 Found 50547 hits with Last Name = 'ma' and Initial = 'f'
Found 50547 hits with Last Name = 'ma' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50176988
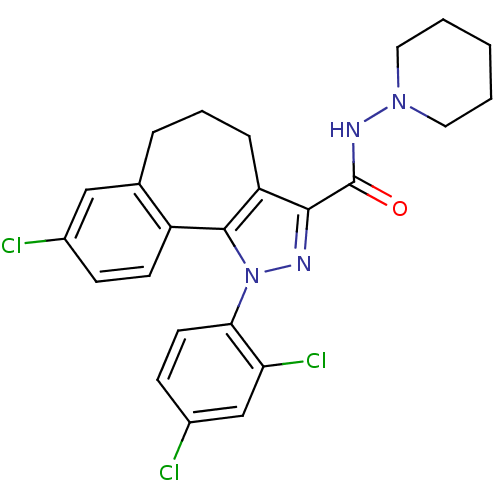
(8-Chloro-1-(2,4-dichloro-phenyl)-1,3a,4,5,6,10b-he...)Show SMILES Clc1ccc(c(Cl)c1)-n1nc(C(=O)NN2CCCCC2)c2CCCc3cc(Cl)ccc3-c12 Show InChI InChI=1S/C24H23Cl3N4O/c25-16-7-9-18-15(13-16)5-4-6-19-22(24(32)29-30-11-2-1-3-12-30)28-31(23(18)19)21-10-8-17(26)14-20(21)27/h7-10,13-14H,1-6,11-12H2,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor (unknown origin) |
J Med Chem 51: 3526-39 (2008)
Article DOI: 10.1021/jm8000778
BindingDB Entry DOI: 10.7270/Q29K4B0X |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86041
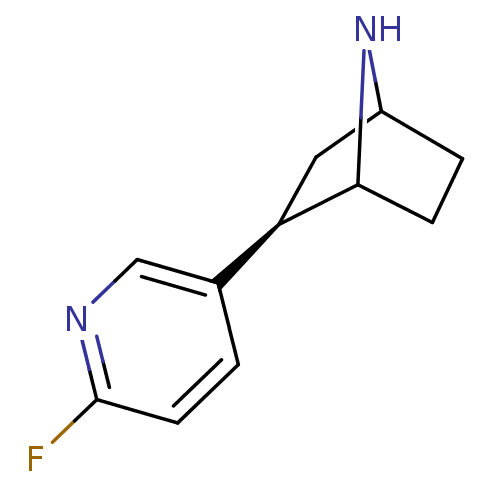
(NFEP)Show SMILES Fc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13FN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50053685
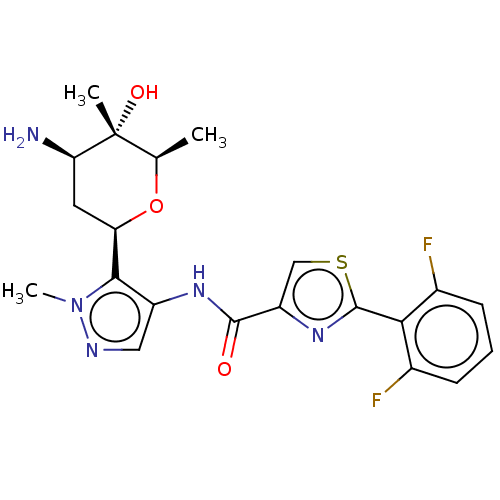
(CHEMBL3318855 | US9328106, 341)Show SMILES C[C@H]1O[C@H](C[C@@H](N)[C@]1(C)O)c1c(NC(=O)c2csc(n2)-c2c(F)cccc2F)cnn1C |r| Show InChI InChI=1S/C21H23F2N5O3S/c1-10-21(2,30)16(24)7-15(31-10)18-13(8-25-28(18)3)26-19(29)14-9-32-20(27-14)17-11(22)5-4-6-12(17)23/h4-6,8-10,15-16,30H,7,24H2,1-3H3,(H,26,29)/t10-,15-,16-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) |
ACS Med Chem Lett 5: 730-1 (2014)
Article DOI: 10.1021/ml5001678
BindingDB Entry DOI: 10.7270/Q2SN0BMH |
More data for this
Ligand-Target Pair | |
cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1
(Xenopus) | BDBM86042

(NEP)Show InChI InChI=1S/C11H14N2/c1-2-8(7-12-5-1)10-6-9-3-4-11(10)13-9/h1-2,5,7,9-11,13H,3-4,6H2/t9?,10-,11?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1
(Xenopus) | BDBM86041

(NFEP)Show SMILES Fc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13FN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1
(Xenopus) | BDBM86040
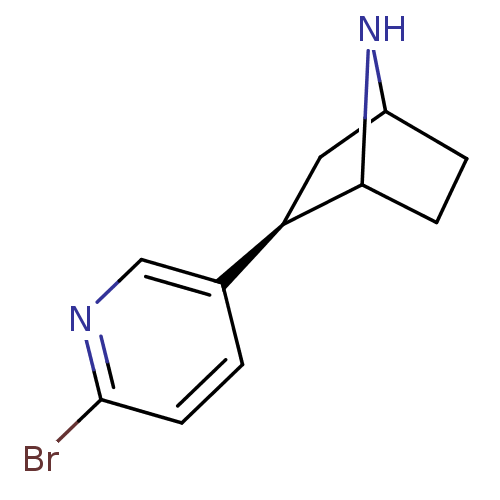
(NBEP)Show SMILES Brc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13BrN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50053679

(CHEMBL3318853 | US9328106, 115)Show SMILES [H][C@]12CC[C@](CC[C@@H]1N)(O2)c1c(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)cnn1C |r| Show InChI InChI=1S/C21H22F2N6O2S/c1-29-17(21-7-5-12(24)14(31-21)6-8-21)13(9-26-29)27-19(30)16-18(25)32-20(28-16)15-10(22)3-2-4-11(15)23/h2-4,9,12,14H,5-8,24-25H2,1H3,(H,27,30)/t12-,14-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) |
ACS Med Chem Lett 5: 730-1 (2014)
Article DOI: 10.1021/ml5001678
BindingDB Entry DOI: 10.7270/Q2SN0BMH |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-4
(Xenopus) | BDBM86042

(NEP)Show InChI InChI=1S/C11H14N2/c1-2-8(7-12-5-1)10-6-9-3-4-11(10)13-9/h1-2,5,7,9-11,13H,3-4,6H2/t9?,10-,11?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-4
(Xenopus) | BDBM86041

