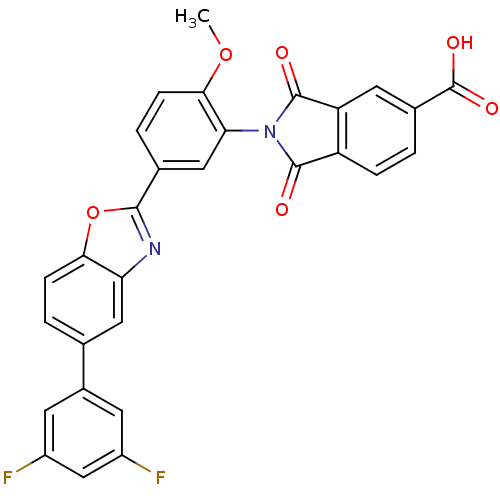 (2-(5-(5-(3,5-difluorophenyl)benzo[d]oxazol-2-yl)-2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd Curated by ChEMBL | Assay Description In vivo inhibitory activity against human Heparanase | Bioorg Med Chem Lett 14: 3269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.03.086 BindingDB Entry DOI: 10.7270/Q2CC1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-phosphate-mannose--protein mannosyltransferase 1 (Candida albicans) | BDBM50149774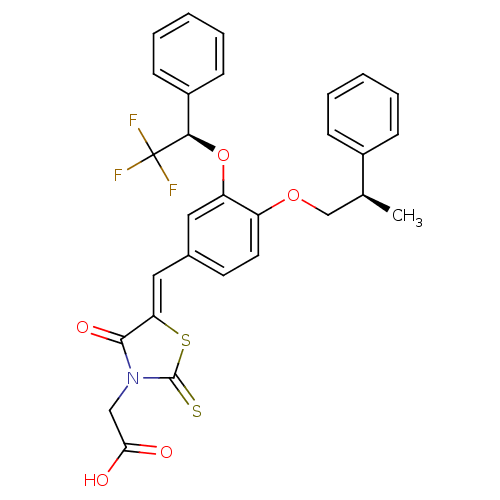 (CHEMBL182467 | {4-Oxo-5-[4-((R)-2-phenyl-propoxy)-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxfordshire OX14 4YS Curated by ChEMBL | Assay Description In vitro inhibition of Candida albicans protein mannosyl transferase 1 | Bioorg Med Chem Lett 14: 3975-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.050 BindingDB Entry DOI: 10.7270/Q22J6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50147537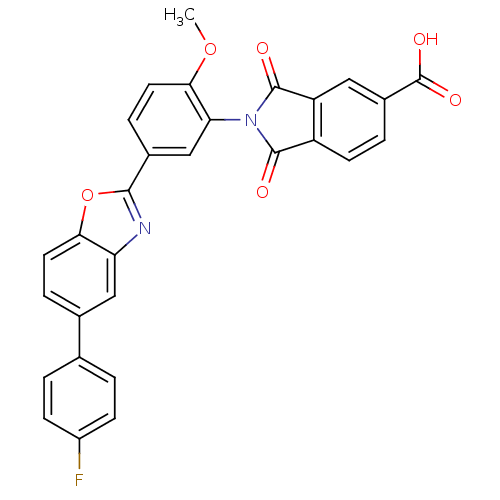 (2-(5-(5-(4-fluorophenyl)benzo[d]oxazol-2-yl)-2-met...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd Curated by ChEMBL | Assay Description In vivo inhibitory activity against human Heparanase | Bioorg Med Chem Lett 14: 3269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.03.086 BindingDB Entry DOI: 10.7270/Q2CC1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50147522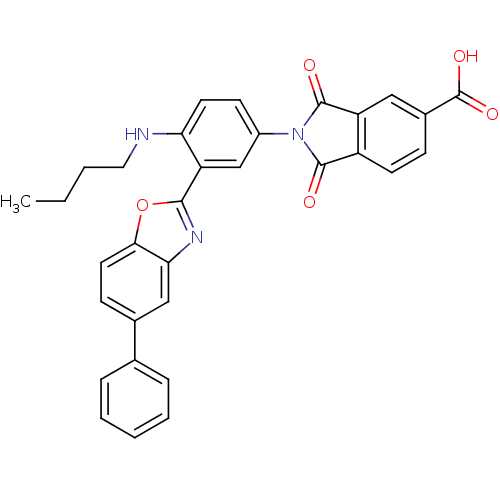 (2-(4-(butylamino)-3-(5-phenylbenzo[d]oxazol-2-yl)p...