 Found 3405 hits with Last Name = 'chang' and Initial = 'e'
Found 3405 hits with Last Name = 'chang' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin B
(Homo sapiens (Human)) | BDBM50213272
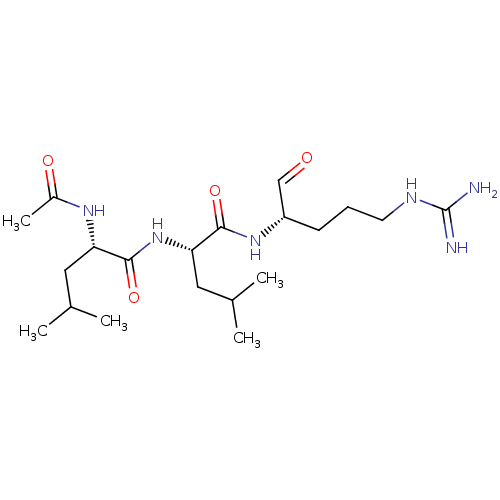
(CHEBI:6426 | Leupeptin)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C=O |r| Show InChI InChI=1S/C20H38N6O4/c1-12(2)9-16(24-14(5)28)19(30)26-17(10-13(3)4)18(29)25-15(11-27)7-6-8-23-20(21)22/h11-13,15-17H,6-10H2,1-5H3,(H,24,28)(H,25,29)(H,26,30)(H4,21,22,23)/t15-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The City University of New York
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B |
Bioorg Med Chem 21: 2975-87 (2013)
Article DOI: 10.1016/j.bmc.2013.03.062
BindingDB Entry DOI: 10.7270/Q2PG1VNW |
More data for this
Ligand-Target Pair | |
4-hydroxy-tetrahydrodipicolinate reductase
(Escherichia coli) | BDBM59098
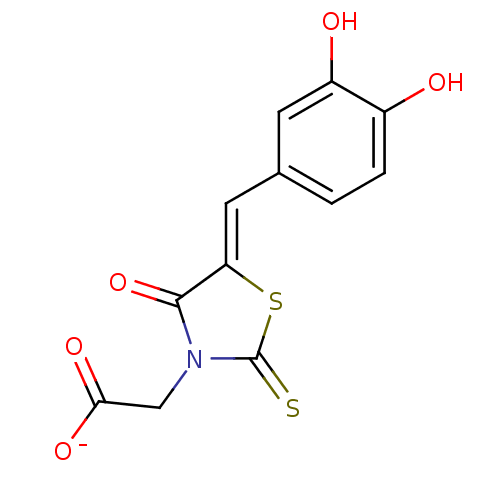
(Bi-ligand, 1)Show InChI InChI=1S/C12H9NO5S2/c14-7-2-1-6(3-8(7)15)4-9-11(18)13(5-10(16)17)12(19)20-9/h1-4,14-15H,5H2,(H,16,17)/p-1/b9-4- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
Quinone-dependent D-lactate dehydrogenase
(Escherichia coli) | BDBM59099
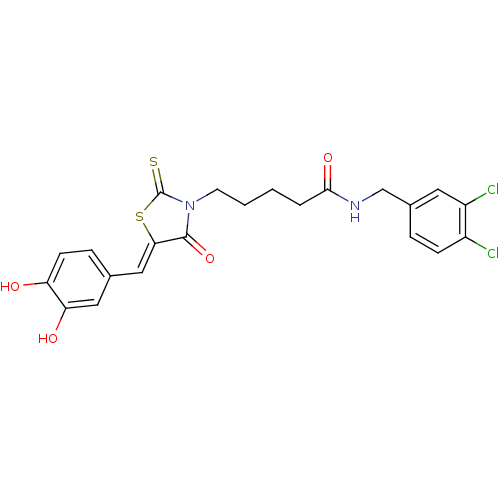
(Bi-ligand, 2)Show SMILES Oc1ccc(\C=C2/SC(=S)N(CCCCC(=O)NCc3ccc(Cl)c(Cl)c3)C2=O)cc1O Show InChI InChI=1S/C22H20Cl2N2O4S2/c23-15-6-4-14(9-16(15)24)12-25-20(29)3-1-2-8-26-21(30)19(32-22(26)31)11-13-5-7-17(27)18(28)10-13/h4-7,9-11,27-28H,1-3,8,12H2,(H,25,29)/b19-11- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
4-hydroxy-tetrahydrodipicolinate reductase
(Escherichia coli) | BDBM59101
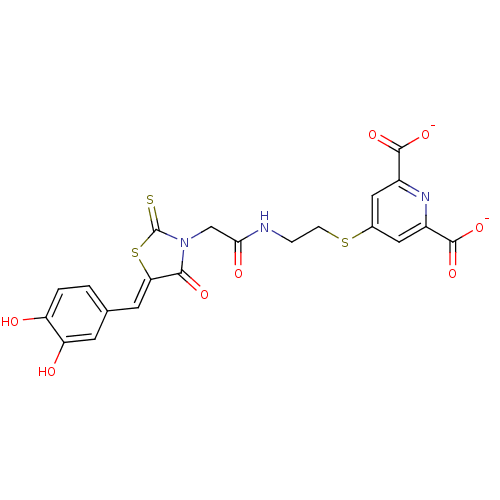
(Bi-ligand, 4)Show SMILES Oc1ccc(\C=C2/SC(=S)N(CC(=O)NCCSc3cc(nc(c3)C([O-])=O)C([O-])=O)C2=O)cc1O Show InChI InChI=1S/C21H17N3O8S3/c25-14-2-1-10(5-15(14)26)6-16-18(28)24(21(33)35-16)9-17(27)22-3-4-34-11-7-12(19(29)30)23-13(8-11)20(31)32/h1-2,5-8,25-26H,3-4,9H2,(H,22,27)(H,29,30)(H,31,32)/p-2/b16-6- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM59100
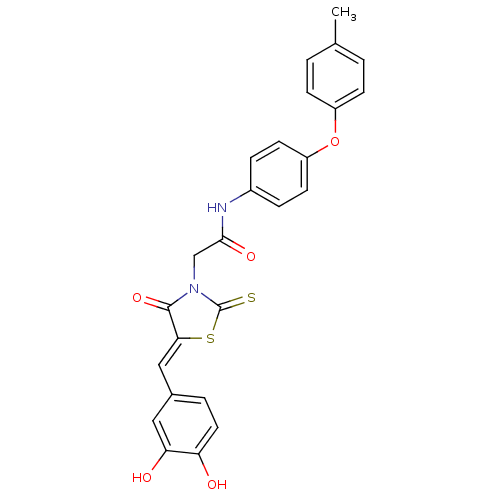
(Bi-ligand, 3)Show SMILES Cc1ccc(Oc2ccc(NC(=O)CN3C(=S)S\C(=C/c4ccc(O)c(O)c4)C3=O)cc2)cc1 Show InChI InChI=1S/C25H20N2O5S2/c1-15-2-7-18(8-3-15)32-19-9-5-17(6-10-19)26-23(30)14-27-24(31)22(34-25(27)33)13-16-4-11-20(28)21(29)12-16/h2-13,28-29H,14H2,1H3,(H,26,30)/b22-13- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 202 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50568138
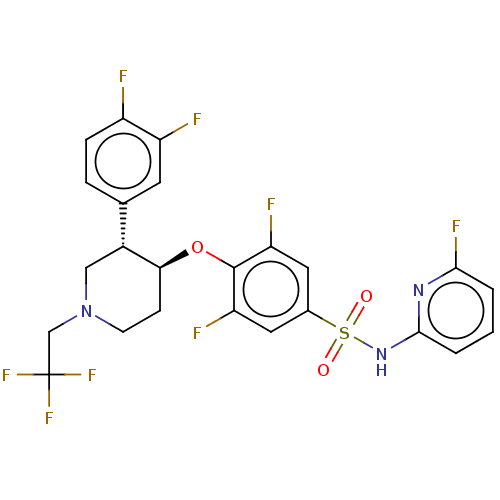
(CHEMBL4854735)Show SMILES Fc1cccc(NS(=O)(=O)c2cc(F)c(O[C@H]3CCN(CC(F)(F)F)C[C@@H]3c3ccc(F)c(F)c3)c(F)c2)n1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]BNZA from Nav1.5 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128133
BindingDB Entry DOI: 10.7270/Q2CJ8J7B |
More data for this
Ligand-Target Pair | |
