

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50044868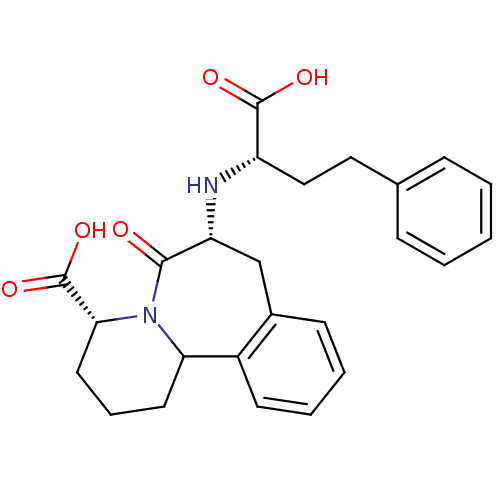 (7-(1-Carboxy-3-phenyl-propylamino)-6-oxo-1,2,3,4,6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50044866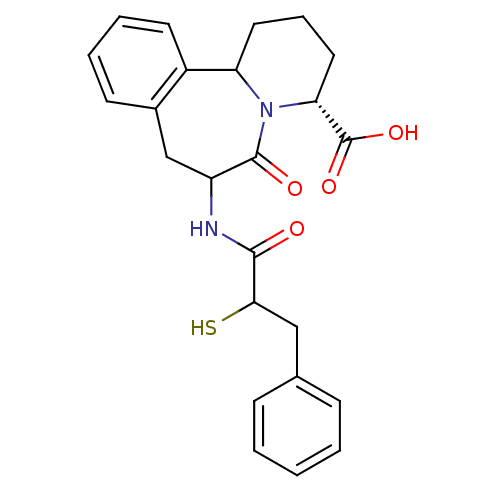 (7-(2-Mercapto-3-phenyl-propionylamino)-6-oxo-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rat kidney neutral endopeptidase (NEP) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50044866 (7-(2-Mercapto-3-phenyl-propionylamino)-6-oxo-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rat kidney neutral endopeptidase (NEP) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50044866 (7-(2-Mercapto-3-phenyl-propionylamino)-6-oxo-1,2,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50044866 (7-(2-Mercapto-3-phenyl-propionylamino)-6-oxo-1,2,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacylase sirtuin-5, mitochondrial (Homo sapiens (Human)) | BDBM50598638 (CHEMBL5199270) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00687 BindingDB Entry DOI: 10.7270/Q2125XQ3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM86210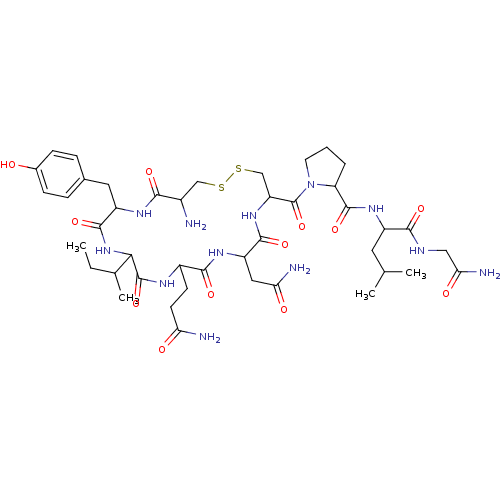 (CAS_50-56-6 | NSC_439302 | Oxytocin) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LCG Bioscience Curated by