 Found 658 hits with Last Name = 'yang' and Initial = 'e'
Found 658 hits with Last Name = 'yang' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM107767
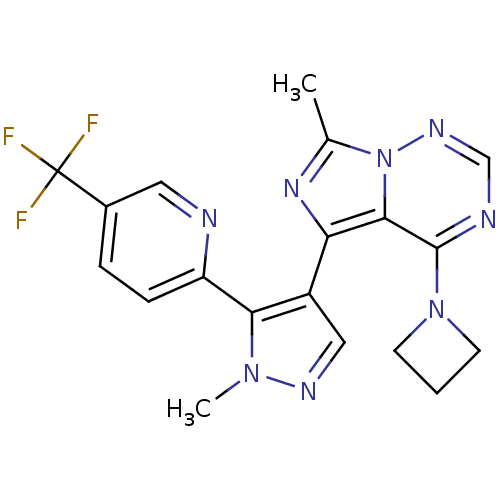
(US11419874, PF-05180999 | US8598155, 2)Show SMILES Cc1nc(-c2cnn(C)c2-c2ccc(cn2)C(F)(F)F)c2c(ncnn12)N1CCC1 Show InChI InChI=1S/C19H17F3N8/c1-11-27-15(17-18(29-6-3-7-29)24-10-26-30(11)17)13-9-25-28(2)16(13)14-5-4-12(8-23-14)19(20,21)22/h4-5,8-10H,3,6-7H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled 4-(azetidin-1-yl)-3-[5-[4-(trifluoromethyl)phenyl]-1H-pyrazol-4-yl]-1-(tritritiomethyl)pyrazolo[3,4-d]pyrimidine from PD... |
J Med Chem 61: 1001-1018 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01466
BindingDB Entry DOI: 10.7270/Q2ZS2ZZ4 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296480

(CHEMBL553249)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C102H157N25O35/c1-14-51(10)81(101(162)127-34-19-23-73(127)100(161)111-54(13)85(146)117-69(43-77(135)136)95(156)124-72(46-80(141)142)98(159)125-71(45-79(139)140)97(158)122-67(40-55-20-16-15-17-21-55)94(155)114-60(28-30-74(104)130)88(149)119-65(38-49(6)7)91(152)112-59(82(105)143)22-18-33-108-102(106)107)126-99(160)68(41-56-24-26-57(129)27-25-56)121-89(150)62(32-35-128)113-83(144)52(11)110-90(151)63(36-47(2)3)116-84(145)53(12)109-87(148)61(29-31-75(131)132)115-92(153)66(39-50(8)9)120-96(157)70(44-78(137)138)123-93(154)64(37-48(4)5)118-86(147)58(103)42-76(133)134/h15-17,20-21,24-27,47-54,58-73,81,128-129H,14,18-19,22-23,28-46,103H2,1-13H3,(H2,104,130)(H2,105,143)(H,109,148)(H,110,151)(H,111,161)(H,112,152)(H,113,144)(H,114,155)(H,115,153)(H,116,145)(H,117,146)(H,118,147)(H,119,149)(H,120,157)(H,121,150)(H,122,158)(H,123,154)(H,124,156)(H,125,159)(H,126,160)(H,131,132)(H,133,134)(H,135,136)(H,137,138)(H,139,140)(H,141,142)(H4,106,107,108)/t51-,52+,53+,54+,58+,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,81+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296473

(CHEMBL558940)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6@@H](-[#8])-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C103H157N25O35/c1-14-51(10)82(102(163)127-33-19-23-73(127)99(160)111-53(12)85(146)117-69(42-78(136)137)94(155)123-72(45-81(142)143)97(158)124-71(44-80(140)141)96(157)121-67(38-55-20-16-15-17-21-55)93(154)114-61(28-30-75(105)131)88(149)119-65(36-49(6)7)90(151)113-60(83(106)144)22-18-32-109-103(107)108)126-98(159)68(39-56-24-26-57(129)27-25-56)125-100(161)74-40-58(130)46-128(74)101(162)54(13)112-89(150)63(34-47(2)3)116-84(145)52(11)110-87(148)62(29-31-76(132)133)115-91(152)66(37-50(8)9)120-95(156)70(43-79(138)139)122-92(153)64(35-48(4)5)118-86(147)59(104)41-77(134)135/h15-17,20-21,24-27,47-54,58-74,82,129-130H,14,18-19,22-23,28-46,104H2,1-13H3,(H2,105,131)(H2,106,144)(H,110,148)(H,111,160)(H,112,150)(H,113,151)(H,114,154)(H,115,152)(H,116,145)(H,117,146)(H,118,147)(H,119,149)(H,120,156)(H,121,157)(H,122,153)(H,123,155)(H,124,158)(H,125,161)(H,126,159)(H,132,133)(H,134,135)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H4,107,108,109)/t51-,52+,53+,54+,58-,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,74+,82+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50018962
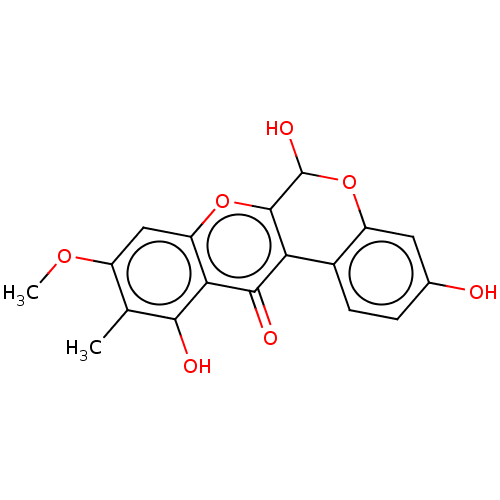
(CHEMBL3287409)Show SMILES COc1cc2oc3C(O)Oc4cc(O)ccc4-c3c(=O)c2c(O)c1C Show InChI InChI=1S/C18H14O7/c1-7-10(23-2)6-12-14(15(7)20)16(21)13-9-4-3-8(19)5-11(9)25-18(22)17(13)24-12/h3-6,18-20,22H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of BACE1 (unknown origin) by Dixon plot analysis |
Bioorg Med Chem Lett 24: 2945-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.060
BindingDB Entry DOI: 10.7270/Q2NV9KSW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50018959
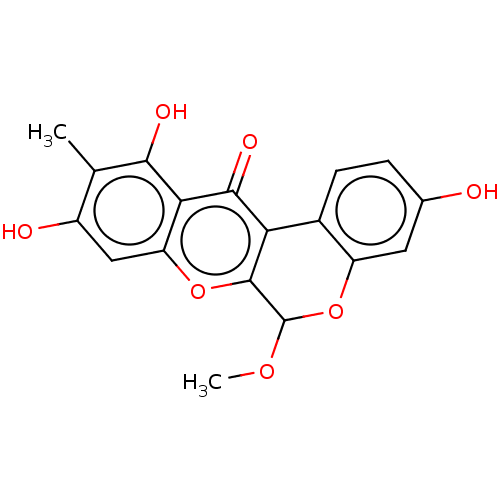
(Boeravinone D)Show SMILES COC1Oc2cc(O)ccc2-c2c1oc1cc(O)c(C)c(O)c1c2=O Show InChI InChI=1S/C18H14O7/c1-7-10(20)6-12-14(15(7)21)16(22)13-9-4-3-8(19)5-11(9)25-18(23-2)17(13)24-12/h3-6,18-21H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of BACE1 (unknown origin) by Dixon plot analysis |
Bioorg Med Chem Lett 24: 2945-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.060
BindingDB Entry DOI: 10.7270/Q2NV9KSW |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50241052

(1,2,3,4,6-Pgg | 1,2,3,4,6-pentakis-O-(3,4,5-trihyd...)Show SMILES Oc1cc(cc(O)c1O)C(=O)OC[C@H]1O[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@@H]1OC(=O)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C41H32O26/c42-17-1-12(2-18(43)28(17)52)36(57)62-11-27-33(64-37(58)13-3-19(44)29(53)20(45)4-13)34(65-38(59)14-5-21(46)30(54)22(47)6-14)35(66-39(60)15-7-23(48)31(55)24(49)8-15)41(63-27)67-40(61)16-9-25(50)32(56)26(51)10-16/h1-10,27,33-35,41-56H,11H2/t27-,33-,34+,35-,41+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) |
Bioorg Med Chem Lett 24: 2945-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.060
BindingDB Entry DOI: 10.7270/Q2NV9KSW |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296481

