 Found 504 hits with Last Name = 'brooks' and Initial = 'j'
Found 504 hits with Last Name = 'brooks' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50025450
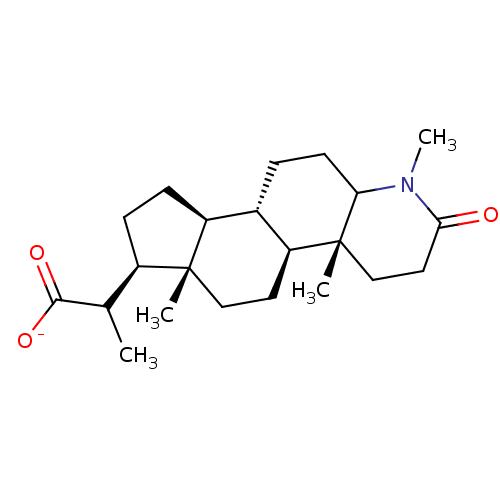
(CHEMBL1790294 | Sodium; 1,4a,6a-trimethyl-2-oxo-he...)Show SMILES [Na+].[H][C@@]12CC[C@H](C(C)C([O-])=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2N(C)C(=O)CC[C@]12C Show InChI InChI=1S/C22H35NO3.Na/c1-13(20(25)26)15-6-7-16-14-5-8-18-22(3,12-10-19(24)23(18)4)17(14)9-11-21(15,16)2;/h13-18H,5-12H2,1-4H3,(H,25,26);/q;+1/p-1/t13?,14-,15+,16-,17-,18?,21+,22+;/m0./s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant value for rat prostatic steroid 5-alpha reductase was determined |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50213061
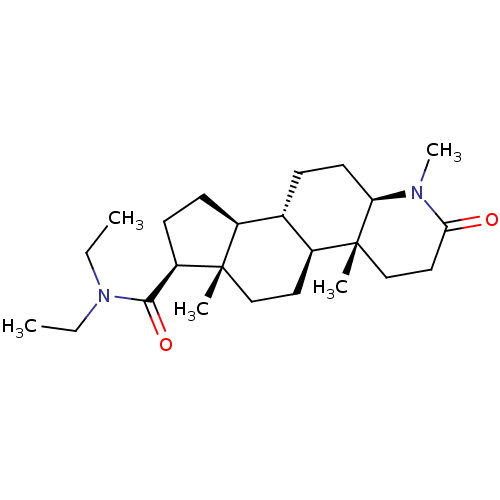
(CHEMBL2298601)Show SMILES CCN(CC)C(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4N(C)C(=O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C24H40N2O2/c1-6-26(7-2)22(28)19-10-9-17-16-8-11-20-24(4,15-13-21(27)25(20)5)18(16)12-14-23(17,19)3/h16-20H,6-15H2,1-5H3/t16-,17-,18-,19+,20+,23-,24+/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant value for rat prostatic steroid 5alpha reductase was determined |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50367296
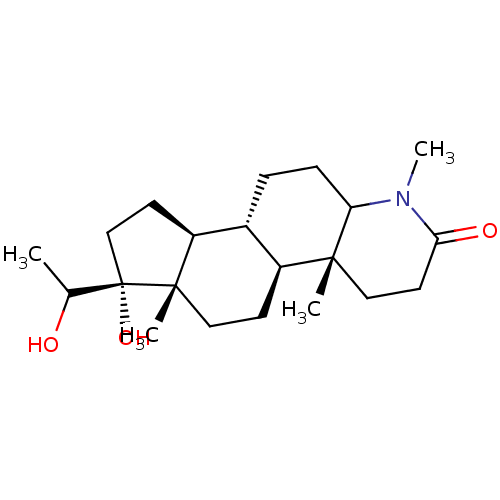
(CHEMBL1790284)Show SMILES CC(O)[C@@]1(O)CC[C@H]2[C@@H]3CCC4N(C)C(=O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C21H35NO3/c1-13(23)21(25)12-8-16-14-5-6-17-19(2,10-9-18(24)22(17)4)15(14)7-11-20(16,21)3/h13-17,23,25H,5-12H2,1-4H3/t13?,14-,15+,16+,17?,19-,20+,21+/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant value for rat prostatic steroid 5-alpha reductase was determined |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50226049

(CHEMBL3349139)Show SMILES [H][C@@]12CC[C@H](C(=O)N(C(C)C)C(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2NC(=O)CC[C@]12C |r| Show InChI InChI=1S/C25H42N2O2/c1-15(2)27(16(3)4)23(29)20-9-8-18-17-7-10-21-25(6,14-12-22(28)26-21)19(17)11-13-24(18,20)5/h15-21H,7-14H2,1-6H3,(H,26,28)/t17-,18-,19-,20+,21?,24-,25+/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant value for rat prostatic steroid 5-alpha reductase was determined |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164644