Quinone-dependent D-lactate dehydrogenase
(Escherichia coli) | BDBM59101

(Bi-ligand, 4)Show SMILES Oc1ccc(\C=C2/SC(=S)N(CC(=O)NCCSc3cc(nc(c3)C([O-])=O)C([O-])=O)C2=O)cc1O Show InChI InChI=1S/C21H17N3O8S3/c25-14-2-1-10(5-15(14)26)6-16-18(28)24(21(33)35-16)9-17(27)22-3-4-34-11-7-12(19(29)30)23-13(8-11)20(31)32/h1-2,5-8,25-26H,3-4,9H2,(H,22,27)(H,29,30)(H,31,32)/p-2/b16-6- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50568135
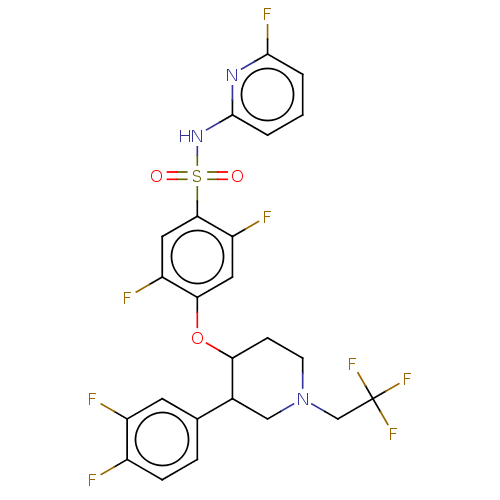
(CHEMBL4849813)Show SMILES Fc1cccc(NS(=O)(=O)c2cc(F)c(OC3CCN(CC(F)(F)F)CC3c3ccc(F)c(F)c3)cc2F)n1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]BNZA from Nav1.5 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128133
BindingDB Entry DOI: 10.7270/Q2CJ8J7B |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM59101

(Bi-ligand, 4)Show SMILES Oc1ccc(\C=C2/SC(=S)N(CC(=O)NCCSc3cc(nc(c3)C([O-])=O)C([O-])=O)C2=O)cc1O Show InChI InChI=1S/C21H17N3O8S3/c25-14-2-1-10(5-15(14)26)6-16-18(28)24(21(33)35-16)9-17(27)22-3-4-34-11-7-12(19(29)30)23-13(8-11)20(31)32/h1-2,5-8,25-26H,3-4,9H2,(H,22,27)(H,29,30)(H,31,32)/p-2/b16-6- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM59099

(Bi-ligand, 2)Show SMILES Oc1ccc(\C=C2/SC(=S)N(CCCCC(=O)NCc3ccc(Cl)c(Cl)c3)C2=O)cc1O Show InChI InChI=1S/C22H20Cl2N2O4S2/c23-15-6-4-14(9-16(15)24)12-25-20(29)3-1-2-8-26-21(30)19(32-22(26)31)11-13-5-7-17(27)18(28)10-13/h4-7,9-11,27-28H,1-3,8,12H2,(H,25,29)/b19-11- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
Quinone-dependent D-lactate dehydrogenase
(Escherichia coli) | BDBM59100

(Bi-ligand, 3)Show SMILES Cc1ccc(Oc2ccc(NC(=O)CN3C(=S)S\C(=C/c4ccc(O)c(O)c4)C3=O)cc2)cc1 Show InChI InChI=1S/C25H20N2O5S2/c1-15-2-7-18(8-3-15)32-19-9-5-17(6-10-19)26-23(30)14-27-24(31)22(34-25(27)33)13-16-4-11-20(28)21(29)12-16/h2-13,28-29H,14H2,1H3,(H,26,30)/b22-13- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
4-hydroxy-tetrahydrodipicolinate reductase
(Escherichia coli) | BDBM59100

(Bi-ligand, 3)Show SMILES Cc1ccc(Oc2ccc(NC(=O)CN3C(=S)S\C(=C/c4ccc(O)c(O)c4)C3=O)cc2)cc1 Show InChI InChI=1S/C25H20N2O5S2/c1-15-2-7-18(8-3-15)32-19-9-5-17(6-10-19)26-23(30)14-27-24(31)22(34-25(27)33)13-16-4-11-20(28)21(29)12-16/h2-13,28-29H,14H2,1H3,(H,26,30)/b22-13- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
4-hydroxy-tetrahydrodipicolinate reductase
(Escherichia coli) | BDBM59099

(Bi-ligand, 2)Show SMILES Oc1ccc(\C=C2/SC(=S)N(CCCCC(=O)NCc3ccc(Cl)c(Cl)c3)C2=O)cc1O Show InChI InChI=1S/C22H20Cl2N2O4S2/c23-15-6-4-14(9-16(15)24)12-25-20(29)3-1-2-8-26-21(30)19(32-22(26)31)11-13-5-7-17(27)18(28)10-13/h4-7,9-11,27-28H,1-3,8,12H2,(H,25,29)/b19-11- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
1-deoxy-D-xylulose 5-phosphate reductoisomerase
(Escherichia coli) | BDBM59098