(CHEMBL562618)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6@@H](F)-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C103H156FN25O34/c1-14-51(10)82(102(163)128-33-19-23-73(128)99(160)112-53(12)85(146)118-69(42-78(136)137)94(155)124-72(45-81(142)143)97(158)125-71(44-80(140)141)96(157)122-67(38-55-20-16-15-17-21-55)93(154)115-61(28-30-75(106)131)88(149)120-65(36-49(6)7)90(151)114-60(83(107)144)22-18-32-110-103(108)109)127-98(159)68(39-56-24-26-58(130)27-25-56)126-100(161)74-40-57(104)46-129(74)101(162)54(13)113-89(150)63(34-47(2)3)117-84(145)52(11)111-87(148)62(29-31-76(132)133)116-91(152)66(37-50(8)9)121-95(156)70(43-79(138)139)123-92(153)64(35-48(4)5)119-86(147)59(105)41-77(134)135/h15-17,20-21,24-27,47-54,57,59-74,82,130H,14,18-19,22-23,28-46,105H2,1-13H3,(H2,106,131)(H2,107,144)(H,111,148)(H,112,160)(H,113,150)(H,114,151)(H,115,154)(H,116,152)(H,117,145)(H,118,146)(H,119,147)(H,120,149)(H,121,156)(H,122,157)(H,123,153)(H,124,155)(H,125,158)(H,126,161)(H,127,159)(H,132,133)(H,134,135)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H4,108,109,110)/t51-,52+,53+,54+,57-,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,74+,82+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50250989

(CHEMBL450745 | eugeniin)Show SMILES Oc1cc(cc(O)c1O)C(=O)O[C@@H]1O[C@@H]2COC(=O)c3cc(O)c(O)c(O)c3-c3c(O)c(O)c(O)cc3C(=O)O[C@H]2[C@H](OC(=O)c2cc(O)c(O)c(O)c2)[C@H]1OC(=O)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C41H30O26/c42-15-1-10(2-16(43)26(15)50)36(57)65-34-33-23(9-62-39(60)13-7-21(48)29(53)31(55)24(13)25-14(40(61)64-33)8-22(49)30(54)32(25)56)63-41(67-38(59)12-5-19(46)28(52)20(47)6-12)35(34)66-37(58)11-3-17(44)27(51)18(45)4-11/h1-8,23,33-35,41-56H,9H2/t23-,33-,34+,35-,41+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 (unknown origin) |
Bioorg Med Chem Lett 24: 2945-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.060
BindingDB Entry DOI: 10.7270/Q2NV9KSW |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296477

(CHEMBL563460)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C102H156N26O35/c1-14-50(10)81(101(163)128-33-19-23-72(128)100(162)112-53(13)85(147)118-68(42-77(136)137)95(157)125-71(45-80(142)143)98(160)126-70(44-79(140)141)97(159)122-65(38-54-20-16-15-17-21-54)93(155)114-59(28-30-73(104)130)88(150)120-63(36-48(6)7)90(152)113-58(82(106)144)22-18-32-109-102(107)108)127-99(161)66(39-55-24-26-56(129)27-25-55)123-94(156)67(41-74(105)131)117-84(146)52(12)111-89(151)61(34-46(2)3)116-83(145)51(11)110-87(149)60(29-31-75(132)133)115-91(153)64(37-49(8)9)121-96(158)69(43-78(138)139)124-92(154)62(35-47(4)5)119-86(148)57(103)40-76(134)135/h15-17,20-21,24-27,46-53,57-72,81,129H,14,18-19,22-23,28-45,103H2,1-13H3,(H2,104,130)(H2,105,131)(H2,106,144)(H,110,149)(H,111,151)(H,112,162)(H,113,152)(H,114,155)(H,115,153)(H,116,145)(H,117,146)(H,118,147)(H,119,148)(H,120,150)(H,121,158)(H,122,159)(H,123,156)(H,124,154)(H,125,157)(H,126,160)(H,127,161)(H,132,133)(H,134,135)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H4,107,108,109)/t50-,51+,52+,53+,57+,58+,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,81+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296478

(CHEMBL557433)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C102H155N25O36/c1-14-50(10)81(101(163)127-33-19-23-72(127)100(162)111-53(13)85(147)117-68(42-77(136)137)95(157)124-71(45-80(142)143)98(160)125-70(44-79(140)141)97(159)121-65(38-54-20-16-15-17-21-54)93(155)113-59(28-30-73(104)129)88(150)119-63(36-48(6)7)90(152)112-58(82(105)144)22-18-32-108-102(106)107)126-99(161)66(39-55-24-26-56(128)27-25-55)122-94(156)67(41-76(134)135)116-84(146)52(12)110-89(151)61(34-46(2)3)115-83(145)51(11)109-87(149)60(29-31-74(130)131)114-91(153)64(37-49(8)9)120-96(158)69(43-78(138)139)123-92(154)62(35-47(4)5)118-86(148)57(103)40-75(132)133/h15-17,20-21,24-27,46-53,57-72,81,128H,14,18-19,22-23,28-45,103H2,1-13H3,(H2,104,129)(H2,105,144)(H,109,149)(H,110,151)(H,111,162)(H,112,152)(H,113,155)(H,114,153)(H,115,145)(H,116,146)(H,117,147)(H,118,148)(H,119,150)(H,120,158)(H,121,159)(H,122,156)(H,123,154)(H,124,157)(H,125,160)(H,126,161)(H,130,131)(H,132,133)(H,134,135)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H4,106,107,108)/t50-,51+,52+,53+,57+,58+,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,81+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296476
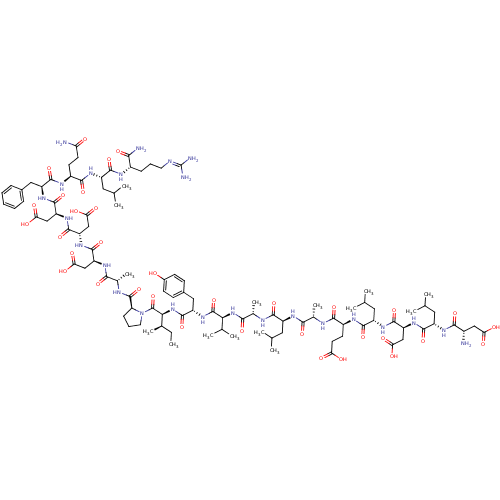
(CHEMBL557420)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C103H159N25O34/c1-16-52(12)82(102(162)128-35-21-25-73(128)100(160)112-54(14)85(145)117-69(43-77(135)136)95(155)123-72(46-80(141)142)98(158)124-71(45-79(139)140)97(157)121-67(40-56-22-18-17-19-23-56)94(154)114-61(30-32-74(105)130)89(149)119-65(38-49(6)7)91(151)113-60(83(106)143)24-20-34-109-103(107)108)127-99(159)68(41-57-26-28-58(129)29-27-57)125-101(161)81(51(10)11)126-86(146)55(15)111-90(150)63(36-47(2)3)116-84(144)53(13)110-88(148)62(31-33-75(131)132)115-92(152)66(39-50(8)9)120-96(156)70(44-78(137)138)122-93(153)64(37-48(4)5)118-87(147)59(104)42-76(133)134/h17-19,22-23,26-29,47-55,59-73,81-82,129H,16,20-21,24-25,30-46,104H2,1-15H3,(H2,105,130)(H2,106,143)(H,110,148)(H,111,150)(H,112,160)(H,113,151)(H,114,154)(H,115,152)(H,116,144)(H,117,145)(H,118,147)(H,119,149)(H,120,156)(H,121,157)(H,122,153)(H,123,155)(H,124,158)(H,125,161)(H,126,146)(H,127,159)(H,131,132)(H,133,134)(H,135,136)(H,137,138)(H,139,140)(H,141,142)(H4,107,108,109)/t52-,53+,54+,55+,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,81+,82+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296479