(NFEP)Show SMILES Fc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13FN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50053683
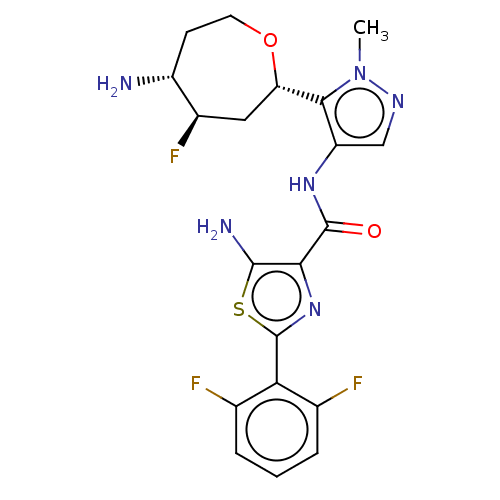
(CHEMBL3318854 | US9328106, 135)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1[C@@H]1C[C@@H](F)[C@H](N)CCO1 |r| Show InChI InChI=1S/C20H21F3N6O2S/c1-29-17(14-7-11(23)12(24)5-6-31-14)13(8-26-29)27-19(30)16-18(25)32-20(28-16)15-9(21)3-2-4-10(15)22/h2-4,8,11-12,14H,5-7,24-25H2,1H3,(H,27,30)/t11-,12-,14+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) |
ACS Med Chem Lett 5: 730-1 (2014)
Article DOI: 10.1021/ml5001678
BindingDB Entry DOI: 10.7270/Q2SN0BMH |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464108
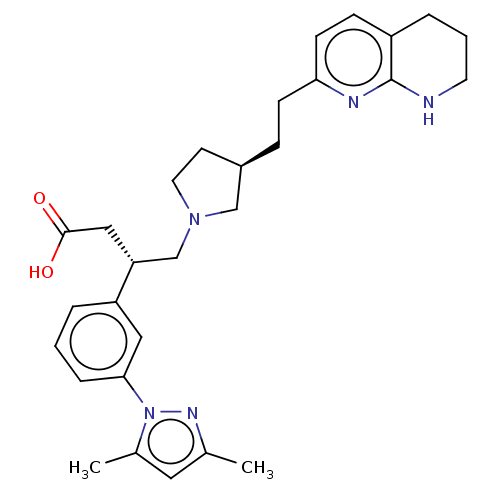
(CHEMBL4241824)Show SMILES Cc1cc(C)n(n1)-c1cccc(c1)[C@@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)CC(O)=O |r| Show InChI InChI=1S/C29H37N5O2/c1-20-15-21(2)34(32-20)27-7-3-5-24(16-27)25(17-28(35)36)19-33-14-12-22(18-33)8-10-26-11-9-23-6-4-13-30-29(23)31-26/h3,5,7,9,11,15-16,22,25H,4,6,8,10,12-14,17-19H2,1-2H3,(H,30,31)(H,35,36)/t22-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to human integrin alphaVbeta6 assessed as dissociation constant up to 48 hrs by liquid scintillation counting |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-4
(Xenopus) | BDBM86040

(NBEP)Show SMILES Brc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13BrN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type IV [15-340]
(Homo sapiens (Human)) | BDBM223209
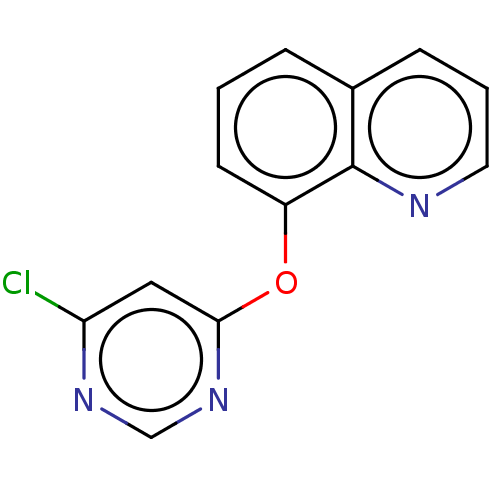
(8-((6-Chloropyrimidin-4-yl)oxy)quinoline (Compound...)Show InChI InChI=1S/C13H8ClN3O/c14-11-7-12(17-8-16-11)18-10-5-1-3-9-4-2-6-15-13(9)10/h1-8H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
B.R. Ambedkar Bihar University
| Assay Description
The docking and scoring of ligands with CAMKIV protein was accomplished using ParDOCK module of Sanjeevini drug design suite, which is based on physi... |
Chem Biol Drug Des 89: 741-754 (2017)
Article DOI: 10.1111/cbdd.12898
BindingDB Entry DOI: 10.7270/Q28C9V30 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86040

(NBEP)Show SMILES Brc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13BrN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464104
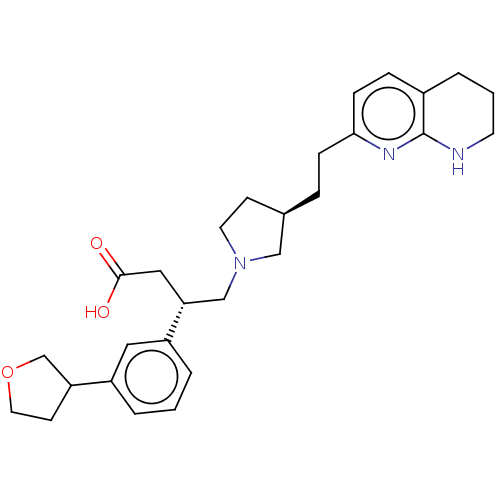
(CHEMBL4244784)Show SMILES OC(=O)C[C@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)c1cccc(c1)C1CCOC1 |r| Show InChI InChI=1S/C28H37N3O3/c32-27(33)16-25(23-4-1-3-22(15-23)24-11-14-34-19-24)18-31-13-10-20(17-31)6-8-26-9-7-21-5-2-12-29-28(21)30-26/h1,3-4,7,9,15,20,24-25H,2,5-6,8,10-14,16-19H2,(H,29,30)(H,32,33)/t20-,24?,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464110
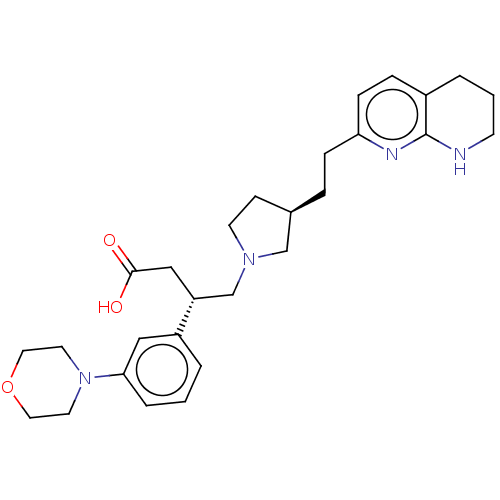
(CHEMBL4238909)Show SMILES OC(=O)C[C@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)c1cccc(c1)N1CCOCC1 |r| Show InChI InChI=1S/C28H38N4O3/c33-27(34)18-24(23-3-1-5-26(17-23)32-13-15-35-16-14-32)20-31-12-10-21(19-31)6-8-25-9-7-22-4-2-11-29-28(22)30-25/h1,3,5,7,9,17,21,24H,2,4,6,8,10-16,18-20H2,(H,29,30)(H,33,34)/t21-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to human integrin alphaVbeta6 assessed as dissociation constant up to 48 hrs by liquid scintillation counting |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86042