(2',3'-Dideoxyadenosine Triphosphate (Ddatp) | 2',3...)Show SMILES Nc1ncnc2n(cnc12)[C@H]1CC[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 |r| Show InChI InChI=1S/C10H16N5O11P3/c11-9-8-10(13-4-12-9)15(5-14-8)7-2-1-6(24-7)3-23-28(19,20)26-29(21,22)25-27(16,17)18/h4-7H,1-3H2,(H,19,20)(H,21,22)(H2,11,12,13)(H2,16,17,18)/t6-,7+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164642
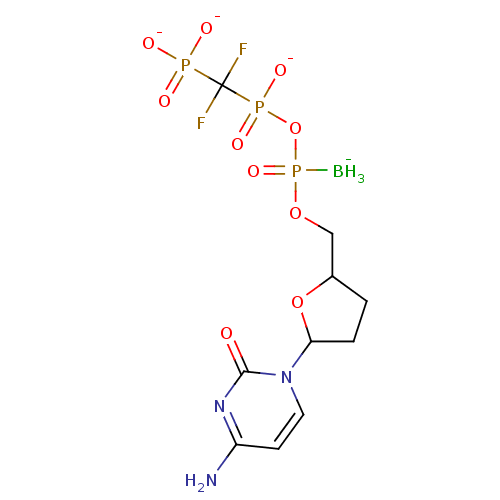
(2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...)Show SMILES [BH3-]P(=O)(OCC1CCC(O1)n1ccc(N)nc1=O)OP([O-])(=O)C(F)(F)P([O-])([O-])=O Show InChI InChI=1S/C10H18BF2N3O10P3/c11-29(23,26-28(21,22)10(12,13)27(18,19)20)24-5-6-1-2-8(25-6)16-4-3-7(14)15-9(16)17/h3-4,6,8H,1-2,5H2,11H3,(H,21,22)(H2,14,15,17)(H2,18,19,20)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50025400
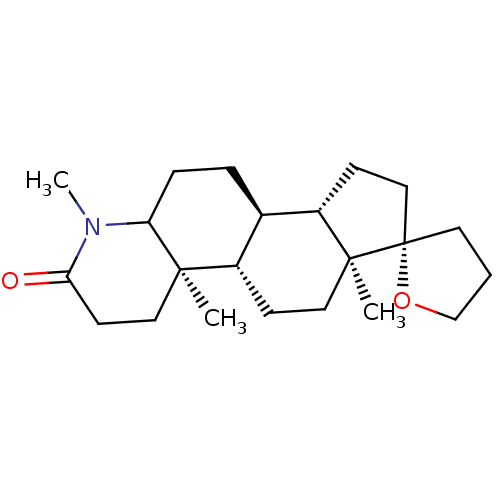
(1'-ethyl-4a',6a'-dimethylspiro[tetrahydrofuran-2,7...)Show SMILES [H][C@@]12CC[C@@]3(CCCO3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2N(C)C(=O)CC[C@]12C Show InChI InChI=1S/C23H37NO2/c1-4-24-19-7-6-16-17(21(19,2)12-10-20(24)25)8-13-22(3)18(16)9-14-23(22)11-5-15-26-23/h16-19H,4-15H2,1-3H3/t16?,17?,18?,19?,21?,22?,23-/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant value for rat prostatic steroid 5-alpha reductase was determined |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164639
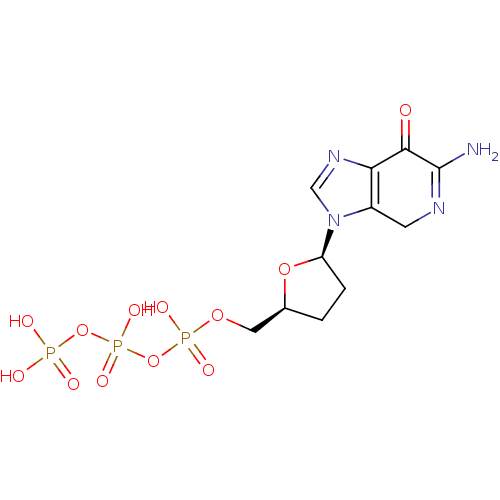
(2'-3'-dideoxy-7-deaza-guaninetriphosphate | CHEMBL...)Show SMILES NC1=NCc2c(ncn2[C@H]2CC[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O2)C1=O |t:1| Show InChI InChI=1S/C11H17N4O12P3/c12-11-10(16)9-7(3-13-11)15(5-14-9)8-2-1-6(25-8)4-24-29(20,21)27-30(22,23)26-28(17,18)19/h5-6,8H,1-4H2,(H2,12,13)(H,20,21)(H,22,23)(H2,17,18,19)/t6-,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50216166