(CHEMBL562806)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6@H](-[#6])-[#8])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C102H157N25O35/c1-15-50(10)80(101(162)127-34-20-24-72(127)99(160)111-52(12)84(145)116-68(42-76(135)136)94(155)122-71(45-79(141)142)97(158)123-70(44-78(139)140)96(157)120-66(39-55-21-17-16-18-22-55)93(154)113-60(29-31-73(104)130)88(149)118-64(37-48(6)7)90(151)112-59(82(105)143)23-19-33-108-102(106)107)125-98(159)67(40-56-25-27-57(129)28-26-56)124-100(161)81(54(14)128)126-85(146)53(13)110-89(150)62(35-46(2)3)115-83(144)51(11)109-87(148)61(30-32-74(131)132)114-91(152)65(38-49(8)9)119-95(156)69(43-77(137)138)121-92(153)63(36-47(4)5)117-86(147)58(103)41-75(133)134/h16-18,21-22,25-28,46-54,58-72,80-81,128-129H,15,19-20,23-24,29-45,103H2,1-14H3,(H2,104,130)(H2,105,143)(H,109,148)(H,110,150)(H,111,160)(H,112,151)(H,113,154)(H,114,152)(H,115,144)(H,116,145)(H,117,147)(H,118,149)(H,119,156)(H,120,157)(H,121,153)(H,122,155)(H,123,158)(H,124,161)(H,125,159)(H,126,146)(H,131,132)(H,133,134)(H,135,136)(H,137,138)(H,139,140)(H,141,142)(H4,106,107,108)/t50-,51+,52+,53+,54+,58+,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,80+,81+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296475
(CHEMBL557344)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6@H](-[#6])-[#6]-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C104H161N25O34/c1-16-52(11)82(127-87(147)56(15)112-91(151)64(37-48(3)4)117-85(145)54(13)111-89(149)63(32-34-76(132)133)116-93(153)67(40-51(9)10)121-97(157)71(45-79(138)139)123-94(154)65(38-49(5)6)119-88(148)60(105)43-77(134)135)102(162)126-69(42-58-27-29-59(130)30-28-58)100(160)128-83(53(12)17-2)103(163)129-36-22-26-74(129)101(161)113-55(14)86(146)118-70(44-78(136)137)96(156)124-73(47-81(142)143)99(159)125-72(46-80(140)141)98(158)122-68(41-57-23-19-18-20-24-57)95(155)115-62(31-33-75(106)131)90(150)120-66(39-50(7)8)92(152)114-61(84(107)144)25-21-35-110-104(108)109/h18-20,23-24,27-30,48-56,60-74,82-83,130H,16-17,21-22,25-26,31-47,105H2,1-15H3,(H2,106,131)(H2,107,144)(H,111,149)(H,112,151)(H,113,161)(H,114,152)(H,115,155)(H,116,153)(H,117,145)(H,118,146)(H,119,148)(H,120,150)(H,121,157)(H,122,158)(H,123,154)(H,124,156)(H,125,159)(H,126,162)(H,127,147)(H,128,160)(H,132,133)(H,134,135)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H4,108,109,110)/t52-,53-,54+,55+,56+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,74+,82+,83+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296474

(CHEMBL558009)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C104H161N25O34/c1-16-53(12)83(103(163)129-35-21-25-75(129)102(162)113-56(15)87(147)119-71(44-79(136)137)97(157)126-74(47-82(142)143)100(160)127-73(46-81(140)141)99(159)123-69(41-57-22-18-17-19-23-57)96(156)115-62(30-32-76(106)131)90(150)121-67(39-51(8)9)92(152)114-61(84(107)144)24-20-34-110-104(108)109)128-101(161)70(42-58-26-28-59(130)29-27-58)124-94(154)65(37-49(4)5)118-86(146)55(14)112-91(151)64(36-48(2)3)117-85(145)54(13)111-89(149)63(31-33-77(132)133)116-93(153)68(40-52(10)11)122-98(158)72(45-80(138)139)125-95(155)66(38-50(6)7)120-88(148)60(105)43-78(134)135/h17-19,22-23,26-29,48-56,60-75,83,130H,16,20-21,24-25,30-47,105H2,1-15H3,(H2,106,131)(H2,107,144)(H,111,149)(H,112,151)(H,113,162)(H,114,152)(H,115,156)(H,116,153)(H,117,145)(H,118,146)(H,119,147)(H,120,148)(H,121,150)(H,122,158)(H,123,159)(H,124,154)(H,125,155)(H,126,157)(H,127,160)(H,128,161)(H,132,133)(H,134,135)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H4,108,109,110)/t53-,54+,55+,56+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,74+,75+,83+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296482

(CHEMBL541769)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C104H159N25O34/c1-14-53(10)83(103(163)129-37-21-26-75(129)100(160)112-55(12)86(146)118-70(45-79(136)137)95(155)124-73(48-82(142)143)98(158)125-72(47-81(140)141)97(157)122-68(42-57-22-16-15-17-23-57)94(154)115-62(31-33-76(106)131)89(149)120-66(40-51(6)7)91(151)114-61(84(107)144)24-20-35-110-104(108)109)127-99(159)69(43-58-27-29-59(130)30-28-58)126-101(161)74-25-18-19-36-128(74)102(162)56(13)113-90(150)64(38-49(2)3)117-85(145)54(11)111-88(148)63(32-34-77(132)133)116-92(152)67(41-52(8)9)121-96(156)71(46-80(138)139)123-93(153)65(39-50(4)5)119-87(147)60(105)44-78(134)135/h15-17,22-23,27-30,49-56,60-75,83,130H,14,18-21,24-26,31-48,105H2,1-13H3,(H2,106,131)(H2,107,144)(H,111,148)(H,112,160)(H,113,150)(H,114,151)(H,115,154)(H,116,152)(H,117,145)(H,118,146)(H,119,147)(H,120,149)(H,121,156)(H,122,157)(H,123,153)(H,124,155)(H,125,158)(H,126,161)(H,127,159)(H,132,133)(H,134,135)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H4,108,109,110)/t53-,54+,55+,56+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,74+,75+,83+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296483
