

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176019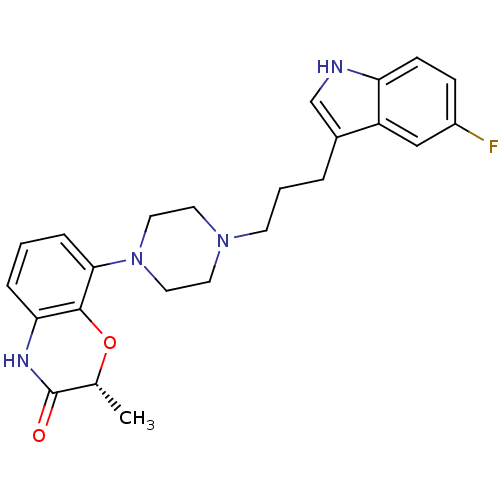 ((R)-8-(4-(3-(5-fluoro-1H-indol-3-yl)propyl)piperaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176024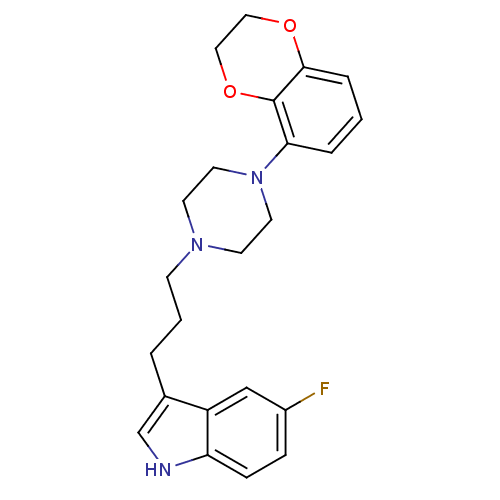 (3-{3-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-pipera...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176044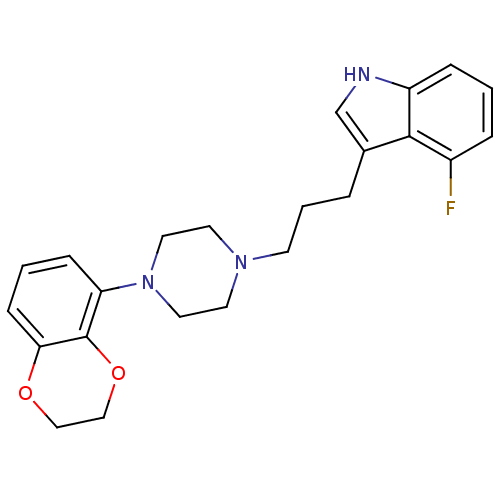 (3-{3-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-pipera...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176049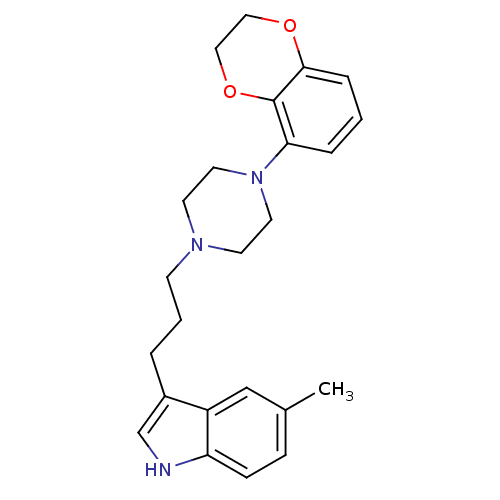 (3-{3-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-pipera...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176035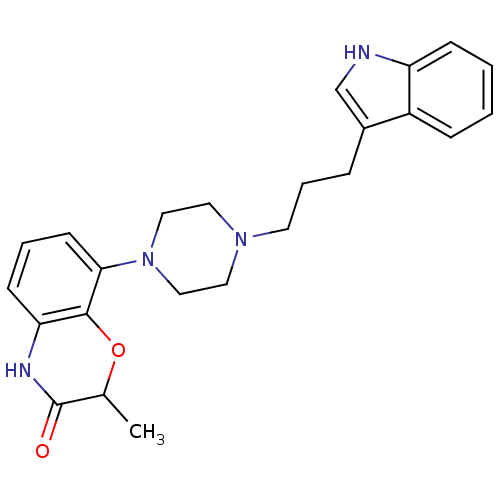 (8-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl}-2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176021 (3-{3-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-pipera...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176042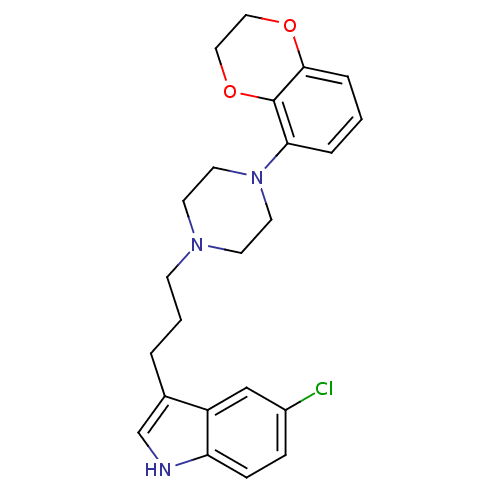 (5-Chloro-3-{3-[4-(2,3-dihydro-benzo[1,4]dioxin-5-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176020 ((R)-8-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176034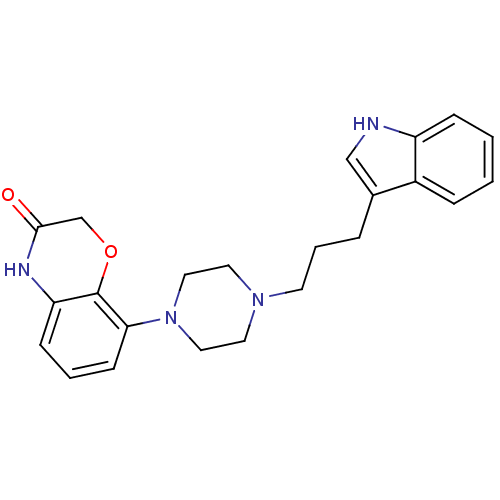 (8-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl}-4H...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176045 (3-{3-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-pipera...