

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50396691 (CHEMBL449572 | US9271961, Jasmonic acid) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 expressed in Escherichia coli BL21(DE3) using phenanthrenequinone as substrate by spectrophotometric analysis | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50396691 (CHEMBL449572 | US9271961, Jasmonic acid) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C4 expressed in Escherichia coli BL21(DE3) using phenanthrenequinone as substrate by spectrophotometric analysis | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50396691 (CHEMBL449572 | US9271961, Jasmonic acid) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C1 expressed in Escherichia coli BL21(DE3) using phenanthrenequinone as substrate by spectrophotometric analysis | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396691 (CHEMBL449572 | US9271961, Jasmonic acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | 1.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 expressed in Escherichia coli BL21(DE3) using phenanthrenequinone as substrate by spectrophotometric analysis | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 1 (Homo sapiens (Human)) | BDBM50124354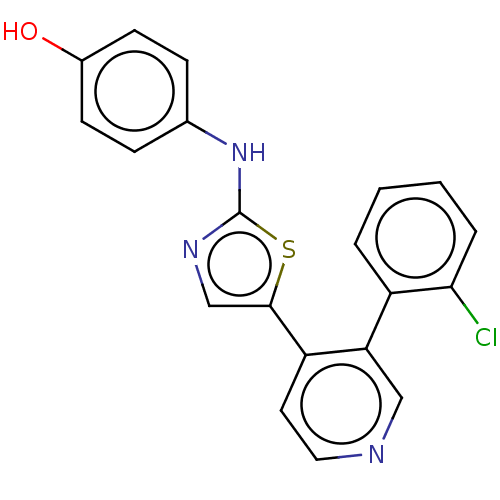 (CHEMBL3623439) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged LIMK1 (unknown origin) expressed in Sf21 cells by HTRF assay using cofilin as a substrate | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50200622 (CHEMBL3917975) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Babraham Research Campus Curated by ChEMBL | Assay Description Inhibition of human recombinant ATX using Rac-1-Palmitoyl-glycero-3-phosphocholine as substrate incubated for 2 hrs using ADHP fluorogenic peroxidase... | Bioorg Med Chem Lett 26: 5403-5410 (2016) Article DOI: 10.1016/j.bmcl.2016.10.036 BindingDB Entry DOI: 10.7270/Q2DJ5HM1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| LIM domain kinase 1 (Homo