(CHEMBL538372)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C102H158N26O34/c1-14-51(10)81(101(162)128-35-19-23-73(128)100(161)112-54(13)85(146)118-69(43-77(135)136)95(156)125-72(46-80(141)142)98(159)126-71(45-79(139)140)97(158)123-67(40-55-20-16-15-17-21-55)94(155)115-60(28-30-74(105)130)88(149)120-65(38-49(6)7)91(152)113-59(82(106)143)22-18-34-109-102(107)108)127-99(160)68(41-56-24-26-57(129)27-25-56)122-89(150)62(32-33-103)114-83(144)52(11)111-90(151)63(36-47(2)3)117-84(145)53(12)110-87(148)61(29-31-75(131)132)116-92(153)66(39-50(8)9)121-96(157)70(44-78(137)138)124-93(154)64(37-48(4)5)119-86(147)58(104)42-76(133)134/h15-17,20-21,24-27,47-54,58-73,81,129H,14,18-19,22-23,28-46,103-104H2,1-13H3,(H2,105,130)(H2,106,143)(H,110,148)(H,111,151)(H,112,161)(H,113,152)(H,114,144)(H,115,155)(H,116,153)(H,117,145)(H,118,146)(H,119,147)(H,120,149)(H,121,157)(H,122,150)(H,123,158)(H,124,154)(H,125,156)(H,126,159)(H,127,160)(H,131,132)(H,133,134)(H,135,136)(H,137,138)(H,139,140)(H,141,142)(H4,107,108,109)/t51-,52+,53+,54+,58+,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,81+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50296472
(CHEMBL558824)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C103H157N25O34/c1-14-52(10)82(102(162)128-36-20-24-73(128)99(159)111-54(12)85(145)117-69(44-78(135)136)94(154)123-72(47-81(141)142)97(157)124-71(46-80(139)140)96(156)121-67(41-56-21-16-15-17-22-56)93(153)114-61(30-32-75(105)130)88(148)119-65(39-50(6)7)90(150)113-60(83(106)143)23-18-34-109-103(107)108)126-98(158)68(42-57-26-28-58(129)29-27-57)125-100(160)74-25-19-35-127(74)101(161)55(13)112-89(149)63(37-48(2)3)116-84(144)53(11)110-87(147)62(31-33-76(131)132)115-91(151)66(40-51(8)9)120-95(155)70(45-79(137)138)122-92(152)64(38-49(4)5)118-86(146)59(104)43-77(133)134/h15-17,21-22,26-29,48-55,59-74,82,129H,14,18-20,23-25,30-47,104H2,1-13H3,(H2,105,130)(H2,106,143)(H,110,147)(H,111,159)(H,112,149)(H,113,150)(H,114,153)(H,115,151)(H,116,144)(H,117,145)(H,118,146)(H,119,148)(H,120,155)(H,121,156)(H,122,152)(H,123,154)(H,124,157)(H,125,160)(H,126,158)(H,131,132)(H,133,134)(H,135,136)(H,137,138)(H,139,140)(H,141,142)(H4,107,108,109)/t52-,53+,54+,55+,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,74+,82+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... |
Bioorg Med Chem Lett 19: 4403-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.083
BindingDB Entry DOI: 10.7270/Q2DJ5FP3 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50088373
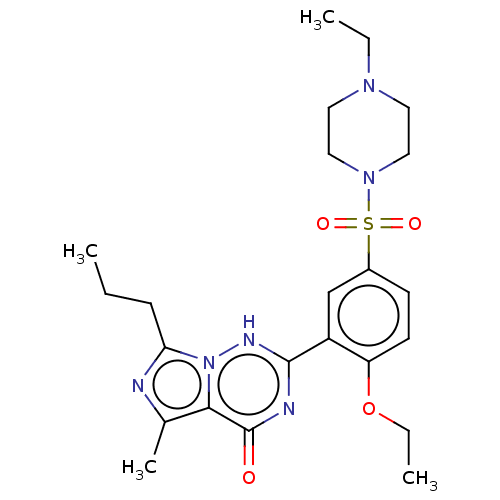
(CHEBI:46295 | Vardenafil | cid_110634)Show SMILES CCCc1nc(C)c2n1[nH]c(nc2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C23H32N6O4S/c1-5-8-20-24-16(4)21-23(30)25-22(26-29(20)21)18-15-17(9-10-19(18)33-7-3)34(31,32)28-13-11-27(6-2)12-14-28/h9-10,15H,5-8,11-14H2,1-4H3,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5A1 (unknwon origin) using [3H]cGMP as substrate after 30 mins by SPA |
J Med Chem 61: 1001-1018 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01466
BindingDB Entry DOI: 10.7270/Q2ZS2ZZ4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM107776
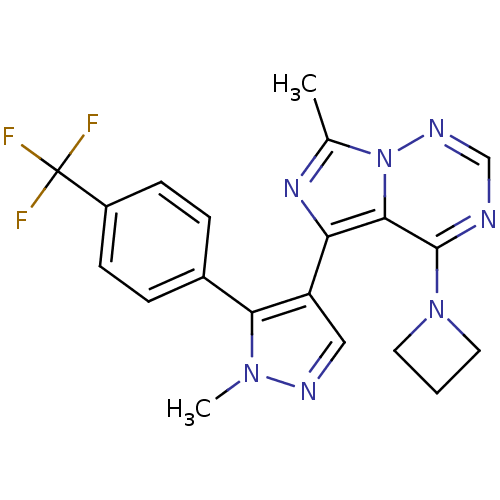
(US8598155, 13)Show SMILES Cc1nc(-c2cnn(C)c2-c2ccc(cc2)C(F)(F)F)c2c(ncnn12)N1CCC1 Show InChI InChI=1S/C20H18F3N7/c1-12-27-16(18-19(29-8-3-9-29)24-11-26-30(12)18)15-10-25-28(2)17(15)13-4-6-14(7-5-13)20(21,22)23/h4-7,10-11H,3,8-9H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length human N-terminal FLAG-tagged PDE2A3 expressed in sf21 cells using [3H]cGMP as substrate after 30 mins by SPA |
J Med Chem 61: 1001-1018 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01466
BindingDB Entry DOI: 10.7270/Q2ZS2ZZ4 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM107776

(US8598155, 13)Show SMILES Cc1nc(-c2cnn(C)c2-c2ccc(cc2)C(F)(F)F)c2c(ncnn12)N1CCC1 Show InChI InChI=1S/C20H18F3N7/c1-12-27-16(18-19(29-8-3-9-29)24-11-26-30(12)18)15-10-25-28(2)17(15)13-4-6-14(7-5-13)20(21,22)23/h4-7,10-11H,3,8-9H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.303 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
The activity of the test substances on human full-length PDE2A3 enzyme was determined using the [3H]-cGMP scintillation proximity assay (SPA) modifie... |
US Patent US8598155 (2013)
BindingDB Entry DOI: 10.7270/Q26T0K8C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50453639
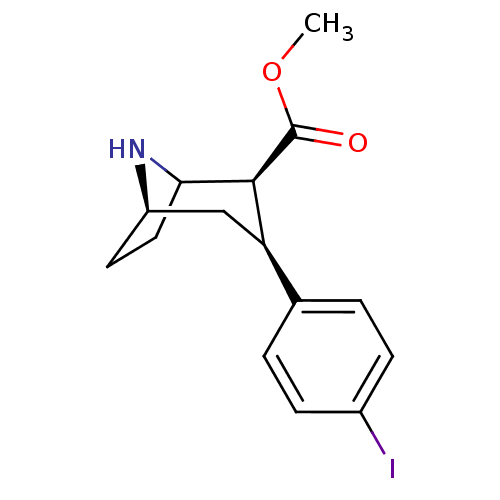
(CHEMBL2112889)Show SMILES [H][C@]12CCC(N1)[C@H]([C@H](C2)c1ccc(I)cc1)C(=O)OC |TLB:9:7:5:3.2,THB:16:6:5:3.2| Show InChI InChI=1S/C15H18INO2/c1-19-15(18)14-12(8-11-6-7-13(14)17-11)9-2-4-10(16)5-3-9/h2-5,11-14,17H,6-8H2,1H3/t11-,12-,13?,14+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Inhibition of [3H]paroxetine binding to 5-hydroxytryptamine (5-HT) transporter |
J Med Chem 37: 1220-3 (1994)
BindingDB Entry DOI: 10.7270/Q2Z60PQ2 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50263791
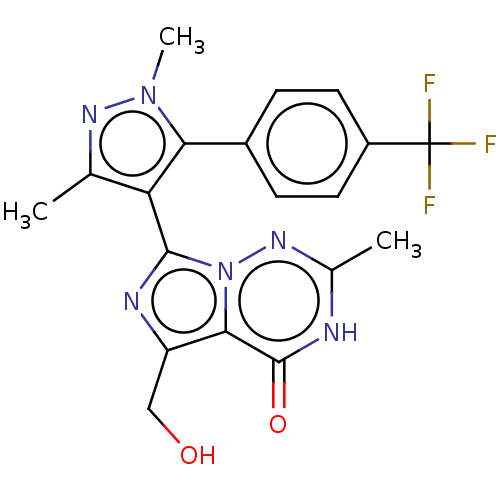
(CHEMBL4062397)Show SMILES Cc1nn(C)c(c1-c1nc(CO)c2n1nc(C)[nH]c2=O)-c1ccc(cc1)C(F)(F)F |(22.28,-12.54,;21.03,-13.44,;21.02,-14.98,;19.55,-15.45,;19.07,-16.91,;18.65,-14.2,;19.56,-12.96,;19.08,-11.49,;19.99,-10.25,;19.09,-9,;19.57,-7.53,;21.07,-7.21,;17.62,-9.47,;17.62,-11.01,;16.28,-11.78,;14.95,-11.01,;13.61,-11.77,;14.96,-9.46,;16.29,-8.7,;16.3,-7.15,;17.11,-14.2,;16.33,-15.53,;14.8,-15.53,;14.02,-14.19,;14.8,-12.86,;16.34,-12.87,;12.47,-14.18,;12.47,-15.72,;11.14,-14.94,;11.72,-12.85,)| Show InChI InChI=1S/C19H17F3N6O2/c1-9-14(17-24-13(8-29)16-18(30)23-10(2)26-28(16)17)15(27(3)25-9)11-4-6-12(7-5-11)19(20,21)22/h4-7,29H,8H2,1-3H3,(H,23,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PDE2A1 using 3',5'-[3H]cGMP as substrate measured after 30 mins by Yttrium silicate scintillation proximity assay |