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176041 ((S)-8-{4-[3-(5-Fluoro-1H-indol-3-yl)-propyl]-piper...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176023 ((S)-8-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50176023 ((S)-8-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-spiperone binding to human dopamine D2 receptor expressed in CHO cells | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50176043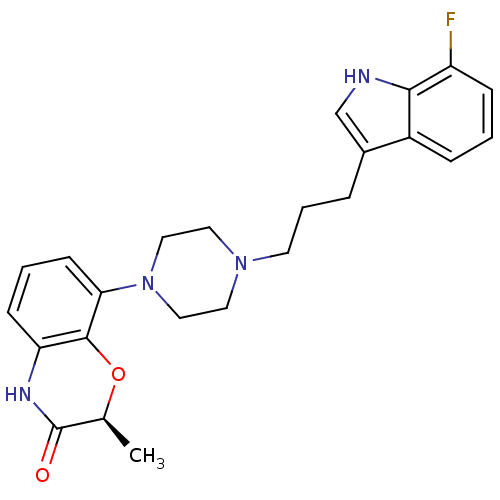 ((S)-8-{4-[3-(7-Fluoro-1H-indol-3-yl)-propyl]-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-spiperone binding to human dopamine D2 receptor expressed in CHO cells | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176028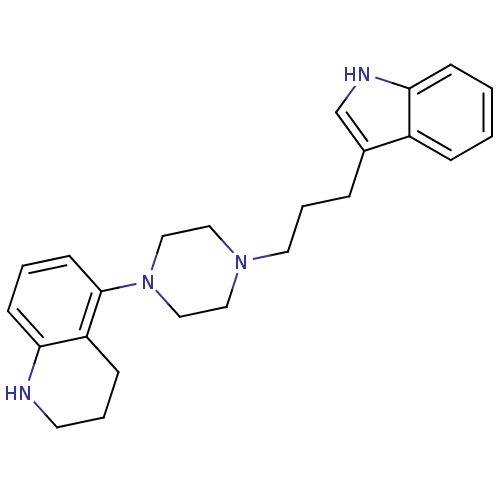 (5-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl}-1,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176032 (3-[3-(4-Chroman-5-yl-piperazin-1-yl)-propyl]-1H-in...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176039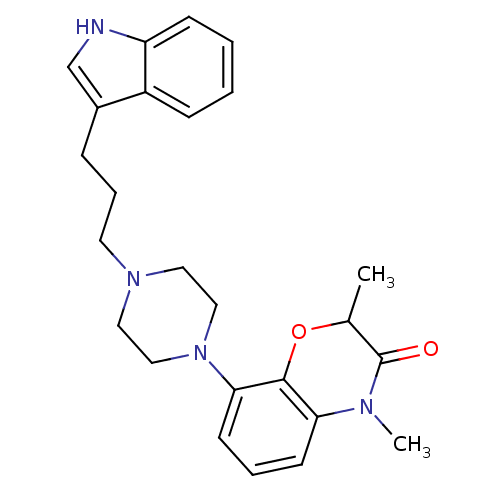 (8-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl}-2,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176025 (4-Chloro-3-{3-[4-(2,3-dihydro-benzo[1,4]dioxin-5-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176033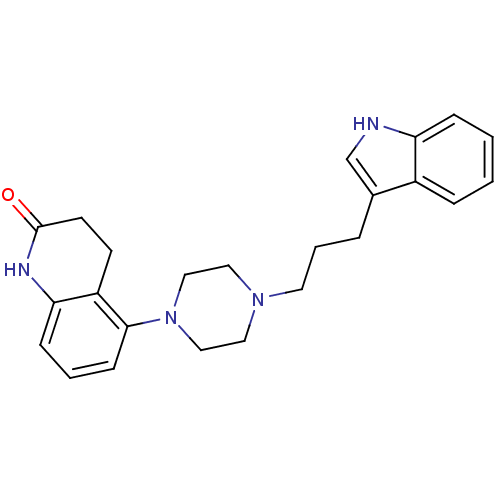 (5-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl}-3,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176029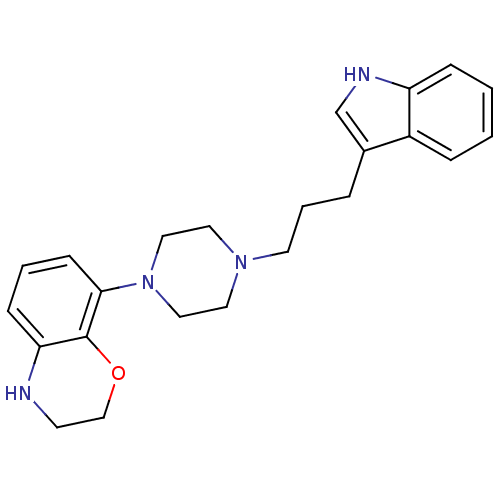 (8-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl}-3,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176036 (3-{3-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-pipera...