sapiens (Human)) | BDBM50124355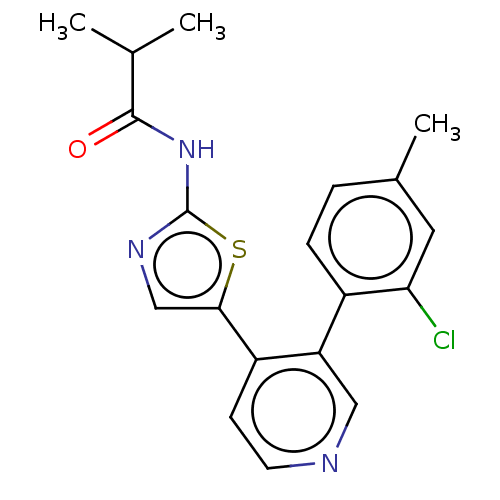 (CHEMBL3623442) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged LIMK1 (unknown origin) expressed in Sf21 cells by HTRF assay using cofilin as a substrate | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 2 (Homo sapiens (Human)) | BDBM50124354 (CHEMBL3623439) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged LIMK2 | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50200572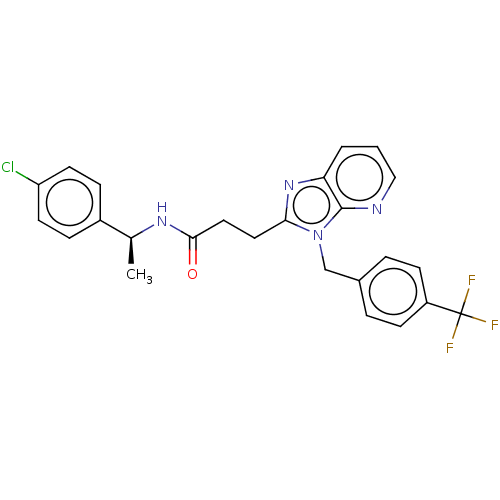 (CHEMBL3889955) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Babraham Research Campus Curated by ChEMBL | Assay Description Inhibition of human recombinant ATX using Rac-1-Palmitoyl-glycero-3-phosphocholine as substrate incubated for 2 hrs using ADHP fluorogenic peroxidase... | Bioorg Med Chem Lett 26: 5403-5410 (2016) Article DOI: 10.1016/j.bmcl.2016.10.036 BindingDB Entry DOI: 10.7270/Q2DJ5HM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50200569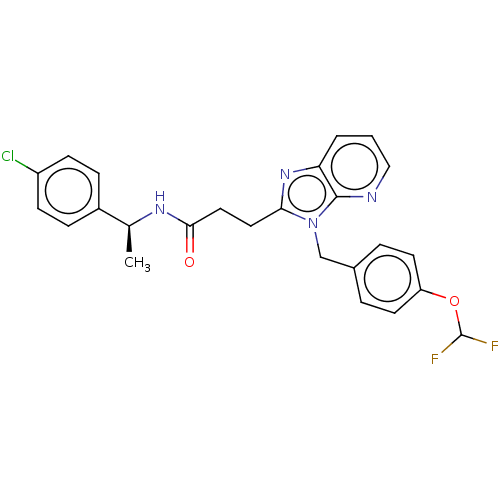 (CHEMBL3946146) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Babraham Research Campus Curated by ChEMBL | Assay Description Inhibition of human recombinant ATX using Rac-1-Palmitoyl-glycero-3-phosphocholine as substrate incubated for 2 hrs using ADHP fluorogenic peroxidase... | Bioorg Med Chem Lett 26: 5403-5410 (2016) Article DOI: 10.1016/j.bmcl.2016.10.036 BindingDB Entry DOI: 10.7270/Q2DJ5HM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50200579 (CHEMBL3917466) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Babraham Research Campus Curated by ChEMBL | Assay Description Inhibition of human recombinant ATX using Rac-1-Palmitoyl-glycero-3-phosphocholine as substrate incubated for 2 hrs using ADHP fluorogenic peroxidase... | Bioorg Med Chem Lett 26: 5403-5410 (2016) Article DOI: 10.1016/j.bmcl.2016.10.036 BindingDB Entry DOI: 10.7270/Q2DJ5HM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50200624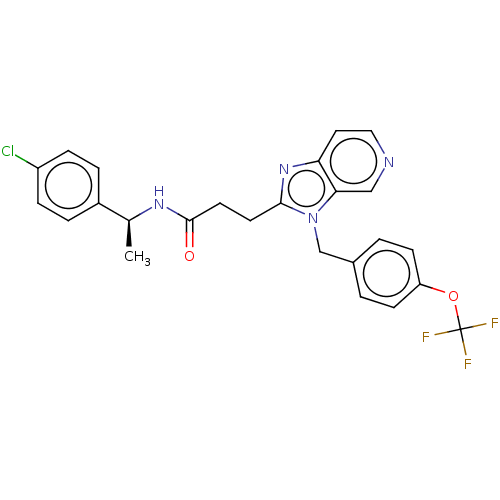 (CHEMBL3912114) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Babraham Research Campus Curated by ChEMBL | Assay Description Inhibition of human recombinant ATX using Rac-1-Palmitoyl-glycero-3-phosphocholine as substrate incubated for 2 hrs using ADHP fluorogenic peroxidase... | Bioorg Med Chem Lett 26: 5403-5410 (2016) Article DOI: 10.1016/j.bmcl.2016.10.036 BindingDB Entry DOI: 10.7270/Q2DJ5HM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50200582 (CHEMBL3944813) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Babraham Research Campus Curated by ChEMBL | Assay Description Inhibition of human recombinant ATX using Rac-1-Palmitoyl-glycero-3-phosphocholine as substrate incubated for 2 hrs using ADHP fluorogenic peroxidase... | Bioorg Med Chem Lett 26: 5403-5410 (2016) Article DOI: 10.1016/j.bmcl.2016.10.036 BindingDB Entry DOI: 10.7270/Q2DJ5HM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 1 (Homo sapiens (Human)) | BDBM50124352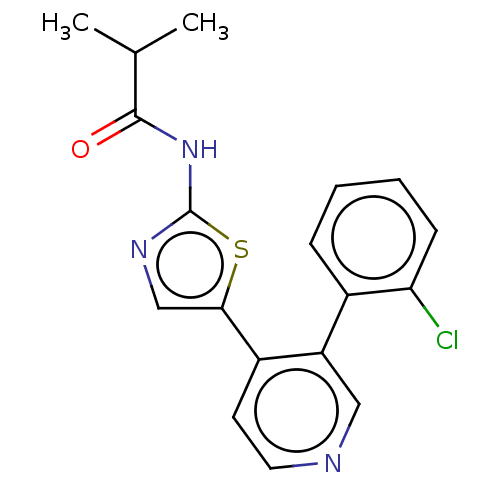 (CHEMBL3623441) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged LIMK1 (unknown origin) expressed in Sf21 cells by HTRF assay using cofilin as a substrate | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50446016 (CHEMBL3103330) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of AKR1C3 (unknown origin) expressed in human HCT116 cells assessed as formation of PR-104H from PR-104A preincubated for 2 hrs | Bioorg Med Chem 22: 967-77 (2014) Article DOI: 10.1016/j.bmc.2013.12.050 BindingDB Entry DOI: 10.7270/Q25H7HQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50187693 (CHEMBL3186509) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Babraham Research Campus Curated by ChEMBL | Assay Description Inhibition of human recombinant ATX using Rac-1-Palmitoyl-glycero-3-phosphocholine as substrate incubated for 2 hrs using ADHP fluorogenic peroxidase... | Bioorg Med Chem Lett 26: 5403-5410 (2016) Article DOI: 10.1016/j.bmcl.2016.10.036 