ACS Med Chem Lett 9: 68-72 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00343
BindingDB Entry DOI: 10.7270/Q2MG7S23 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50006784
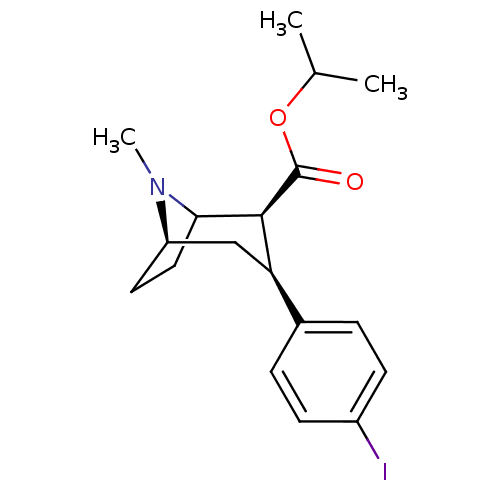
(3-(4-Iodo-phenyl)-8-methyl-8-aza-bicyclo[3.2.1]oct...)Show SMILES CC(C)OC(=O)[C@@H]1C2CC[C@H](C[C@@H]1c1ccc(I)cc1)N2C |TLB:21:20:6.12.11:8.9,THB:4:6:20:8.9,13:12:20:8.9| Show InChI InChI=1S/C18H24INO2/c1-11(2)22-18(21)17-15(12-4-6-13(19)7-5-12)10-14-8-9-16(17)20(14)3/h4-7,11,14-17H,8-10H2,1-3H3/t14-,15-,16?,17+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Inhibition of [3H]WIN-35428 binding to dopamine (DA) transporter |
J Med Chem 37: 1220-3 (1994)
BindingDB Entry DOI: 10.7270/Q2Z60PQ2 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50166893
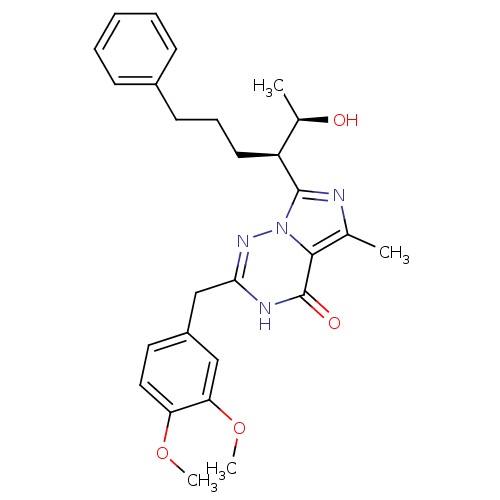
(2-(3,4-Dimethoxy-benzyl)-7-[(R)-1-((R)-1-hydroxy-e...)Show SMILES COc1ccc(Cc2nn3c(nc(C)c3c(=O)[nH]2)[C@@H](CCCc2ccccc2)[C@@H](C)O)cc1OC Show InChI InChI=1S/C27H32N4O4/c1-17-25-27(33)29-24(16-20-13-14-22(34-3)23(15-20)35-4)30-31(25)26(28-17)21(18(2)32)12-8-11-19-9-6-5-7-10-19/h5-7,9-10,13-15,18,21,32H,8,11-12,16H2,1-4H3,(H,29,30,33)/t18-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human FLAG-tagged PDE2A3 expressed in sf21 cells using [3H]cGMP as substrate by scintillation proximity assay |
J Med Chem 60: 5673-5698 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00397
BindingDB Entry DOI: 10.7270/Q2VX0JNT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50333656
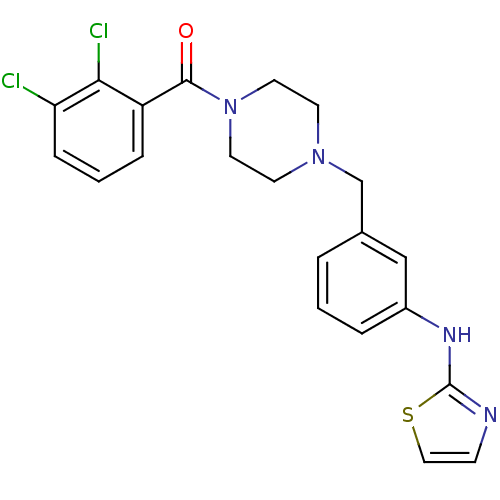
((2,3-dichlorophenyl)(4-(3-(thiazol-2-ylamino)benzy...)Show SMILES Clc1cccc(C(=O)N2CCN(Cc3cccc(Nc4nccs4)c3)CC2)c1Cl Show InChI InChI=1S/C21H20Cl2N4OS/c22-18-6-2-5-17(19(18)23)20(28)27-10-8-26(9-11-27)14-15-3-1-4-16(13-15)25-21-24-7-12-29-21/h1-7,12-13H,8-11,14H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged Aurora A kinase expressed in insect cells |
Bioorg Med Chem 19: 907-16 (2011)
Article DOI: 10.1016/j.bmc.2010.11.064
BindingDB Entry DOI: 10.7270/Q2V40VGC |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM107778
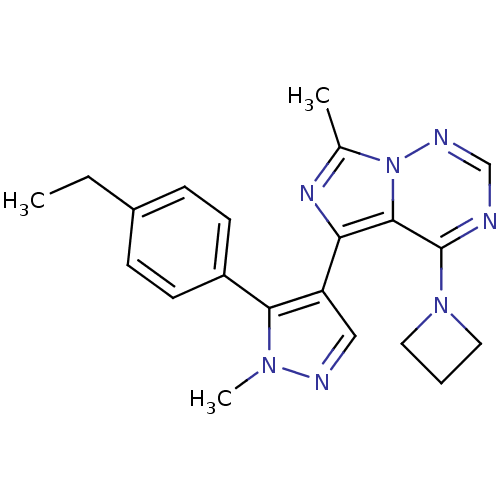
(US8598155, 15)Show SMILES CCc1ccc(cc1)-c1c(cnn1C)-c1nc(C)n2ncnc(N3CCC3)c12 Show InChI InChI=1S/C21H23N7/c1-4-15-6-8-16(9-7-15)19-17(12-23-26(19)3)18-20-21(27-10-5-11-27)22-13-24-28(20)14(2)25-18/h6-9,12-13H,4-5,10-11H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.517 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
The activity of the test substances on human full-length PDE2A3 enzyme was determined using the [3H]-cGMP scintillation proximity assay (SPA) modifie... |
US Patent US8598155 (2013)
BindingDB Entry DOI: 10.7270/Q26T0K8C |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM107849
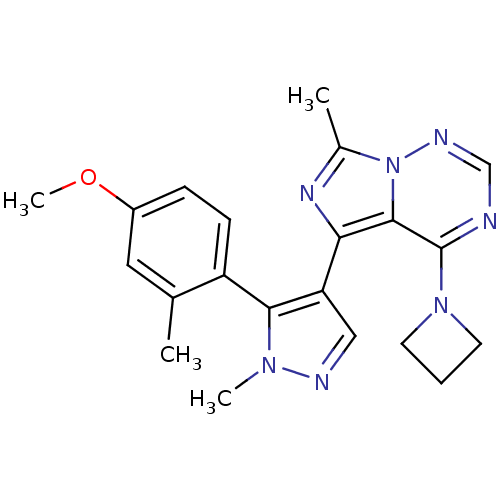
(US8598155, 8)Show SMILES COc1ccc(-c2c(cnn2C)-c2nc(C)n3ncnc(N4CCC4)c23)c(C)c1 |(6.83,-.36,;5.18,-1.42,;3.85,-.65,;2.52,-1.42,;1.18,-.65,;1.18,.89,;-.15,1.66,;-1.62,1.19,;-2.52,2.43,;-1.62,3.68,;-.15,3.2,;1.18,3.97,;-2.39,-.15,;-1.48,-1.39,;-2.39,-2.64,;-1.62,-3.97,;-3.85,-2.16,;-5.18,-2.93,;-6.52,-2.16,;-6.52,-.62,;-5.18,.15,;-5.18,1.69,;-6.52,2.46,;-5.75,3.79,;-4.41,3.02,;-3.85,-.62,;2.52,1.66,;2.52,3.2,;3.85,.89,)| Show InChI InChI=1S/C21H23N7O/c1-13-10-15(29-4)6-7-16(13)19-17(11-23-26(19)3)18-20-21(27-8-5-9-27)22-12-24-28(20)14(2)25-18/h6-7,10-12H,5,8-9H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.578 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
The activity of the test substances on human full-length PDE2A3 enzyme was determined using the [3H]-cGMP scintillation proximity assay (SPA) modifie... |
US Patent US8598155 (2013)
BindingDB Entry DOI: 10.7270/Q26T0K8C |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM14192
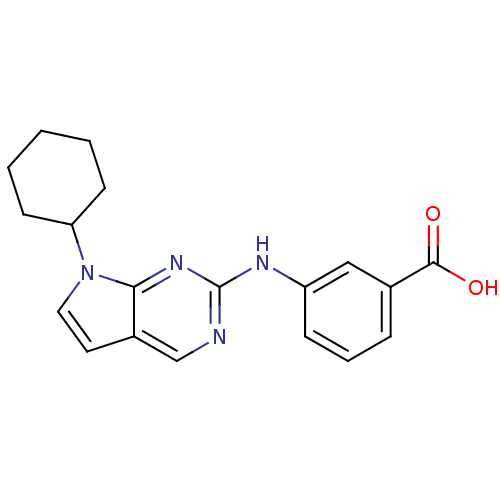
(3-({7-cyclohexyl-7H-pyrrolo[2,3-d]pyrimidin-2-yl}a...)Show InChI InChI=1S/C19H20N4O2/c24-18(25)13-5-4-6-15(11-13)21-19-20-12-14-9-10-23(17(14)22-19)16-7-2-1-3-8-16/h4-6,9-12,16H,1-3,7-8H2,(H,24,25)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged Aurora A kinase expressed in insect cells |
Bioorg Med Chem 19: 907-16 (2011)
Article DOI: 10.1016/j.bmc.2010.11.064
BindingDB Entry DOI: 10.7270/Q2V40VGC |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50286695
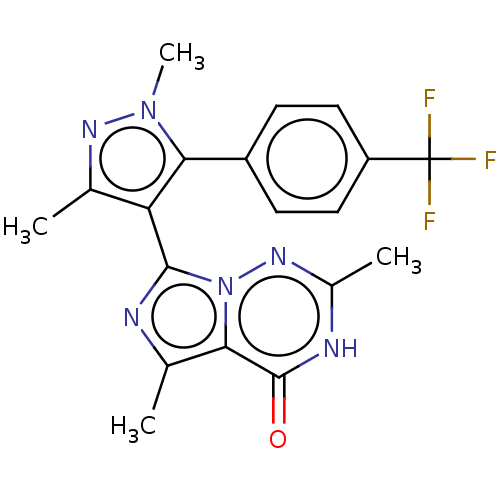
(CHEMBL4160171)Show SMILES Cc1nn(C)c(c1-c1nc(C)c2n1nc(C)[nH]c2=O)-c1ccc(cc1)C(F)(F)F |(18.8,-12.08,;20.34,-12.07,;21.23,-10.82,;22.7,-11.29,;23.94,-10.38,;22.71,-12.83,;21.25,-13.32,;20.78,-14.79,;21.69,-16.02,;20.8,-17.27,;21.28,-18.73,;19.33,-16.8,;19.33,-15.27,;18,-14.49,;16.67,-15.27,;15.33,-14.5,;16.67,-16.81,;18,-17.57,;18,-19.11,;23.95,-13.73,;23.8,-15.26,;25.05,-16.16,;26.45,-15.53,;26.6,-13.98,;25.35,-13.09,;27.7,-16.42,;27.55,-17.95,;29.11,-15.79,;29.03,-17.19,)| Show InChI InChI=1S/C42H56N4O4/c1-28-18-19-32(41(50)45-21-20-30-14-8-10-16-33(30)25-45)23-35(28)39(48)43-36(22-29-12-6-5-7-13-29)38(47)27-46-26-34-17-11-9-15-31(34)24-37(46)40(49)44-42(2,3)4/h5-8,10,12-14,16,18-19,23,30-31,33-34,36-38,47H,9,11,15,17,20-22,24-27H2,1-4H3,(H,43,48)(H,44,49)/t30?,31-,33?,34+,36-,37-,38+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PDE2A1 using 3',5'-[3H]cGMP as substrate measured after 30 mins by Yttrium silicate scintillation proximity assay |
