

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50355500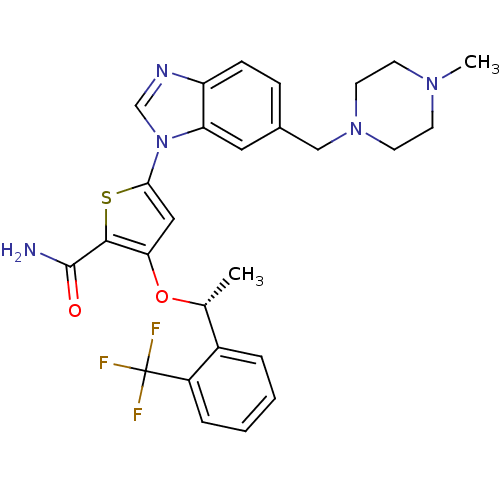 (CHEMBL1908394 | US9695172, GSK461364) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center Curated by ChEMBL | Assay Description Inhibition of human recombinant His6-tagged PLK1 (1 to 603 residues) using casein as substrate preincubated for 60 mins followed by polo box peptide ... | J Med Chem 60: 7863-7875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00996 BindingDB Entry DOI: 10.7270/Q26112G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50239566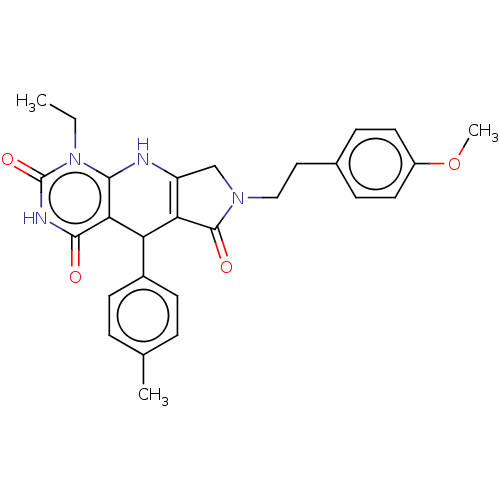 (CHEMBL4103140) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRD4 bromodomain 1 (42 to 168 residues)(unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence an... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50239567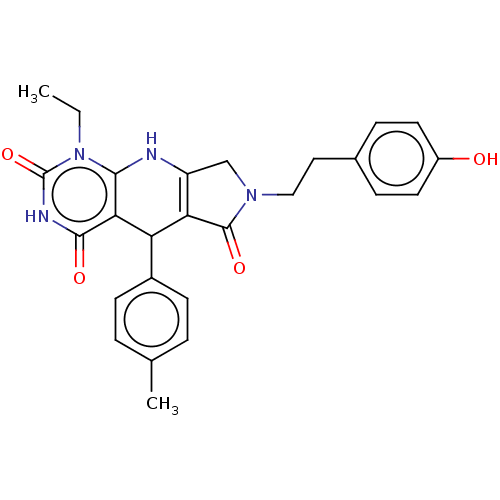 (CHEMBL4085204) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRD4 bromodomain 1 (42 to 168 residues)(unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence an... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50239568 (CHEMBL4092949) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRD4 bromodomain 1 (42 to 168 residues)(unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence an... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50239586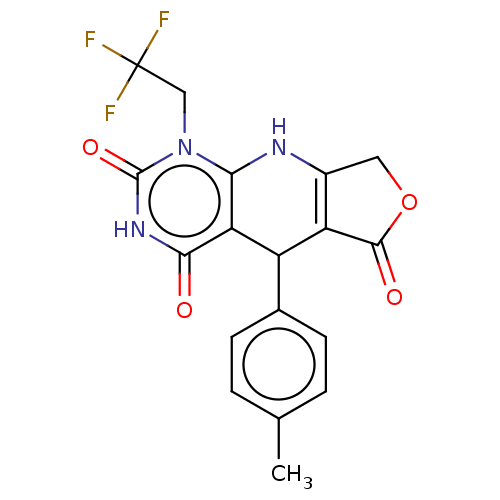 (CHEMBL4072799) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRD4 bromodomain 1 (42 to 168 residues)(unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence an... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50239565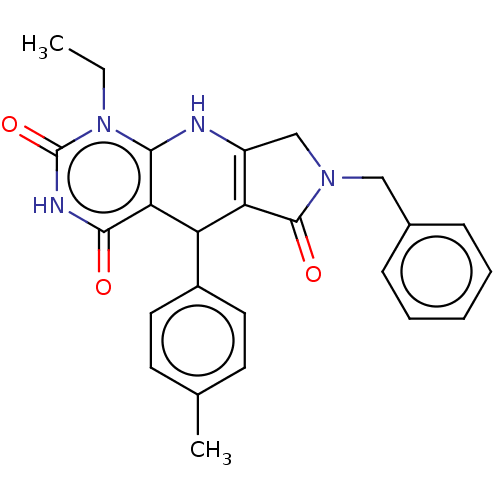 (CHEMBL4065286) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRD4 bromodomain 1 (42 to 168 residues)(unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence an... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50239567 (CHEMBL4085204) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRDT bromodomain 1 (29 to 134 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence a... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50239566 (CHEMBL4103140) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRDT bromodomain 1 (29 to 134 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence a... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50239568 (CHEMBL4092949) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRDT bromodomain 1 (29 to 134 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence a... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50239565 (CHEMBL4065286) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRDT bromodomain 1 (29 to 134 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence a... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50239588 (CHEMBL4077905) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRD4 bromodomain 1 (42 to 168 residues)(unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence an... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50239585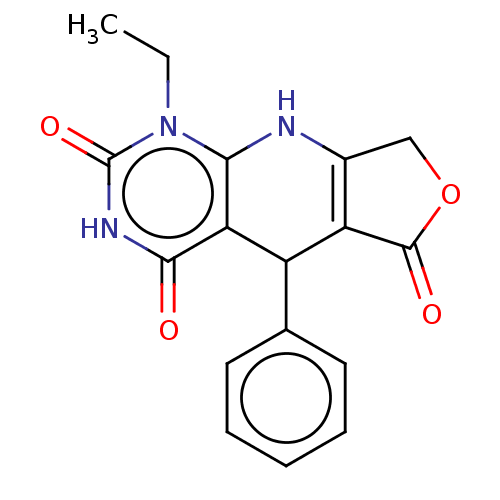 (CHEMBL4094028) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRD4 bromodomain 1 (42 to 168 residues)(unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence an... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50239562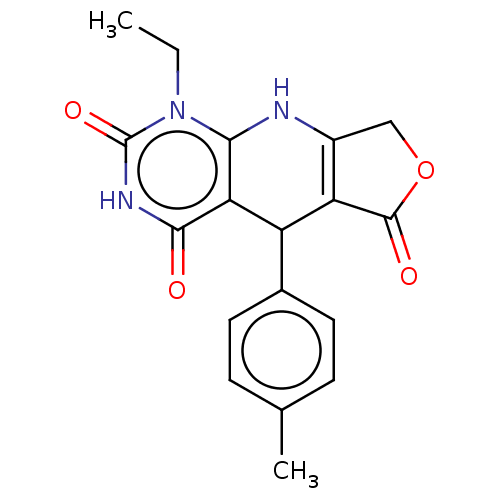 (CHEMBL4104179) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRD4 bromodomain 1 (42 to 168 residues)(unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence an... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50239562 (CHEMBL4104179) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRDT bromodomain 1 (29 to 134 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence a... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50239586 (CHEMBL4072799) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRDT bromodomain 1 (29 to 134 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence a... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50239588 (CHEMBL4077905) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRDT bromodomain 1 (29 to 134 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence a... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50239574 (CHEMBL4094424) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRD4 bromodomain 1 (42 to 168 residues)(unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence an... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50239571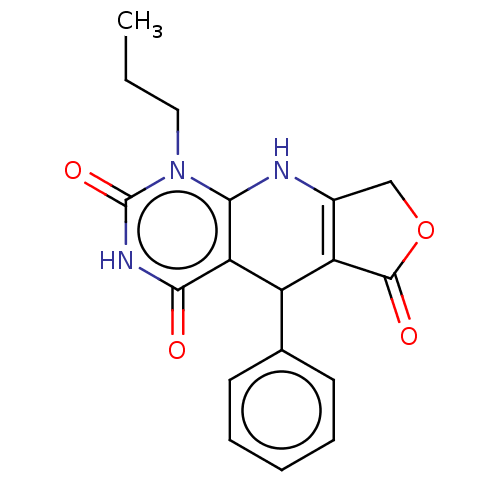 (CHEMBL4088746) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRD4 bromodomain 1 (42 to 168 residues)(unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence an... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50239585 (CHEMBL4094028) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRDT bromodomain 1 (29 to 134 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence a... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50239571 (CHEMBL4088746) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRDT bromodomain 1 (29 to 134 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence a... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50239577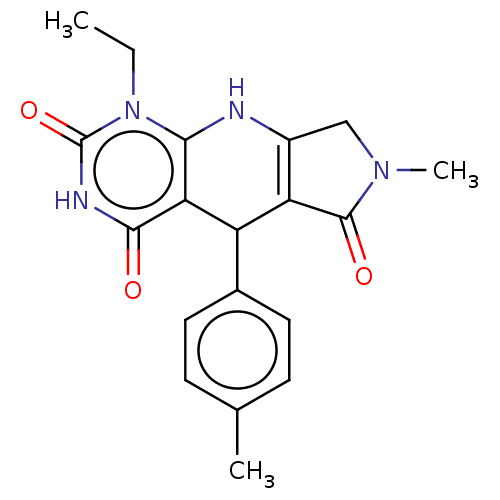 (CHEMBL4064204) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRD4 bromodomain 1 (42 to 168 residues)(unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence an... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50239574 (CHEMBL4094424) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Affinity constant of compound was evaluated in human brain | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50239577 (CHEMBL4064204) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRDT bromodomain 1 (29 to 134 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence a... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50239576 (CHEMBL4093510) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory effect on human lymphoblast (Molt/4F) thymidylate synthase | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50239564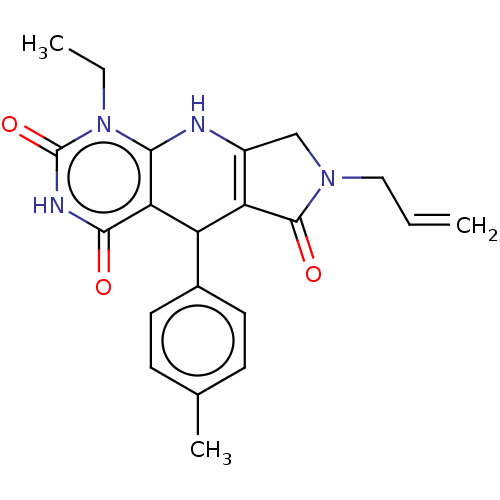 (CHEMBL4089152) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRD4 bromodomain 1 (42 to 168 residues)(unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence an... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50239576 (CHEMBL4093510) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRDT bromodomain 1 (29 to 134 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence a... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50239570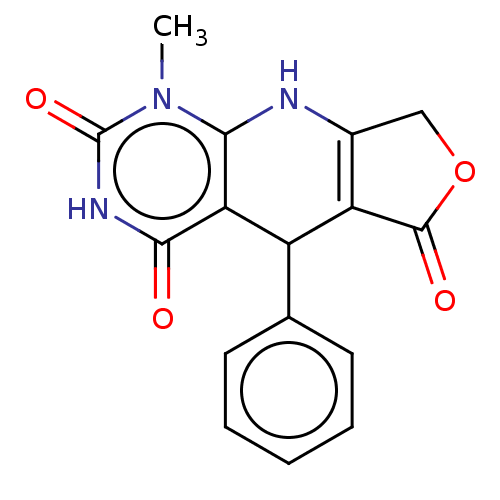 (CHEMBL4066490) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRDT bromodomain 1 (29 to 134 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence a... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50239575 (CHEMBL4066935) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRD4 bromodomain 1 (42 to 168 residues)(unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence an... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50239587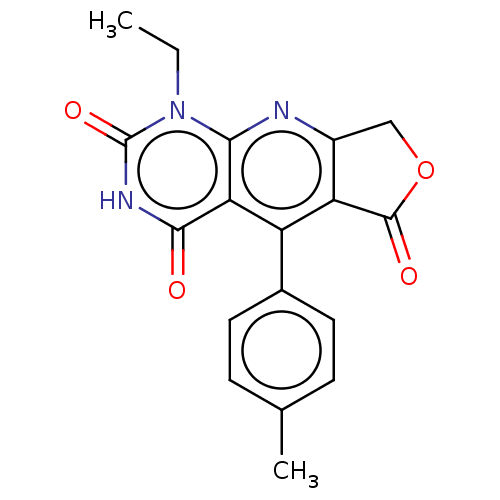 (CHEMBL4067951) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory effect on murine leukemia (L1210) thymidylate synthase | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50239575 (CHEMBL4066935) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRDT bromodomain 1 (29 to 134 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence a... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50239587 (CHEMBL4067951) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRDT bromodomain 1 (29 to 134 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence a... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50239573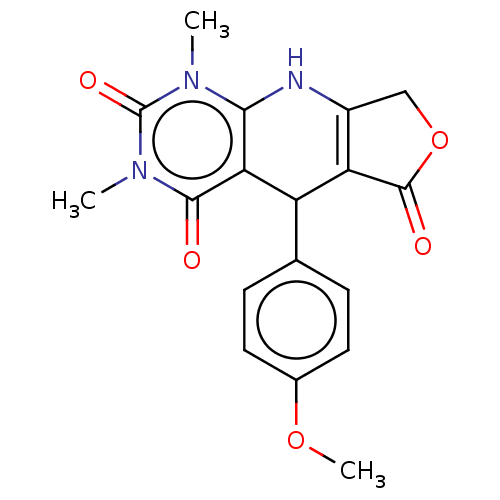 (CHEMBL4102160) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRDT bromodomain 1 (29 to 134 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence a... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50239572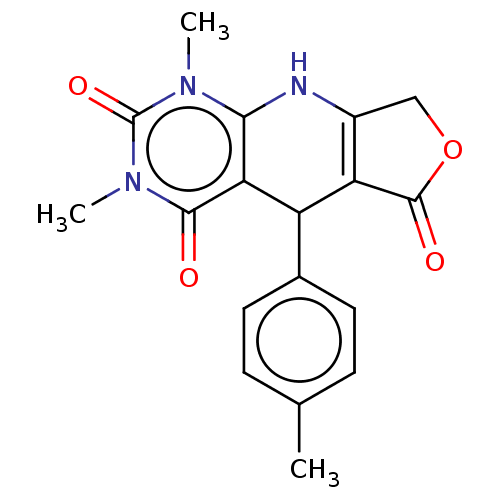 (CHEMBL4060512) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRDT bromodomain 1 (29 to 134 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence a... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50239578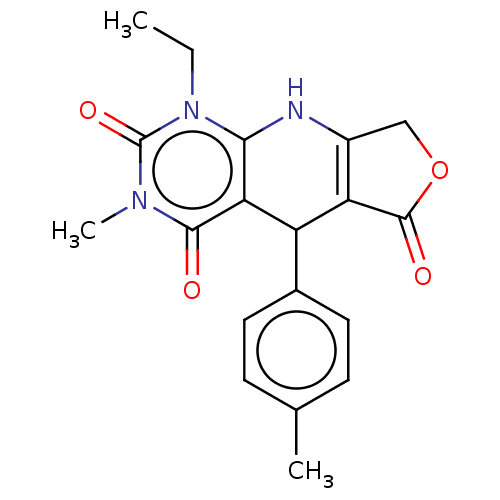 (CHEMBL4085767) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory effect on Lactobacillus casei thymidylate synthase | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50239569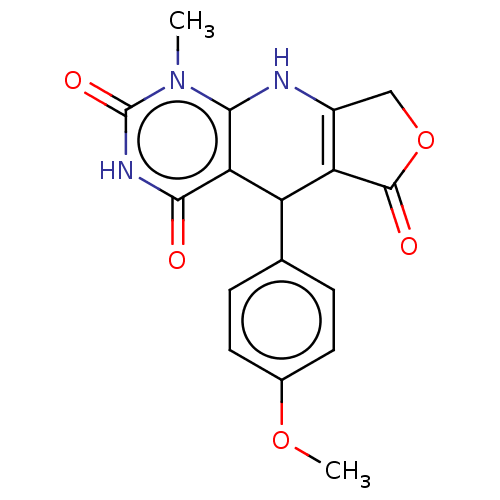 (CHEMBL4086263) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRDT bromodomain 1 (29 to 134 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence a... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain testis-specific protein (Homo sapiens (Human)) | BDBM50239578 (CHEMBL4085767) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of BI-BODIPY binding to BRDT bromodomain 1 (29 to 134 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) by fluorescence a... | J Med Chem 60: 4805-4817 (2017) Article DOI: 10.1021/acs.jmedchem.6b01336 BindingDB Entry DOI: 10.7270/Q2PC34JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/potassium-transporting ATPase subunit alpha-4/beta-1 (Rattus norvegicus) | BDBM50255109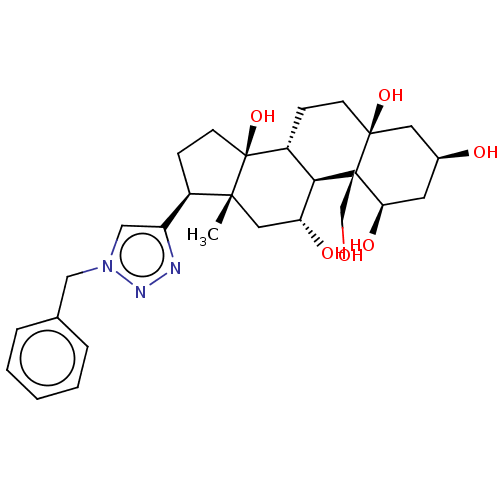 (CHEMBL4081196) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Institute for Therapeutics Discovery and Development, College of Pharmacy , University of Minnesota , Minneapolis , Minnesota 55414 , United States. Curated by ChEMBL | Assay Description Inhibition of recombinant rat Na+/K+-ATPase alpha4/beta1 expressed in baculovirus infected insect Sf9 cell membranes using [gamma-32P]ATP as substrat... | J Med Chem 61: 1800-1820 (2018) Article DOI: 10.1021/acs.jmedchem.7b00925 BindingDB Entry DOI: 10.7270/Q2T43WJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/potassium-transporting ATPase subunit alpha-4/beta-1 (Rattus norvegicus) | BDBM50255111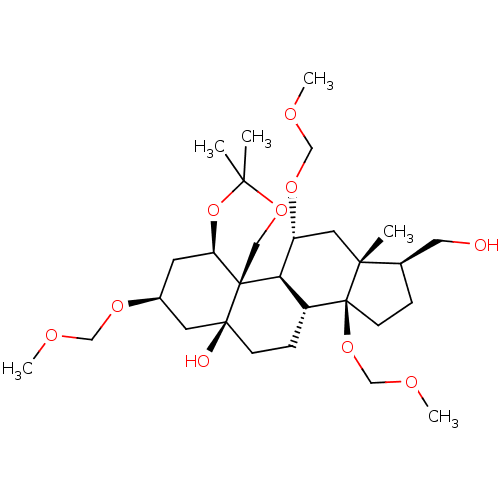 (CHEMBL4092961) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Institute for Therapeutics Discovery and Development, College of Pharmacy , University of Minnesota , Minneapolis , Minnesota 55414 , United States. Curated by ChEMBL | Assay Description Inhibition of recombinant rat Na+/K+-ATPase alpha4/beta1 expressed in baculovirus infected insect Sf9 cell membranes using [gamma-32P]ATP as substrat... | J Med Chem 61: 1800-1820 (2018) Article DOI: 10.1021/acs.jmedchem.7b00925 BindingDB Entry DOI: 10.7270/Q2T43WJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/potassium-transporting ATPase subunit alpha-4/beta-1 (Rattus norvegicus) | BDBM50255122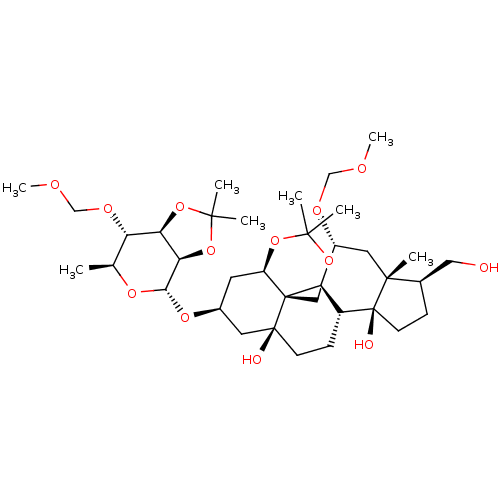 (CHEMBL4059538) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Institute for Therapeutics Discovery and Development, College of Pharmacy , University of Minnesota , Minneapolis , Minnesota 55414 , United States. Curated by ChEMBL | Assay Description Inhibition of recombinant rat Na+/K+-ATPase alpha4/beta1 expressed in baculovirus infected insect Sf9 cell membranes using [gamma-32P]ATP as substrat... | J Med Chem 61: 1800-1820 (2018) Article DOI: 10.1021/acs.jmedchem.7b00925 BindingDB Entry DOI: 10.7270/Q2T43WJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM25121 (4-{[(7R)-8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal GST-tagged PLK1 (1 to 603 residues) expressed in baculovirus expression system using casein as substrate a... | J Med Chem 60: 7863-7875 (2017) Article DOI: 10.1021/acs.jmedchem.7b00996 BindingDB Entry DOI: 10.7270/Q26112G9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM50059889 ((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of CDK5/P25 (unknown origin)-mediated phosphorylation of peptide substrate incubated for 15 mins prior to substrate addition measured afte... | J Med Chem 56: 3768-82 (2013) Article DOI: 10.1021/jm301234k BindingDB Entry DOI: 10.7270/Q25T3MV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK2/Cyclin A1 (unknown origin) using histone H1 as substrate preincubated for 20 mins followed by 33P-ATP addition and measured after ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113232 BindingDB Entry DOI: 10.7270/Q22J6GMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/potassium-transporting ATPase subunit alpha-4/beta-1 (Rattus norvegicus) | BDBM50255138 (CHEMBL4066361) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Institute for Therapeutics Discovery and Development, College of Pharmacy , University of Minnesota , Minneapolis , Minnesota 55414 , United States. Curated by ChEMBL | Assay Description Inhibition of recombinant rat Na+/K+-ATPase alpha4/beta1 expressed in baculovirus infected insect Sf9 cell membranes using [gamma-32P]ATP as substrat... | J Med Chem 61: 1800-1820 (2018) Article DOI: 10.1021/acs.jmedchem.7b00925 BindingDB Entry DOI: 10.7270/Q2T43WJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-O (Homo sapiens) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK2/Cyclin-O (unknown origin) using histone H1 as substrate preincubated for 20 mins followed by 33P-ATP addition and measured after 2... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113232 BindingDB Entry DOI: 10.7270/Q22J6GMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM50433369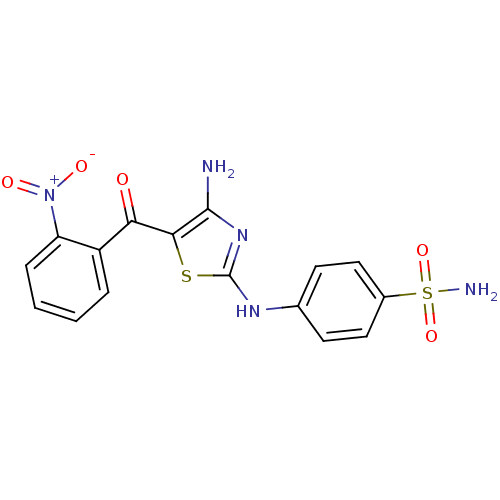 (CHEMBL2377825) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of CDK5/P25 (unknown origin)-mediated phosphorylation of peptide substrate incubated for 15 mins prior to substrate addition measured afte... | J Med Chem 56: 3768-82 (2013) Article DOI: 10.1021/jm301234k BindingDB Entry DOI: 10.7270/Q25T3MV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) using histone H1 as substrate preincubated for 20 mins followed by 33P-ATP addition and measured after 2... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113232 BindingDB Entry DOI: 10.7270/Q22J6GMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 1 (Homo sapiens (Human)) | BDBM50059889 ((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lee Moffitt Cancer Center and Research Institute Curated by ChEMBL | Assay Description Inhibition of CDK1/Cyclin A (unknown origin)-mediated phosphorylation of peptide substrate incubated for 15 mins prior to substrate addition measured... | J Med Chem 56: 3768-82 (2013) Article DOI: 10.1021/jm301234k BindingDB Entry DOI: 10.7270/Q25T3MV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/potassium-transporting ATPase subunit alpha-4/beta-3 (Rattus norvegicus) | BDBM50286739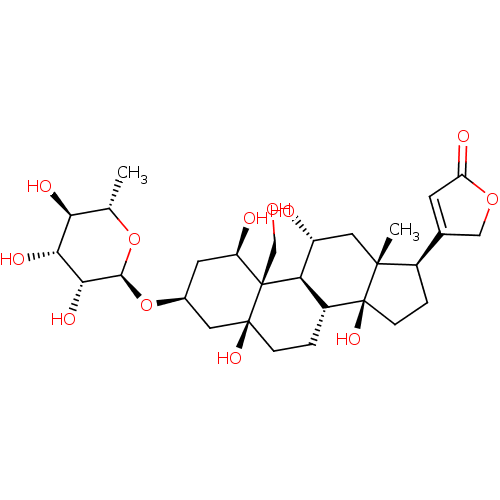 (4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Institute for Therapeutics Discovery and Development, College of Pharmacy , University of Minnesota , Minneapolis , Minnesota 55414 , United States. Curated by ChEMBL | Assay Description Inhibition of recombinant rat Na+/K+-ATPase alpha4/beta3 expressed in baculovirus infected Sf9 cell membranes using [gamma-32P]ATP as substrate prein... | J Med Chem 61: 1800-1820 (2018) Article DOI: 10.1021/acs.jmedchem.7b00925 BindingDB Entry DOI: 10.7270/Q2T43WJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50563174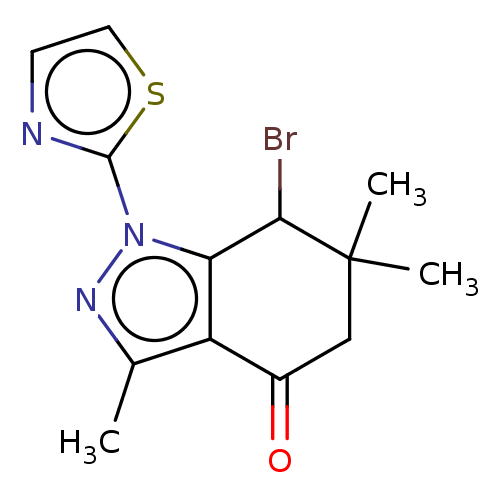 (CHEMBL1708376) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CDK2/Cyclin E (unknown origin) using histone H1 as substrate preincubated for 20 mins followed by 33P-ATP addition and measured after 2... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113232 BindingDB Entry DOI: 10.7270/Q22J6GMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/potassium-transporting ATPase subunit alpha-4/beta-1 (Rattus norvegicus) | BDBM50255120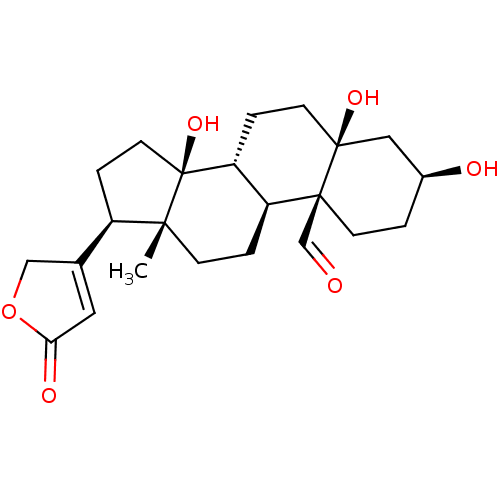 (CHEBI:38178 | STROPHANTHIDIN) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Institute for Therapeutics Discovery and Development, College of Pharmacy , University of Minnesota , Minneapolis , Minnesota 55414 , United States. Curated by ChEMBL | Assay Description Inhibition of recombinant rat Na+/K+-ATPase alpha4/beta1 expressed in baculovirus infected insect Sf9 cell membranes using [gamma-32P]ATP as substrat... | J Med Chem 61: 1800-1820 (2018) Article DOI: 10.1021/acs.jmedchem.7b00925 BindingDB Entry DOI: 10.7270/Q2T43WJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 306 total ) | Next | Last >> |