(CHEMBL3349181)Show SMILES [H][C@@]12CC[C@H](C(=O)N(CC)CC)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@]2([H])NC(=O)CC[C@]12C |r| Show InChI InChI=1S/C23H38N2O2/c1-5-25(6-2)21(27)18-9-8-16-15-7-10-19-23(4,14-12-20(26)24-19)17(15)11-13-22(16,18)3/h15-19H,5-14H2,1-4H3,(H,24,26)/t15-,16-,17-,18+,19-,22-,23+/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant value for rat prostatic steroid 5-alpha reductase was determined |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164652
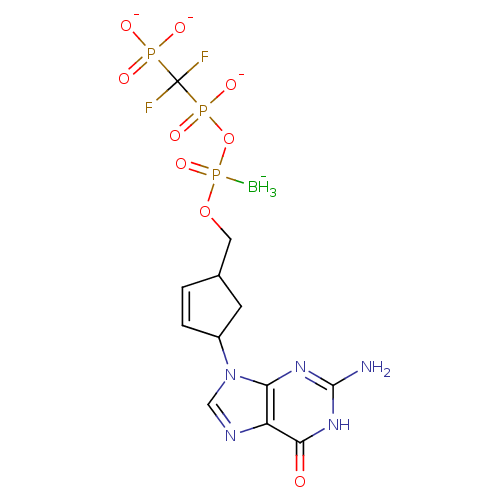
(2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...)Show SMILES [BH3-]P(=O)(OCC1CC(C=C1)n1cnc2c1nc(N)[nH]c2=O)OP([O-])(=O)C(F)(F)P([O-])([O-])=O |c:8| Show InChI InChI=1S/C12H18BF2N5O9P3/c13-32(27,29-31(25,26)12(14,15)30(22,23)24)28-4-6-1-2-7(3-6)20-5-17-8-9(20)18-11(16)19-10(8)21/h1-2,5-7H,3-4H2,13H3,(H,25,26)(H2,22,23,24)(H3,16,18,19,21)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164654
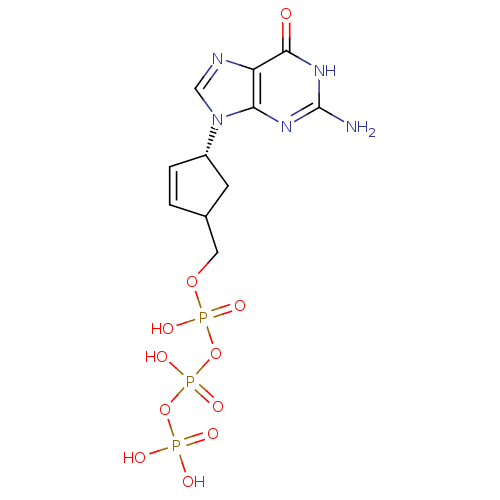
(CHEMBL370031 | [[[4-(2-amino-6-oxo-3,9-dihydropuri...)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1CC(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)C=C1 |c:30| Show InChI InChI=1S/C11H16N5O11P3/c12-11-14-9-8(10(17)15-11)13-5-16(9)7-2-1-6(3-7)4-25-29(21,22)27-30(23,24)26-28(18,19)20/h1-2,5-7H,3-4H2,(H,21,22)(H,23,24)(H2,18,19,20)(H3,12,14,15,17)/t6?,7-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164637
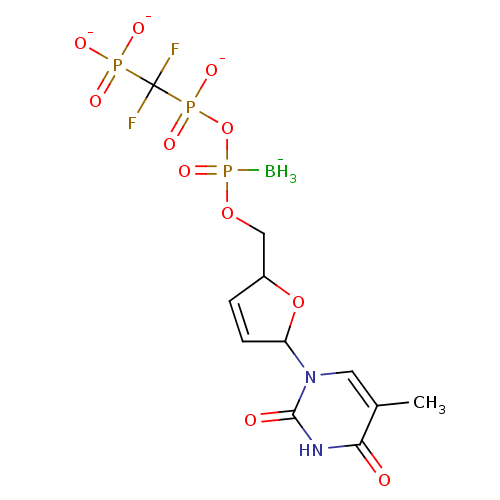
(2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...)Show SMILES [BH3-]P(=O)(OCC1OC(C=C1)n1cc(C)c(=O)[nH]c1=O)OP([O-])(=O)C(F)(F)P([O-])([O-])=O |c:8| Show InChI InChI=1S/C11H17BF2N2O11P3/c1-6-4-16(10(18)15-9(6)17)8-3-2-7(26-8)5-25-30(12,24)27-29(22,23)11(13,14)28(19,20)21/h2-4,7-8H,5H2,1,12H3,(H,22,23)(H,15,17,18)(H2,19,20,21)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164638

(2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...)Show SMILES [BH3-]P(=O)(OCC1OC(CC1F)n1cc(C)c(=O)[nH]c1=O)OP([O-])(=O)C(F)(F)P([O-])([O-])=O Show InChI InChI=1S/C11H18BF3N2O11P3/c1-5-3-17(10(19)16-9(5)18)8-2-6(13)7(27-8)4-26-31(12,25)28-30(23,24)11(14,15)29(20,21)22/h3,6-8H,2,4H2,1,12H3,(H,23,24)(H,16,18,19)(H2,20,21,22)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164647

(2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...)Show SMILES [BH3-]P(=O)(OCC1CCC(O1)n1cnc2c(N)ncnc12)OP([O-])(=O)C(F)(F)P([O-])([O-])=O Show InChI InChI=1S/C11H18BF2N5O9P3/c12-31(25,28-30(23,24)11(13,14)29(20,21)22)26-3-6-1-2-7(27-6)19-5-18-8-9(15)16-4-17-10(8)19/h4-7H,1-3H2,12H3,(H,23,24)(H2,15,16,17)(H2,20,21,22)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50145605