(Bi-ligand, 1)Show InChI InChI=1S/C12H9NO5S2/c14-7-2-1-6(3-8(7)15)4-9-11(18)13(5-10(16)17)12(19)20-9/h1-4,14-15H,5H2,(H,16,17)/p-1/b9-4- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
Quinone-dependent D-lactate dehydrogenase
(Escherichia coli) | BDBM59098

(Bi-ligand, 1)Show InChI InChI=1S/C12H9NO5S2/c14-7-2-1-6(3-8(7)15)4-9-11(18)13(5-10(16)17)12(19)20-9/h1-4,14-15H,5H2,(H,16,17)/p-1/b9-4- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc
| Assay Description
All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. |
Chem Biol 11: 185-94 (2004)
Article DOI: 10.1016/j.chembiol.2004.02.012
BindingDB Entry DOI: 10.7270/Q2K9360M |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50491873
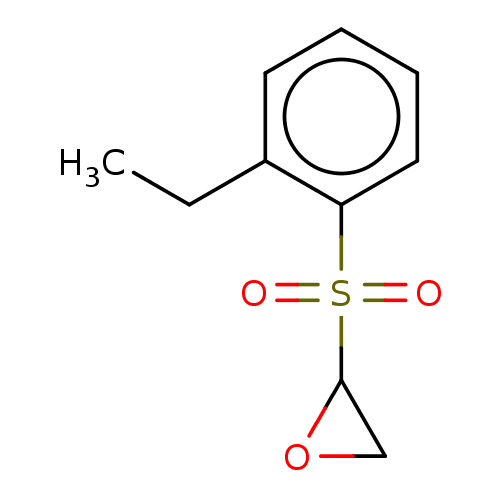
(CHEMBL2387617)Show InChI InChI=1S/C10H12O3S/c1-2-8-5-3-4-6-9(8)14(11,12)10-7-13-10/h3-6,10H,2,7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The City University of New York
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B using Z-RR-para-nitroanilide as substrate assessed as reversible equilibrium binding constant by Kitz-Wilson pl... |
Bioorg Med Chem 21: 2975-87 (2013)
Article DOI: 10.1016/j.bmc.2013.03.062
BindingDB Entry DOI: 10.7270/Q2PG1VNW |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM145281
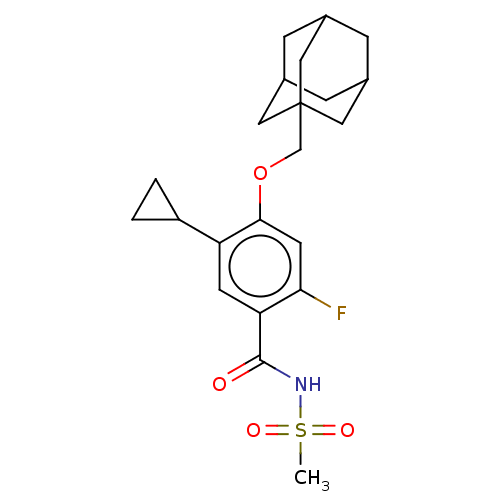
(US8952169, 60 | US9771376, Example 60)Show SMILES CS(=O)(=O)NC(=O)c1cc(C2CC2)c(OCC23CC4CC(CC(C4)C2)C3)cc1F |TLB:25:16:23:19.20.21,THB:15:16:23:19.20.21,25:20:23:16.24.17,24:16:19:23.22.21,24:22:19:16.25.17| Show InChI InChI=1S/C22H28FNO4S/c1-29(26,27)24-21(25)18-7-17(16-2-3-16)20(8-19(18)23)28-12-22-9-13-4-14(10-22)6-15(5-13)11-22/h7-8,13-16H,2-6,9-12H2,1H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium channel protein type 7 subunit alpha
(Homo sapiens (Human)) | BDBM145285
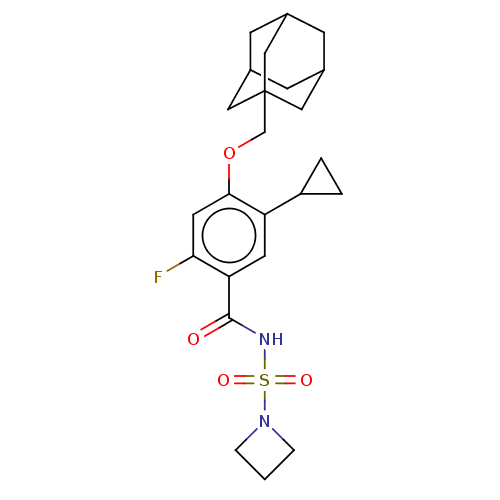
(US8952169, 64 | US9771376, Example 64)Show SMILES Fc1cc(OCC23CC4CC(CC(C4)C2)C3)c(cc1C(=O)NS(=O)(=O)N1CCC1)C1CC1 |TLB:5:6:9:13.12.11,15:6:13:9.10.11,THB:15:10:13:6.14.7,14:6:9:13.12.11,14:12:9:6.15.7| Show InChI InChI=1S/C24H31FN2O4S/c25-21-10-22(31-14-24-11-15-6-16(12-24)8-17(7-15)13-24)19(18-2-3-18)9-20(21)23(28)26-32(29,30)27-4-1-5-27/h9-10,15-18H,1-8,11-14H2,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human Nav1.7 expressed in HEK cells by whole cell voltage clamp analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00049
BindingDB Entry DOI: 10.7270/Q2WW7NKS |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 7 subunit alpha
(Homo sapiens (Human)) | BDBM70937
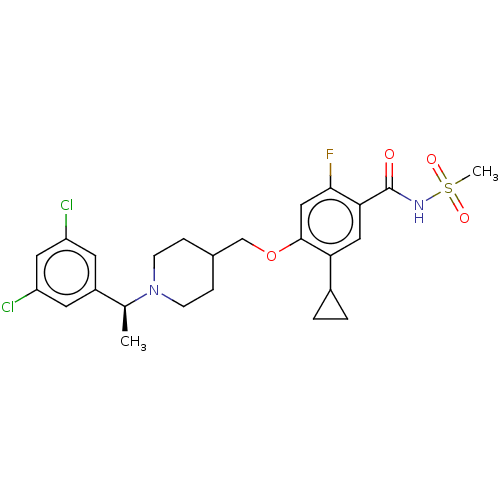
(US9546164, 100 | US9694002, 100)Show SMILES C[C@H](N1CCC(COc2cc(F)c(cc2C2CC2)C(=O)NS(C)(=O)=O)CC1)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C25H29Cl2FN2O4S/c1-15(18-9-19(26)11-20(27)10-18)30-7-5-16(6-8-30)14-34-24-13-23(28)22(12-21(24)17-3-4-17)25(31)29-35(2,32)33/h9-13,15-17H,3-8,14H2,1-2H3,(H,29,31)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human Nav1.7 expressed in HEK cells by whole cell voltage clamp analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00049