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd Curated by ChEMBL | Assay Description In vivo inhibitory activity against human Heparanase | Bioorg Med Chem Lett 14: 3269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.03.086 BindingDB Entry DOI: 10.7270/Q2CC1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50147539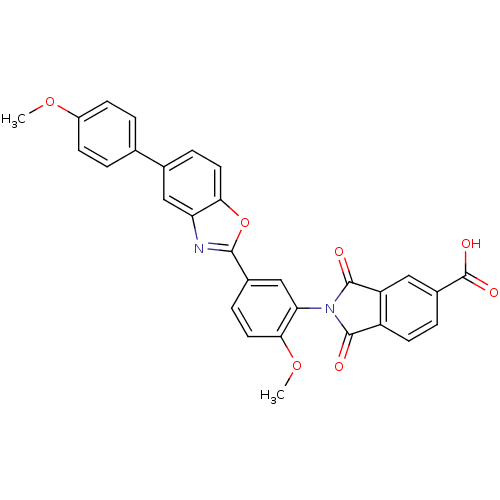 (2-(2-methoxy-5-(5-(4-methoxyphenyl)benzo[d]oxazol-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd Curated by ChEMBL | Assay Description In vivo inhibitory activity against human Heparanase | Bioorg Med Chem Lett 14: 3269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.03.086 BindingDB Entry DOI: 10.7270/Q2CC1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50495159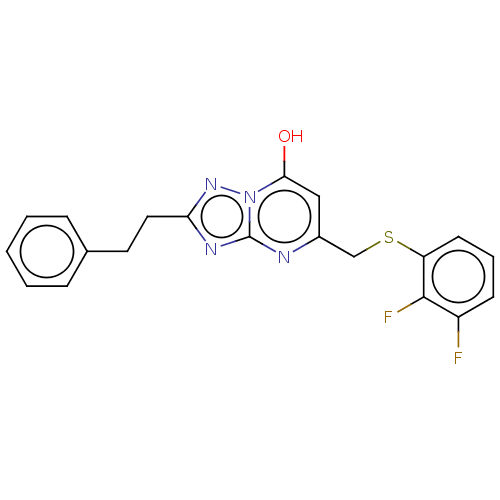 (CHEMBL3105079) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR2 receptor expressed in CHO cells assessed as inhibition of IL8-induced [35S]GTPgammaS binding by SPA me... | Bioorg Med Chem Lett 24: 72-6 (2014) Article DOI: 10.1016/j.bmcl.2013.11.074 BindingDB Entry DOI: 10.7270/Q2QR513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50147517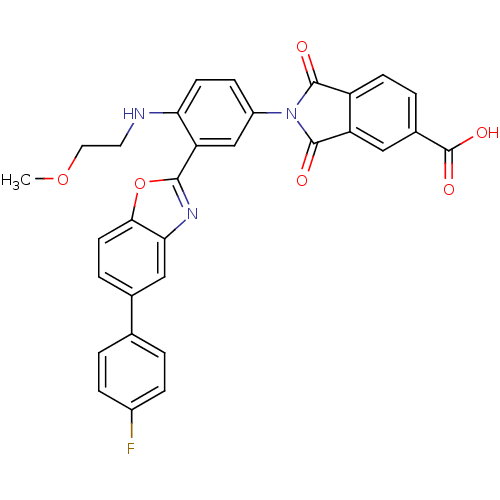 (2-[3-[5-(4-Fluoro-phenyl)-benzooxazol-2-yl]-4-(2-m...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd Curated by ChEMBL | Assay Description In vivo inhibitory activity against human Heparanase | Bioorg Med Chem Lett 14: 3269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.03.086 BindingDB Entry DOI: 10.7270/Q2CC1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-phosphate-mannose--protein mannosyltransferase 1 (Candida albicans) | BDBM50149764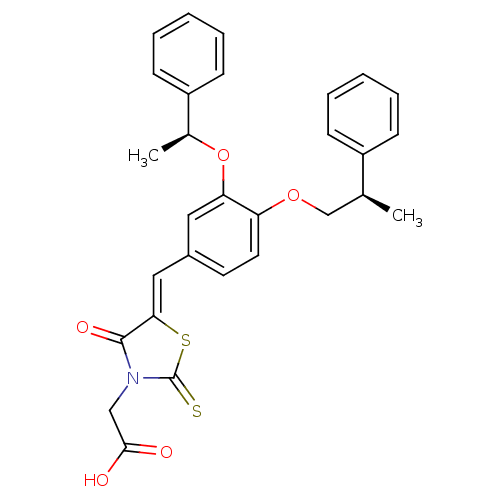 (CHEMBL185312 | {4-Oxo-5-[3-((S)-1-phenyl-ethoxy)-4...