

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100528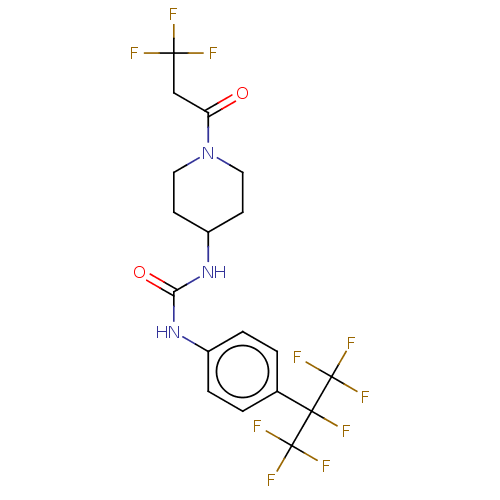 (CHEMBL3327081) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50507816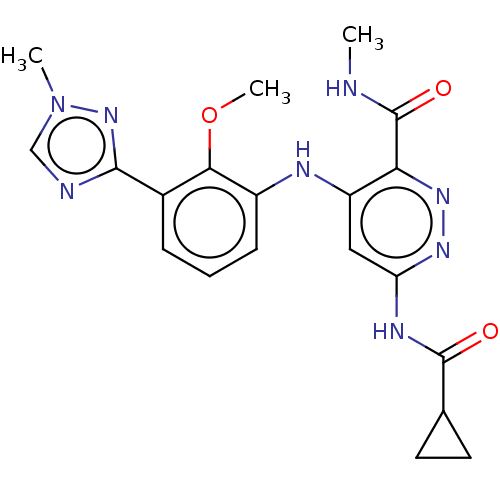 (Bms-986165 | Deucravacitinib) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of TYK2 in human Jurkat cells assessed as reduction in IFN-alpha stimulated TYK2 phosphorylation by caspase3/7 reagent-based Western blot ... | Eur J Med Chem 163: 413-427 (2019) Article DOI: 10.1016/j.ejmech.2018.11.072 BindingDB Entry DOI: 10.7270/Q2736V7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100535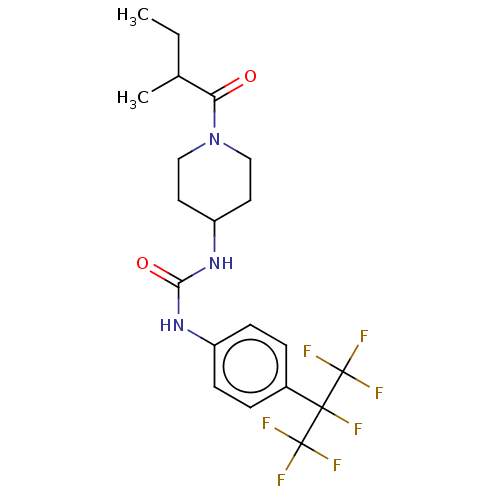 (CHEMBL3327073) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408198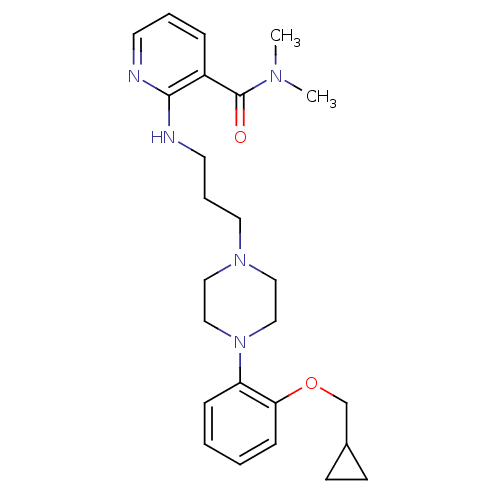 (CHEMBL91278) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H4 (GUINEA PIG) | BDBM22904 ((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by PDSP Ki Database | Mol Pharmacol 59: 434-41 (2001) Article DOI: 10.1124/mol.59.3.434 BindingDB Entry DOI: 10.7270/Q2Z899ZR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408201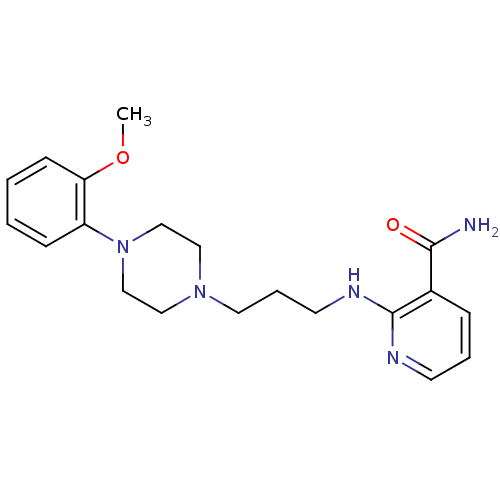 (CHEMBL88512) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50060964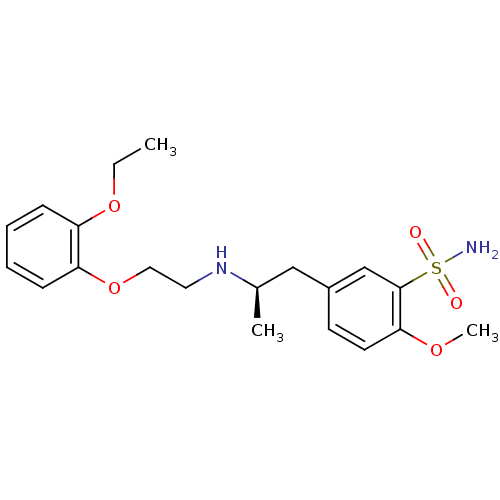 ((R)-5-(2-((2-(2-ethoxyphenoxy)ethyl)amino)propyl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H4 (GUINEA PIG) | BDBM7966 (2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by PDSP Ki Database | Mol Pharmacol 59: 434-41 (2001) Article DOI: 10.1124/mol.59.3.434 BindingDB Entry DOI: 10.7270/Q2Z899ZR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor H4 (GUINEA PIG) | BDBM22904 ((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by PDSP Ki Database | Mol Pharmacol 59: 434-41 (2001) Article DOI: 10.1124/mol.59.3.434 BindingDB Entry DOI: 10.7270/Q2Z899ZR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22904 ((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by PDSP Ki Database | Mol Pharmacol 59: 434-41 (2001) Article DOI: 10.1124/mol.59.3.434 BindingDB Entry DOI: 10.7270/Q2Z899ZR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100521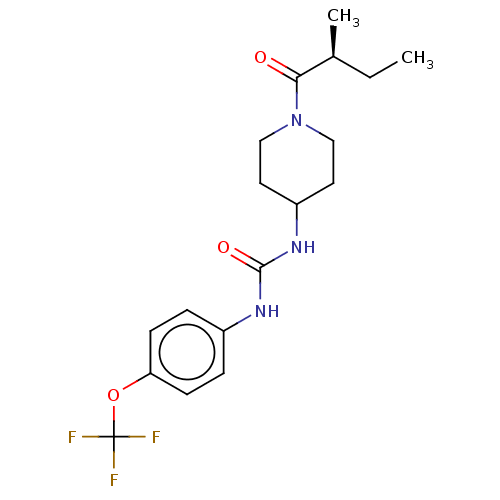 (CHEMBL3327078 | US10377744, Compound No. 2696) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50201006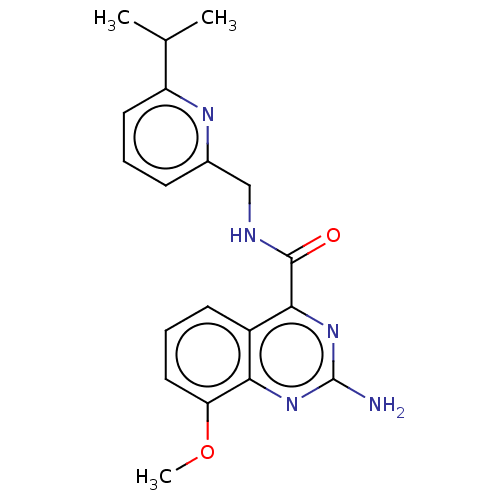 (CHEMBL3923709 | US10138212, Example 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50201019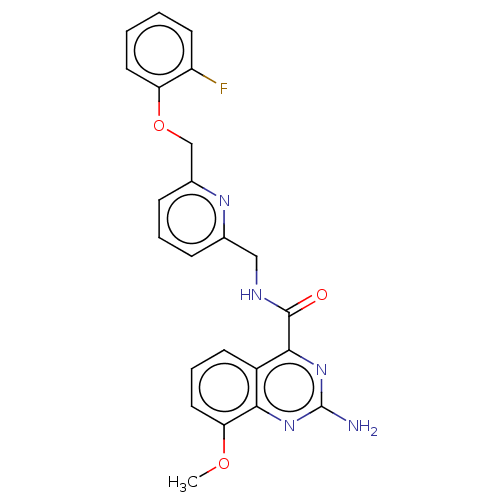 (CHEMBL3973920 | US10138212, Example 44) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139771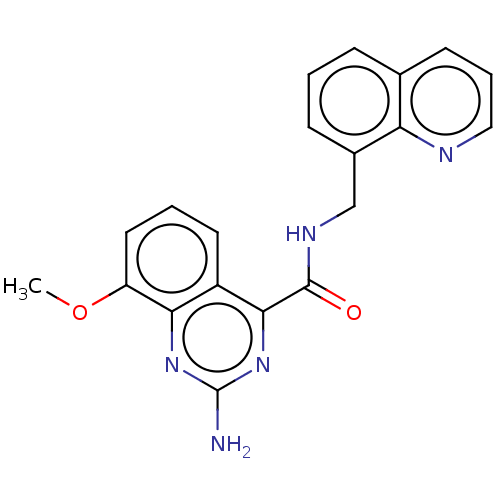 (CHEMBL3765580 | US10138212, Example 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303246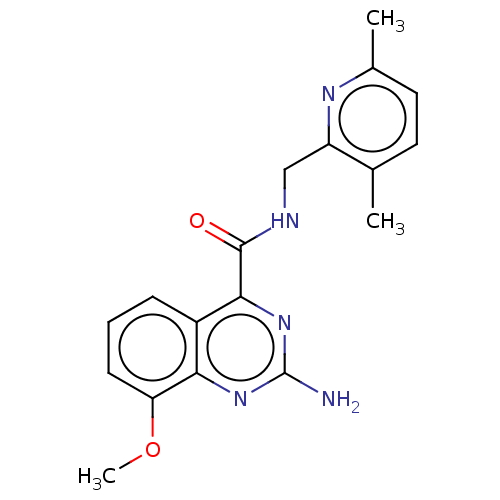 (2-amino-N-[(3,6- dimethyl-2- pyridyl)methyl]-8- me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303248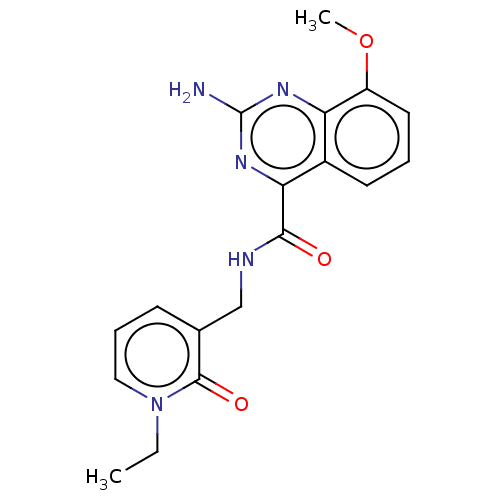 (2-amino-N-[(1-ethyl-2- oxo-3-pyridyl)methyl]-8- me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50200981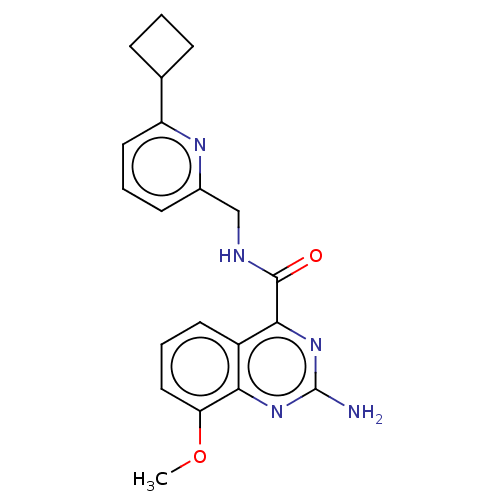 (CHEMBL3960148 | US10138212, Example 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139773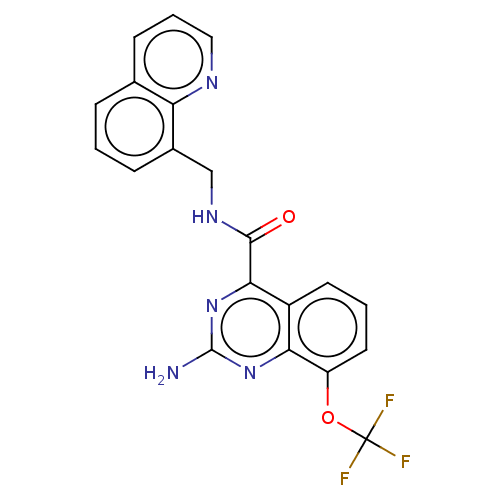 (CHEMBL3765379 | US10138212, Example 101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139771 (CHEMBL3765580 | US10138212, Example 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139773 (CHEMBL3765379 | US10138212, Example 101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100519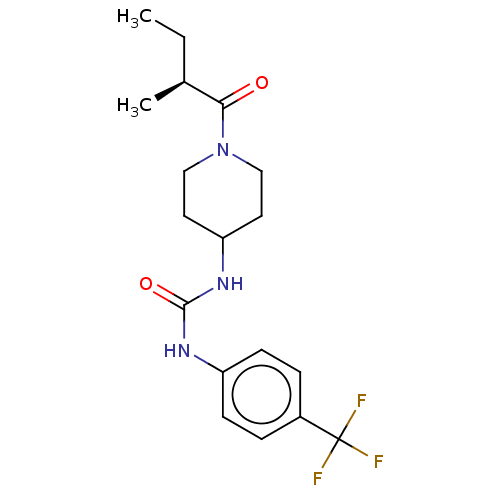 (CHEMBL3327067 | US10377744, Compound No. 2391) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100531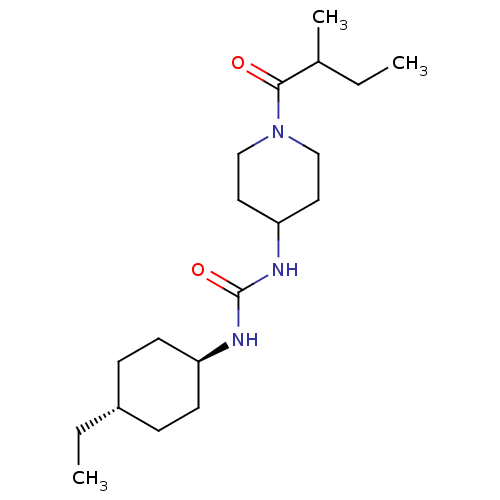 (CHEMBL3325465) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity of compound was determined against to human cannabinoid receptor 2 in chinese hamster ovary cells | J Med Chem 46: 2110-6 (2003) Article DOI: 10.1021/jm020329q BindingDB Entry DOI: 10.7270/Q2J67HPG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139748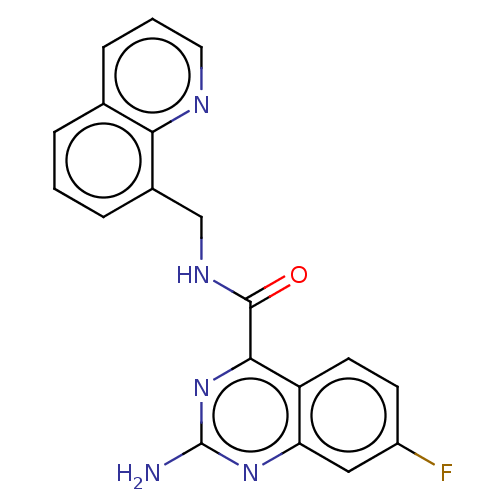 (CHEMBL3763717) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM471689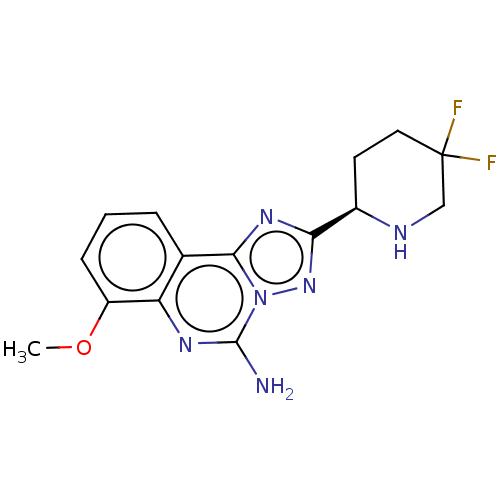 (US10822338, Example 58B) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2A receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10822338 (2020) BindingDB Entry DOI: 10.7270/Q2KS6VMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303251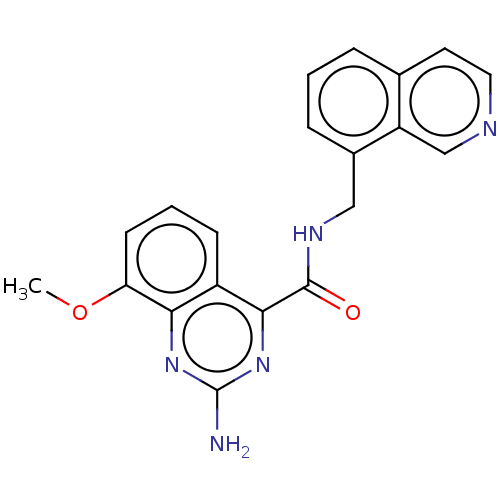 (2-amino-N-(8- isoquinolylmethyl)-8- methoxy-quinaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303181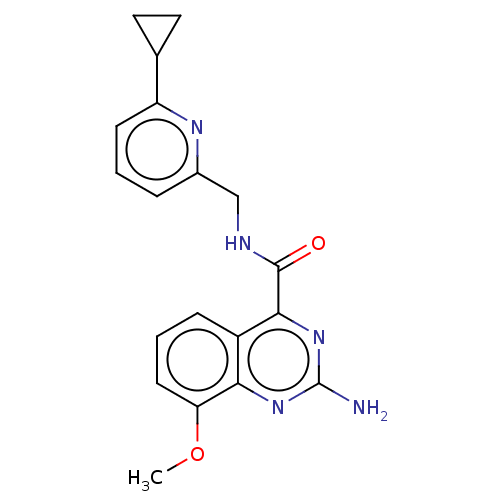 (2-amino-N-[(6- cyclopropyl-2- pyridyl)methyl]-8- m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303280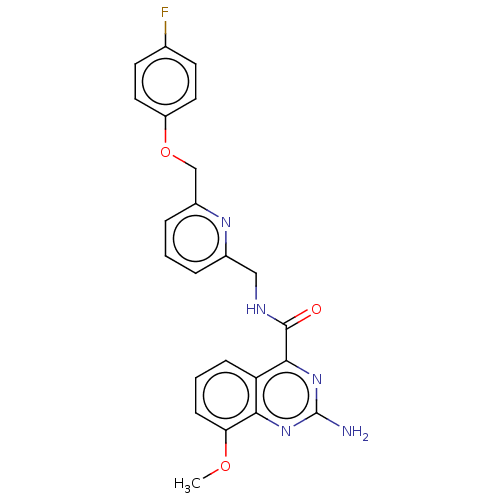 (2-amino-N-[[6-[(4- fluorophenoxy)methyl]- 2-pyridy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM471619 ((R)-7-methoxy-2-(1-(3,3,3- trifluoro-2- (trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2A receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10822338 (2020) BindingDB Entry DOI: 10.7270/Q2KS6VMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM471691 (US10822338, Example 35C) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2A receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10822338 (2020) BindingDB Entry DOI: 10.7270/Q2KS6VMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100520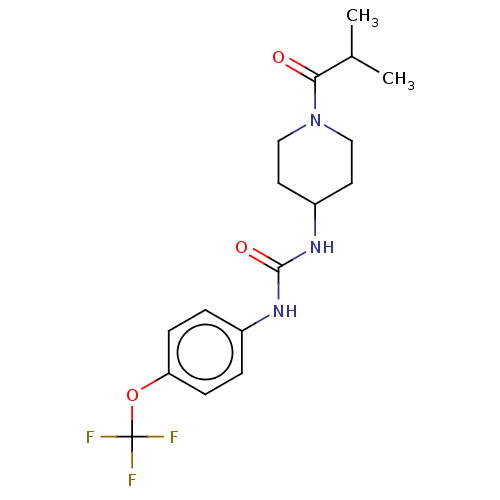 (CHEMBL3327077 | US10377744, Compound No. 2422 | US...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100534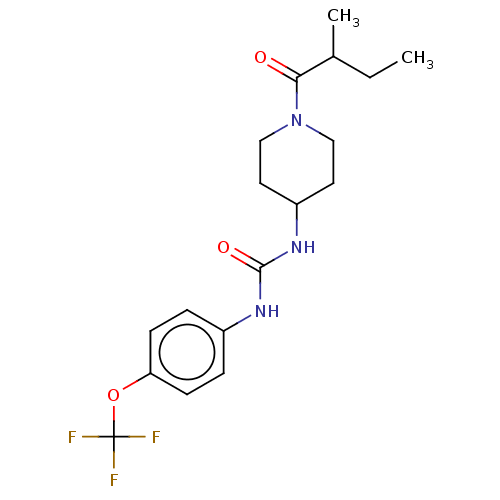 (CHEMBL3327074) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100541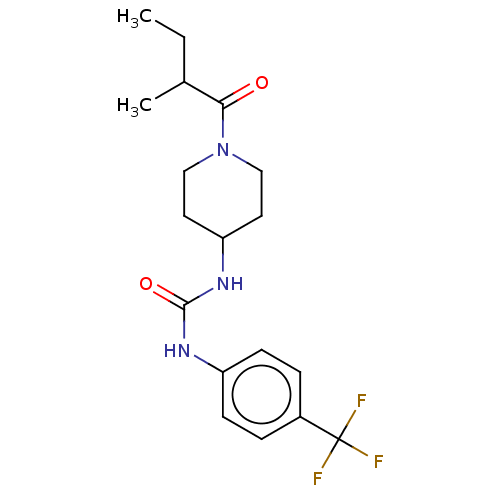 (CHEMBL3327066) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50125092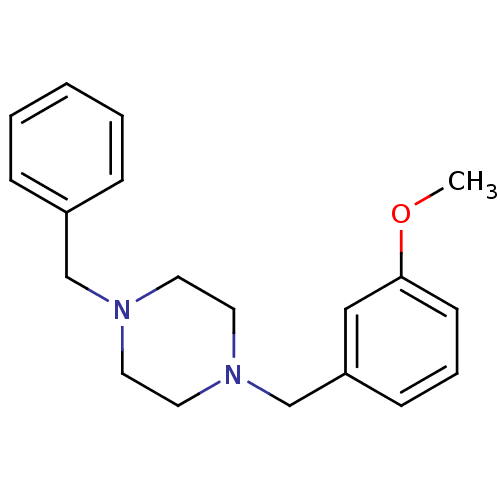 (1-Benzyl-4-(3-methoxy-benzyl)-piperazine | CHEMBL1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity towards Sigma-1 receptor | Bioorg Med Chem Lett 13: 749-51 (2003) BindingDB Entry DOI: 10.7270/Q2JQ10C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408248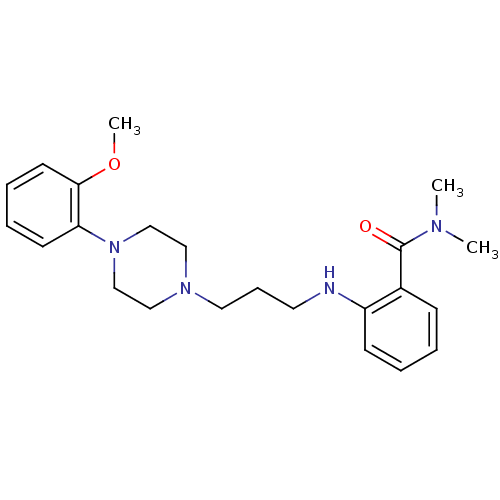 (CHEMBL330060) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50408246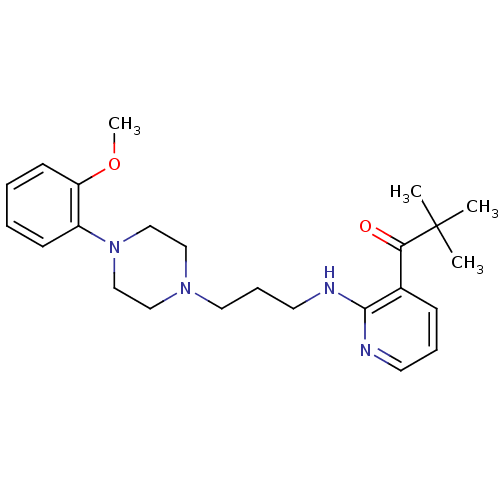 (CHEMBL92261) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by ChEMBL | Assay Description In vitro for the displacement of [3H]prazosin binding to bovine Alpha-1A adrenergic receptor | J Med Chem 40: 2674-87 (1997) Checked by Author Article DOI: 10.1021/jm970166j BindingDB Entry DOI: 10.7270/Q2R78GD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM471642 (US10822338, Example 24B) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2A receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10822338 (2020) BindingDB Entry DOI: 10.7270/Q2KS6VMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM471620 ((R)-2-(1-(2,2- difluoroethyl)piperidin-3- yl)-7-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2A receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10822338 (2020) BindingDB Entry DOI: 10.7270/Q2KS6VMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50463294 (CHEMBL4249256) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human KOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 26: 4254-4263 (2018) Article DOI: 10.1016/j.bmc.2018.07.020 BindingDB Entry DOI: 