(NEP)Show InChI InChI=1S/C11H14N2/c1-2-8(7-12-5-1)10-6-9-3-4-11(10)13-9/h1-2,5,7,9-11,13H,3-4,6H2/t9?,10-,11?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50105834
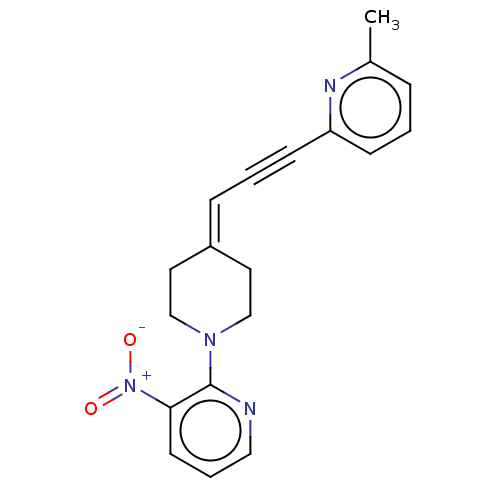
(CHEMBL3597594)Show SMILES [#6]-c1cccc(n1)C#C\[#6]=[#6]-1/[#6]-[#6]-[#7](-[#6]-[#6]-1)-c1ncccc1-[#7+](-[#8-])=O Show InChI InChI=1S/C19H18N4O2/c1-15-5-2-7-17(21-15)8-3-6-16-10-13-22(14-11-16)19-18(23(24)25)9-4-12-20-19/h2,4-7,9,12H,10-11,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Modena and Reggio Emilia
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEP from mGluR5 receptor in Sprague-Dawley rat forebrain membrane after 60 mins by liquid scintillation spectrometry |
Bioorg Med Chem 23: 3040-58 (2015)
Article DOI: 10.1016/j.bmc.2015.05.008
BindingDB Entry DOI: 10.7270/Q2PK0HX0 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50146585
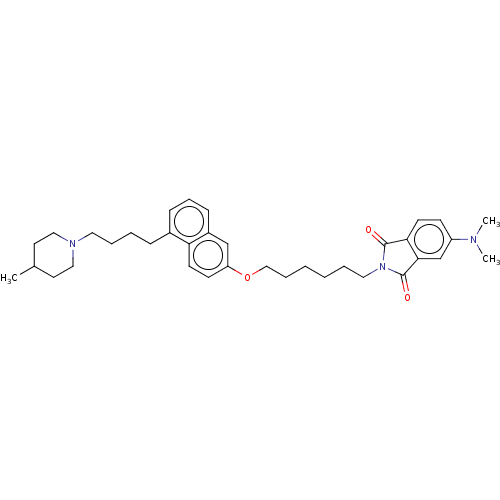
(CHEMBL3763396)Show SMILES CC1CCN(CCCCc2cccc3cc(OCCCCCCN4C(=O)c5ccc(cc5C4=O)N(C)C)ccc23)CC1 Show InChI InChI=1S/C36H47N3O3/c1-27-18-22-38(23-19-27)20-8-6-11-28-12-10-13-29-25-31(15-17-32(28)29)42-24-9-5-4-7-21-39-35(40)33-16-14-30(37(2)3)26-34(33)36(39)41/h10,12-17,25-27H,4-9,11,18-24H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50146585

(CHEMBL3763396)Show SMILES CC1CCN(CCCCc2cccc3cc(OCCCCCCN4C(=O)c5ccc(cc5C4=O)N(C)C)ccc23)CC1 Show InChI InChI=1S/C36H47N3O3/c1-27-18-22-38(23-19-27)20-8-6-11-28-12-10-13-29-25-31(15-17-32(28)29)42-24-9-5-4-7-21-39-35(40)33-16-14-30(37(2)3)26-34(33)36(39)41/h10,12-17,25-27H,4-9,11,18-24H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464123

(CHEMBL4242263)Show SMILES Cc1cc(C)n(n1)-c1cc(cc(c1)N1CCOCC1)[C@@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)CC(O)=O |r| Show InChI InChI=1S/C33H44N6O3/c1-23-16-24(2)39(36-23)31-18-27(17-30(20-31)38-12-14-42-15-13-38)28(19-32(40)41)22-37-11-9-25(21-37)5-7-29-8-6-26-4-3-10-34-33(26)35-29/h6,8,16-18,20,25,28H,3-5,7,9-15,19,21-22H2,1-2H3,(H,34,35)(H,40,41)/t25-,28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464124
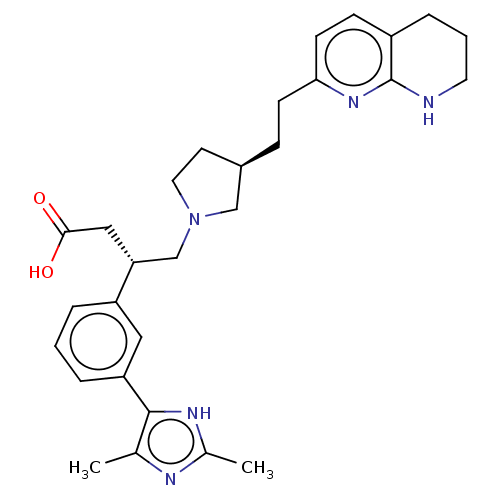
(CHEMBL4249629)Show SMILES Cc1nc(C)c([nH]1)-c1cccc(c1)[C@@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)CC(O)=O |r| Show InChI InChI=1S/C29H37N5O2/c1-19-28(32-20(2)31-19)24-6-3-5-23(15-24)25(16-27(35)36)18-34-14-12-21(17-34)8-10-26-11-9-22-7-4-13-30-29(22)33-26/h3,5-6,9,11,15,21,25H,4,7-8,10,12-14,16-18H2,1-2H3,(H,30,33)(H,31,32)(H,35,36)/t21-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464119
(CHEMBL4241584)Show SMILES OC(=O)C[C@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)c1cccc(c1)-n1ccnn1 |r| Show InChI InChI=1S/C26H32N6O2/c33-25(34)16-22(21-3-1-5-24(15-21)32-14-12-28-30-32)18-31-13-10-19(17-31)6-8-23-9-7-20-4-2-11-27-26(20)29-23/h1,3,5,7,9,12,14-15,19,22H,2,4,6,8,10-11,13,16-18H2,(H,27,29)(H,33,34)/t19-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464108