(4-amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydrofura...)Show InChI InChI=1S/C9H13N3O3/c10-7-3-4-12(9(14)11-7)8-2-1-6(5-13)15-8/h3-4,6,8,13H,1-2,5H2,(H2,10,11,14)/t6-,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50370655
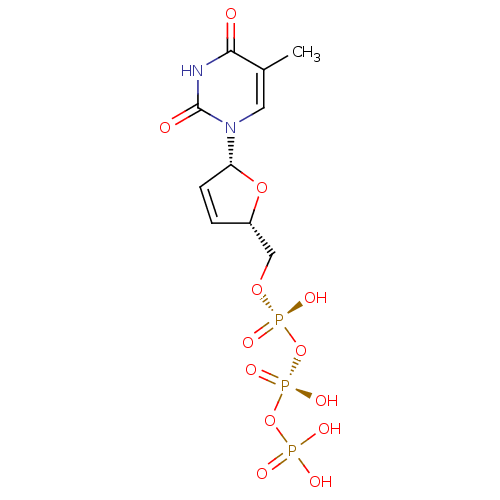
(CHEMBL485652)Show SMILES Cc1cn([C@@H]2O[C@H](CO[P@@](O)(=O)O[P@@](O)(=O)OP(O)(O)=O)C=C2)c(=O)[nH]c1=O |r,c:21| Show InChI InChI=1S/C10H15N2O13P3/c1-6-4-12(10(14)11-9(6)13)8-3-2-7(23-8)5-22-27(18,19)25-28(20,21)24-26(15,16)17/h2-4,7-8H,5H2,1H3,(H,18,19)(H,20,21)(H,11,13,14)(H2,15,16,17)/t7-,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164648
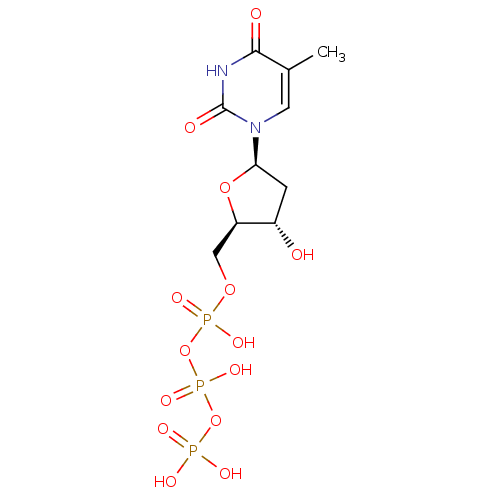
(2'-deoxythymidine triphosphate | 5'-TTP | CHEMBL36...)Show SMILES Cc1cn([C@H]2C[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H17N2O14P3/c1-5-3-12(10(15)11-9(5)14)8-2-6(13)7(24-8)4-23-28(19,20)26-29(21,22)25-27(16,17)18/h3,6-8,13H,2,4H2,1H3,(H,19,20)(H,21,22)(H,11,14,15)(H2,16,17,18)/t6-,7+,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164653
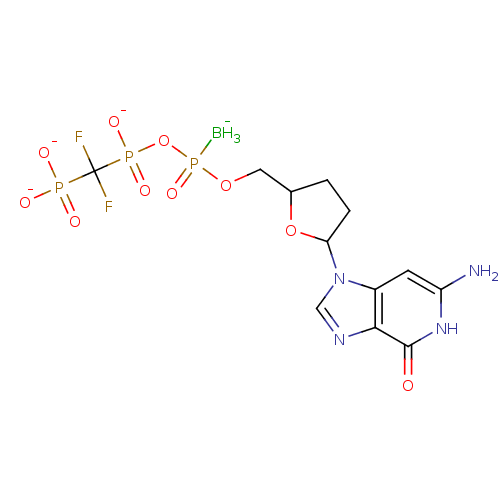
(2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...)Show SMILES [BH3-]P(=O)(OCC1CCC(O1)n1cnc2c1cc(N)[nH]c2=O)OP([O-])(=O)C(F)(F)P([O-])([O-])=O Show InChI InChI=1S/C12H19BF2N4O10P3/c13-32(26,29-31(24,25)12(14,15)30(21,22)23)27-4-6-1-2-9(28-6)19-5-17-10-7(19)3-8(16)18-11(10)20/h3,5-6,9H,1-2,4H2,13H3,(H,24,25)(H3,16,18,20)(H2,21,22,23)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164650
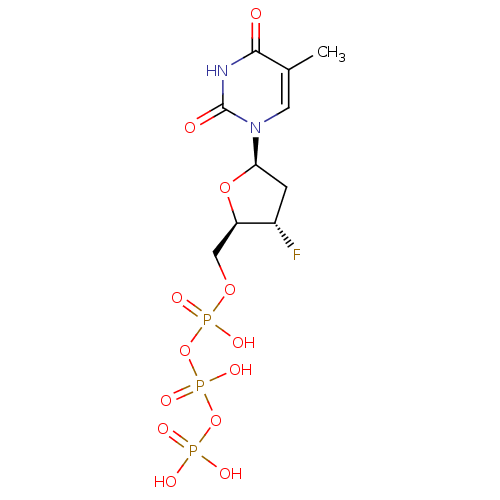
(CHEMBL191725 | [[[3-fluoro-5-(5-methyl-2,4-dioxo-1...)Show SMILES Cc1cn([C@H]2C[C@H](F)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C10H16FN2O13P3/c1-5-3-13(10(15)12-9(5)14)8-2-6(11)7(24-8)4-23-28(19,20)26-29(21,22)25-27(16,17)18/h3,6-8H,2,4H2,1H3,(H,19,20)(H,21,22)(H,12,14,15)(H2,16,17,18)/t6-,7+,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50370476

(Combivir | ZIDOVUDINE TRIPHOSPHATE)Show SMILES Cc1cn([C@H]2C[C@H](N=[N+]=[N-])[C@@H](CO[P@@](O)(=O)O[P@@](O)(=O)OP(O)(O)=O)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H16N5O13P3/c1-5-3-15(10(17)12-9(5)16)8-2-6(13-14-11)7(26-8)4-25-30(21,22)28-31(23,24)27-29(18,19)20/h3,6-8H,2,4H2,1H3,(H,21,22)(H,23,24)(H,12,16,17)(H2,18,19,20)/t6-,7+,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164640

(CHEMBL190609 | [[[[3-azido-5-(5-methyl-2,4-dioxo-1...)Show SMILES [BH3-]P(=O)(OCC1OC(CC1N=[N+]=[N-])n1cc(C)c(=O)[nH]c1=O)OP([O-])(=O)C(F)(F)P([O-])([O-])=O Show InChI InChI=1S/C11H18BF2N5O11P3/c1-5-3-19(10(21)16-9(5)20)8-2-6(17-18-15)7(29-8)4-28-33(12,27)30-32(25,26)11(13,14)31(22,23)24/h3,6-8H,2,4H2,1,12H3,(H,25,26)(H,16,20,21)(H2,22,23,24)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50225982

(CHEMBL3349117)Show SMILES [H][C@@]12CC[C@H](C(=O)OC)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2NC(=O)CC[C@]12C |r| Show InChI InChI=1S/C20H31NO3/c1-19-10-8-14-12(13(19)5-6-15(19)18(23)24-3)4-7-16-20(14,2)11-9-17(22)21-16/h12-16H,4-11H2,1-3H3,(H,21,22)/t12-,13-,14-,15+,16?,19-,20+/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat prostatic steroid 5-alpha-reductase |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50025359