ACS Med Chem Lett 9: 68-72 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00343
BindingDB Entry DOI: 10.7270/Q2MG7S23 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50041282
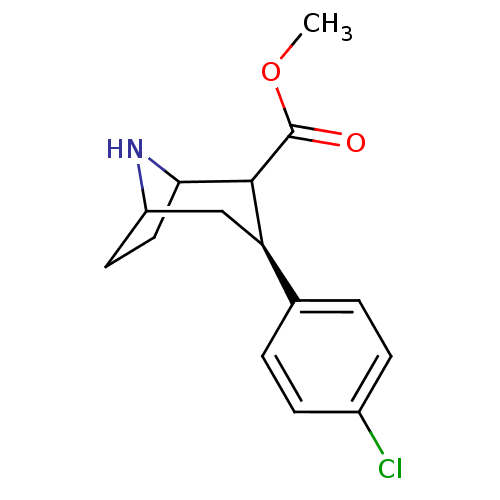
((3S,8R)-3-(4-Chloro-phenyl)-8-aza-bicyclo[3.2.1]oc...)Show SMILES COC(=O)C1C2CCC(C[C@@H]1c1ccc(Cl)cc1)N2 |TLB:11:10:18:6.7,THB:2:4:18:6.7| Show InChI InChI=1S/C15H18ClNO2/c1-19-15(18)14-12(8-11-6-7-13(14)17-11)9-2-4-10(16)5-3-9/h2-5,11-14,17H,6-8H2,1H3/t11?,12-,13?,14?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Inhibition of [3H]WIN-35428 binding to dopamine (DA) transporter |
J Med Chem 37: 1220-3 (1994)
BindingDB Entry DOI: 10.7270/Q2Z60PQ2 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50434022
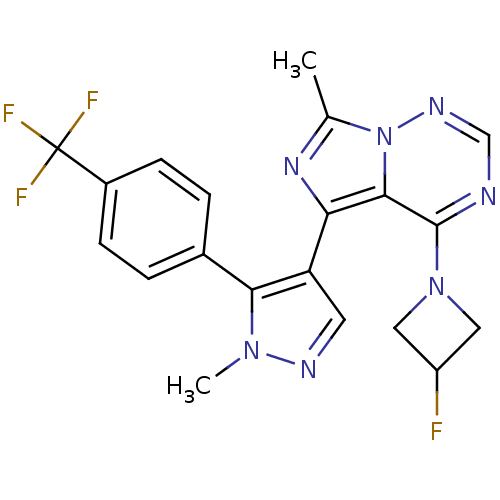
(CHEMBL2381188 | US8598155, 52)Show SMILES Cc1nc(-c2cnn(C)c2-c2ccc(cc2)C(F)(F)F)c2c(ncnn12)N1CC(F)C1 Show InChI InChI=1S/C20H17F4N7/c1-11-28-16(18-19(25-10-27-31(11)18)30-8-14(21)9-30)15-7-26-29(2)17(15)12-3-5-13(6-4-12)20(22,23)24/h3-7,10,14H,8-9H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.633 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
The activity of the test substances on human full-length PDE2A3 enzyme was determined using the [3H]-cGMP scintillation proximity assay (SPA) modifie... |
US Patent US8598155 (2013)
BindingDB Entry DOI: 10.7270/Q26T0K8C |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453639

(CHEMBL2112889)Show SMILES [H][C@]12CCC(N1)[C@H]([C@H](C2)c1ccc(I)cc1)C(=O)OC |TLB:9:7:5:3.2,THB:16:6:5:3.2| Show InChI InChI=1S/C15H18INO2/c1-19-15(18)14-12(8-11-6-7-13(14)17-11)9-2-4-10(16)5-3-9/h2-5,11-14,17H,6-8H2,1H3/t11-,12-,13?,14+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Inhibition of [3H]WIN-35428 binding to dopamine (DA) transporter |
J Med Chem 37: 1220-3 (1994)
BindingDB Entry DOI: 10.7270/Q2Z60PQ2 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged Aurora A kinase expressed in insect cells |
Bioorg Med Chem 19: 907-16 (2011)
Article DOI: 10.1016/j.bmc.2010.11.064
BindingDB Entry DOI: 10.7270/Q2V40VGC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM107819
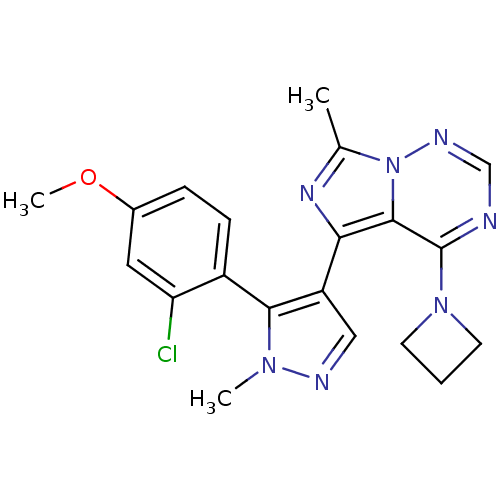
(US8598155, 57)Show SMILES COc1ccc(-c2c(cnn2C)-c2nc(C)n3ncnc(N4CCC4)c23)c(Cl)c1 |(4.96,-4.08,;5.36,-2.59,;4.27,-1.5,;2.78,-1.9,;1.7,-.81,;2.09,.68,;1.01,1.77,;-.46,1.29,;-1.36,2.54,;-.46,3.78,;1.01,3.31,;2.34,4.08,;-1.23,-.04,;-.32,-1.29,;-1.23,-2.53,;-.46,-3.87,;-2.69,-2.06,;-4.03,-2.83,;-5.36,-2.06,;-5.36,-.52,;-4.03,.25,;-4.03,1.79,;-2.94,2.88,;-4.03,3.97,;-5.12,2.88,;-2.69,-.52,;3.58,1.08,;3.98,2.56,;4.67,-.01,)| Show InChI InChI=1S/C20H20ClN7O/c1-12-25-17(19-20(27-7-4-8-27)22-11-24-28(12)19)15-10-23-26(2)18(15)14-6-5-13(29-3)9-16(14)21/h5-6,9-11H,4,7-8H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.761 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
The activity of the test substances on human full-length PDE2A3 enzyme was determined using the [3H]-cGMP scintillation proximity assay (SPA) modifie... |
US Patent US8598155 (2013)
BindingDB Entry DOI: 10.7270/Q26T0K8C |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM107773
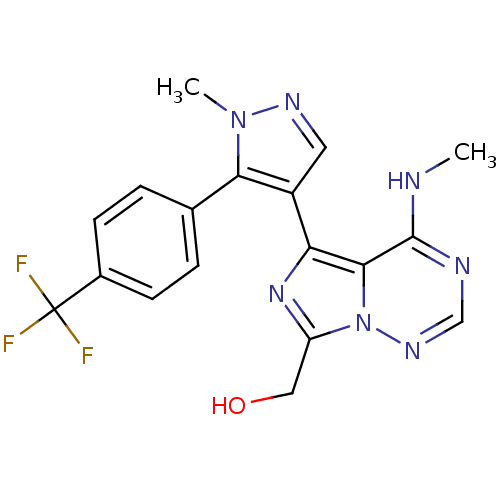
(US8598155, 9)Show SMILES CNc1ncnn2c(CO)nc(-c3cnn(C)c3-c3ccc(cc3)C(F)(F)F)c12 Show InChI InChI=1S/C18H16F3N7O/c1-22-17-16-14(26-13(8-29)28(16)25-9-23-17)12-7-24-27(2)15(12)10-3-5-11(6-4-10)18(19,20)21/h3-7,9,29H,8H2,1-2H3,(H,22,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.763 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
The activity of the test substances on human full-length PDE2A3 enzyme was determined using the [3H]-cGMP scintillation proximity assay (SPA) modifie... |
US Patent US8598155 (2013)
BindingDB Entry DOI: 10.7270/Q26T0K8C |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM107799
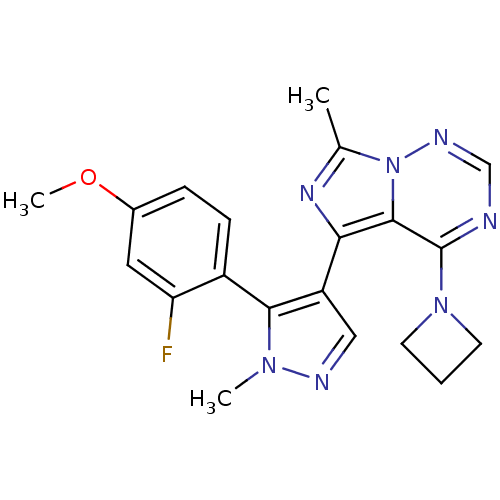
(US8598155, 36)Show SMILES COc1ccc(-c2c(cnn2C)-c2nc(C)n3ncnc(N4CCC4)c23)c(F)c1 |(4.96,-4.08,;5.36,-2.59,;4.27,-1.5,;2.78,-1.9,;1.7,-.81,;2.09,.68,;1.01,1.77,;-.46,1.29,;-1.36,2.54,;-.46,3.78,;1.01,3.31,;2.34,4.08,;-1.23,-.04,;-.32,-1.29,;-1.23,-2.53,;-.46,-3.87,;-2.69,-2.06,;-4.03,-2.83,;-5.36,-2.06,;-5.36,-.52,;-4.03,.25,;-4.03,1.79,;-2.94,2.88,;-4.03,3.97,;-5.12,2.88,;-2.69,-.52,;3.58,1.08,;3.98,2.56,;4.67,-.01,)| Show InChI InChI=1S/C20H20FN7O/c1-12-25-17(19-20(27-7-4-8-27)22-11-24-28(12)19)15-10-23-26(2)18(15)14-6-5-13(29-3)9-16(14)21/h5-6,9-11H,4,7-8H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.825 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
The activity of the test substances on human full-length PDE2A3 enzyme was determined using the [3H]-cGMP scintillation proximity assay (SPA) modifie... |
US Patent US8598155 (2013)