10.7270/Q28W3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50200984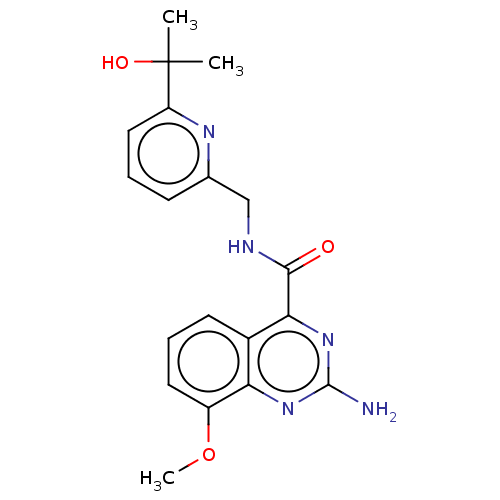 (CHEMBL3932655 | US10138212, Example 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM303252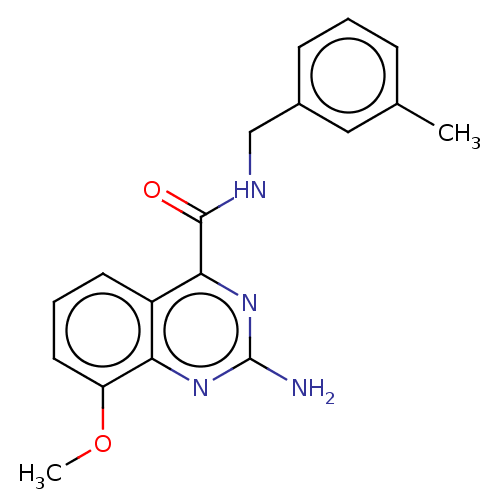 (2-amino-8-methoxy-N- (m- tolylmethyl)quinazoline- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50200989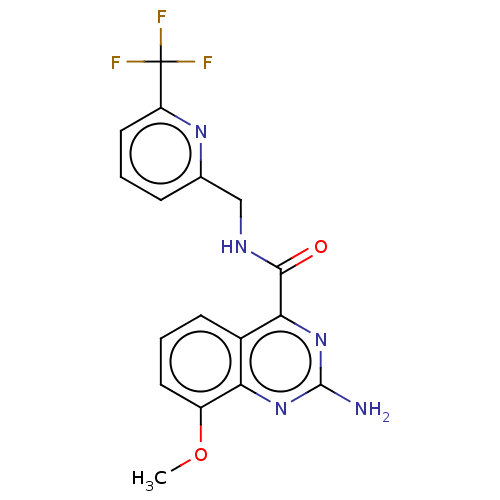 (CHEMBL3906827 | US10138212, Example 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2a receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10138212 (2018) BindingDB Entry DOI: 10.7270/Q2WM1GGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM471649 (US10822338, Example 29C) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2A receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10822338 (2020) BindingDB Entry DOI: 10.7270/Q2KS6VMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM471628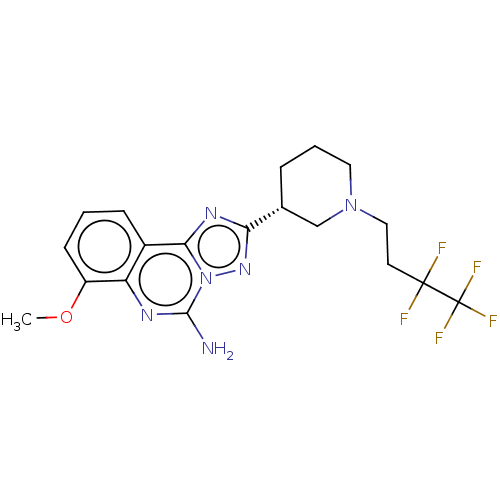 ((R)-7-methoxy-2-(1- (3,3,4,4,4- pentafluorobutyl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding affinities of compounds of the invention for the human A2A receptor were determined in a competition binding assay using Scintillation Proxim... | US Patent US10822338 (2020) BindingDB Entry DOI: 