PDSP Ki Database | J Pharmacol Exp Ther 306: 253-61 (2003) Article DOI: 10.1124/jpet.103.049395 BindingDB Entry DOI: 10.7270/Q2MC8XKT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description In vitro inhibition constant for Aurora-A | J Med Chem 49: 955-70 (2006) Article DOI: 10.1021/jm050786h BindingDB Entry DOI: 10.7270/Q24J0FXV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50347563 (CHEMBL1801740) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human Angiotensin receptor 1 | J Med Chem 54: 4219-33 (2011) Article DOI: 10.1021/jm200409s BindingDB Entry DOI: 10.7270/Q2SB463J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM86210 (CAS_50-56-6 | NSC_439302 | Oxytocin) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LCG Bioscience Curated by PDSP Ki Database | J Pharmacol Exp Ther 306: 253-61 (2003) Article DOI: 10.1124/jpet.103.049395 BindingDB Entry DOI: 10.7270/Q2MC8XKT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26295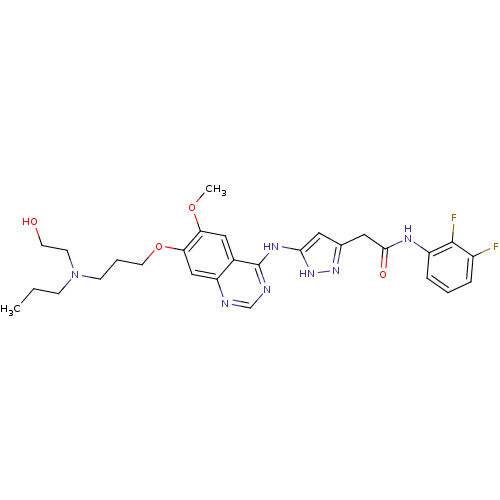 (CHEMBL214849 | N-(2,3-difluorophenyl)-2-{3-[(7-{3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26292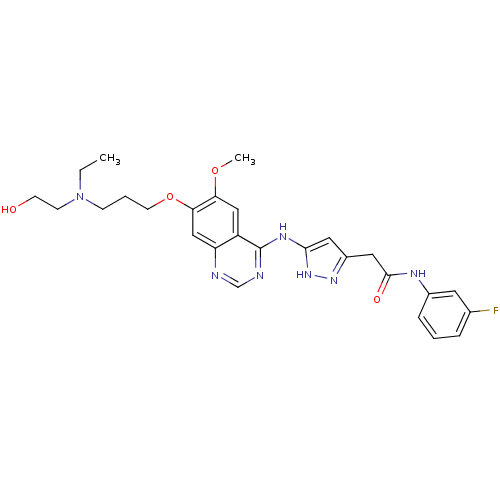 (2-{3-[(7-{3-[ethyl(2-hydroxyethyl)amino]propoxy}-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26296 (CHEMBL216053 | N-(2,3-difluorophenyl)-2-{3-[(7-{3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26291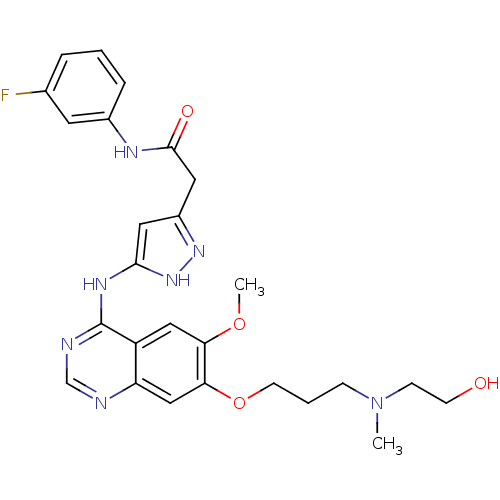 (N-(3-fluorophenyl)-2-{3-[(7-{3-[(2-hydroxyethyl)(m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26290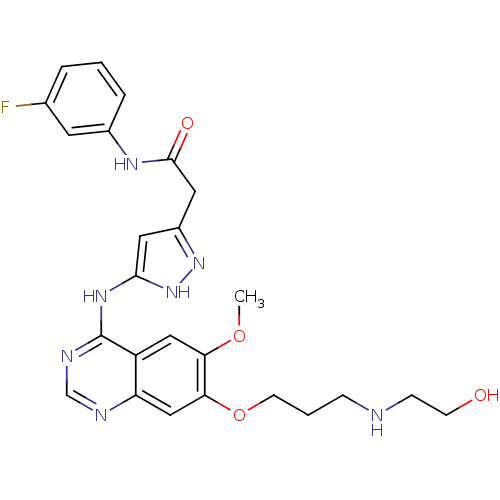 (N-(3-fluorophenyl)-2-{3-[(7-{3-[(2-hydroxyethyl)am...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26289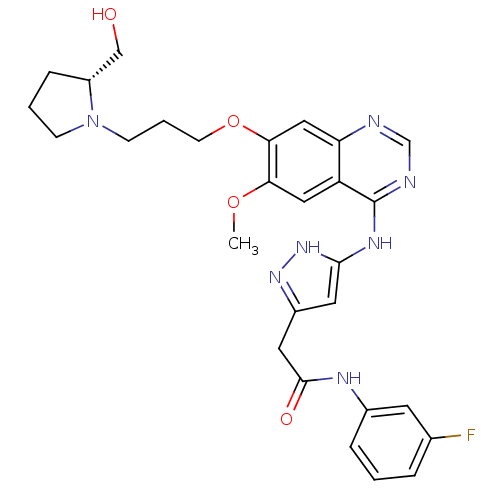 (N-(3-fluorophenyl)-2-{3-[(7-{3-[(2R)-2-(hydroxymet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26288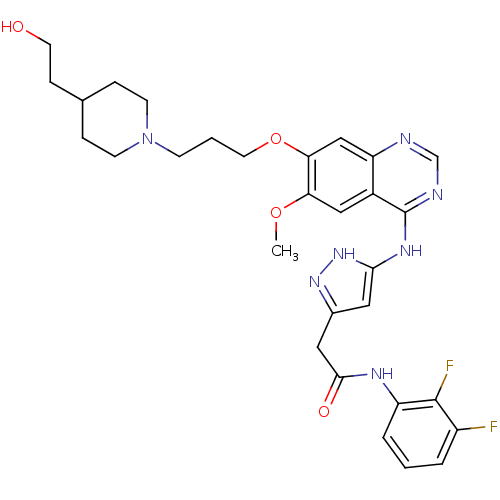 (N-(2,3-difluorophenyl)-2-{3-[(7-{3-[4-(2-hydroxyet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26287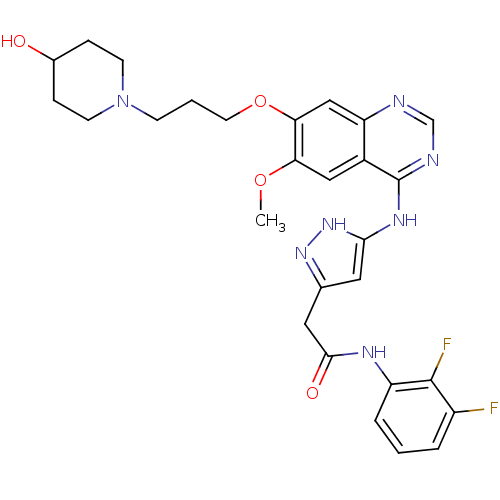 (N-(2,3-difluorophenyl)-2-[3-({7-[3-(4-hydroxypiper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26286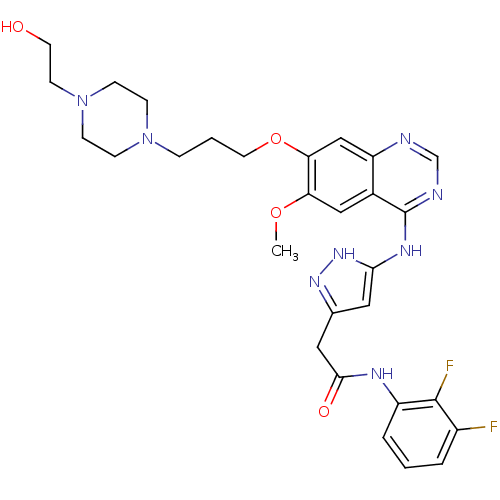 (N-(2,3-difluorophenyl)-2-{3-[(7-{3-[4-(2-hydroxyet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26285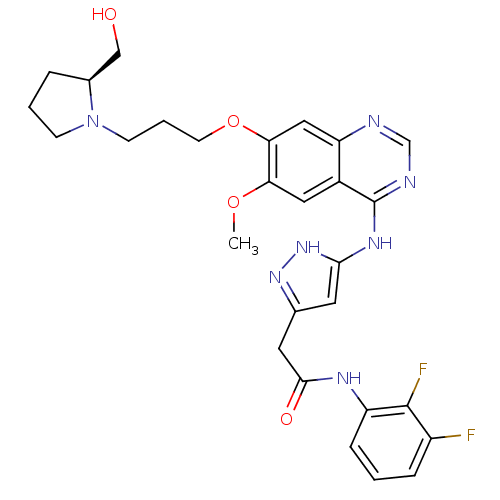 (N-(2,3-difluorophenyl)-2-{3-[(7-{3-[(2S)-2-(hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26284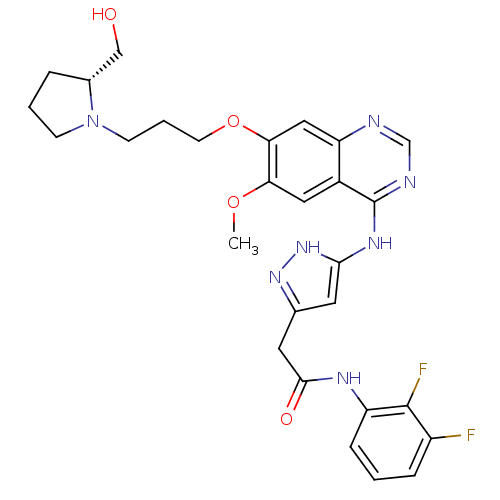 (N-(2,3-difluorophenyl)-2-{3-[(7-{3-[(2R)-2-(hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26277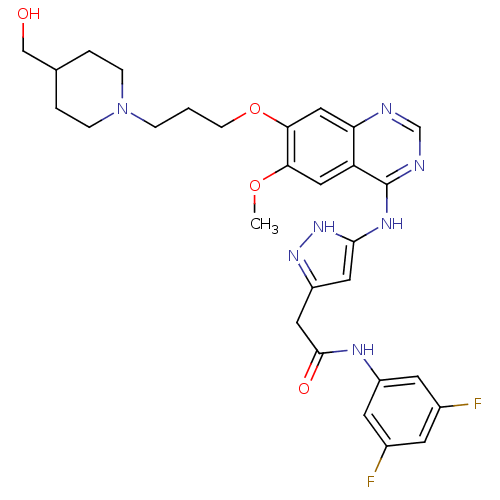 (N-(3,5-difluorophenyl)-2-{3-[(7-{3-[4-(hydroxymeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26294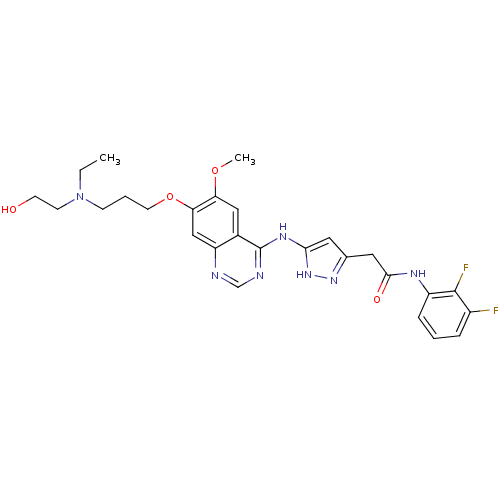 (CHEMBL214848 | N-(2,3-difluorophenyl)-2-{3-[(7-{3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26302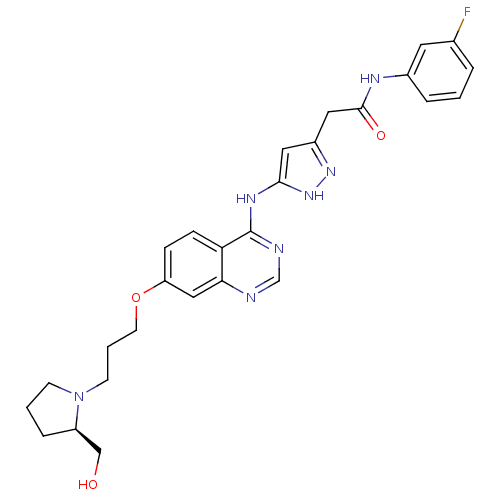 (N-(3-fluorophenyl)-2-{3-[(7-{3-[(2R)-2-(hydroxymet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26297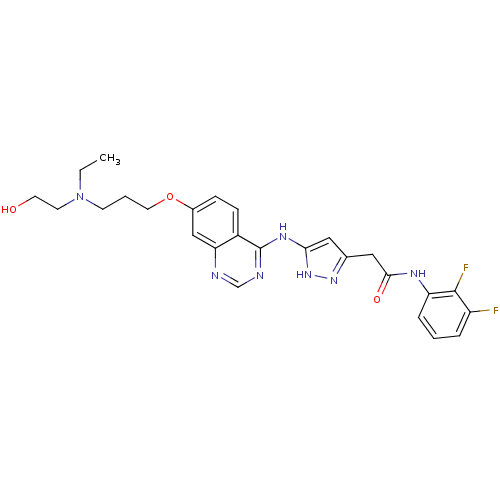 (CHEMBL215322 | N-(2,3-difluorophenyl)-2-{3-[(7-{3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26298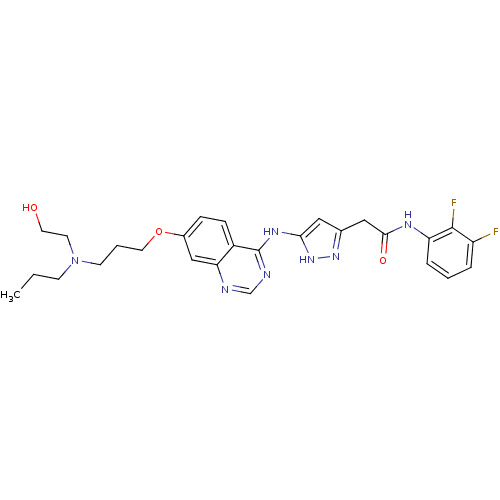 (N-(2,3-difluorophenyl)-2-{3-[(7-{3-[(2-hydroxyethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26299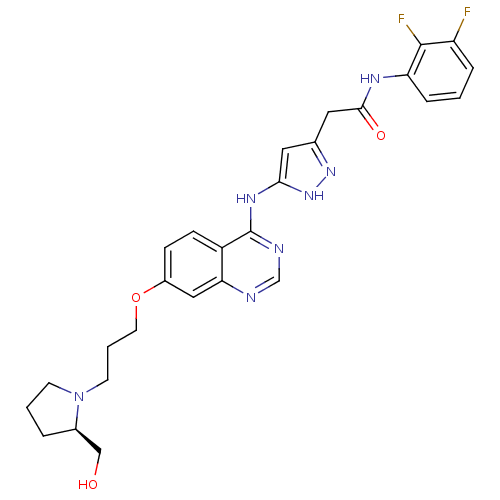 (N-(2,3-difluorophenyl)-2-{3-[(7-{3-[(2R)-2-(hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26300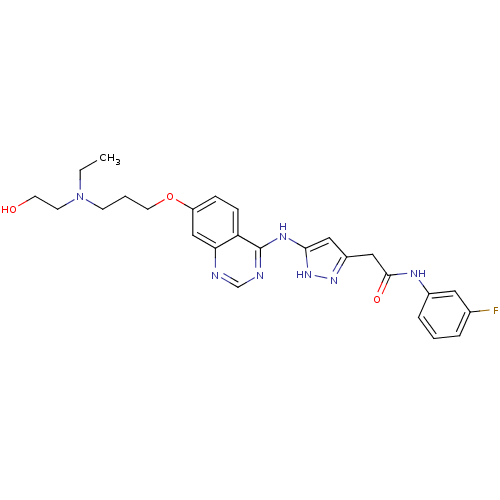 (2-{3-[(7-{3-[ethyl(2-hydroxyethyl)amino]propoxy}qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26301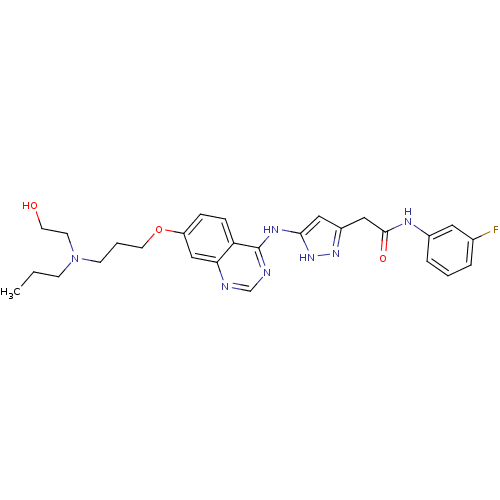 (N-(3-fluorophenyl)-2-{3-[(7-{3-[(2-hydroxyethyl)(p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT2A (Homo sapiens (Human)) | BDBM50234923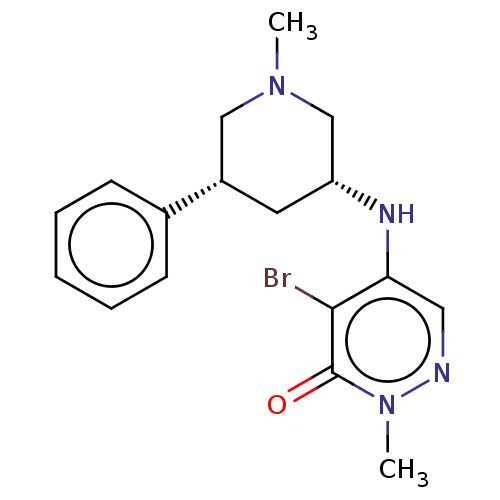 (CHEMBL4069412) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to human partial length GCN5 expressed in bacterial expression system by BROMOscan method | J Med Chem 60: 695-709 (2017) Article DOI: 10.1021/acs.jmedchem.6b01566 BindingDB Entry DOI: 10.7270/Q2W09863 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone acetyltransferase KAT2B (Homo sapiens (Human)) | BDBM50234923 (CHEMBL4069412) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to human partial length PCAF bromodomain expressed in mammalian expression system by BROMOscan method | J Med Chem 60: 695-709 (2017) Article DOI: 10.1021/acs.jmedchem.6b01566 BindingDB Entry DOI: 10.7270/Q2W09863 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT2A (Homo sapiens (Human)) | BDBM50234923 (CHEMBL4069412) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to human partial length GCN5 expressed in bacterial expression system by BROMOscan method | J Med Chem 60: 695-709 (2017) Article DOI: 10.1021/acs.jmedchem.6b01566 BindingDB Entry DOI: 10.7270/Q2W09863 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone acetyltransferase KAT2B (Homo sapiens (Human)) | BDBM50234923 (CHEMBL4069412) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to human partial length PCAF bromodomain expressed in mammalian expression system by BROMOscan method | J Med Chem 60: 695-709 (2017) Article DOI: 10.1021/acs.jmedchem.6b01566 BindingDB Entry DOI: 10.7270/Q2W09863 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM86210 (CAS_50-56-6 | NSC_439302 | Oxytocin) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LCG Bioscience Curated by PDSP Ki Database | J Pharmacol Exp Ther 306: 253-61 (2003) Article DOI: 10.1124/jpet.103.049395 BindingDB Entry DOI: 10.7270/Q2MC8XKT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50044867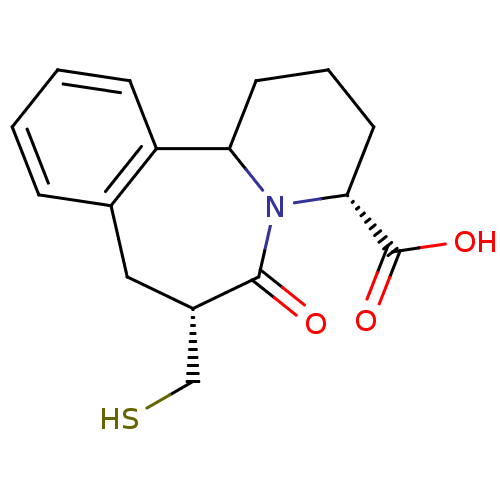 (7-Mercaptomethyl-6-oxo-1,2,3,4,6,7,8,12b-octahydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rat kidney neutral endopeptidase (NEP) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50024096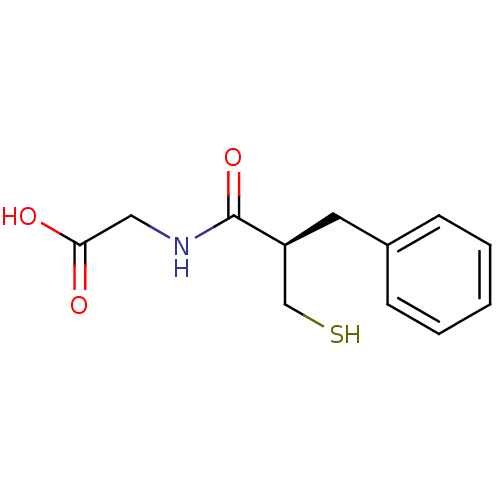 (((R)-2-Mercaptomethyl-3-phenyl-propionylamino)-ace...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rat kidney neutral endopeptidase (NEP) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26276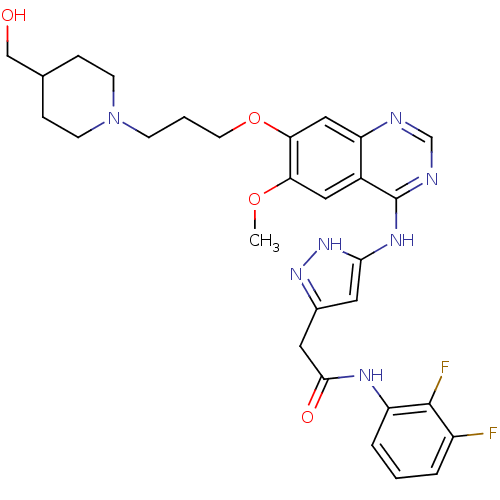 (N-(2,3-difluorophenyl)-2-{3-[(7-{3-[4-(hydroxymeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26293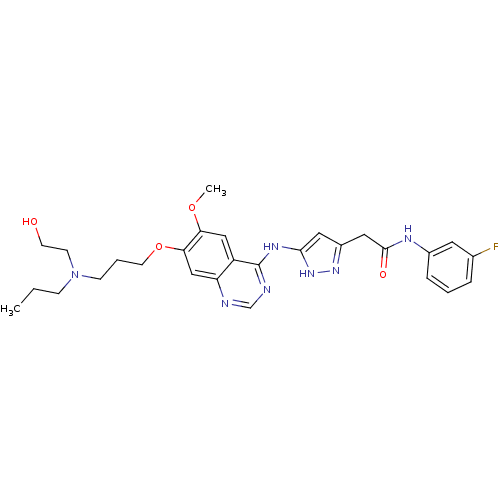 (CHEMBL217804 | N-(3-fluorophenyl)-2-{3-[(7-{3-[(2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26274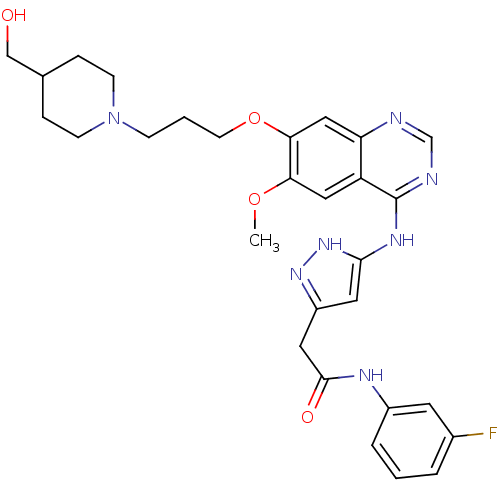 (CHEMBL216769 | N-(3-fluorophenyl)-2-{3-[(7-{3-[4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50044869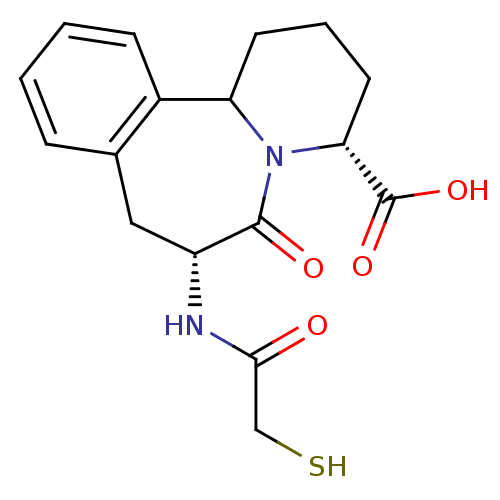 (7-(2-Mercapto-acetylamino)-6-oxo-1,2,3,4,6,7,8,12b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM86209 (Atosiban | CAS_90779-69-4 | NSC_0) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LCG Bioscience Curated by PDSP Ki Database | J Pharmacol Exp Ther 306: 253-61 (2003) Article DOI: 10.1124/jpet.103.049395 BindingDB Entry DOI: 10.7270/Q2MC8XKT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase C (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description In vitro inhibition constant for Aurora-C | J Med Chem 49: 955-70 (2006) Article DOI: 10.1021/jm050786h BindingDB Entry DOI: 10.7270/Q24J0FXV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50044869 (7-(2-Mercapto-acetylamino)-6-oxo-1,2,3,4,6,7,8,12b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rat kidney neutral endopeptidase (NEP) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacylase sirtuin-5, mitochondrial (Homo sapiens (Human)) | BDBM50540057 (CHEMBL4636862) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00687 