BindingDB Entry DOI: 10.7270/Q2DJ5HM1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| LIM domain kinase 1 (Homo sapiens (Human)) | BDBM50124400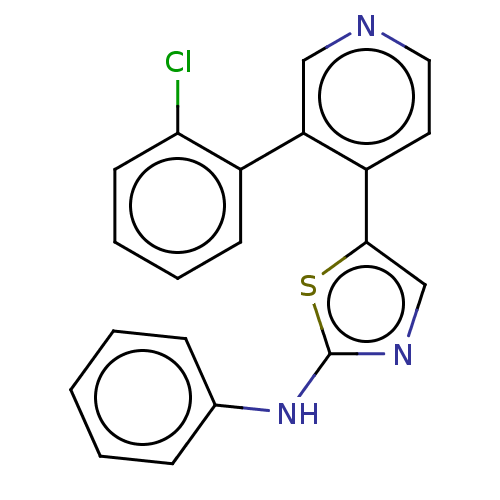 (CHEMBL3623438) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged LIMK1 (unknown origin) expressed in Sf21 cells by HTRF assay using cofilin as a substrate | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 2 (Homo sapiens (Human)) | BDBM50124355 (CHEMBL3623442) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-tagged LIMK2 | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 1 (Homo sapiens (Human)) | BDBM50124399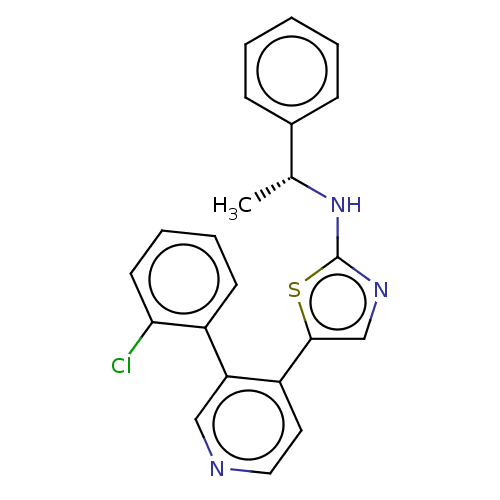 (CHEMBL3623437) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged LIMK1 (unknown origin) expressed in Sf21 cells by HTRF assay using cofilin as a substrate | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50200664 (CHEMBL3983022) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Babraham Research Campus Curated by ChEMBL | Assay Description Inhibition of human recombinant ATX using Rac-1-Palmitoyl-glycero-3-phosphocholine as substrate incubated for 2 hrs using ADHP fluorogenic peroxidase... | Bioorg Med Chem Lett 26: 5403-5410 (2016) Article DOI: 10.1016/j.bmcl.2016.10.036 BindingDB Entry DOI: 10.7270/Q2DJ5HM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50200650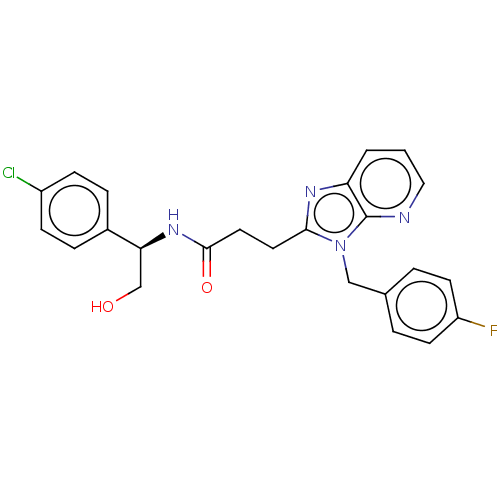 (CHEMBL3926932) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Babraham Research Campus Curated by ChEMBL | Assay Description Inhibition of human recombinant ATX using Rac-1-Palmitoyl-glycero-3-phosphocholine as substrate incubated for 2 hrs using ADHP fluorogenic peroxidase... | Bioorg Med Chem Lett 26: 5403-5410 (2016) Article DOI: 10.1016/j.bmcl.2016.10.036 BindingDB Entry DOI: 10.7270/Q2DJ5HM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 1 (Homo sapiens (Human)) | BDBM50124397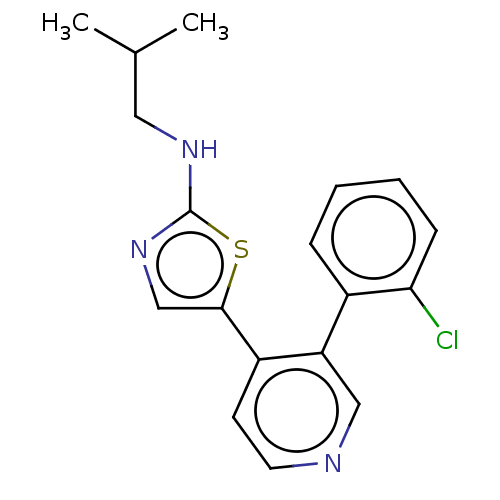 (CHEMBL3623435) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged LIMK1 (unknown origin) expressed in Sf21 cells by HTRF assay using cofilin as a substrate | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50200666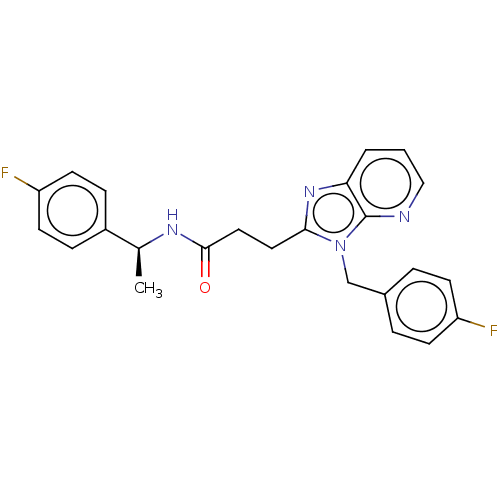 (CHEMBL3916898) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Babraham Research Campus Curated by ChEMBL | Assay Description Inhibition of human recombinant ATX using Rac-1-Palmitoyl-glycero-3-phosphocholine as substrate incubated for 2 hrs using ADHP fluorogenic peroxidase... | Bioorg Med Chem Lett 26: 5403-5410 (2016) Article DOI: 10.1016/j.bmcl.2016.10.036 BindingDB Entry DOI: 10.7270/Q2DJ5HM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396653 (CHEMBL2172122) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 1 (Homo sapiens (Human)) | BDBM50124353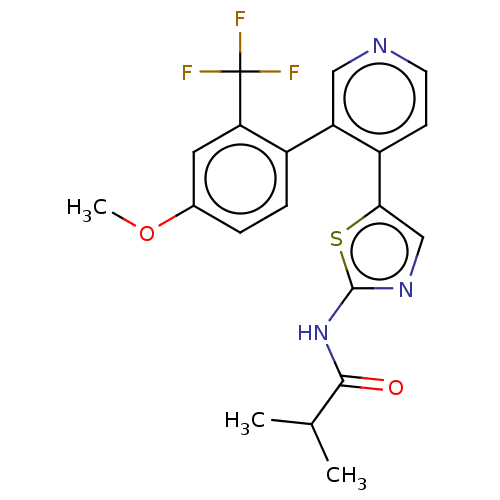 (CHEMBL3623443) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged LIMK1 (unknown origin) expressed in Sf21 cells by HTRF assay using cofilin as a substrate | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50200578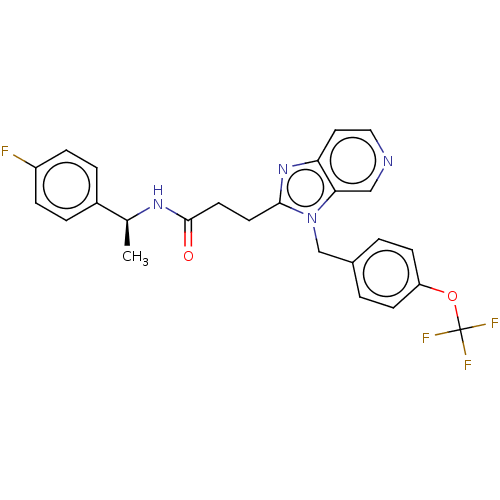 (CHEMBL3982059) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Babraham Research Campus Curated by ChEMBL | Assay Description Inhibition of human recombinant ATX using Rac-1-Palmitoyl-glycero-3-phosphocholine as substrate incubated for 2 hrs using ADHP fluorogenic peroxidase... | Bioorg Med Chem Lett 26: 5403-5410 (2016) Article DOI: 10.1016/j.bmcl.2016.10.036 BindingDB Entry DOI: 10.7270/Q2DJ5HM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396661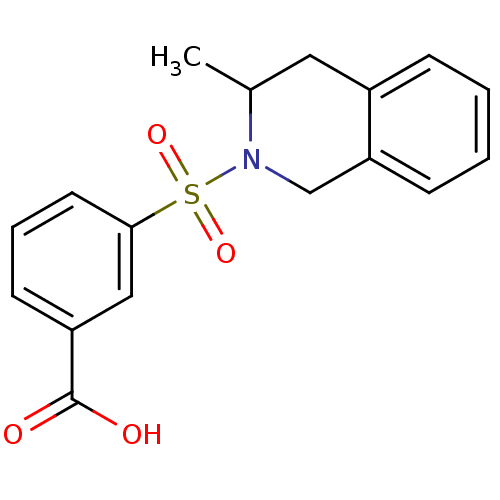 (CHEMBL2172110) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396695 (CHEMBL2172121) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396694 (CHEMBL2172112) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396693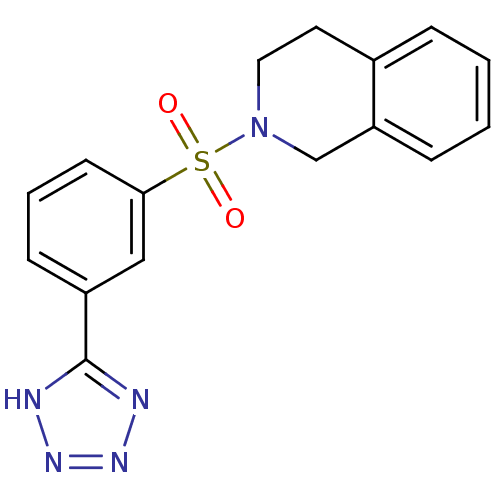 (CHEMBL2172067) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50446017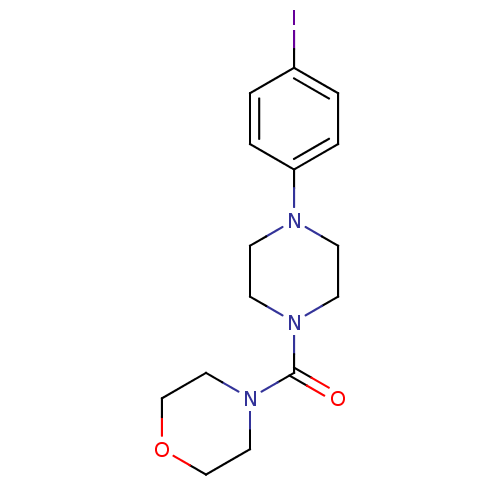 (CHEMBL3103349) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of AKR1C3 (unknown origin) expressed in human HCT116 cells assessed as formation of PR-104H from PR-104A preincubated for 2 hrs | Bioorg Med Chem 22: 967-77 (2014) Article DOI: 10.1016/j.bmc.2013.12.050 BindingDB Entry DOI: 10.7270/Q25H7HQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50200651 (CHEMBL3964183) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Babraham Research Campus Curated by ChEMBL | Assay Description Inhibition of human recombinant ATX using Rac-1-Palmitoyl-glycero-3-phosphocholine as substrate incubated for 2 hrs using ADHP fluorogenic peroxidase... | Bioorg Med Chem Lett 26: 5403-5410 (2016) Article DOI: 10.1016/j.bmcl.2016.10.036 BindingDB Entry DOI: 10.7270/Q2DJ5HM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50200651 (CHEMBL3964183) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Babraham Research Campus Curated by ChEMBL | Assay Description Inhibition of human recombinant ATX using Rac-1-Palmitoyl-glycero-3-phosphocholine as substrate incubated for 2 hrs using ADHP fluorogenic peroxidase... | Bioorg Med Chem Lett 26: 5403-5410 (2016) Article DOI: 10.1016/j.bmcl.2016.10.036 BindingDB Entry DOI: 10.7270/Q2DJ5HM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50200580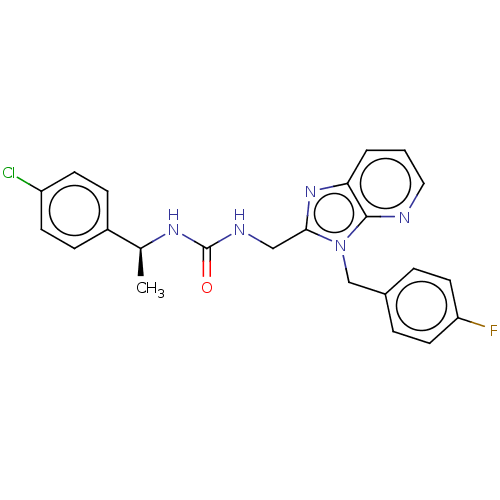 (CHEMBL3907343) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Babraham Research Campus Curated by ChEMBL | Assay Description Inhibition of human recombinant ATX using Rac-1-Palmitoyl-glycero-3-phosphocholine as substrate incubated for 2 hrs using ADHP fluorogenic peroxidase... | Bioorg Med Chem Lett 26: 5403-5410 (2016) Article DOI: 10.1016/j.bmcl.2016.10.036 BindingDB Entry DOI: 10.7270/Q2DJ5HM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50430719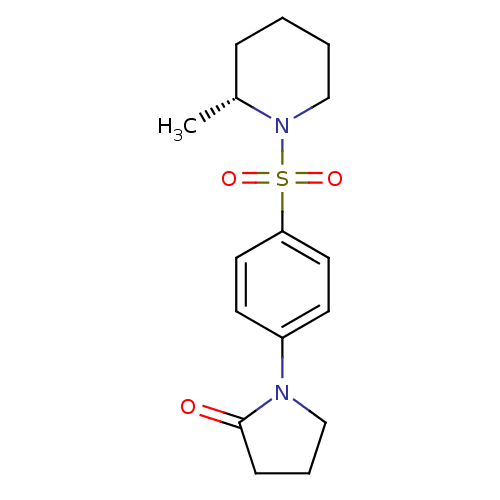 (CHEMBL2333522) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) overexpressed in human HCT116 cells assessed