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxfordshire OX14 4YS Curated by ChEMBL | Assay Description In vitro inhibition of Candida albicans protein mannosyl transferase 1 | Bioorg Med Chem Lett 14: 3975-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.050 BindingDB Entry DOI: 10.7270/Q22J6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-phosphate-mannose--protein mannosyltransferase 1 (Candida albicans) | BDBM50149744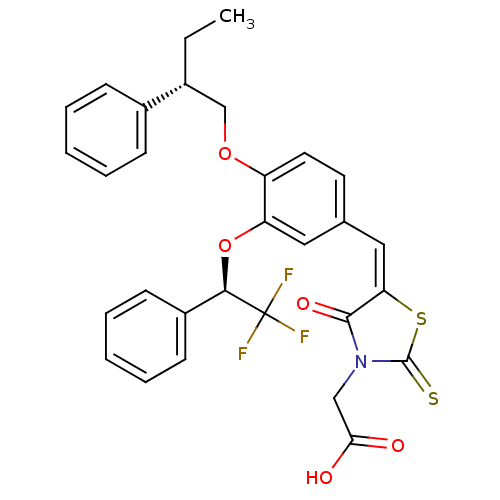 (CHEMBL182588 | {4-Oxo-5-[1-[4-((S)-2-phenyl-butoxy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxfordshire OX14 4YS Curated by ChEMBL | Assay Description In vitro inhibition of Candida albicans protein mannosyl transferase 1 | Bioorg Med Chem Lett 14: 3975-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.050 BindingDB Entry DOI: 10.7270/Q22J6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50048184 (CHEMBL3310789) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human recombinant CXCR2 receptor expressed in CHO cell membranes by SPA based [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 24: 3285-90 (2014) Article DOI: 10.1016/j.bmcl.2014.06.011 BindingDB Entry DOI: 10.7270/Q2930VTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50495165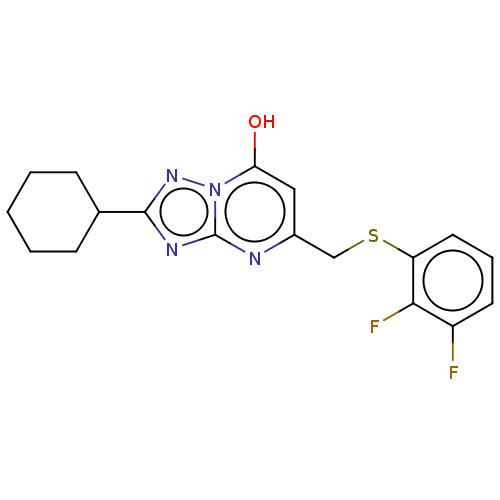 (CHEMBL3105077) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR2 receptor expressed in CHO cells assessed as inhibition of IL8-induced [35S]GTPgammaS binding by SPA me... | Bioorg Med Chem Lett 24: 72-6 (2014) Article DOI: 10.1016/j.bmcl.2013.11.074 BindingDB Entry DOI: 10.7270/Q2QR513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50147536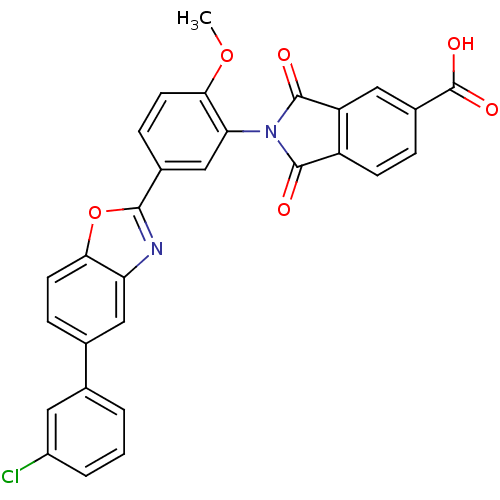 (2-(5-(5-(3-chlorophenyl)benzo[d]oxazol-2-yl)-2-met...