(CHEMBL4241824)Show SMILES Cc1cc(C)n(n1)-c1cccc(c1)[C@@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)CC(O)=O |r| Show InChI InChI=1S/C29H37N5O2/c1-20-15-21(2)34(32-20)27-7-3-5-24(16-27)25(17-28(35)36)19-33-14-12-22(18-33)8-10-26-11-9-23-6-4-13-30-29(23)31-26/h3,5,7,9,11,15-16,22,25H,4,6,8,10,12-14,17-19H2,1-2H3,(H,30,31)(H,35,36)/t22-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphavbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 62: 7543-7556 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00819
BindingDB Entry DOI: 10.7270/Q2S75KQH |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464108

(CHEMBL4241824)Show SMILES Cc1cc(C)n(n1)-c1cccc(c1)[C@@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)CC(O)=O |r| Show InChI InChI=1S/C29H37N5O2/c1-20-15-21(2)34(32-20)27-7-3-5-24(16-27)25(17-28(35)36)19-33-14-12-22(18-33)8-10-26-11-9-23-6-4-13-30-29(23)31-26/h3,5,7,9,11,15-16,22,25H,4,6,8,10,12-14,17-19H2,1-2H3,(H,30,31)(H,35,36)/t22-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464118
(CHEMBL4249172)Show SMILES OC(=O)C[C@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)c1cccc(O[C@H]2CCOC2)c1 |r| Show InChI InChI=1S/C28H37N3O4/c32-27(33)16-23(22-3-1-5-25(15-22)35-26-11-14-34-19-26)18-31-13-10-20(17-31)6-8-24-9-7-21-4-2-12-29-28(21)30-24/h1,3,5,7,9,15,20,23,26H,2,4,6,8,10-14,16-19H2,(H,29,30)(H,32,33)/t20-,23-,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50459886

(CHEMBL261010)Show InChI InChI=1S/C13H15ClN4/c1-17-6-8-18(9-7-17)13-12(14)15-10-4-2-3-5-11(10)16-13/h2-5H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]granisetron from human 5-HT3A receptor expressed in HEK293 cells by scintillation counting method |
Bioorg Med Chem Lett 27: 3207-3218 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.073
BindingDB Entry DOI: 10.7270/Q2MC92NR |
More data for this
Ligand-Target Pair | |
Apelin
(Homo sapiens (Human)) | BDBM50009575
(CHEMBL3234446)Show SMILES CSCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C96H156N34O20S/c1-55(2)47-67(83(140)127-71(53-131)86(143)125-69(50-58-51-109-54-115-58)85(142)116-60(26-11-13-38-98)78(135)114-52-76(133)128-43-18-31-72(128)87(144)122-66(36-46-151-3)91(148)130-45-20-33-74(130)89(146)126-70(92(149)150)49-57-23-8-5-9-24-57)124-81(138)63(29-16-41-112-95(105)106)120-88(145)73-32-19-44-129(73)90(147)65(30-17-42-113-96(107)108)121-82(139)64(34-35-75(100)132)119-80(137)61(27-14-39-110-93(101)102)117-79(136)62(28-15-40-111-94(103)104)118-84(141)68(48-56-21-6-4-7-22-56)123-77(134)59(99)25-10-12-37-97/h4-9,21-24,51,54-55,59-74,131H,10-20,25-50,52-53,97-99H2,1-3H3,(H2,100,132)(H,109,115)(H,114,135)(H,116,142)(H,117,136)(H,118,141)(H,119,137)(H,120,145)(H,121,139)(H,122,144)(H,123,134)(H,124,138)(H,125,143)(H,126,146)(H,127,140)(H,149,150)(H4,101,102,110)(H4,103,104,111)(H4,105,106,112)(H4,107,108,113)/t59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of [125I]-pE13F from human apelin receptor expressed in CHO cell membranes after 1 hr by gamma counting analysis |
J Med Chem 57: 2908-19 (2014)
Article DOI: 10.1021/jm401789v
BindingDB Entry DOI: 10.7270/Q21J9C9F |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50368278
(CHEMBL1790497)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)OC(C)(C)C)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C50H75N9O8/c1-11-33(6)43(47(64)53-29-35-20-15-16-22-52-35)57-44(61)37(32(4)5)27-42(60)38(24-31(2)3)55-46(63)41(26-36-28-51-30-54-36)58(10)48(65)39(25-34-18-13-12-14-19-34)56-45(62)40-21-17-23-59(40)49(66)67-50(7,8)9/h12-16,18-20,22,28,30-33,37-43,60H,11,17,21,23-27,29H2,1-10H3,(H,51,54)(H,53,64)(H,55,63)(H,56,62)(H,57,61)/t33-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition constants using recombinant human renin assay |
J Med Chem 34: 2107-12 (1991)
BindingDB Entry DOI: 10.7270/Q2HQ40J6 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50368274
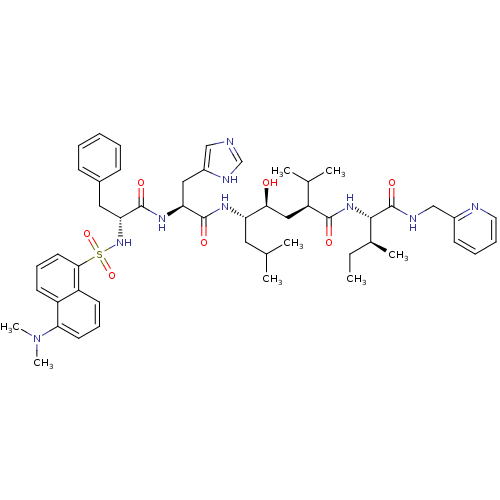
(CHEMBL1790492)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](Cc1ccccc1)NS(=O)(=O)c1cccc2c(cccc12)N(C)C)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C51H69N9O7S/c1-9-34(6)47(51(65)54-30-36-19-13-14-24-53-36)58-48(62)40(33(4)5)28-45(61)41(25-32(2)3)56-49(63)42(27-37-29-52-31-55-37)57-50(64)43(26-35-17-11-10-12-18-35)59-68(66,67)46-23-16-20-38-39(46)21-15-22-44(38)60(7)8/h10-24,29,31-34,40-43,45,47,59,61H,9,25-28,30H2,1-8H3,(H,52,55)(H,54,65)(H,56,63)(H,57,64)(H,58,62)/t34-,40-,41-,42-,43+,45-,47-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition constants using recombinant human renin assay |
J Med Chem 34: 2107-12 (1991)
BindingDB Entry DOI: 10.7270/Q2HQ40J6 |
More data for this
Ligand-Target Pair | |
Cholinergic receptor, nicotinic alpha 1
(Xenopus) | BDBM86040