BindingDB Entry DOI: 10.7270/Q2WW7NKS |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM145591
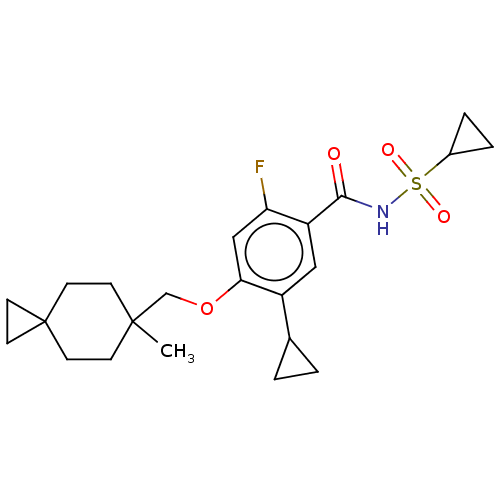
(US8952169, 389)Show SMILES CC1(COc2cc(F)c(cc2C2CC2)C(=O)NS(=O)(=O)C2CC2)CCC2(CC2)CC1 Show InChI InChI=1S/C23H30FNO4S/c1-22(6-8-23(9-7-22)10-11-23)14-29-20-13-19(24)18(12-17(20)15-2-3-15)21(26)25-30(27,28)16-4-5-16/h12-13,15-16H,2-11,14H2,1H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM47139
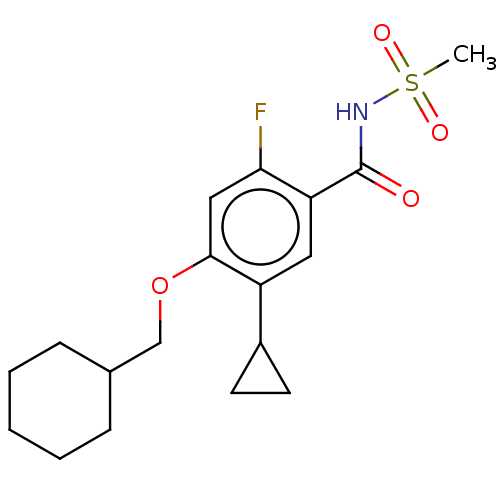
(US8952169, 175 | US8952169, 295)Show SMILES CS(=O)(=O)NC(=O)c1cc(C2CC2)c(OCC2CCCCC2)cc1F Show InChI InChI=1S/C18H24FNO4S/c1-25(22,23)20-18(21)15-9-14(13-7-8-13)17(10-16(15)19)24-11-12-5-3-2-4-6-12/h9-10,12-13H,2-8,11H2,1H3,(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium channel protein type 7 subunit alpha
(Homo sapiens (Human)) | BDBM71064
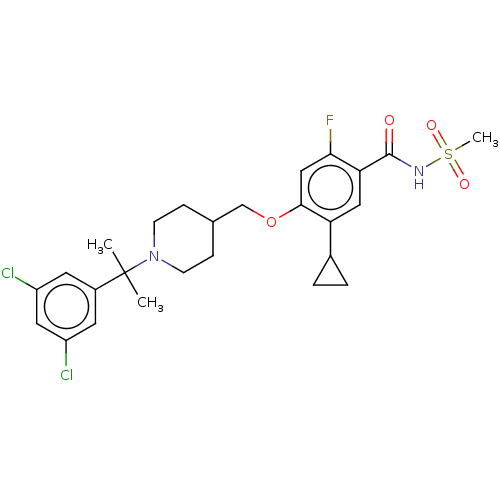
(US9546164, 209 | US9694002, 209)Show SMILES CC(C)(N1CCC(COc2cc(F)c(cc2C2CC2)C(=O)NS(C)(=O)=O)CC1)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C26H31Cl2FN2O4S/c1-26(2,18-10-19(27)12-20(28)11-18)31-8-6-16(7-9-31)15-35-24-14-23(29)22(13-21(24)17-4-5-17)25(32)30-36(3,33)34/h10-14,16-17H,4-9,15H2,1-3H3,(H,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]GX-545 Nav1.7 (unknown origin) expressed in HEK cells by liquid scintillation counting based radioligand competition assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00049
BindingDB Entry DOI: 10.7270/Q2WW7NKS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560806
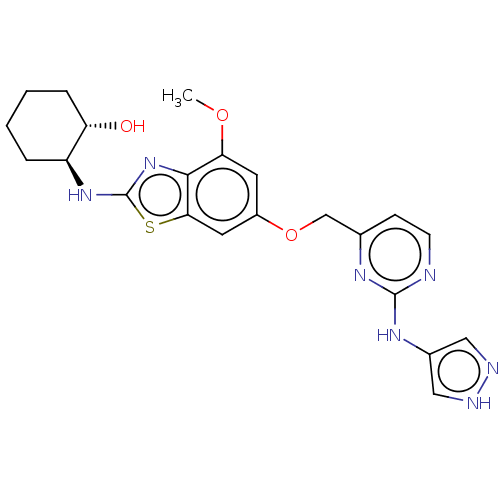
(CHEMBL4758092)Show SMILES COc1cc(OCc2ccnc(Nc3cn[nH]c3)n2)cc2sc(N[C@H]3CCCC[C@@H]3O)nc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50460035
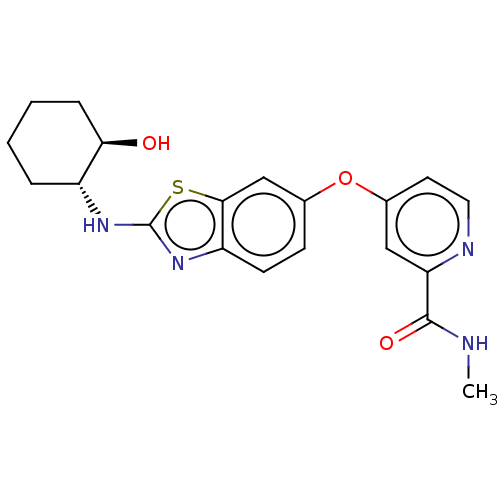
(CHEMBL4227505)Show SMILES CNC(=O)c1cc(Oc2ccc3nc(N[C@@H]4CCCC[C@H]4O)sc3c2)ccn1 |r| Show InChI InChI=1S/C20H22N4O3S/c1-21-19(26)16-10-13(8-9-22-16)27-12-6-7-15-18(11-12)28-20(24-15)23-14-4-2-3-5-17(14)25/h6-11,14,17,25H,2-5H2,1H3,(H,21,26)(H,23,24)/t14-,17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CSF1R (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM268527
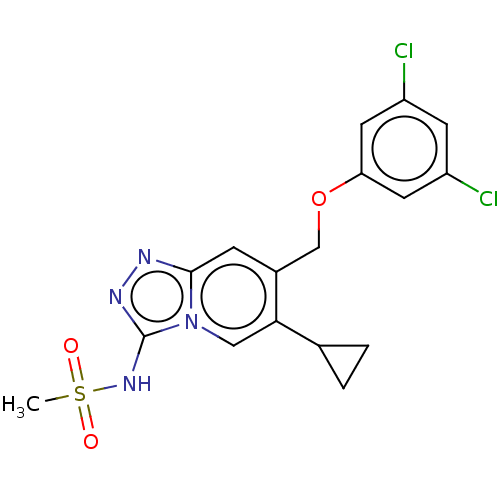
(N-(6-cyclopropyl-7-((3,5-dichlorophenoxy)methyl)-[...)Show SMILES CS(=O)(=O)Nc1nnc2cc(COc3cc(Cl)cc(Cl)c3)c(cn12)C1CC1 Show InChI InChI=1S/C17H16Cl2N4O3S/c1-27(24,25)22-17-21-20-16-4-11(15(8-23(16)17)10-2-3-10)9-26-14-6-12(18)5-13(19)7-14/h4-8,10H,2-3,9H2,1H3,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of inactivated state of recombinant human NaV1.7 expressed in HEK293 cell membranes coexpressing Nav beta1 subunit assessed as decrease in... |