BindingDB Entry DOI: 10.7270/Q2125XQ3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM158154 (US10081622, Compound 11 | US10370379, Entrectinib ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of recombinant ALK (unknown origin) | J Med Chem 59: 3392-408 (2016) Article DOI: 10.1021/acs.jmedchem.6b00064 BindingDB Entry DOI: 10.7270/Q27M09TG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| NAD-dependent protein deacylase sirtuin-5, mitochondrial (Homo sapiens (Human)) | BDBM50598639 (CHEMBL5209031) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00687 BindingDB Entry DOI: 10.7270/Q2125XQ3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50044867 (7-Mercaptomethyl-6-oxo-1,2,3,4,6,7,8,12b-octahydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26278 (N-(3-chlorophenyl)-2-{3-[(7-{3-[4-(hydroxymethyl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26282 (2-{3-[(7-{3-[4-(hydroxymethyl)piperidin-1-yl]propo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | -45.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26283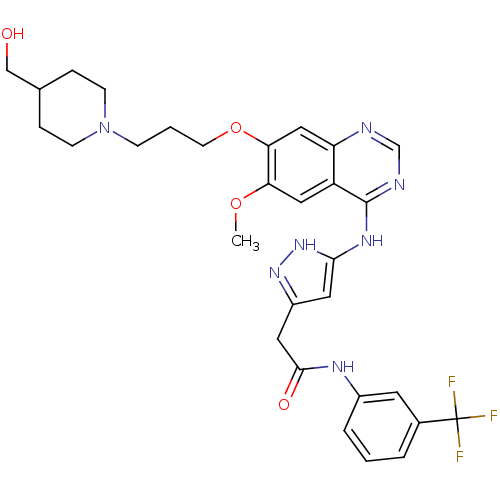 (2-{3-[(7-{3-[4-(hydroxymethyl)piperidin-1-yl]propo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4409 total ) | Next | Last >> |