BindingDB Entry DOI: 10.7270/Q26T0K8C |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50333657
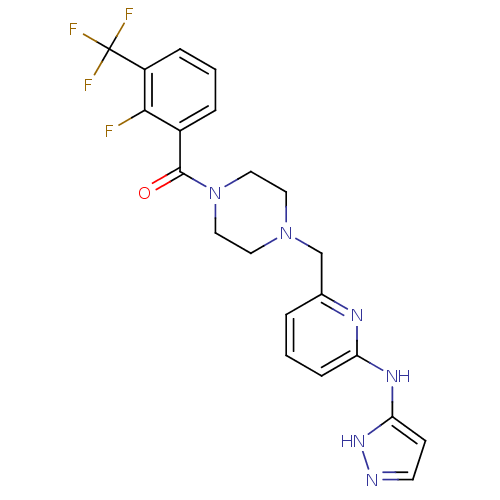
((4-((6-(1H-pyrazol-3-ylamino)pyridin-2-yl)methyl)p...)Show SMILES Fc1c(cccc1C(F)(F)F)C(=O)N1CCN(Cc2cccc(Nc3ccn[nH]3)n2)CC1 Show InChI InChI=1S/C21H20F4N6O/c22-19-15(4-2-5-16(19)21(23,24)25)20(32)31-11-9-30(10-12-31)13-14-3-1-6-17(27-14)28-18-7-8-26-29-18/h1-8H,9-13H2,(H2,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-tagged Aurora A kinase expressed in insect cells |
Bioorg Med Chem 19: 907-16 (2011)
Article DOI: 10.1016/j.bmc.2010.11.064
BindingDB Entry DOI: 10.7270/Q2V40VGC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50344779
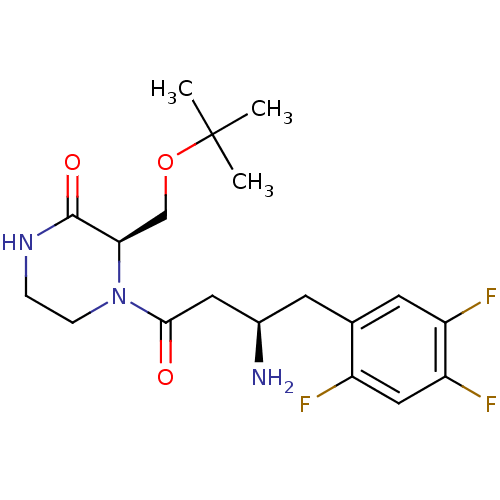
((R)-4-((R)-3-amino-4-(2,4,5-trifluorophenyl)butano...)Show SMILES CC(C)(C)OC[C@H]1N(CCNC1=O)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C19H26F3N3O3/c1-19(2,3)28-10-16-18(27)24-4-5-25(16)17(26)8-12(23)6-11-7-14(21)15(22)9-13(11)20/h7,9,12,16H,4-6,8,10,23H2,1-3H3,(H,24,27)/t12-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Dong-A Pharm. Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DPP-4 assessed as H-Gly-Pro-AMC cleavage after 1 hr by fluorescence assay |
Bioorg Med Chem Lett 21: 3809-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.029
BindingDB Entry DOI: 10.7270/Q2XS5VQK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM107814
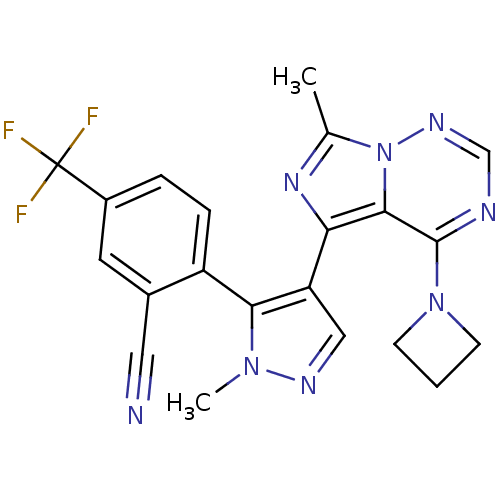
(US8598155, 51)Show SMILES Cc1nc(-c2cnn(C)c2-c2ccc(cc2C#N)C(F)(F)F)c2c(ncnn12)N1CCC1 |(-1,-3.97,;-1.77,-2.64,;-.87,-1.39,;-1.77,-.15,;-1,1.19,;-1.91,2.43,;-1,3.68,;.46,3.2,;1.79,3.97,;.46,1.66,;1.55,.57,;1.15,-.91,;2.24,-2,;3.73,-1.6,;4.13,-.12,;3.04,.97,;3.44,2.46,;3.83,3.95,;4.82,-2.69,;5.91,-3.78,;3.73,-3.78,;5.91,-1.6,;-3.24,-.62,;-4.57,.15,;-5.91,-.62,;-5.91,-2.16,;-4.57,-2.93,;-3.24,-2.16,;-4.57,1.69,;-3.48,2.78,;-4.57,3.86,;-5.66,2.78,)| Show InChI InChI=1S/C21H17F3N8/c1-12-29-17(19-20(31-6-3-7-31)26-11-28-32(12)19)16-10-27-30(2)18(16)15-5-4-14(21(22,23)24)8-13(15)9-25/h4-5,8,10-11H,3,6-7H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.959 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
The activity of the test substances on human full-length PDE2A3 enzyme was determined using the [3H]-cGMP scintillation proximity assay (SPA) modifie... |
US Patent US8598155 (2013)
BindingDB Entry DOI: 10.7270/Q26T0K8C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50210257
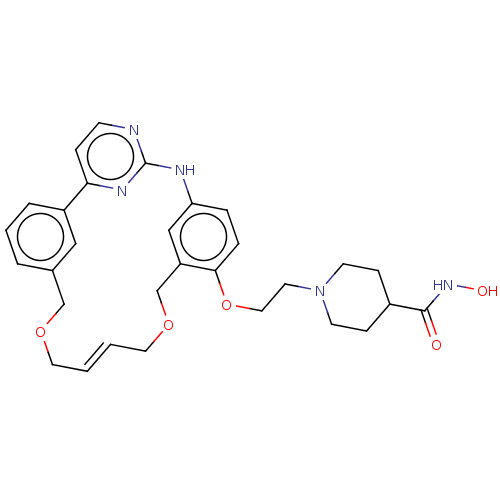
(CHEMBL3961053)Show SMILES ONC(=O)C1CCN(CCOc2ccc3Nc4nccc(n4)-c4cccc(COC\C=C\COCc2c3)c4)CC1 |t:31| Show InChI InChI=1S/C30H35N5O5/c36-29(34-37)23-9-12-35(13-10-23)14-17-40-28-7-6-26-19-25(28)21-39-16-2-1-15-38-20-22-4-3-5-24(18-22)27-8-11-31-30(32-26)33-27/h1-8,11,18-19,23,37H,9-10,12-17,20-21H2,(H,34,36)(H,31,32,33)/b2-1+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) preincubated for 20 mins followed by [33P]ATP addition measured after 120 mins by Hotspot assay |
J Med Chem 59: 8233-62 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00157
BindingDB Entry DOI: 10.7270/Q2W95C5G |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM107845
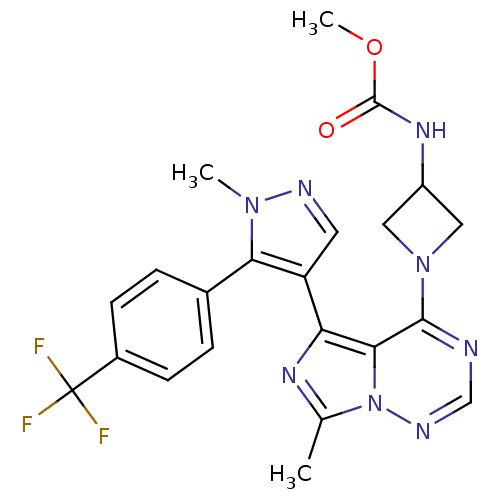
(US8598155, 83)Show SMILES COC(=O)NC1CN(C1)c1ncnn2c(C)nc(-c3cnn(C)c3-c3ccc(cc3)C(F)(F)F)c12 Show InChI InChI=1S/C22H21F3N8O2/c1-12-29-17(16-8-27-31(2)18(16)13-4-6-14(7-5-13)22(23,24)25)19-20(26-11-28-33(12)19)32-9-15(10-32)30-21(34)35-3/h4-8,11,15H,9-10H2,1-3H3,(H,30,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.975 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
The activity of the test substances on human full-length PDE2A3 enzyme was determined using the [3H]-cGMP scintillation proximity assay (SPA) modifie... |
US Patent US8598155 (2013)
BindingDB Entry DOI: 10.7270/Q26T0K8C |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM107797
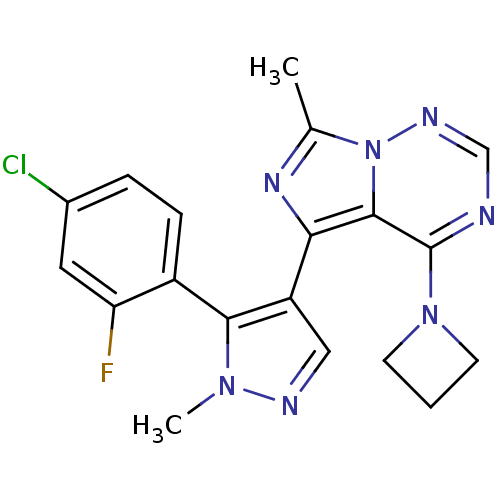
(US8598155, 34)Show SMILES Cc1nc(-c2cnn(C)c2-c2ccc(Cl)cc2F)c2c(ncnn12)N1CCC1 |(-.46,-3.97,;-1.23,-2.64,;-.32,-1.39,;-1.23,-.15,;-.46,1.19,;-1.36,2.43,;-.46,3.68,;1.01,3.2,;2.34,3.97,;1.01,1.66,;2.09,.57,;1.7,-.91,;2.78,-2,;4.27,-1.6,;5.36,-2.69,;4.67,-.12,;3.58,.97,;3.98,2.46,;-2.69,-.62,;-4.03,.15,;-5.36,-.62,;-5.36,-2.16,;-4.03,-2.93,;-2.69,-2.16,;-4.03,1.69,;-2.94,2.78,;-4.03,3.86,;-5.12,2.78,)| Show InChI InChI=1S/C19H17ClFN7/c1-11-25-16(18-19(27-6-3-7-27)22-10-24-28(11)18)14-9-23-26(2)17(14)13-5-4-12(20)8-15(13)21/h4-5,8-10H,3,6-7H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
The activity of the test substances on human full-length PDE2A3 enzyme was determined using the [3H]-cGMP scintillation proximity assay (SPA) modifie... |
US Patent US8598155 (2013)
BindingDB Entry DOI: 10.7270/Q26T0K8C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50210177

(ONX-0803 | Pacritinib | SB-1518 | SB1518)Show SMILES C(CN1CCCC1)Oc1ccc2Nc3nccc(n3)-c3cccc(COC\C=C\COCc1c2)c3 |t:29| Show InChI InChI=1S/C28H32N4O3/c1-2-13-32(12-1)14-17-35-27-9-8-25-19-24(27)21-34-16-4-3-15-33-20-22-6-5-7-23(18-22)26-10-11-29-28(30-25)31-26/h3-11,18-19H,1-2,12-17,20-21H2,(H,29,30,31)/b4-3+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) preincubated for 20 mins followed by [33P]ATP addition measured after 120 mins by Hotspot assay |