J Med Chem 61: 4810-4831 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01826
BindingDB Entry DOI: 10.7270/Q2BV7K92 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560807
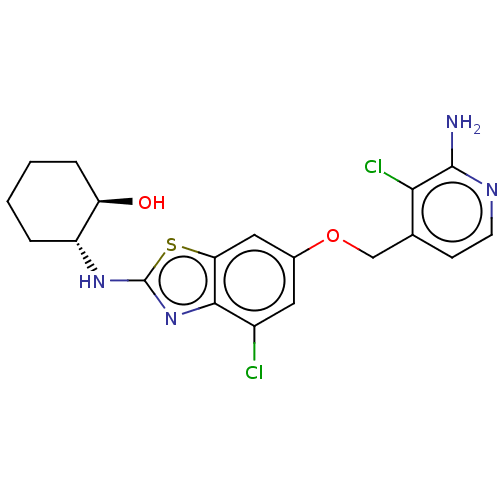
(CHEMBL4751287)Show SMILES Nc1nccc(COc2cc(Cl)c3nc(N[C@@H]4CCCC[C@H]4O)sc3c2)c1Cl |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560804
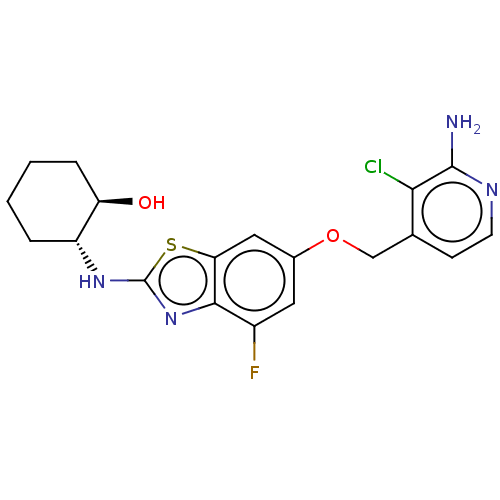
(CHEMBL4763875)Show SMILES Nc1nccc(COc2cc(F)c3nc(N[C@@H]4CCCC[C@H]4O)sc3c2)c1Cl |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560798
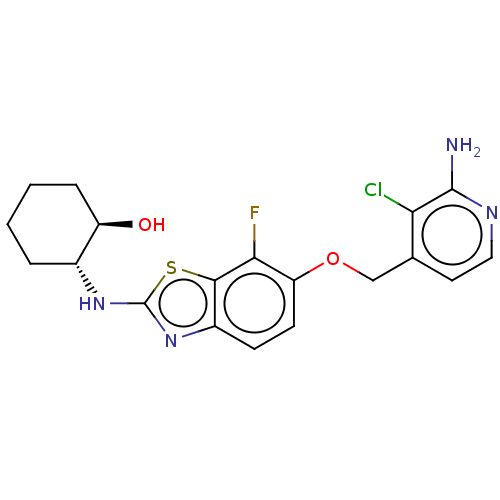
(CHEMBL4795462)Show SMILES Nc1nccc(COc2ccc3nc(N[C@@H]4CCCC[C@H]4O)sc3c2F)c1Cl |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560786
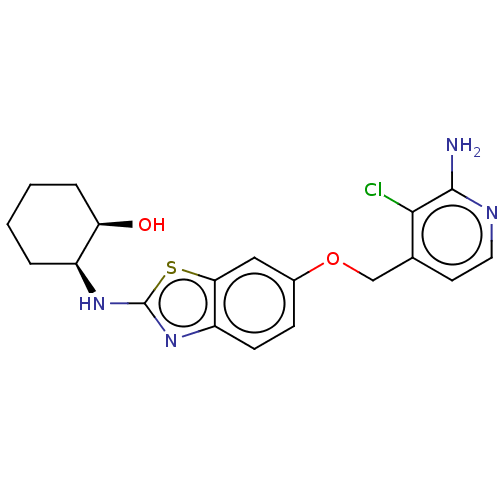
(CHEMBL4798876)Show SMILES Nc1nccc(COc2ccc3nc(N[C@H]4CCCC[C@H]4O)sc3c2)c1Cl |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 7 subunit alpha
(Homo sapiens (Human)) | BDBM145285

(US8952169, 64 | US9771376, Example 64)Show SMILES Fc1cc(OCC23CC4CC(CC(C4)C2)C3)c(cc1C(=O)NS(=O)(=O)N1CCC1)C1CC1 |TLB:5:6:9:13.12.11,15:6:13:9.10.11,THB:15:10:13:6.14.7,14:6:9:13.12.11,14:12:9:6.15.7| Show InChI InChI=1S/C24H31FN2O4S/c25-21-10-22(31-14-24-11-15-6-16(12-24)8-17(7-15)13-24)19(18-2-3-18)9-20(21)23(28)26-32(29,30)27-4-1-5-27/h9-10,15-18H,1-8,11-14H2,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]GX-545 Nav1.7 (unknown origin) expressed in HEK cells by liquid scintillation counting based radioligand competition assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00049
BindingDB Entry DOI: 10.7270/Q2WW7NKS |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 7 subunit alpha
(Homo sapiens (Human)) | BDBM145369
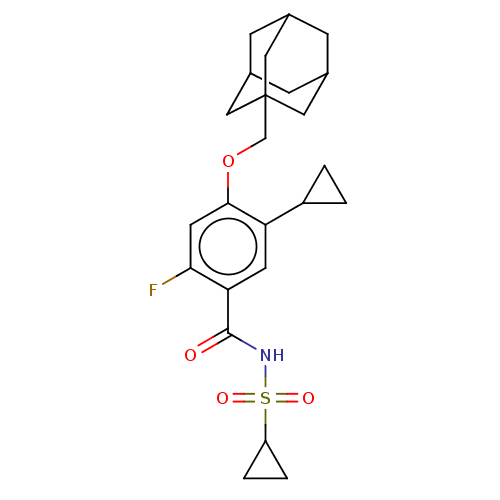
(US8952169, 148 | US9771376, Example 148)Show SMILES Fc1cc(OCC23CC4CC(CC(C4)C2)C3)c(cc1C(=O)NS(=O)(=O)C1CC1)C1CC1 |TLB:15:6:13:9.10.11,THB:5:6:13:9.10.11,15:10:13:6.14.7,14:6:9:13.12.11,14:12:9:6.15.7| Show InChI InChI=1S/C24H30FNO4S/c25-21-9-22(30-13-24-10-14-5-15(11-24)7-16(6-14)12-24)19(17-1-2-17)8-20(21)23(27)26-31(28,29)18-3-4-18/h8-9,14-18H,1-7,10-13H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]GX-545 Nav1.7 (unknown origin) expressed in HEK cells by liquid scintillation counting based radioligand competition assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00049
BindingDB Entry DOI: 10.7270/Q2WW7NKS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560791
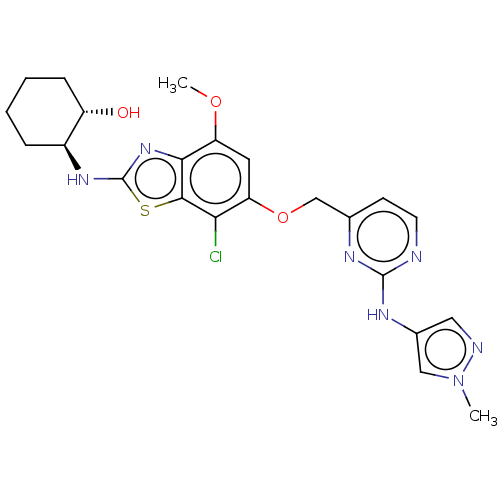
(CHEMBL4740157)Show SMILES COc1cc(OCc2ccnc(Nc3cnn(C)c3)n2)c(Cl)c2sc(N[C@H]3CCCC[C@@H]3O)nc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560801
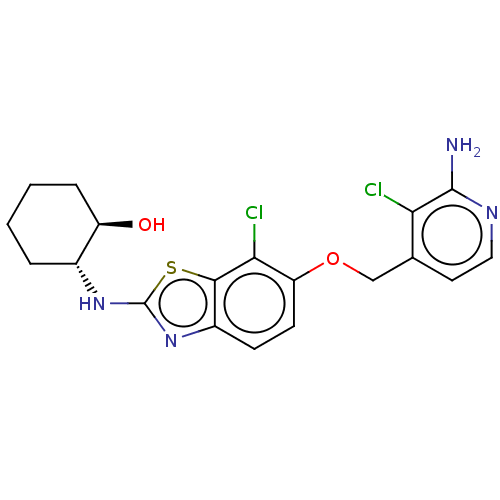
(CHEMBL4798601)Show SMILES Nc1nccc(COc2ccc3nc(N[C@@H]4CCCC[C@H]4O)sc3c2Cl)c1Cl |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560788
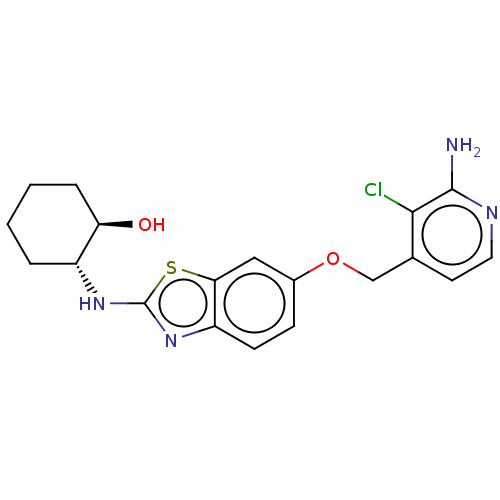