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd Curated by ChEMBL | Assay Description In vivo inhibitory activity against human Heparanase | Bioorg Med Chem Lett 14: 3269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.03.086 BindingDB Entry DOI: 10.7270/Q2CC1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50147528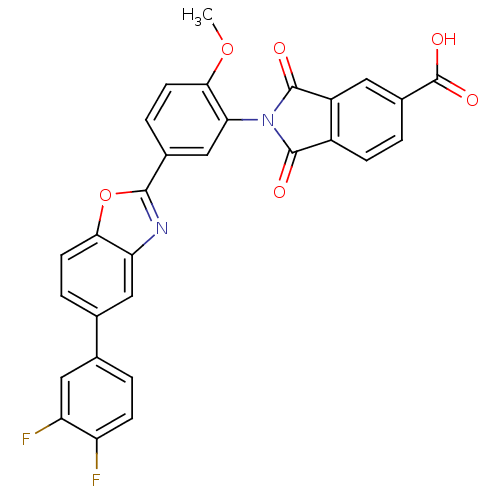 (2-(5-(5-(3,4-difluorophenyl)benzo[d]oxazol-2-yl)-2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd Curated by ChEMBL | Assay Description In vivo inhibitory activity against human Heparanase | Bioorg Med Chem Lett 14: 3269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.03.086 BindingDB Entry DOI: 10.7270/Q2CC1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-phosphate-mannose--protein mannosyltransferase 1 (Candida albicans) | BDBM50149741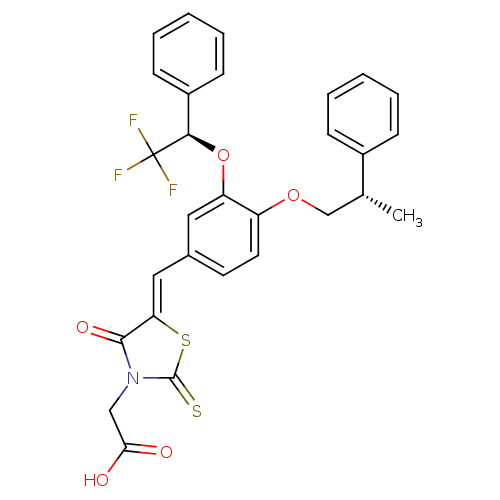 (CHEMBL369344 | {4-Oxo-5-[1-[4-((S)-2-phenyl-propox...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxfordshire OX14 4YS Curated by ChEMBL | Assay Description In vitro inhibition of Candida albicans protein mannosyl transferase 1 | Bioorg Med Chem Lett 14: 3975-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.050 BindingDB Entry DOI: 10.7270/Q22J6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-phosphate-mannose--protein mannosyltransferase 1 (Candida albicans) | BDBM50149768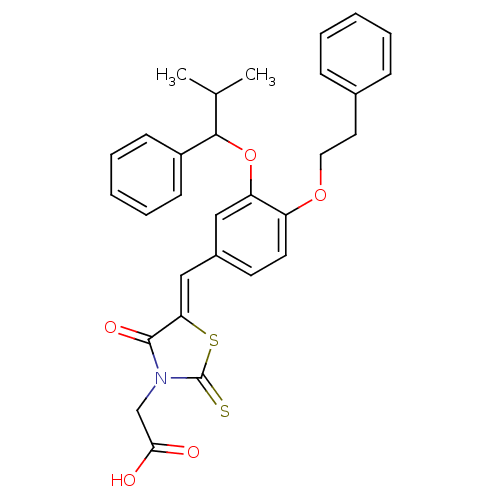 (CHEMBL424794 | {5-[1-[3-(2-Methyl-1-phenyl-propoxy...