(1,4a,6a-Trimethyl-2-oxo-hexadecahydro-indeno[5,4-f...)Show SMILES [H][C@@]12CC[C@H](C(=O)OC)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2N(C)C(=O)CC[C@]12C |r| Show InChI InChI=1S/C21H33NO3/c1-20-11-9-15-13(14(20)6-7-16(20)19(24)25-4)5-8-17-21(15,2)12-10-18(23)22(17)3/h13-17H,5-12H2,1-4H3 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Apparent inhibitory constant value for rat prostatic 5-alpha reductase was determined |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50138406
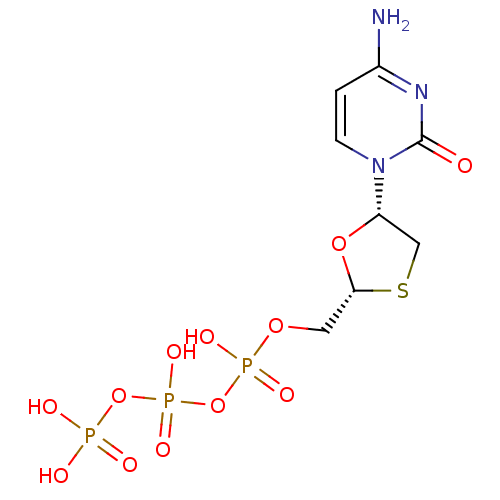
(3TC Triphosphate | CHEMBL1230 | LAMIVUDINE | Lamiv...)Show SMILES Nc1ccn([C@@H]2CS[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O2)c(=O)n1 |r| Show InChI InChI=1S/C8H14N3O12P3S/c9-5-1-2-11(8(12)10-5)6-4-27-7(21-6)3-20-25(16,17)23-26(18,19)22-24(13,14)15/h1-2,6-7H,3-4H2,(H,16,17)(H,18,19)(H2,9,10,12)(H2,13,14,15)/t6-,7+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164655
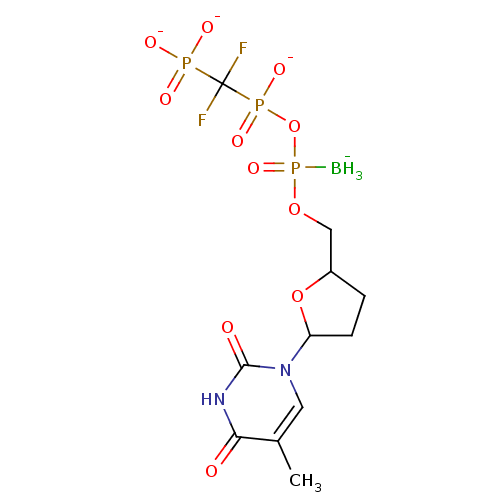
(2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...)Show SMILES [BH3-]P(=O)(OCC1CCC(O1)n1cc(C)c(=O)[nH]c1=O)OP([O-])(=O)C(F)(F)P([O-])([O-])=O Show InChI InChI=1S/C11H19BF2N2O11P3/c1-6-4-16(10(18)15-9(6)17)8-3-2-7(26-8)5-25-30(12,24)27-29(22,23)11(13,14)28(19,20)21/h4,7-8H,2-3,5H2,1,12H3,(H,22,23)(H,15,17,18)(H2,19,20,21)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164651
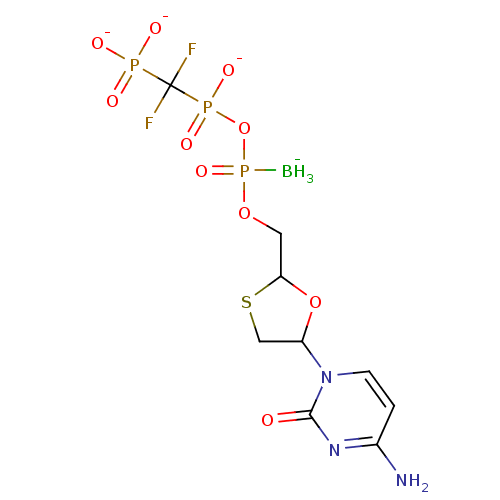
(2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...)Show SMILES [BH3-]P(=O)(OCC1OC(CS1)n1ccc(N)nc1=O)OP([O-])(=O)C(F)(F)P([O-])([O-])=O Show InChI InChI=1S/C9H16BF2N3O10P3S/c10-28(22,25-27(20,21)9(11,12)26(17,18)19)23-3-7-24-6(4-29-7)15-2-1-5(13)14-8(15)16/h1-2,6-7H,3-4H2,10H3,(H,20,21)(H2,13,14,16)(H2,17,18,19)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 314 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164656

(2',3'-Dideoxynucleoside5'-alpha-P-Borano-beta,gamm...)Show SMILES [BH3-]P(=O)(OCC1CCC(O1)n1ccc(=O)[nH]c1=O)OP([O-])(=O)C(F)(F)P([O-])([O-])=O Show InChI InChI=1S/C10H17BF2N2O11P3/c11-29(23,26-28(21,22)10(12,13)27(18,19)20)24-5-6-1-2-8(25-6)15-4-3-7(16)14-9(15)17/h3-4,6,8H,1-2,5H2,11H3,(H,21,22)(H,14,16,17)(H2,18,19,20)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 438 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164645
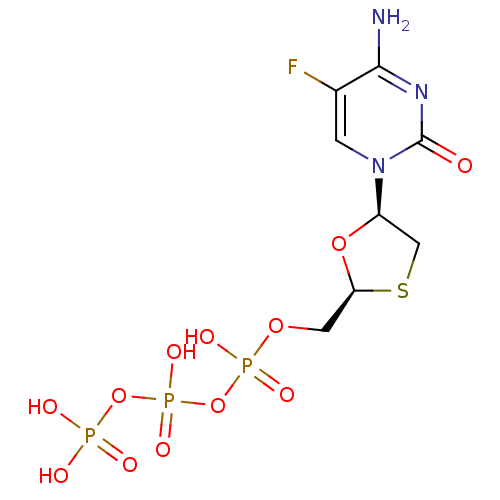
(CHEMBL192771 | [[[5-(4-amino-5-fluoro-2-oxo-1H-pyr...)Show SMILES Nc1nc(=O)n(cc1F)[C@H]1CS[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 Show InChI InChI=1S/C8H13FN3O12P3S/c9-4-1-12(8(13)11-7(4)10)5-3-28-6(22-5)2-21-26(17,18)24-27(19,20)23-25(14,15)16/h1,5-6H,2-3H2,(H,17,18)(H,19,20)(H2,10,11,13)(H2,14,15,16)/t5-,6+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164646