(CHEMBL4759047)Show SMILES Nc1nccc(COc2ccc3nc(N[C@@H]4CCCC[C@H]4O)sc3c2)c1Cl |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 7 subunit alpha
(Homo sapiens (Human)) | BDBM145281

(US8952169, 60 | US9771376, Example 60)Show SMILES CS(=O)(=O)NC(=O)c1cc(C2CC2)c(OCC23CC4CC(CC(C4)C2)C3)cc1F |TLB:25:16:23:19.20.21,THB:15:16:23:19.20.21,25:20:23:16.24.17,24:16:19:23.22.21,24:22:19:16.25.17| Show InChI InChI=1S/C22H28FNO4S/c1-29(26,27)24-21(25)18-7-17(16-2-3-16)20(8-19(18)23)28-12-22-9-13-4-14(10-22)6-15(5-13)11-22/h7-8,13-16H,2-6,9-12H2,1H3,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]GX-545 Nav1.7 (unknown origin) expressed in HEK cells by liquid scintillation counting based radioligand competition assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00049
BindingDB Entry DOI: 10.7270/Q2WW7NKS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560812
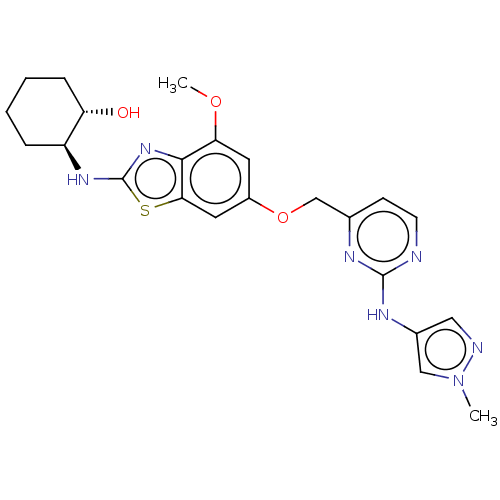
(CHEMBL4748740)Show SMILES COc1cc(OCc2ccnc(Nc3cnn(C)c3)n2)cc2sc(N[C@H]3CCCC[C@@H]3O)nc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 7 subunit alpha
(Homo sapiens (Human)) | BDBM70937

(US9546164, 100 | US9694002, 100)Show SMILES C[C@H](N1CCC(COc2cc(F)c(cc2C2CC2)C(=O)NS(C)(=O)=O)CC1)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C25H29Cl2FN2O4S/c1-15(18-9-19(26)11-20(27)10-18)30-7-5-16(6-8-30)14-34-24-13-23(28)22(12-21(24)17-3-4-17)25(31)29-35(2,32)33/h9-13,15-17H,3-8,14H2,1-2H3,(H,29,31)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]GX-545 Nav1.7 (unknown origin) expressed in HEK cells by liquid scintillation counting based radioligand competition assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00049
BindingDB Entry DOI: 10.7270/Q2WW7NKS |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 7 subunit alpha
(Homo sapiens (Human)) | BDBM70936
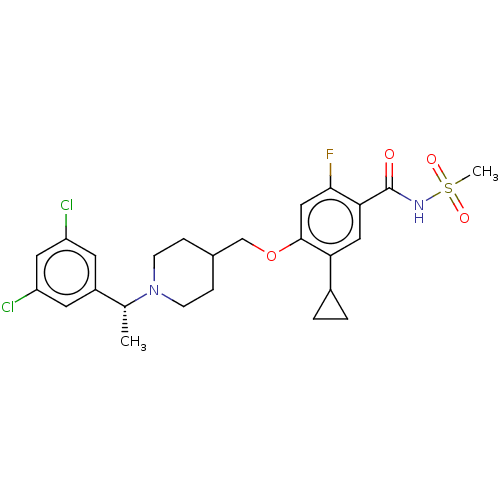
(US9546164, 99 | US9694002, 99)Show SMILES C[C@@H](N1CCC(COc2cc(F)c(cc2C2CC2)C(=O)NS(C)(=O)=O)CC1)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C25H29Cl2FN2O4S/c1-15(18-9-19(26)11-20(27)10-18)30-7-5-16(6-8-30)14-34-24-13-23(28)22(12-21(24)17-3-4-17)25(31)29-35(2,32)33/h9-13,15-17H,3-8,14H2,1-2H3,(H,29,31)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]GX-545 Nav1.7 (unknown origin) expressed in HEK cells by liquid scintillation counting based radioligand competition assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00049
BindingDB Entry DOI: 10.7270/Q2WW7NKS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 5
(Homo sapiens (Human)) | BDBM128404
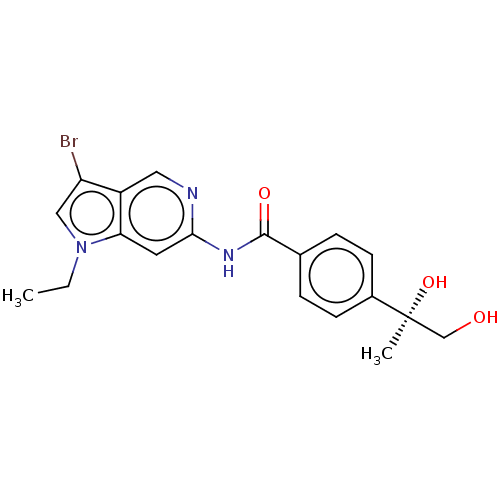
(US8802695, 20)Show SMILES CCn1cc(Br)c2cnc(NC(=O)c3ccc(cc3)[C@@](C)(O)CO)cc12 |r| Show InChI InChI=1S/C19H20BrN3O3/c1-3-23-10-15(20)14-9-21-17(8-16(14)23)22-18(25)12-4-6-13(7-5-12)19(2,26)11-24/h4-10,24,26H,3,11H2,1-2H3,(H,21,22,25)/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Takeda Pharmaceutical Company Limited