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxfordshire OX14 4YS Curated by ChEMBL | Assay Description In vitro inhibition of Candida albicans protein mannosyl transferase 1 | Bioorg Med Chem Lett 14: 3975-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.050 BindingDB Entry DOI: 10.7270/Q22J6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50048244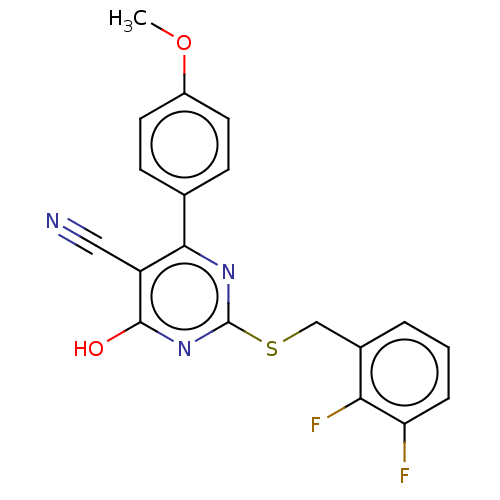 (CHEMBL3310774) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human recombinant CXCR2 receptor expressed in CHO cell membranes by SPA based [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 24: 3285-90 (2014) Article DOI: 10.1016/j.bmcl.2014.06.011 BindingDB Entry DOI: 10.7270/Q2930VTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50147535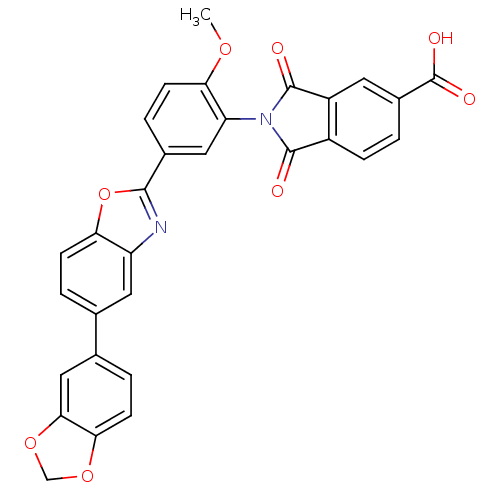 (2-(5-(5-(benzo[d][1,3]dioxol-5-yl)benzo[d]oxazol-2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd Curated by ChEMBL | Assay Description In vivo inhibitory activity against human Heparanase | Bioorg Med Chem Lett 14: 3269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.03.086 BindingDB Entry DOI: 10.7270/Q2CC1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50048249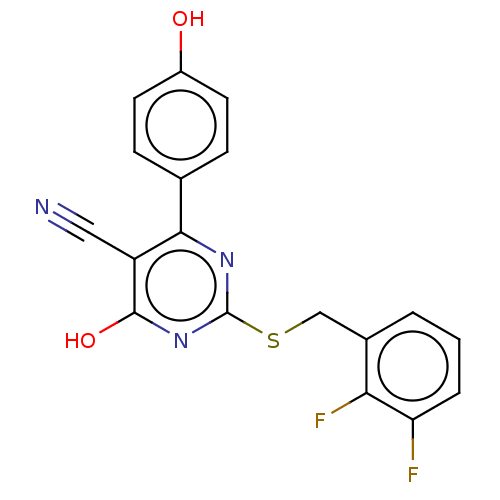 (CHEMBL3310777) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human recombinant CXCR2 receptor expressed in CHO cell membranes by SPA based [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 24: 3285-90 (2014) Article DOI: 10.1016/j.bmcl.2014.06.011 BindingDB Entry DOI: 10.7270/Q2930VTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50147519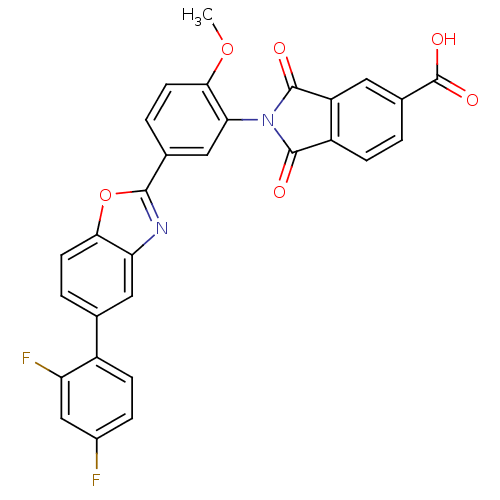 (2-(5-(5-(2,4-difluorophenyl)benzo[d]oxazol-2-yl)-2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd Curated by ChEMBL | Assay Description In vivo inhibitory activity against human Heparanase | Bioorg Med Chem Lett 14: 