as inhibition of aerobic reduction of dinitrobenzamide... | Eur J Med Chem 62: 738-44 (2013) Article DOI: 10.1016/j.ejmech.2013.01.047 BindingDB Entry DOI: 10.7270/Q2H133CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396658 (CHEMBL2172114) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396659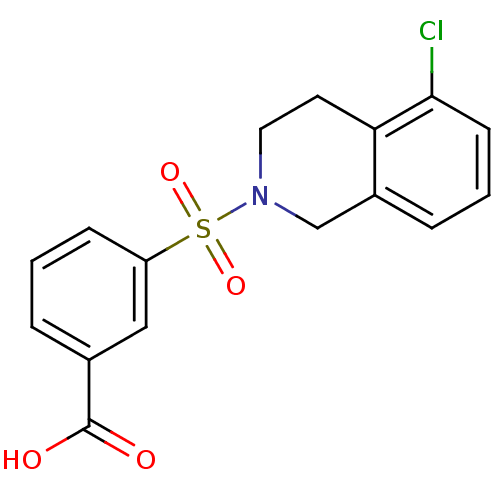 (CHEMBL2172113) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50446018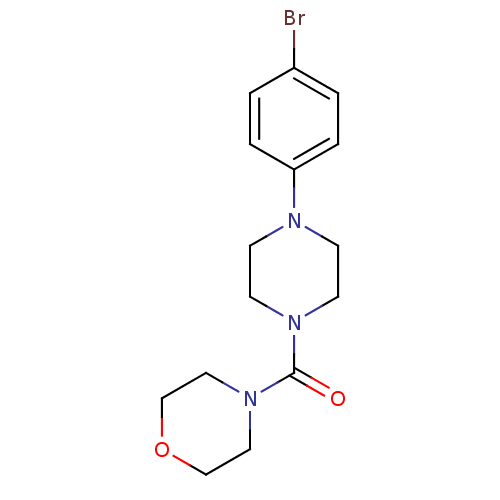 (CHEMBL3103348) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of AKR1C3 (unknown origin) expressed in human HCT116 cells assessed as formation of PR-104H from PR-104A preincubated for 2 hrs | Bioorg Med Chem 22: 967-77 (2014) Article DOI: 10.1016/j.bmc.2013.12.050 BindingDB Entry DOI: 10.7270/Q25H7HQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396653 (CHEMBL2172122) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of AKR1C3 overexpressed in human HCT116 cells assessed as inhibition of PR-104A conversion to hydroxylamine after 2 hrs by spectrophotomet... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396692 (CHEMBL2172087) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50446015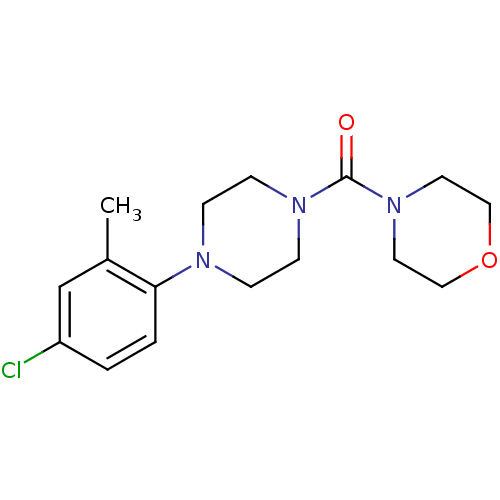 (CHEMBL3103331) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of AKR1C3 (unknown origin) expressed in human HCT116 cells assessed as formation of PR-104H from PR-104A preincubated for 2 hrs | Bioorg Med Chem 22: 967-77 (2014) Article DOI: 10.1016/j.bmc.2013.12.050 BindingDB Entry DOI: 10.7270/Q25H7HQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396704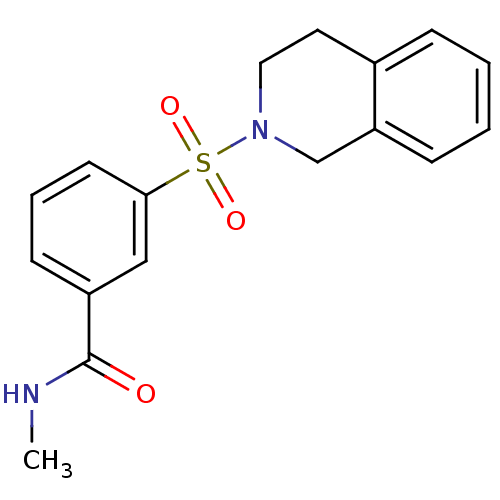 (CHEMBL2172072) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of AKR1C3 overexpressed in human HCT116 cells assessed as inhibition of PR-104A conversion to hydroxylamine after 2 hrs by spectrophotomet... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50446014 (CHEMBL3103332) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of AKR1C3 (unknown origin) expressed in human HCT116 cells assessed as formation of PR-104H from PR-104A preincubated for 2 hrs | Bioorg Med Chem 22: 967-77 (2014) Article DOI: 10.1016/j.bmc.2013.12.050 BindingDB Entry DOI: 10.7270/Q25H7HQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LIM domain kinase 1 (Homo sapiens (Human)) | BDBM50124363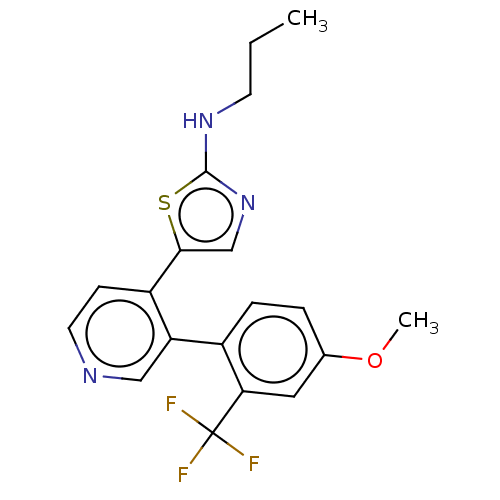 (CHEMBL3622874) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Research Technology Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal 6His-tagged LIMK1 (unknown origin) expressed in Sf21 cells by HTRF assay using cofilin as a substrate | J Med Chem 58: 8309-13 (2015) Article DOI: 10.1021/acs.jmedchem.5b01242 BindingDB Entry DOI: 10.7270/Q27P917N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396650 (CHEMBL2172220) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396676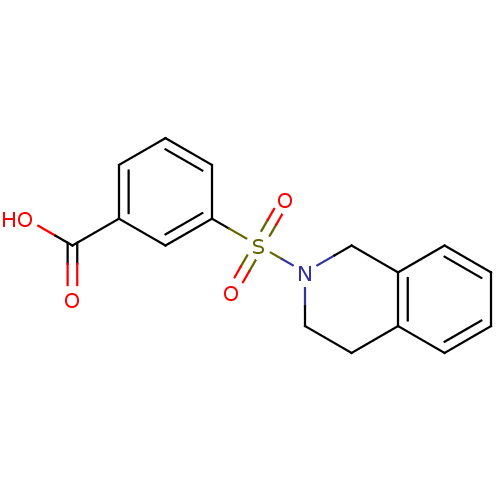 (CHEMBL1566492) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396707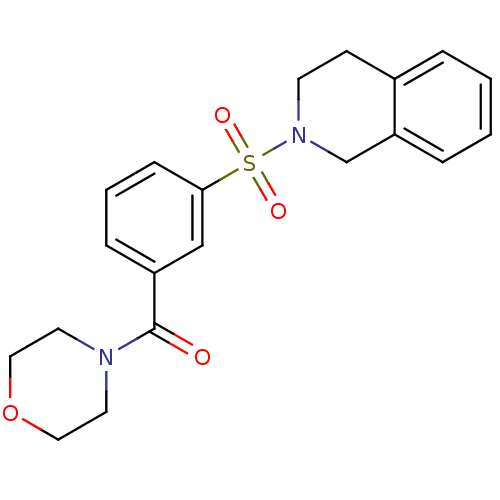 (CHEMBL2172069) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of AKR1C3 overexpressed in human HCT116 cells assessed as inhibition of PR-104A conversion to hydroxylamine after 2 hrs by spectrophotomet... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50200622 (CHEMBL3917975) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Babraham Research Campus Curated by ChEMBL | Assay Description Inhibition of ATX in human plasma assessed as decrease in hydrolysis of lysophosphatidylcholine by measuring choline release after 24 hrs by horserad... | Bioorg Med Chem Lett 26: 5403-5410 (2016) Article DOI: 10.1016/j.bmcl.2016.10.036 BindingDB Entry DOI: 10.7270/Q2DJ5HM1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396657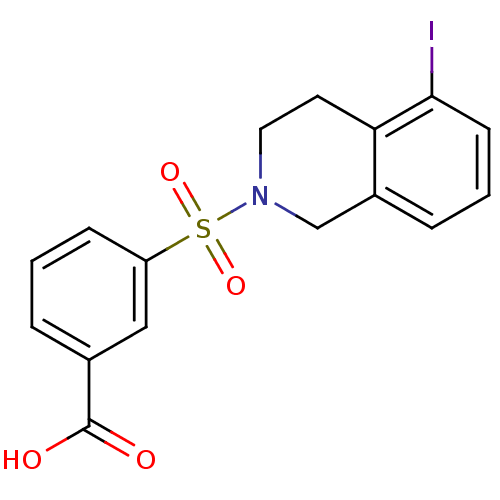 (CHEMBL2172115) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396719 (CHEMBL2172088) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 719 total ) | Next | Last >> |