US Patent
| Assay Description
The test compounds (2.5 μL) dissolved in DMSO were added to wells containing 37.5 μL of the reaction solution (25 mM HEPES (pH 7.5), 10 mM ... |
US Patent US8802695 (2014)
BindingDB Entry DOI: 10.7270/Q27W69WD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 5
(Homo sapiens (Human)) | BDBM128406
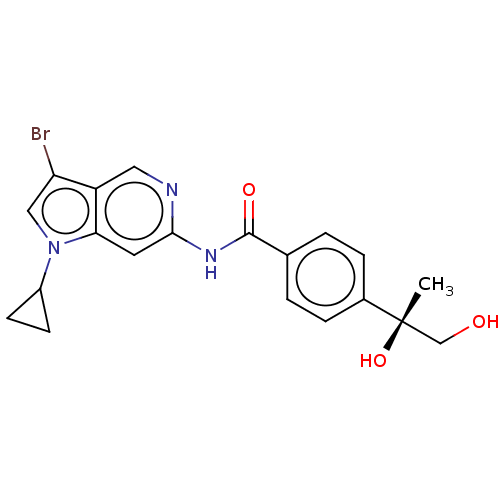
(US8802695, 22)Show SMILES C[C@](O)(CO)c1ccc(cc1)C(=O)Nc1cc2n(cc(Br)c2cn1)C1CC1 |r| Show InChI InChI=1S/C20H20BrN3O3/c1-20(27,11-25)13-4-2-12(3-5-13)19(26)23-18-8-17-15(9-22-18)16(21)10-24(17)14-6-7-14/h2-5,8-10,14,25,27H,6-7,11H2,1H3,(H,22,23,26)/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Takeda Pharmaceutical Company Limited
US Patent
| Assay Description
The test compounds (2.5 μL) dissolved in DMSO were added to wells containing 37.5 μL of the reaction solution (25 mM HEPES (pH 7.5), 10 mM ... |
US Patent US8802695 (2014)
BindingDB Entry DOI: 10.7270/Q27W69WD |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 2 subunit alpha
(Homo sapiens (Human)) | BDBM145591

(US8952169, 389)Show SMILES CC1(COc2cc(F)c(cc2C2CC2)C(=O)NS(=O)(=O)C2CC2)CCC2(CC2)CC1 Show InChI InChI=1S/C23H30FNO4S/c1-22(6-8-23(9-7-22)10-11-23)14-29-20-13-19(24)18(12-17(20)15-2-3-15)21(26)25-30(27,28)16-4-5-16/h12-13,15-16H,2-11,14H2,1H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50097721
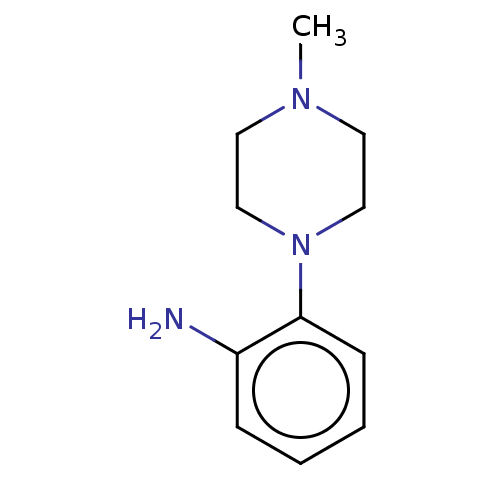
(CHEMBL1879790 | EN300-11843)Show InChI InChI=1S/C29H38FN5O3S/c1-4-6-7-8-9-10-15-33(3)26(36)20-35-19-24(16-23-17-31-28(38)34(5-2)18-23)27(37)32-29(35)39-21-22-11-13-25(30)14-12-22/h11-14,17-19H,4-10,15-16,20-21H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM50465470
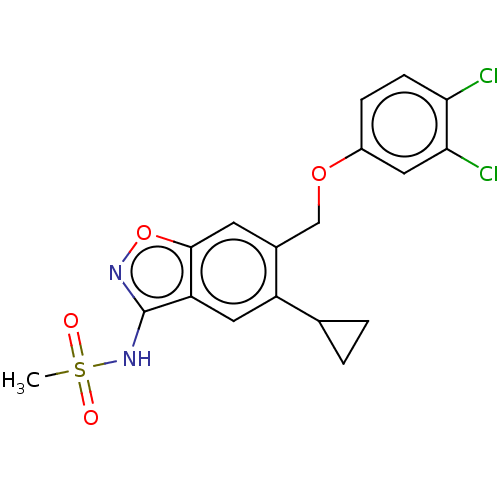
(CHEMBL4277472)Show SMILES CS(=O)(=O)Nc1noc2cc(COc3ccc(Cl)c(Cl)c3)c(cc12)C1CC1 Show InChI InChI=1S/C18H16Cl2N2O4S/c1-27(23,24)22-18-14-8-13(10-2-3-10)11(6-17(14)26-21-18)9-25-12-4-5-15(19)16(20)7-12/h4-8,10H,2-3,9H2,1H3,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of inactivated state of recombinant human NaV1.7 expressed in HEK293 cell membranes coexpressing Nav beta1 subunit assessed as decrease in... |
J Med Chem 61: 4810-4831 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01826
BindingDB Entry DOI: 10.7270/Q2BV7K92 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM268527

(N-(6-cyclopropyl-7-((3,5-dichlorophenoxy)methyl)-[...)Show SMILES CS(=O)(=O)Nc1nnc2cc(COc3cc(Cl)cc(Cl)c3)c(cn12)C1CC1 Show InChI InChI=1S/C17H16Cl2N4O3S/c1-27(24,25)22-17-21-20-16-4-11(15(8-23(16)17)10-2-3-10)9-26-14-6-12(18)5-13(19)7-14/h4-8,10H,2-3,9H2,1H3,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Xenon Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of inactivated state of recombinant wild type human NaV1.7 expressed in HEK293 cell membranes coexpressing Nav beta1 subunit assessed as d... |