(CHEMBL192371 | [[[5-(2,4-dioxo-1H-pyrimidin-1-yl)t...)Show SMILES OP(O)(=O)OP(O)(=O)OP(O)(=O)OC[C@@H]1CC[C@@H](O1)n1ccc(=O)[nH]c1=O Show InChI InChI=1S/C9H15N2O13P3/c12-7-3-4-11(9(13)10-7)8-2-1-6(22-8)5-21-26(17,18)24-27(19,20)23-25(14,15)16/h3-4,6,8H,1-2,5H2,(H,17,18)(H,19,20)(H,10,12,13)(H2,14,15,16)/t6-,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50164643

(({[({[5-(4-amino-5-fluoro-2-oxo-1,2-dihydropyrimid...)Show SMILES [BH3-]P(=O)(OCC1OC(CS1)n1cc(F)c(N)nc1=O)OP([O-])(=O)C(F)(F)P([O-])([O-])=O Show InChI InChI=1S/C9H15BF3N3O10P3S/c10-29(23,26-28(21,22)9(12,13)27(18,19)20)24-2-6-25-5(3-30-6)16-1-4(11)7(14)15-8(16)17/h1,5-6H,2-3H2,10H3,(H,21,22)(H2,14,15,17)(H2,18,19,20)/q-1/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biota, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against HIV-1 reverse transcriptase |
J Med Chem 48: 2695-700 (2005)
Article DOI: 10.1021/jm040101y
BindingDB Entry DOI: 10.7270/Q2G73FG5 |
More data for this
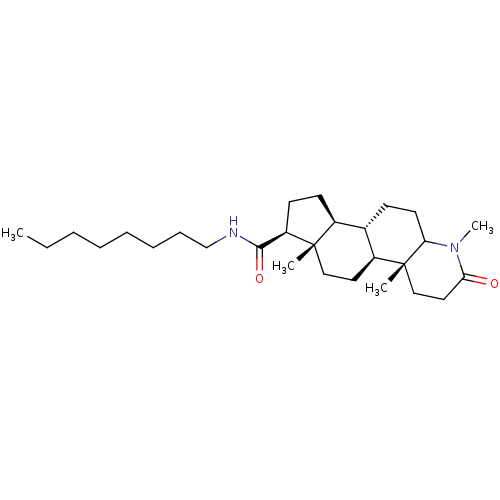
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50025367

(4a,6a-Dimethyl-2-oxo-hexadecahydro-indeno[5,4-f]qu...)Show SMILES [H][C@@]12CC[C@H](C(=O)NCCCCCCCC)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2NC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30N2O2/c1-18-9-7-13-11(12(18)4-5-14(18)17(20)23)3-6-15-19(13,2)10-8-16(22)21-15/h11-15H,3-10H2,1-2H3,(H2,20,23)(H,21,22) | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat prostatic steroid 5-alpha-reductase |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50225985
(CHEMBL3349105)Show SMILES [H][C@@]12CC[C@H](C(=O)NCCCCCCCC)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2N(C)C(=O)CC[C@]12C |r| Show InChI InChI=1S/C28H48N2O2/c1-5-6-7-8-9-10-19-29-26(32)23-13-12-21-20-11-14-24-28(3,18-16-25(31)30(24)4)22(20)15-17-27(21,23)2/h20-24H,5-19H2,1-4H3,(H,29,32)/t20-,21-,22-,23+,24?,27-,28+/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat prostatic steroid 5-alpha-reductase |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50025454

(1,4,4a,6a-Tetramethyl-2-oxo-hexadecahydro-indeno[5...)Show SMILES [H][C@@]12CC[C@H](C(=O)NC(C)(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2N(C)C(=O)CC[C@]12C |r| Show InChI InChI=1S/C25H42N2O2/c1-7-27(8-2)23(29)20-11-10-18-17-9-12-21-25(5,16(3)15-22(28)26(21)6)19(17)13-14-24(18,20)4/h16-21H,7-15H2,1-6H3/t16-,17?,18?,19?,20?,21+,24?,25?/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat prostatic steroid 5-alpha-reductase |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50025341

(4a,6a-Dimethyl-7-(3-methyl-butyryl)-hexadecahydro-...)Show SMILES [H][C@@]12CC[C@H](C(=O)C(C)CC)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2NC(=O)CC[C@]12C |r| Show InChI InChI=1S/C23H37NO2/c1-14(2)13-19(25)18-7-6-16-15-5-8-20-23(4,12-10-21(26)24-20)17(15)9-11-22(16,18)3/h14-18,20H,5-13H2,1-4H3,(H,24,26) | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat prostatic steroid 5-alpha-reductase |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM50025425

(17-Hydroxy-10,13-dimethyl-4,5,6,7,8,9,10,11,12,13,...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2CC(=O)C=C[C@]12C |r,c:22| Show InChI InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h7,9,12,14-17,21H,3-6,8,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro antagonist activity against rat prostatic androgen receptor (AR) |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50025450

(CHEMBL1790294 | Sodium; 1,4a,6a-trimethyl-2-oxo-he...)Show SMILES [Na+].[H][C@@]12CC[C@H](C(C)C([O-])=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2N(C)C(=O)CC[C@]12C Show InChI InChI=1S/C22H35NO3.Na/c1-13(20(25)26)15-6-7-16-14-5-8-18-22(3,12-10-19(24)23(18)4)17(14)9-11-21(15,16)2;/h13-18H,5-12H2,1-4H3,(H,25,26);/q;+1/p-1/t13?,14-,15+,16-,17-,18?,21+,22+;/m0./s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat prostatic steroid 5-alpha-reductase |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50025332