3269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.03.086 BindingDB Entry DOI: 10.7270/Q2CC1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-phosphate-mannose--protein mannosyltransferase 1 (Candida albicans) | BDBM50149749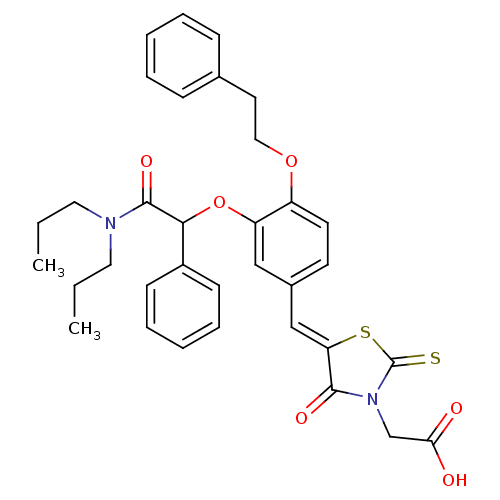 (CHEMBL182291 | {5-[3-(Dipropylcarbamoyl-phenyl-met...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxfordshire OX14 4YS Curated by ChEMBL | Assay Description In vitro inhibition of Candida albicans protein mannosyl transferase 1 | Bioorg Med Chem Lett 14: 3975-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.050 BindingDB Entry DOI: 10.7270/Q22J6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50147518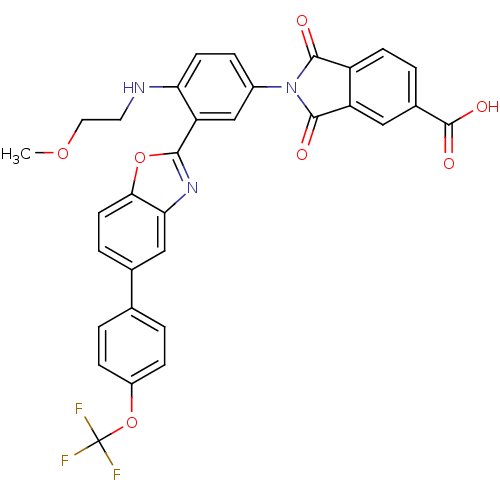 (2-{4-(2-Methoxy-ethylamino)-3-[5-(4-trifluorometho...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd Curated by ChEMBL | Assay Description In vivo inhibitory activity against human Heparanase | Bioorg Med Chem Lett 14: 3269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.03.086 BindingDB Entry DOI: 10.7270/Q2CC1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50495171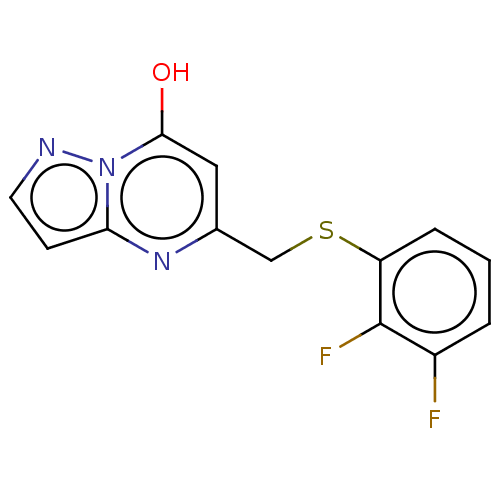 (CHEMBL3105085) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR2 receptor expressed in CHO cells assessed as inhibition of IL8-induced [35S]GTPgammaS binding by SPA me... | Bioorg Med Chem Lett 24: 72-6 (2014) Article DOI: 10.1016/j.bmcl.2013.11.074 BindingDB Entry DOI: 10.7270/Q2QR513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50495182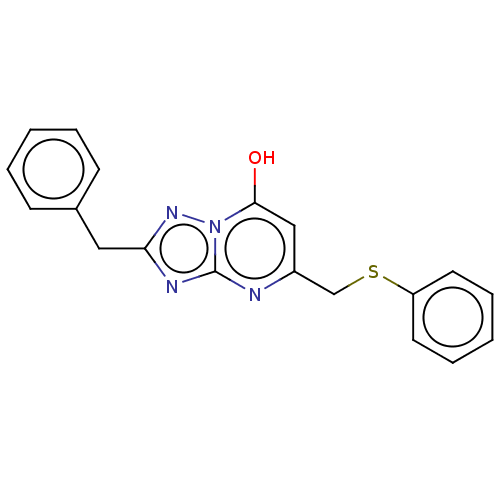 (CHEMBL3104900) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR2 receptor expressed in CHO cells assessed as inhibition