(NBEP)Show SMILES Brc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13BrN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50184183
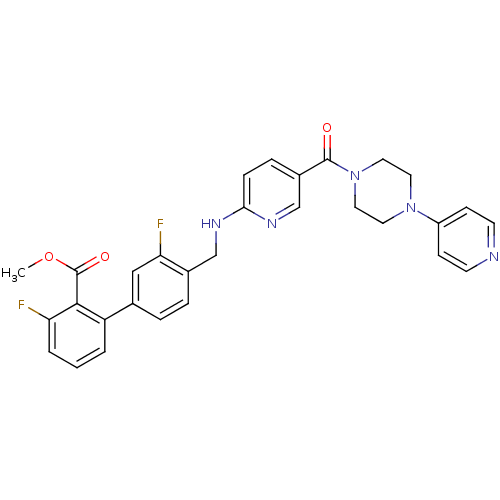
(3,3'-difluoro-4'-{[5-(4-pyridin-4-yl-piperazine-1-...)Show SMILES COC(=O)c1c(F)cccc1-c1ccc(CNc2ccc(cn2)C(=O)N2CCN(CC2)c2ccncc2)c(F)c1 Show InChI InChI=1S/C30H27F2N5O3/c1-40-30(39)28-24(3-2-4-25(28)31)20-5-6-21(26(32)17-20)18-34-27-8-7-22(19-35-27)29(38)37-15-13-36(14-16-37)23-9-11-33-12-10-23/h2-12,17,19H,13-16,18H2,1H3,(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Heptares Therapeutics Limited
Curated by ChEMBL
| Assay Description
Antagonist activity at human bradykinin B1 receptor expressed in human HEK293 cells assessed as calcium flux by FLIPR assay |
J Med Chem 54: 4283-311 (2011)
Article DOI: 10.1021/jm200371q
BindingDB Entry DOI: 10.7270/Q23N23RZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50094037
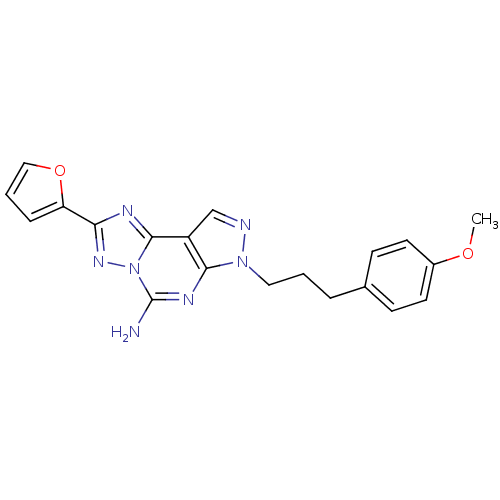
(2-Furan-2-yl-7-[3-(4-methoxy-phenyl)-propyl]-7H-py...)Show SMILES COc1ccc(CCCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)cc1 Show InChI InChI=1S/C20H19N7O2/c1-28-14-8-6-13(7-9-14)4-2-10-26-18-15(12-22-26)19-23-17(16-5-3-11-29-16)25-27(19)20(21)24-18/h3,5-9,11-12H,2,4,10H2,1H3,(H2,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milano-Bicocca
Curated by ChEMBL
| Assay Description
Inhibitory activity against human adenosine A2A receptor expressed in HEK-293 cells by displacement of [3H]-SCH-58,261 |
J Med Chem 43: 4359-62 (2000)
BindingDB Entry DOI: 10.7270/Q2Z037C8 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50156147
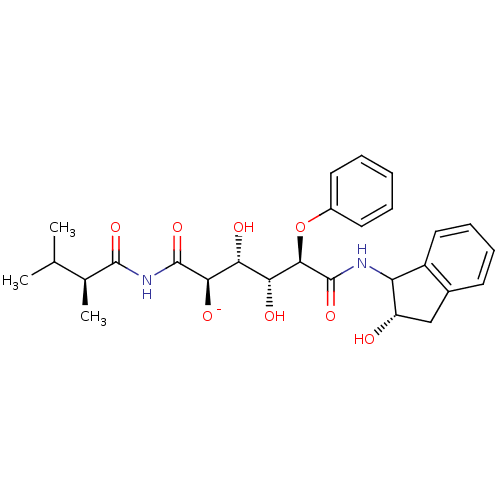
(1-(2,3-Dimethyl-butyrylamino)-3,4-dihydroxy-5-(2-h...)Show SMILES CC(C)[C@H](C)C(=O)NC(=O)[C@H]([O-])[C@H](O)[C@@H](O)[C@@H](Oc1ccccc1)C(=O)NC1[C@@H](O)Cc2ccccc12 Show InChI InChI=1S/C27H33N2O8/c1-14(2)15(3)25(34)29-26(35)23(33)21(31)22(32)24(37-17-10-5-4-6-11-17)27(36)28-20-18-12-8-7-9-16(18)13-19(20)30/h4-12,14-15,19-24,30-32H,13H2,1-3H3,(H,28,36)(H,29,34,35)/q-1/t15-,19-,20?,21+,22+,23+,24+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Inhibition constant for human immunodeficiency virus type 1 protease |
J Med Chem 47: 5953-61 (2004)
Article DOI: 10.1021/jm0499110
BindingDB Entry DOI: 10.7270/Q2FQ9XDV |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464120
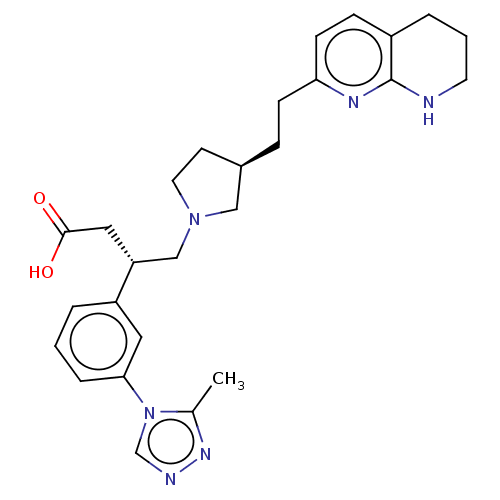
(CHEMBL4237919)Show SMILES Cc1nncn1-c1cccc(c1)[C@@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)CC(O)=O |r| Show InChI InChI=1S/C27H34N6O2/c1-19-31-29-18-33(19)25-6-2-4-22(14-25)23(15-26(34)35)17-32-13-11-20(16-32)7-9-24-10-8-21-5-3-12-28-27(21)30-24/h2,4,6,8,10,14,18,20,23H,3,5,7,9,11-13,15-17H2,1H3,(H,28,30)(H,34,35)/t20-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50012322