(1,4a,6a-Trimethyl-7-(2-methyl-butyryl)-hexadecahyd...)Show SMILES [H][C@@]12CC[C@H](C(=O)C(C)CC)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2N(C)C(=O)CC[C@]12C |r| Show InChI InChI=1S/C24H39NO2/c1-6-15(2)22(27)19-9-8-17-16-7-10-20-24(4,14-12-21(26)25(20)5)18(16)11-13-23(17,19)3/h15-20H,6-14H2,1-5H3 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat prostatic steroid 5-alpha-reductase |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro antagonist activity against rat prostatic androgen receptor (AR) |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM50025417

(7-Hydroxy-1,4a,6a-trimethyl-1,4a,4b,5,6,6a,7,8,9,9...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])N(C)C(=O)C=C[C@]12C |r,c:24| Show InChI InChI=1S/C19H29NO2/c1-18-11-9-17(22)20(3)15(18)6-4-12-13-5-7-16(21)19(13,2)10-8-14(12)18/h9,11-16,21H,4-8,10H2,1-3H3/t12?,13?,14?,15-,16?,18?,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro antagonist activity against rat prostatic androgen receptor (AR) |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50025415
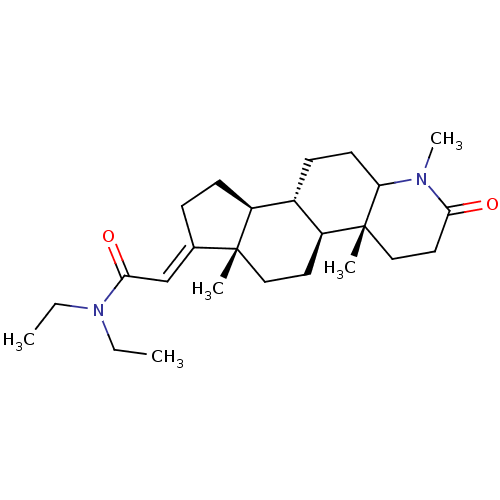
(CHEMBL3349288 | N,N-Diethyl-2-(1,4a,6a-trimethyl-2...)Show SMILES [H][C@@]12CC\C(=C/C(=O)N(CC)CC)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2N(C)C(=O)CC[C@]12C |r| Show InChI InChI=1S/C25H40N2O2/c1-6-27(7-2)23(29)16-17-8-10-19-18-9-11-21-25(4,15-13-22(28)26(21)5)20(18)12-14-24(17,19)3/h16,18-21H,6-15H2,1-5H3/b17-16+ | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat prostatic steroid 5-alpha-reductase |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50025353

(7-(1-Hydroxy-ethyl)-1,4a,6a-trimethyl-hexadecahydr...)Show SMILES [H][C@@]12C[C@@H](C)[C@H](C(C)O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2N(C)C(=O)CC[C@]12C Show InChI InChI=1S/C21H35NO2/c1-13(23)15-6-7-16-14-5-8-18-21(3,12-10-19(24)22(18)4)17(14)9-11-20(15,16)2/h13-18,23H,5-12H2,1-4H3 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat prostatic steroid 5-alpha-reductase |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM8885
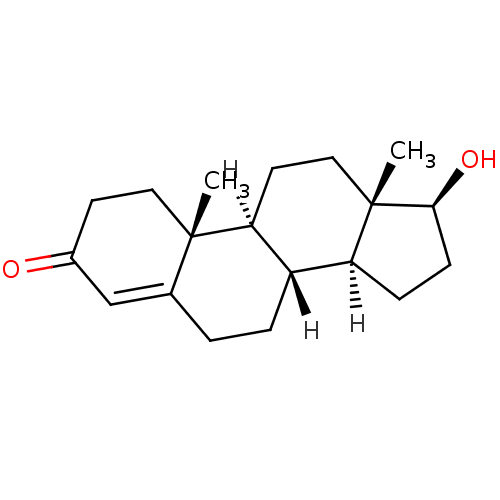
((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:18| Show InChI InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-17,21H,3-10H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro antagonist activity against rat prostatic androgen receptor (AR) |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50025375
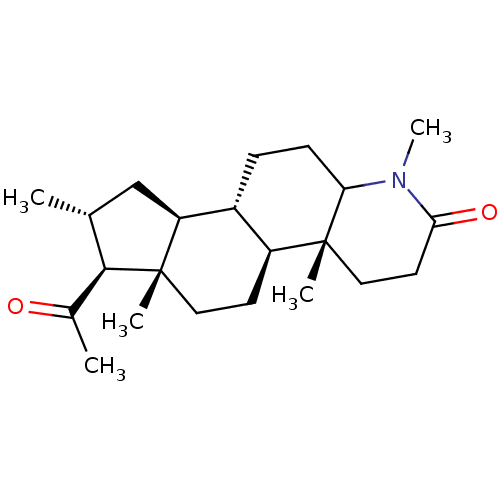
(7-Acetyl-1,4a,6a,8-tetramethyl-hexadecahydro-inden...)Show SMILES [H][C@@]12C[C@@H](C)[C@H](C(C)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2N(C)C(=O)CC[C@]12C Show InChI InChI=1S/C22H35NO2/c1-13-12-17-15-6-7-18-21(3,11-9-19(25)23(18)5)16(15)8-10-22(17,4)20(13)14(2)24/h13,15-18,20H,6-12H2,1-5H3/t13-,15?,16?,17?,18?,20?,21?,22?/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat prostatic steroid 5-alpha-reductase |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50025413
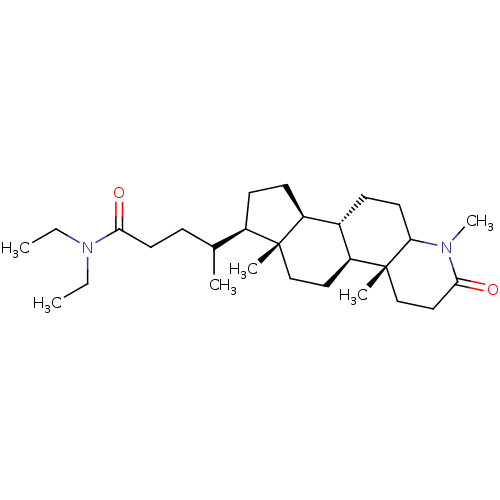
(4-(1,4a,6a-Trimethyl-2-oxo-hexadecahydro-indeno[5,...)Show SMILES [H][C@@]12CC[C@H](C(C)CCC(=O)N(CC)CC)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2N(C)C(=O)CC[C@]12C Show InChI InChI=1S/C28H48N2O2/c1-7-30(8-2)26(32)14-9-19(3)21-11-12-22-20-10-13-24-28(5,18-16-25(31)29(24)6)23(20)15-17-27(21,22)4/h19-24H,7-18H2,1-6H3 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat prostatic steroid 5-alpha-reductase |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50405303