J Med Chem 59: 8233-62 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00157
BindingDB Entry DOI: 10.7270/Q2W95C5G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM107812
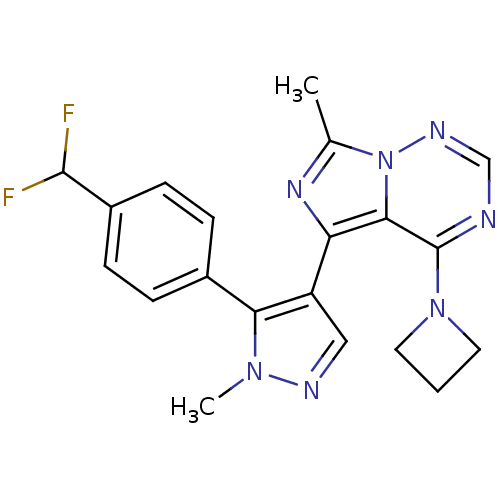
(US8598155, 49)Show SMILES Cc1nc(-c2cnn(C)c2-c2ccc(cc2)C(F)F)c2c(ncnn12)N1CCC1 Show InChI InChI=1S/C20H19F2N7/c1-12-26-16(18-20(28-8-3-9-28)23-11-25-29(12)18)15-10-24-27(2)17(15)13-4-6-14(7-5-13)19(21)22/h4-7,10-11,19H,3,8-9H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.01 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
The activity of the test substances on human full-length PDE2A3 enzyme was determined using the [3H]-cGMP scintillation proximity assay (SPA) modifie... |
US Patent US8598155 (2013)
BindingDB Entry DOI: 10.7270/Q26T0K8C |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM107829
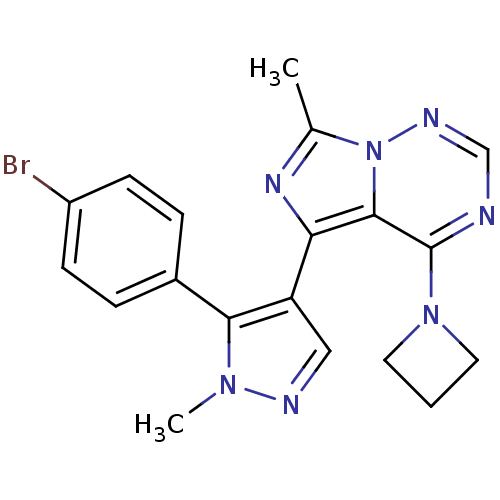
(US8598155, 67)Show SMILES Cc1nc(-c2cnn(C)c2-c2ccc(Br)cc2)c2c(ncnn12)N1CCC1 Show InChI InChI=1S/C19H18BrN7/c1-12-24-16(18-19(26-8-3-9-26)21-11-23-27(12)18)15-10-22-25(2)17(15)13-4-6-14(20)7-5-13/h4-7,10-11H,3,8-9H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.02 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
The activity of the test substances on human full-length PDE2A3 enzyme was determined using the [3H]-cGMP scintillation proximity assay (SPA) modifie... |
US Patent US8598155 (2013)
BindingDB Entry DOI: 10.7270/Q26T0K8C |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM107843
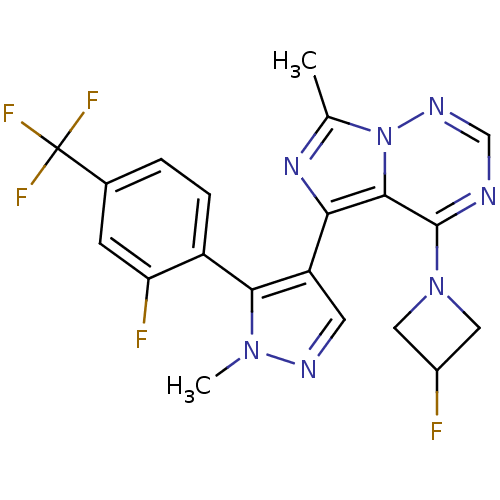
(US8598155, 81)Show SMILES Cc1nc(-c2cnn(C)c2-c2ccc(cc2F)C(F)(F)F)c2c(ncnn12)N1CC(F)C1 |(-1,-3.22,;-1.77,-1.88,;-.87,-.64,;-1.77,.61,;-1,1.94,;-1.91,3.19,;-1,4.43,;.46,3.96,;1.79,4.73,;.46,2.42,;1.55,1.33,;1.15,-.16,;2.24,-1.25,;3.73,-.85,;4.13,.64,;3.04,1.73,;3.44,3.22,;4.82,-1.94,;5.91,-3.03,;3.73,-3.03,;5.91,-.85,;-3.24,.13,;-4.57,.9,;-5.91,.13,;-5.91,-1.41,;-4.57,-2.18,;-3.24,-1.41,;-4.57,2.44,;-3.48,3.53,;-4.57,4.62,;-4.57,6.16,;-5.66,3.53,)| Show InChI InChI=1S/C20H16F5N7/c1-10-29-16(18-19(26-9-28-32(10)18)31-7-12(21)8-31)14-6-27-30(2)17(14)13-4-3-11(5-15(13)22)20(23,24)25/h3-6,9,12H,7-8H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
The activity of the test substances on human full-length PDE2A3 enzyme was determined using the [3H]-cGMP scintillation proximity assay (SPA) modifie... |
US Patent US8598155 (2013)
BindingDB Entry DOI: 10.7270/Q26T0K8C |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM107817
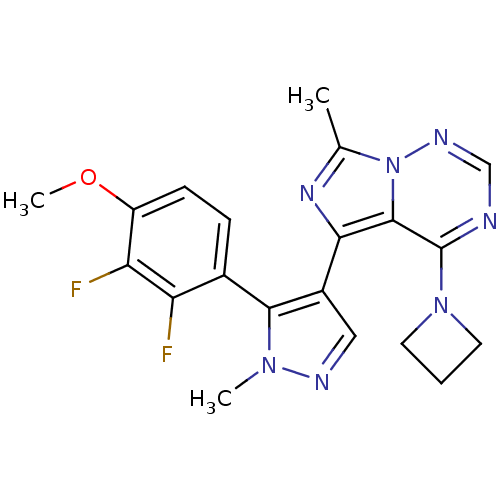
(US8598155, 55)Show SMILES COc1ccc(-c2c(cnn2C)-c2nc(C)n3ncnc(N4CCC4)c23)c(F)c1F |(4.56,-4.08,;4.96,-2.59,;3.87,-1.5,;2.39,-1.9,;1.3,-.81,;1.7,.68,;.61,1.77,;-.86,1.29,;-1.76,2.54,;-.86,3.78,;.61,3.31,;1.94,4.08,;-1.63,-.04,;-.72,-1.29,;-1.63,-2.53,;-.86,-3.87,;-3.09,-2.06,;-4.43,-2.83,;-5.76,-2.06,;-5.76,-.52,;-4.43,.25,;-4.43,1.79,;-3.34,2.88,;-4.43,3.97,;-5.52,2.88,;-3.09,-.52,;3.18,1.08,;3.58,2.56,;4.27,-.01,;5.76,.39,)| Show InChI InChI=1S/C20H19F2N7O/c1-11-26-17(19-20(28-7-4-8-28)23-10-25-29(11)19)13-9-24-27(2)18(13)12-5-6-14(30-3)16(22)15(12)21/h5-6,9-10H,4,7-8H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
The activity of the test substances on human full-length PDE2A3 enzyme was determined using the [3H]-cGMP scintillation proximity assay (SPA) modifie... |
US Patent US8598155 (2013)
BindingDB Entry DOI: 10.7270/Q26T0K8C |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50453638
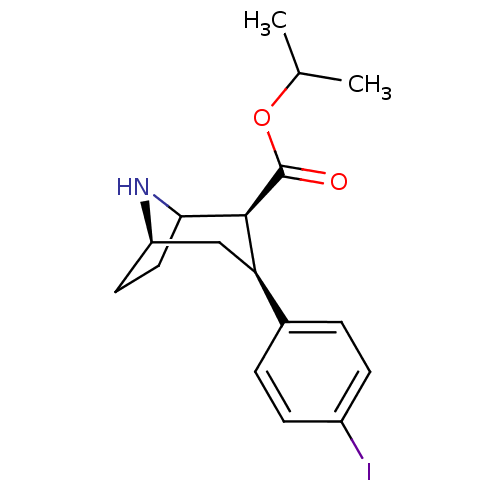
(CHEMBL2112877)Show SMILES [H][C@]12CCC(N1)[C@H]([C@H](C2)c1ccc(I)cc1)C(=O)OC(C)C |TLB:9:7:5:3.2,THB:16:6:5:3.2| Show InChI InChI=1S/C17H22INO2/c1-10(2)21-17(20)16-14(9-13-7-8-15(16)19-13)11-3-5-12(18)6-4-11/h3-6,10,13-16,19H,7-9H2,1-2H3/t13-,14-,15?,16+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Inhibition of [3H]WIN-35428 binding to dopamine (DA) transporter |
J Med Chem 37: 1220-3 (1994)
BindingDB Entry DOI: 10.7270/Q2Z60PQ2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50035738

((R)-3-(4-Chloro-phenyl)-8-methyl-8-aza-bicyclo[3.2...)Show SMILES COC(=O)C1C2CCC(CC1c1ccc(Cl)cc1)N2C |TLB:11:10:18:6.7,THB:2:4:18:6.7| Show InChI InChI=1S/C16H20ClNO2/c1-18-12-7-8-14(18)15(16(19)20-2)13(9-12)10-3-5-11(17)6-4-10/h3-6,12-15H,7-9H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by ChEMBL
| Assay Description
Inhibition of [3H]WIN-35428 binding to dopamine (DA) transporter |
J Med Chem 37: 1220-3 (1994)
BindingDB Entry DOI: 10.7270/Q2Z60PQ2 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM107830
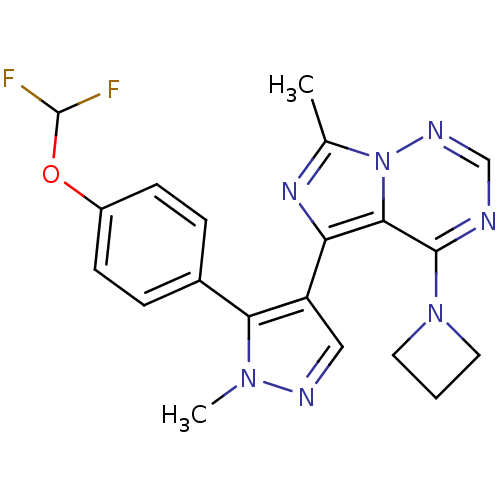
(US8598155, 68)Show SMILES Cc1nc(-c2cnn(C)c2-c2ccc(OC(F)F)cc2)c2c(ncnn12)N1CCC1 Show InChI InChI=1S/C20H19F2N7O/c1-12-26-16(18-19(28-8-3-9-28)23-11-25-29(12)18)15-10-24-27(2)17(15)13-4-6-14(7-5-13)30-20(21)22/h4-7,10-11,20H,3,8-9H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
The activity of the test substances on human full-length PDE2A3 enzyme was determined using the [3H]-cGMP scintillation proximity assay (SPA) modifie... |
US Patent US8598155 (2013)
BindingDB Entry DOI: 10.7270/Q26T0K8C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data






















