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50029150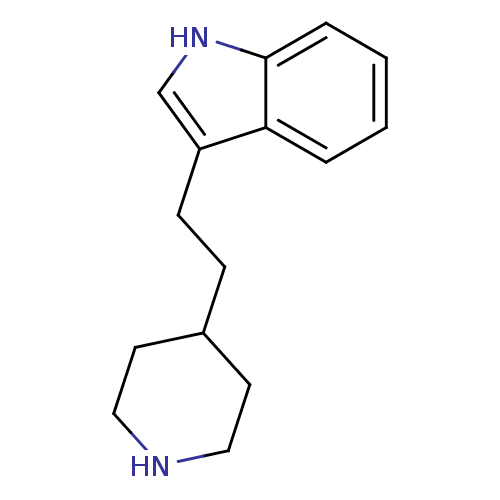 (3-(2-Piperidin-4-yl-ethyl)-1H-indole | CHEMBL27652...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176031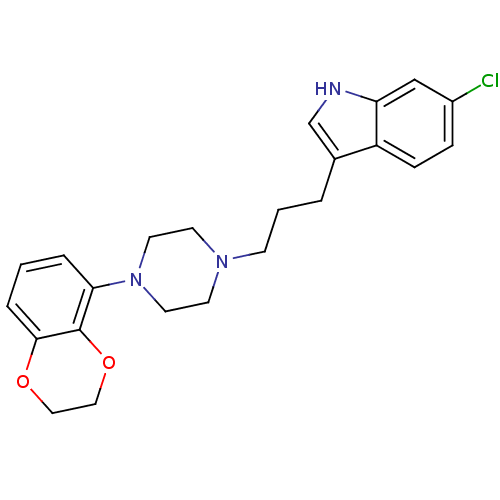 (6-Chloro-3-{3-[4-(2,3-dihydro-benzo[1,4]dioxin-5-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-spiperone binding to human dopamine D2 receptor expressed in CHO cells | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50176031 (6-Chloro-3-{3-[4-(2,3-dihydro-benzo[1,4]dioxin-5-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-spiperone binding to human dopamine D2 receptor expressed in CHO cells | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176040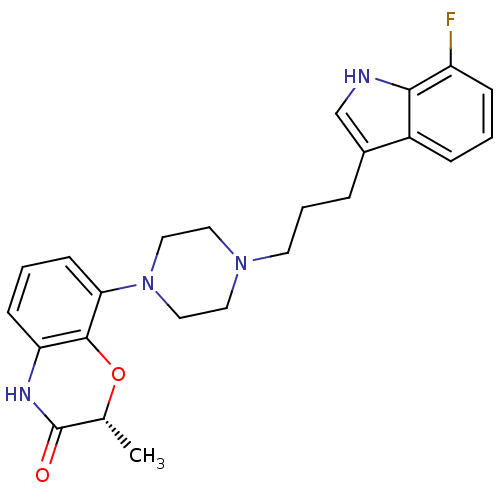 ((R)-8-{4-[3-(7-Fluoro-1H-indol-3-yl)-propyl]-piper...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50354607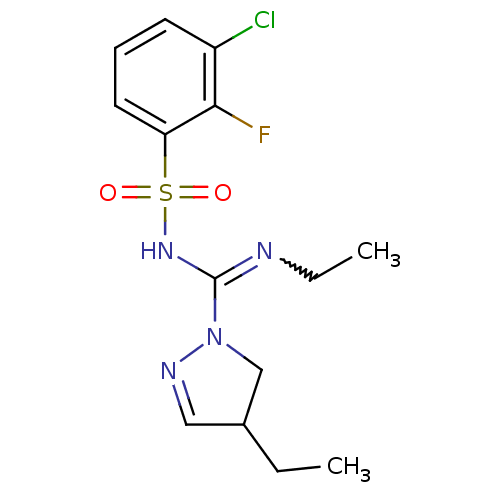 (CHEMBL1834337) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting | J Med Chem 54: 7030-54 (2011) Article DOI: 10.1021/jm200466r BindingDB Entry DOI: 10.7270/Q2J103JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176022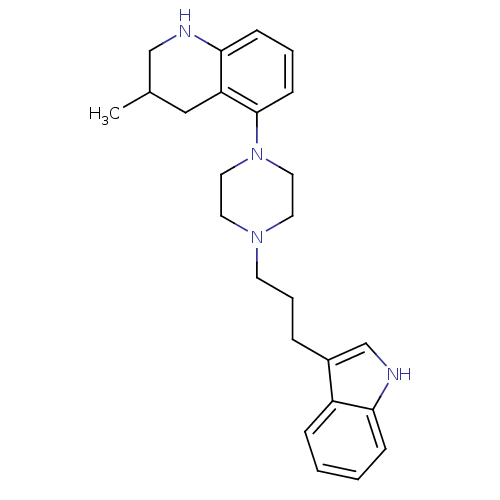 (5-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl}-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50354585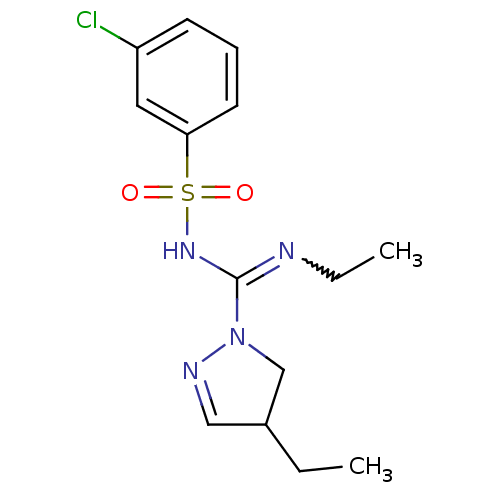 (CHEMBL1834226) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting | J Med Chem 54: 7030-54 (2011) Article DOI: 10.1021/jm200466r BindingDB Entry DOI: 10.7270/Q2J103JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50354585 (CHEMBL1834226) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting | J Med Chem 54: 7030-54 (2011) Article DOI: 10.1021/jm200466r BindingDB Entry DOI: 10.7270/Q2J103JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176026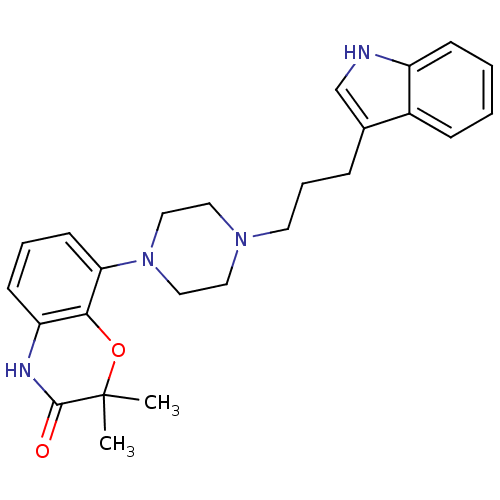 (8-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl}-2,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176043 ((S)-8-{4-[3-(7-Fluoro-1H-indol-3-yl)-propyl]-piper...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50354608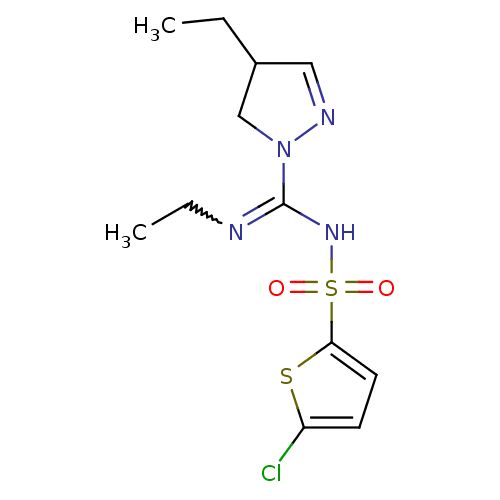 (CHEMBL1834342) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting | J Med Chem 54: 7030-54 (2011) Article DOI: 10.1021/jm200466r BindingDB Entry DOI: 10.7270/Q2J103JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50176034 (8-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl}-4H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-spiperone binding to human dopamine D2 receptor expressed in CHO cells | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50176035 (8-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl}-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-spiperone binding to human dopamine D2 receptor expressed