(CHEMBL2021336)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4N(C)C(=O)CC[C@]4(C)[C@H]3CC[C@]12C Show InChI InChI=1S/C26H44N2O2/c1-16(2)28(17(3)4)24(30)21-10-9-19-18-8-11-22-26(6,15-13-23(29)27(22)7)20(18)12-14-25(19,21)5/h16-22H,8-15H2,1-7H3/t18-,19-,20-,21+,22+,25-,26+/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat prostatic steroid 5-alpha-reductase |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50025399
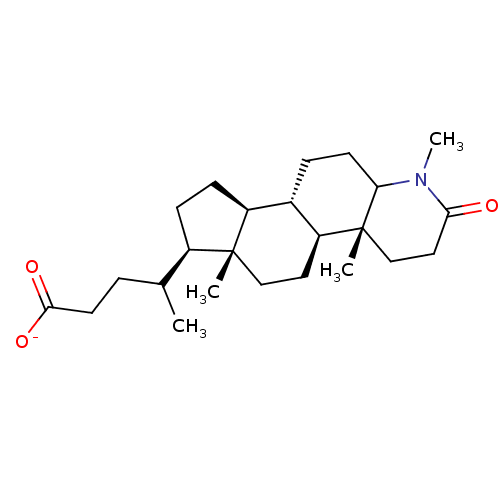
(CHEMBL1790293 | Sodium; 4-(1,4a,6a-trimethyl-2-oxo...)Show SMILES [Na+].[H][C@@]12CC[C@H](C(C)CCC([O-])=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2N(C)C(=O)CC[C@]12C Show InChI InChI=1S/C24H39NO3.Na/c1-15(5-10-22(27)28)17-7-8-18-16-6-9-20-24(3,14-12-21(26)25(20)4)19(16)11-13-23(17,18)2;/h15-20H,5-14H2,1-4H3,(H,27,28);/q;+1/p-1/t15?,16-,17+,18-,19-,20?,23+,24+;/m0./s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat prostatic steroid 5-alpha-reductase |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50025337
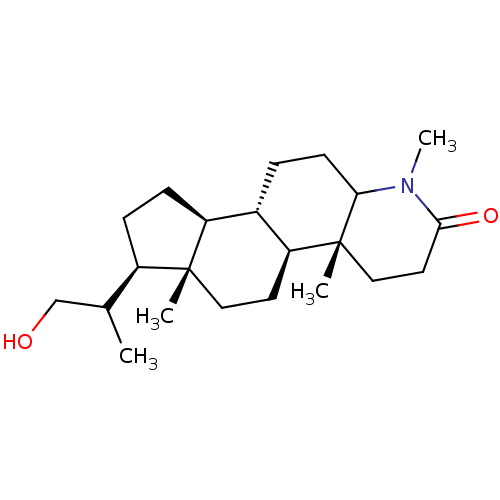
(7-(2-Hydroxy-1-methyl-ethyl)-1,4a,6a-trimethyl-hex...)Show SMILES [H][C@@]12CC[C@H](C(C)CO)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2N(C)C(=O)CC[C@]12C Show InChI InChI=1S/C22H37NO2/c1-14(13-24)16-6-7-17-15-5-8-19-22(3,12-10-20(25)23(19)4)18(15)9-11-21(16,17)2/h14-19,24H,5-13H2,1-4H3 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat prostatic steroid 5-alpha-reductase |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50025379
(1,4a,6a-Trimethyl-2-oxo-hexadecahydro-indeno[5,4-f...)Show SMILES [H][C@@]12CC[C@H](C(=O)NCC(OC)OC)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2N(C)C(=O)CC[C@]12C |r| Show InChI InChI=1S/C24H40N2O4/c1-23-12-10-17-15(6-9-19-24(17,2)13-11-20(27)26(19)3)16(23)7-8-18(23)22(28)25-14-21(29-4)30-5/h15-19,21H,6-14H2,1-5H3,(H,25,28) | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat prostatic steroid 5-alpha-reductase |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50025330

(1',4a',6a'-trimethylspiro[tetrahydrofuran-2,7'-per...)Show SMILES [H][C@@]12CC[C@@]3(CCCO3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@@H](C)CC2N(C)C(=O)CC[C@]12C Show InChI InChI=1S/C22H35NO2/c1-20-11-9-19(24)23(3)18(20)6-5-15-16(20)7-12-21(2)17(15)8-13-22(21)10-4-14-25-22/h15-18H,4-14H2,1-3H3/t15?,16?,17?,18?,20?,21?,22-/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat prostatic steroid 5-alpha-reductase |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50025455
(4a,6a-Dimethyl-2-oxo-hexadecahydro-indeno[5,4-f]qu...)Show SMILES [H][C@@]12CC[C@H](C(=O)NC(C)(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2NC(=O)CC[C@]12C |r| Show InChI InChI=1S/C23H38N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h14-18H,6-13H2,1-5H3,(H,24,26)(H,25,27) | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat prostatic steroid 5-alpha-reductase |
J Med Chem 29: 2298-315 (1986)
BindingDB Entry DOI: 10.7270/Q2XG9RQR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data