(CHEMBL264524 | N-(2-Hydroxy-1,1-bis-hydroxymethyl-...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)NC(CO)(CO)CO)C(C)C)C(=O)NCc1cccc[n+]1[O-] Show InChI InChI=1S/C50H76N10O11/c1-8-33(6)43(47(68)52-26-36-17-12-13-20-60(36)71)56-44(65)37(32(4)5)24-42(64)38(21-31(2)3)54-46(67)41(23-35-25-51-30-53-35)58(7)48(69)39(22-34-15-10-9-11-16-34)55-45(66)40-18-14-19-59(40)49(70)57-50(27-61,28-62)29-63/h9-13,15-17,20,25,30-33,37-43,61-64H,8,14,18-19,21-24,26-29H2,1-7H3,(H,51,53)(H,52,68)(H,54,67)(H,55,66)(H,56,65)(H,57,70)/t33?,37-,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human renin by radio-immuno assay of angiotensin I (ANG I) |
J Med Chem 34: 2107-12 (1991)
BindingDB Entry DOI: 10.7270/Q2HQ40J6 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50122243
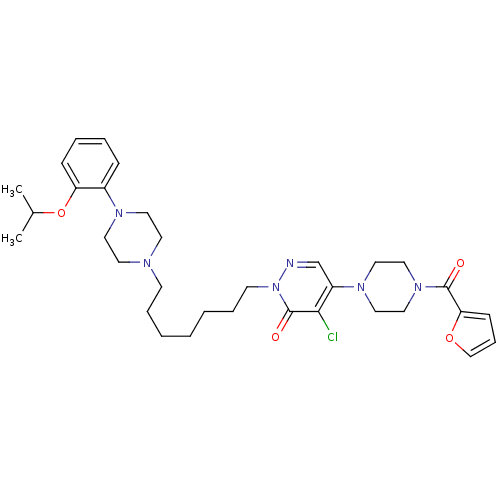
(4-Chloro-5-[4-(furan-2-carbonyl)-piperazin-1-yl]-2...)Show SMILES CC(C)Oc1ccccc1N1CCN(CCCCCCCn2ncc(N3CCN(CC3)C(=O)c3ccco3)c(Cl)c2=O)CC1 Show InChI InChI=1S/C33H45ClN6O4/c1-26(2)44-29-12-7-6-11-27(29)37-18-16-36(17-19-37)14-8-4-3-5-9-15-40-33(42)31(34)28(25-35-40)38-20-22-39(23-21-38)32(41)30-13-10-24-43-30/h6-7,10-13,24-26H,3-5,8-9,14-23H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa
Curated by ChEMBL
| Assay Description
Binding affinity towards Alpha-1 adrenergic receptor in rat cerebral cortex using [3H]prazosin |
Bioorg Med Chem Lett 13: 171-3 (2002)
BindingDB Entry DOI: 10.7270/Q2TM79GX |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86040

(NBEP)Show SMILES Brc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13BrN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 268: 1403-10 (1994)
BindingDB Entry DOI: 10.7270/Q2154FJQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50007692
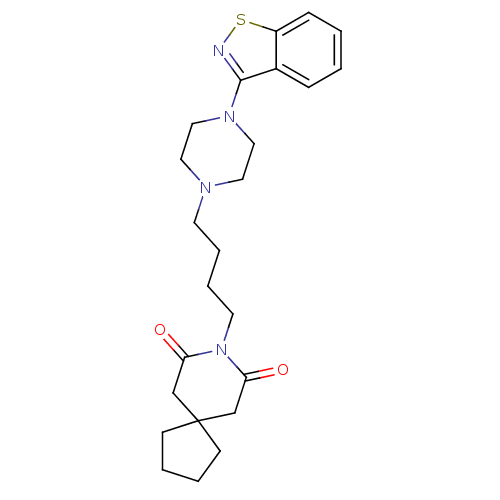
(8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...)Show SMILES O=C1CC2(CCCC2)CC(=O)N1CCCCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C24H32N4O2S/c29-21-17-24(9-3-4-10-24)18-22(30)28(21)12-6-5-11-26-13-15-27(16-14-26)23-19-7-1-2-8-20(19)31-25-23/h1-2,7-8H,3-6,9-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 268: 1403-10 (1994)
BindingDB Entry DOI: 10.7270/Q2154FJQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50451823
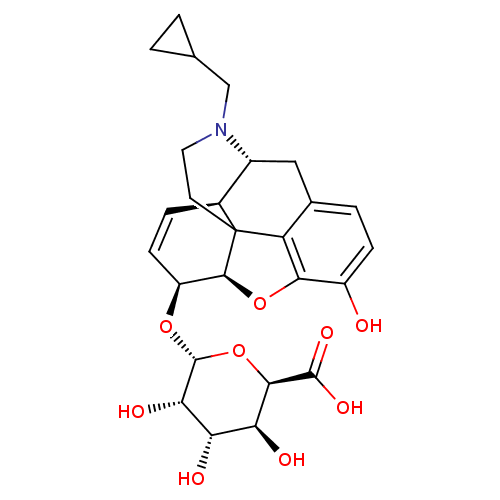
(CHEMBL2112840)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CCC14[C@@]5([H])C=C[C@@H]2O[C@@H]1O[C@H]([C@@H](O)[C@H](O)[C@@H]1O)C(O)=O)ccc3O |c:23,THB:3:4:17:9.15.14,10:9:17:4.5.6| Show InChI InChI=1S/C26H31NO9/c28-15-5-3-12-9-14-13-4-6-16(34-25-20(31)18(29)19(30)22(36-25)24(32)33)23-26(13,17(12)21(15)35-23)7-8-27(14)10-11-1-2-11/h3-6,11,13-14,16,18-20,22-23,25,28-31H,1-2,7-10H2,(H,32,33)/t13-,14+,16-,18-,19-,20-,22+,23-,25+,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ultrafine UFC Ltd
Curated by ChEMBL
| Assay Description
mu-1 receptor binding affinity in rat brain by 3H [d-Ala2, d-Leu5] enkephalin displacement. |
Bioorg Med Chem Lett 13: 1207-14 (2003)
BindingDB Entry DOI: 10.7270/Q2251JQC |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86045
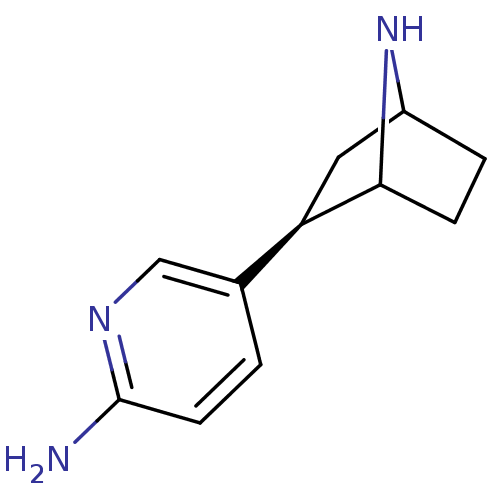
(NNEP)Show SMILES Nc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H15N3/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2,(H2,12,13)/t8?,9-,10?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464113
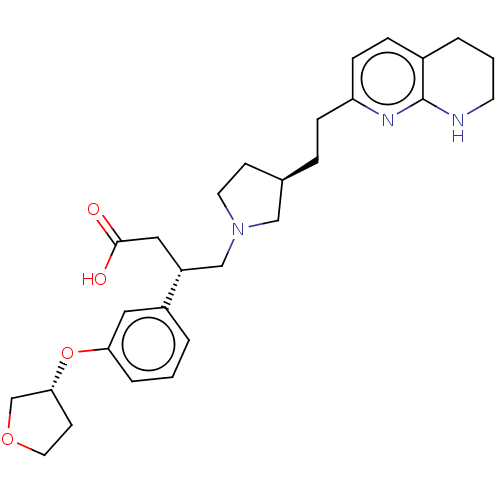
(CHEMBL4239085)Show SMILES OC(=O)C[C@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)c1cccc(O[C@@H]2CCOC2)c1 |r| Show InChI InChI=1S/C28H37N3O4/c32-27(33)16-23(22-3-1-5-25(15-22)35-26-11-14-34-19-26)18-31-13-10-20(17-31)6-8-24-9-7-21-4-2-12-29-28(21)30-24/h1,3,5,7,9,15,20,23,26H,2,4,6,8,10-14,16-19H2,(H,29,30)(H,32,33)/t20-,23-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464097
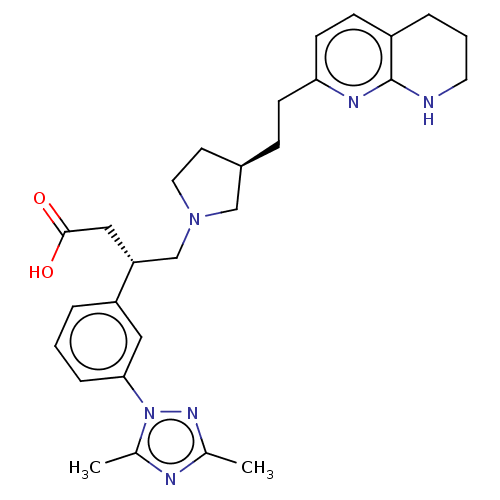
(CHEMBL4237868)Show SMILES Cc1nc(C)n(n1)-c1cccc(c1)[C@@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)CC(O)=O |r| Show InChI InChI=1S/C28H36N6O2/c1-19-30-20(2)34(32-19)26-7-3-5-23(15-26)24(16-27(35)36)18-33-14-12-21(17-33)8-10-25-11-9-22-6-4-13-29-28(22)31-25/h3,5,7,9,11,15,21,24H,4,6,8,10,12-14,16-18H2,1-2H3,(H,29,31)(H,35,36)/t21-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464110