of IL8-induced [35S]GTPgammaS binding by SPA me... | Bioorg Med Chem Lett 24: 72-6 (2014) Article DOI: 10.1016/j.bmcl.2013.11.074 BindingDB Entry DOI: 10.7270/Q2QR513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50495174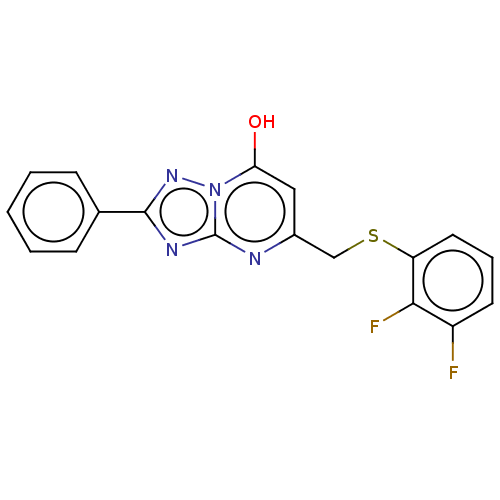 (CHEMBL3105078) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR2 receptor expressed in CHO cells assessed as inhibition of IL8-induced [35S]GTPgammaS binding by SPA me... | Bioorg Med Chem Lett 24: 72-6 (2014) Article DOI: 10.1016/j.bmcl.2013.11.074 BindingDB Entry DOI: 10.7270/Q2QR513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-phosphate-mannose--protein mannosyltransferase 1 (Candida albicans) | BDBM50149758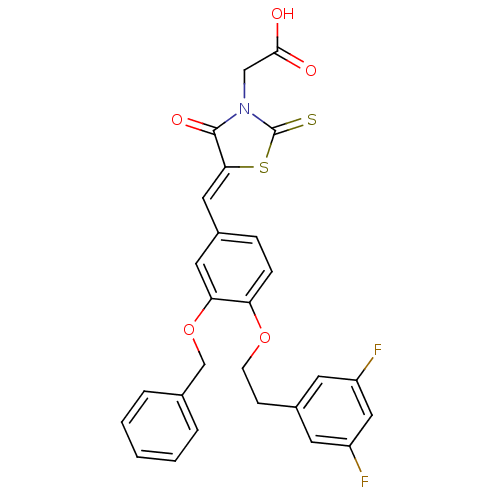 ((5-{3-Benzyloxy-4-[2-(3,5-difluoro-phenyl)-ethoxy]...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxfordshire OX14 4YS Curated by ChEMBL | Assay Description In vitro inhibition of Candida albicans protein mannosyl transferase 1 | Bioorg Med Chem Lett 14: 3975-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.050 BindingDB Entry DOI: 10.7270/Q22J6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50495162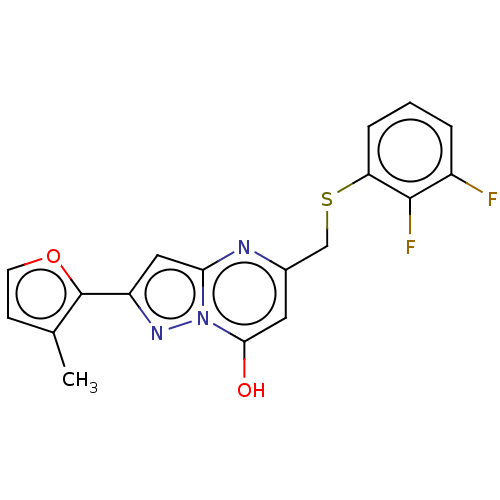 (CHEMBL3105082) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR2 receptor expressed in CHO cells assessed as inhibition of IL8-induced [35S]GTPgammaS binding by SPA me... | Bioorg Med Chem Lett 24: 72-6 (2014) Article DOI: 10.1016/j.bmcl.2013.11.074 BindingDB Entry DOI: 10.7270/Q2QR513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50147530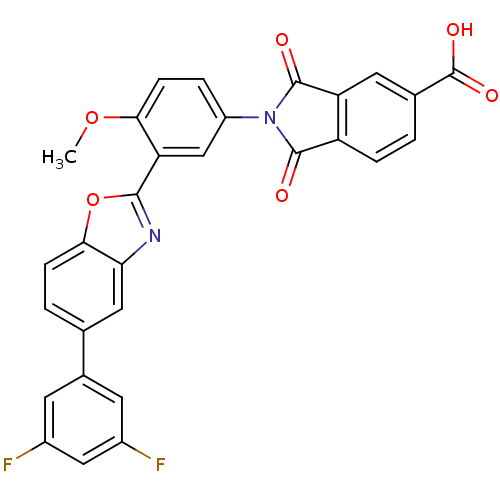 (2-(3-(5-(3,5-difluorophenyl)benzo[d]oxazol-2-yl)-4...