in CHO cells | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50176041 ((S)-8-{4-[3-(5-Fluoro-1H-indol-3-yl)-propyl]-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-spiperone binding to human dopamine D2 receptor expressed in CHO cells | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50176037 (3-{4-[4-(2,3-Dihydro-benzo[1,4]dioxin-5-yl)-pipera...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-spiperone binding to human dopamine D2 receptor expressed in CHO cells | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50354609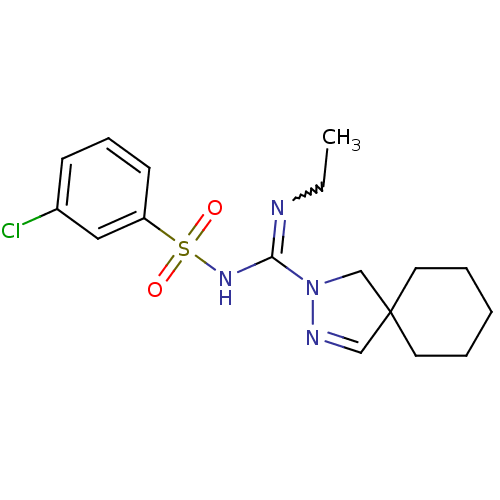 (CHEMBL1834348) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting | J Med Chem 54: 7030-54 (2011) Article DOI: 10.1021/jm200466r BindingDB Entry DOI: 10.7270/Q2J103JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50176040 ((R)-8-{4-[3-(7-Fluoro-1H-indol-3-yl)-propyl]-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-spiperone binding to human dopamine D2 receptor expressed in CHO cells | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50354610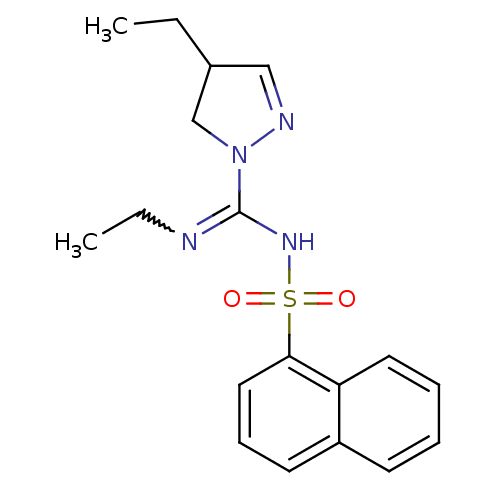 (CHEMBL1834338) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting | J Med Chem 54: 7030-54 (2011) Article DOI: 10.1021/jm200466r BindingDB Entry DOI: 10.7270/Q2J103JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50354611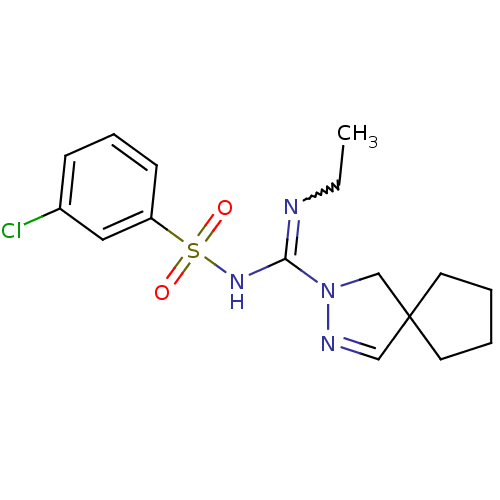 (CHEMBL1834347) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting | J Med Chem 54: 7030-54 (2011) Article DOI: 10.1021/jm200466r BindingDB Entry DOI: 10.7270/Q2J103JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50176019 ((R)-8-(4-(3-(5-fluoro-1H-indol-3-yl)propyl)piperaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-spiperone binding to human dopamine D2 receptor expressed in CHO cells | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50004108 ((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharmaceuticals Curated by PDSP Ki Database | Bioorg Med Chem Lett 18: 188-93 (2008) Article DOI: 10.1016/j.bmcl.2007.10.101 BindingDB Entry DOI: 10.7270/Q2C827W3 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50354612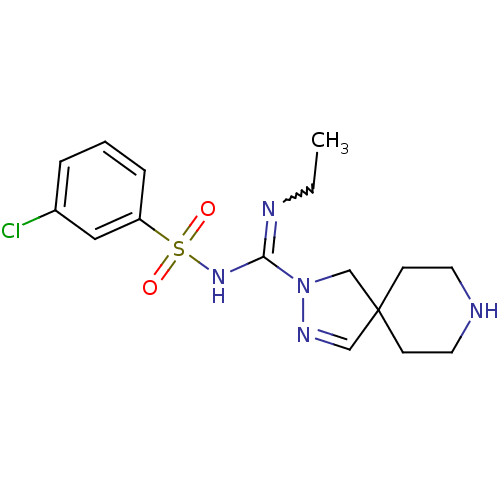 (CHEMBL1834350) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting | J Med Chem 54: 7030-54 (2011) Article DOI: 10.1021/jm200466r BindingDB Entry DOI: 10.7270/Q2J103JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50176026 (8-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl}-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-spiperone binding to human dopamine D2 receptor expressed in CHO cells | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176027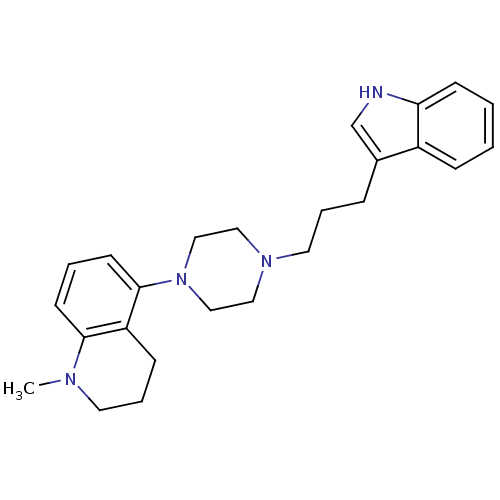 (5-{4-[3-(1H-Indol-3-yl)-propyl]-piperazin-1-yl}-1-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50230537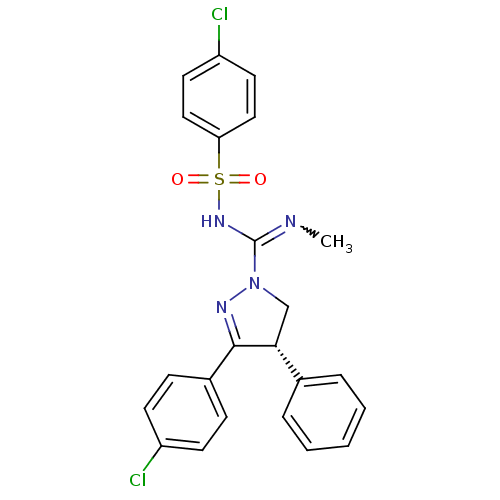 ((S)-3-(4-chlorophenyl)-N'-(4-chlorophenylsulfonyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description