(CHEMBL4238909)Show SMILES OC(=O)C[C@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)c1cccc(c1)N1CCOCC1 |r| Show InChI InChI=1S/C28H38N4O3/c33-27(34)18-24(23-3-1-5-26(17-23)32-13-15-35-16-14-32)20-31-12-10-21(19-31)6-8-25-9-7-22-4-2-11-29-28(22)30-25/h1,3,5,7,9,17,21,24H,2,4,6,8,10-16,18-20H2,(H,29,30)(H,33,34)/t21-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-6
(Homo sapiens (Human)) | BDBM50464100
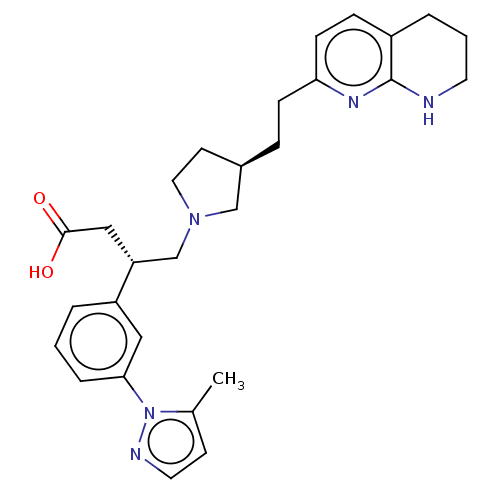
(CHEMBL4243367)Show SMILES Cc1ccnn1-c1cccc(c1)[C@@H](CN1CC[C@@H](CCc2ccc3CCCNc3n2)C1)CC(O)=O |r| Show InChI InChI=1S/C28H35N5O2/c1-20-11-14-30-33(20)26-6-2-4-23(16-26)24(17-27(34)35)19-32-15-12-21(18-32)7-9-25-10-8-22-5-3-13-29-28(22)31-25/h2,4,6,8,10-11,14,16,21,24H,3,5,7,9,12-13,15,17-19H2,1H3,(H,29,31)(H,34,35)/t21-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Binding affinity to alphaVbeta6 (unknown origin) by radioligand binding assay |
J Med Chem 61: 8417-8443 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00959
BindingDB Entry DOI: 10.7270/Q24T6N25 |
More data for this
Ligand-Target Pair | |
Vasoactive intestinal polypeptide receptor 1
(Homo sapiens (Human)) | BDBM50435130
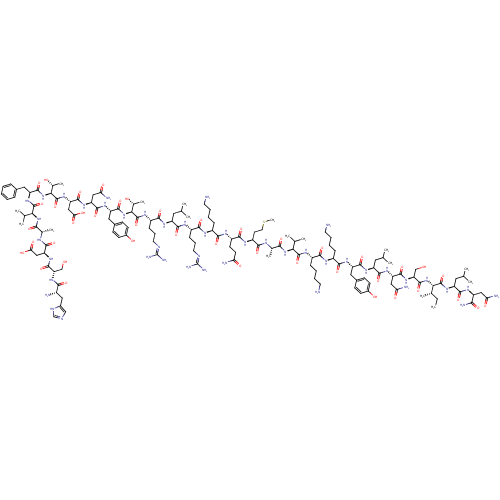
(CHEMBL1893324)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)Cc1cnc[nH]1)C(C)C)[C@@H](C)O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(N)=O Show InChI InChI=1S/C147H238N44O42S/c1-18-75(12)115(143(231)182-97(56-72(6)7)131(219)174-94(118(156)206)61-108(153)199)189-140(228)106(68-193)186-135(223)102(63-110(155)201)179-132(220)96(55-71(4)5)176-133(221)98(58-81-37-41-84(196)42-38-81)177-126(214)88(33-23-26-49-149)168-124(212)89(34-24-27-50-150)172-141(229)113(73(8)9)187-119(207)76(13)165-122(210)93(47-53-234-17)171-128(216)92(45-46-107(152)198)170-123(211)87(32-22-25-48-148)167-125(213)90(35-28-51-162-146(157)158)169-130(218)95(54-70(2)3)175-127(215)91(36-29-52-163-147(159)160)173-144(232)116(78(15)194)190-137(225)99(59-82-39-43-85(197)44-40-82)178-134(222)101(62-109(154)200)180-136(224)104(65-112(204)205)184-145(233)117(79(16)195)191-138(226)100(57-80-30-20-19-21-31-80)183-142(230)114(74(10)11)188-120(208)77(14)166-129(217)103(64-111(202)203)181-139(227)105(67-192)185-121(209)86(151)60-83-66-161-69-164-83/h19-21,30-31,37-44,66,69-79,86-106,113-117,192-197H,18,22-29,32-36,45-65,67-68,148-151H2,1-17H3,(H2,152,198)(H2,153,199)(H2,154,200)(H2,155,201)(H2,156,206)(H,161,164)(H,165,210)(H,166,217)(H,167,213)(H,168,212)(H,169,218)(H,170,211)(H,171,216)(H,172,229)(H,173,232)(H,174,219)(H,175,215)(H,176,221)(H,177,214)(H,178,222)(H,179,220)(H,180,224)(H,181,227)(H,182,231)(H,183,230)(H,184,233)(H,185,209)(H,186,223)(H,187,207)(H,188,208)(H,189,228)(H,190,225)(H,191,226)(H,202,203)(H,204,205)(H4,157,158,162)(H4,159,160,163)/t75-,76-,77-,78+,79+,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,113-,114-,115-,116-,117-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human VPAC1 receptor by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50053688
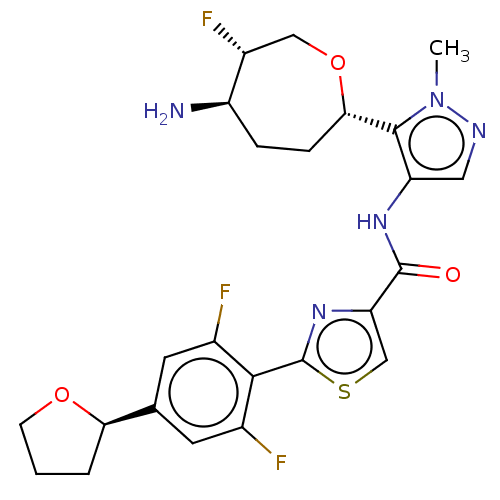