10.7270/Q2KS6VMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50463297 (CHEMBL4246433) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human MOR expressed in CHO cell membranes after 30 mins by liquid scintillation counting analysis | Bioorg Med Chem 26: 4254-4263 (2018) Article DOI: 10.1016/j.bmc.2018.07.020 BindingDB Entry DOI: 10.7270/Q28W3GZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376456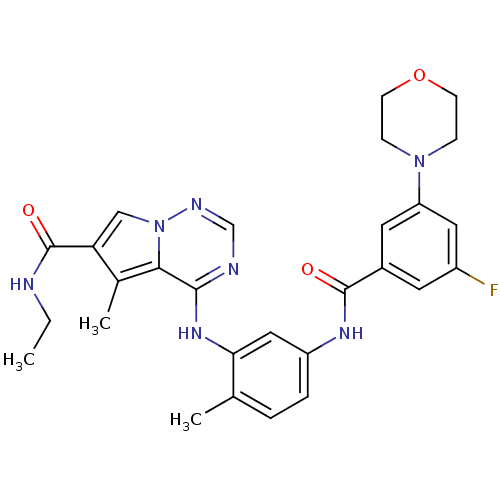 (CHEMBL262592) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of p38alpha | Bioorg Med Chem Lett 18: 2739-44 (2008) Article DOI: 10.1016/j.bmcl.2008.02.067 BindingDB Entry DOI: 10.7270/Q29887X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100539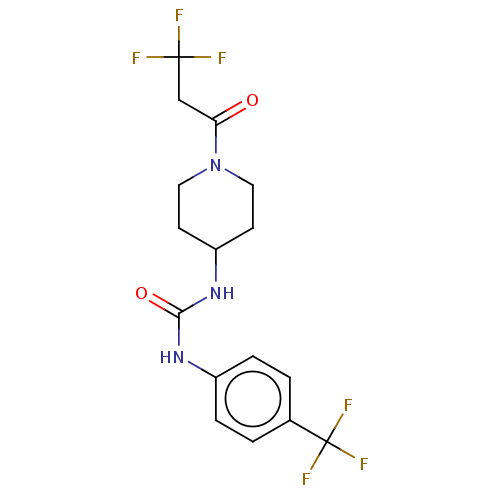 (CHEMBL3327069) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376433 (CHEMBL258895) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of p38alpha | Bioorg Med Chem Lett 18: 2739-44 (2008) Article DOI: 10.1016/j.bmcl.2008.02.067 BindingDB Entry DOI: 10.7270/Q29887X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100522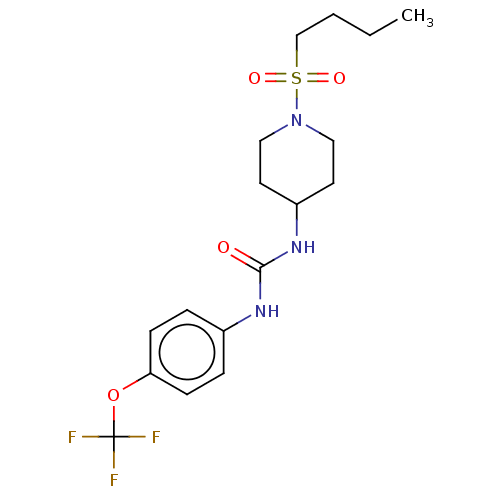 (CHEMBL3327087) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 14756 total ) | Next | Last >> |