Displacement of CP-55,940 binding from recombinant human cannabinoid receptor 1 expressed in CHO cells | J Med Chem 47: 627-43 (2004) Article DOI: 10.1021/jm031019q BindingDB Entry DOI: 10.7270/Q2610ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50354613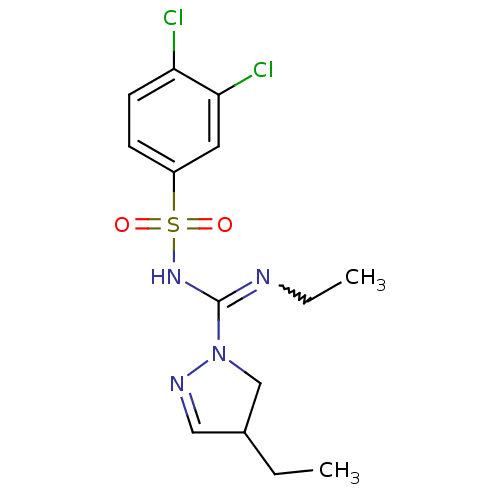 (CHEMBL1834333) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting | J Med Chem 54: 7030-54 (2011) Article DOI: 10.1021/jm200466r BindingDB Entry DOI: 10.7270/Q2J103JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50354585 (CHEMBL1834226) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Healthcare Products B.V. Curated by ChEMBL | Assay Description Displacement of [3H]-methyllysergic acid diethylamide from human 5-HT6 receptor expressed in CHO cells after 30 mins by liquid scintillation counting | J Med Chem 54: 7030-54 (2011) Article DOI: 10.1021/jm200466r BindingDB Entry DOI: 10.7270/Q2J103JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50176048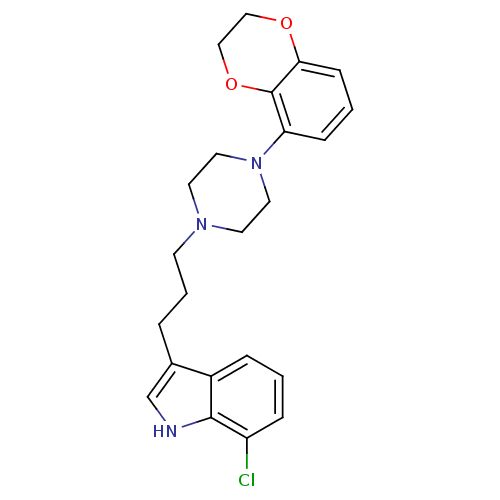 (7-Chloro-3-{3-[4-(2,3-dihydro-benzo[1,4]dioxin-5-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Solvay Pharma Curated by ChEMBL | Assay Description In vitro inhibitory constant against [3H]-paroxetine binding to rat frontal cortex membrane serotonin reuptake site | J Med Chem 48: 6855-69 (2005) Article DOI: 10.1021/jm050148z BindingDB Entry DOI: 10.7270/Q21C1WFP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 286 total ) | Next | Last >> |