(CHEMBL3318856 | US9328106, 380)Show SMILES Cn1ncc(NC(=O)c2csc(n2)-c2c(F)cc(cc2F)[C@H]2CCCO2)c1[C@@H]1CC[C@@H](N)[C@H](F)CO1 |r| Show InChI InChI=1S/C24H26F3N5O3S/c1-32-22(20-5-4-16(28)15(27)10-35-20)17(9-29-32)30-23(33)18-11-36-24(31-18)21-13(25)7-12(8-14(21)26)19-3-2-6-34-19/h7-9,11,15-16,19-20H,2-6,10,28H2,1H3,(H,30,33)/t15-,16-,19-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) |
ACS Med Chem Lett 5: 730-1 (2014)
Article DOI: 10.1021/ml5001678
BindingDB Entry DOI: 10.7270/Q2SN0BMH |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50015490
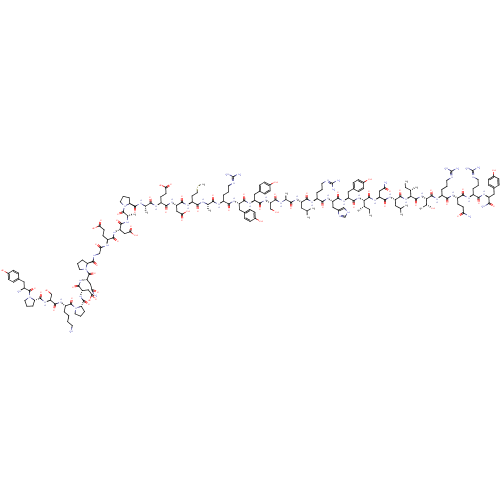
(CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C189H285N55O57S/c1-15-93(7)148(179(295)234-128(81-140(193)254)168(284)226-123(74-92(5)6)171(287)239-149(94(8)16-2)180(296)240-150(99(13)247)181(297)222-115(31-22-67-208-189(202)203)156(272)220-117(56-59-139(192)253)161(277)218-113(29-20-65-206-187(198)199)157(273)224-121(151(195)267)76-101-38-48-107(249)49-39-101)238-172(288)126(79-104-44-54-110(252)55-45-104)229-167(283)127(80-105-86-204-90-210-105)230-159(275)114(30-21-66-207-188(200)201)219-164(280)122(73-91(3)4)225-154(270)96(10)212-173(289)133(88-245)236-166(282)125(78-103-42-52-109(251)53-43-103)228-165(281)124(77-102-40-50-108(250)51-41-102)227-158(274)112(28-19-64-205-186(196)197)216-152(268)95(9)211-155(271)119(62-72-302-14)221-169(285)130(84-146(263)264)232-162(278)118(58-61-144(259)260)217-153(269)97(11)213-176(292)136-33-24-68-241(136)182(298)98(12)214-163(279)129(83-145(261)262)231-160(276)116(57-60-143(257)258)215-142(256)87-209-175(291)135-32-23-70-243(135)185(301)132(82-141(194)255)235-170(286)131(85-147(265)266)233-177(293)138-35-26-71-244(138)184(300)120(27-17-18-63-190)223-174(290)134(89-246)237-178(294)137-34-25-69-242(137)183(299)111(191)75-100-36-46-106(248)47-37-100/h36-55,86,90-99,111-138,148-150,245-252H,15-35,56-85,87-89,190-191H2,1-14H3,(H2,192,253)(H2,193,254)(H2,194,255)(H2,195,267)(H,204,210)(H,209,291)(H,211,271)(H,212,289)(H,213,292)(H,214,279)(H,215,256)(H,216,268)(H,217,269)(H,218,277)(H,219,280)(H,220,272)(H,221,285)(H,222,297)(H,223,290)(H,224,273)(H,225,270)(H,226,284)(H,227,274)(H,228,281)(H,229,283)(H,230,275)(H,231,276)(H,232,278)(H,233,293)(H,234,295)(H,235,286)(H,236,282)(H,237,294)(H,238,288)(H,239,287)(H,240,296)(H,257,258)(H,259,260)(H,261,262)(H,263,264)(H,265,266)(H4,196,197,205)(H4,198,199,206)(H4,200,201,207)(H4,202,203,208)/t93-,94-,95-,96-,97-,98-,99+,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,148-,149-,150-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human neuropeptide Y receptor type 2 by radioligand displacement assay |
Eur J Med Chem 63: 85-94 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.044
BindingDB Entry DOI: 10.7270/Q2JH3NKC |
More data for this
Ligand-Target Pair | |
cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1
(Xenopus) | BDBM86045

(NNEP)Show SMILES Nc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H15N3/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2,(H2,12,13)/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data