J Med Chem 61: 4810-4831 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01826
BindingDB Entry DOI: 10.7270/Q2BV7K92 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560790
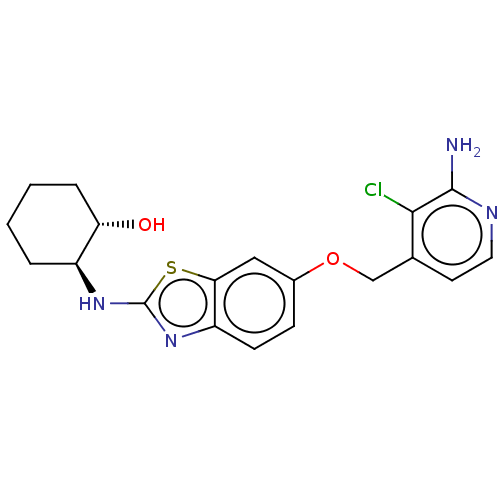
(CHEMBL4741283)Show SMILES Nc1nccc(COc2ccc3nc(N[C@H]4CCCC[C@@H]4O)sc3c2)c1Cl |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50560789
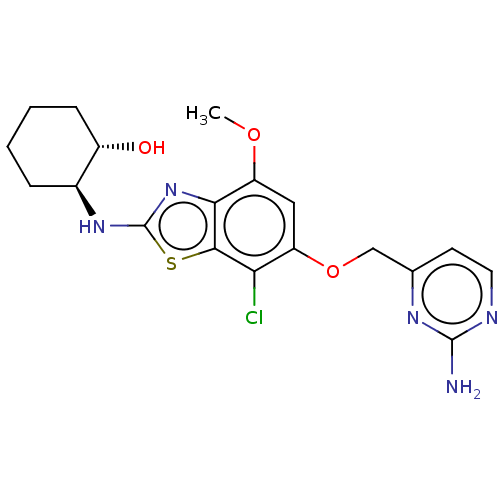
(CHEMBL4779878)Show SMILES COc1cc(OCc2ccnc(N)n2)c(Cl)c2sc(N[C@H]3CCCC[C@@H]3O)nc12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AlexaFluor-labeled tracer 236 binding to recombinant human His-tagged CSF1R (538 to 910 residues) preincubated for 20 mins followed by ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00936
BindingDB Entry DOI: 10.7270/Q2Q81HSS |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 7 subunit alpha
(Homo sapiens (Human)) | BDBM145375
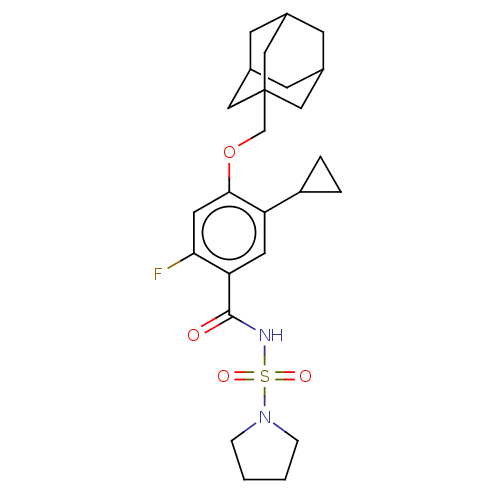
(US8952169, 154)Show SMILES Fc1cc(OCC23CC4CC(CC(C4)C2)C3)c(cc1C(=O)NS(=O)(=O)N1CCCC1)C1CC1 |TLB:15:6:13:9.10.11,THB:5:6:13:9.10.11,15:10:13:6.14.7,14:6:9:13.12.11,14:12:9:6.15.7| Show InChI InChI=1S/C25H33FN2O4S/c26-22-11-23(32-15-25-12-16-7-17(13-25)9-18(8-16)14-25)20(19-3-4-19)10-21(22)24(29)27-33(30,31)28-5-1-2-6-28/h10-11,16-19H,1-9,12-15H2,(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]GX-545 Nav1.7 (unknown origin) expressed in HEK cells by liquid scintillation counting based radioligand competition assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00049
BindingDB Entry DOI: 10.7270/Q2WW7NKS |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Mus musculus) | BDBM145591

(US8952169, 389)Show SMILES CC1(COc2cc(F)c(cc2C2CC2)C(=O)NS(=O)(=O)C2CC2)CCC2(CC2)CC1 Show InChI InChI=1S/C23H30FNO4S/c1-22(6-8-23(9-7-22)10-11-23)14-29-20-13-19(24)18(12-17(20)15-2-3-15)21(26)25-30(27,28)16-4-5-16/h12-13,15-16H,2-11,14H2,1H3,(H,25,26) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium channel protein type 7 subunit alpha
(Homo sapiens (Human)) | BDBM145376
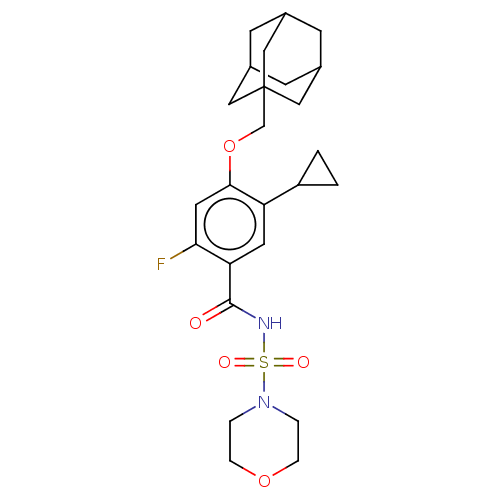
(US8952169, 155)Show SMILES Fc1cc(OCC23CC4CC(CC(C4)C2)C3)c(cc1C(=O)NS(=O)(=O)N1CCOCC1)C1CC1 |TLB:15:6:13:9.10.11,THB:5:6:13:9.10.11,15:10:13:6.14.7,14:6:9:13.12.11,14:12:9:6.15.7| Show InChI InChI=1S/C25H33FN2O5S/c26-22-11-23(33-15-25-12-16-7-17(13-25)9-18(8-16)14-25)20(19-1-2-19)10-21(22)24(29)27-34(30,31)28-3-5-32-6-4-28/h10-11,16-19H,1-9,12-15H2,(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]GX-545 Nav1.7 (unknown origin) expressed in HEK cells by liquid scintillation counting based radioligand competition assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00049
BindingDB Entry DOI: 10.7270/Q2WW7NKS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data