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd Curated by ChEMBL | Assay Description In vivo inhibitory activity against human Heparanase | Bioorg Med Chem Lett 14: 3269-73 (2004) Article DOI: 10.1016/j.bmcl.2004.03.086 BindingDB Entry DOI: 10.7270/Q2CC1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50048252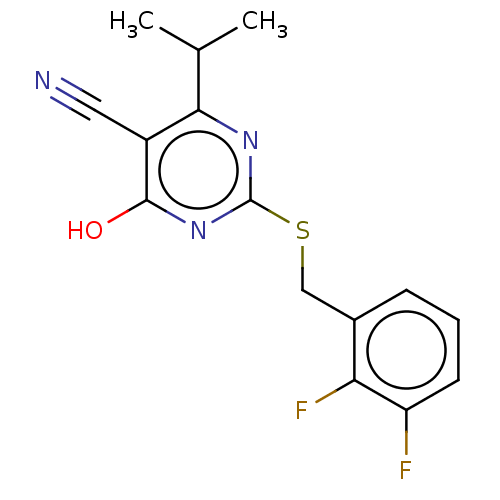 (CHEMBL3310780) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human recombinant CXCR2 receptor expressed in CHO cell membranes by SPA based [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 24: 3285-90 (2014) Article DOI: 10.1016/j.bmcl.2014.06.011 BindingDB Entry DOI: 10.7270/Q2930VTJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50495175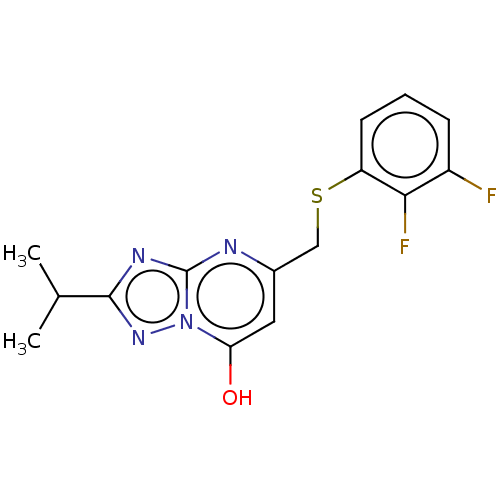 (CHEMBL3104914) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR2 receptor expressed in CHO cells assessed as inhibition of IL8-induced [35S]GTPgammaS binding by SPA me... | Bioorg Med Chem Lett 24: 72-6 (2014) Article DOI: 10.1016/j.bmcl.2013.11.074 BindingDB Entry DOI: 10.7270/Q2QR513C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-phosphate-mannose--protein mannosyltransferase 1 (Candida albicans) | BDBM50149776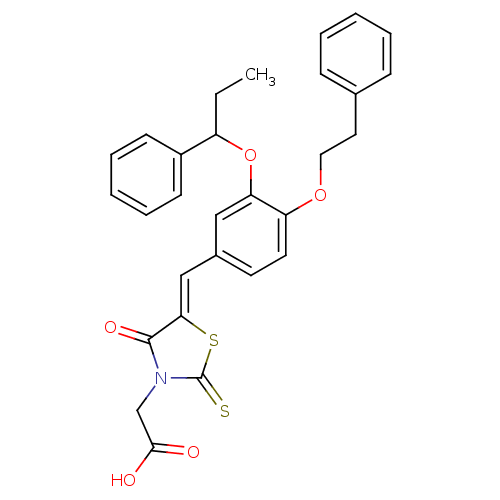 (CHEMBL367136 | {4-Oxo-5-[4-phenethyloxy-3-(1-pheny...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Oxfordshire OX14 4YS Curated by ChEMBL | Assay Description In vitro inhibition of Candida albicans protein mannosyl transferase 1 | Bioorg Med Chem Lett 14: 3975-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.050 BindingDB Entry DOI: 10.7270/Q22J6B96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 177 total ) | Next | Last >> |