 Found 3374 hits with Last Name = 'hwang' and Initial = 'j'
Found 3374 hits with Last Name = 'hwang' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.270 | -50.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem Lett 18: 5567-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.002
BindingDB Entry DOI: 10.7270/Q2W0948B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18699
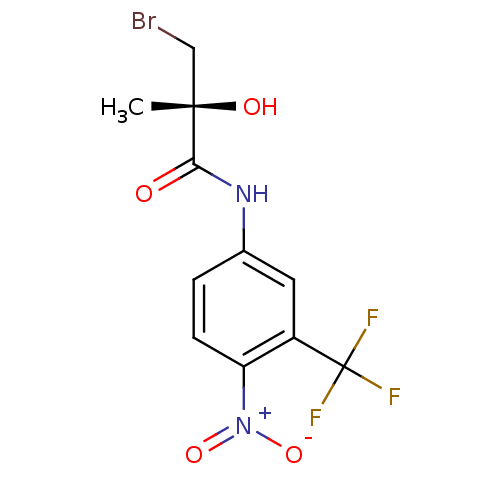
((2R)-3-bromo-2-hydroxy-2-methyl-N-[4-nitro-3-(trif...)Show SMILES C[C@](O)(CBr)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C11H10BrF3N2O4/c1-10(19,5-12)9(18)16-6-2-3-8(17(20)21)7(4-6)11(13,14)15/h2-4,19H,5H2,1H3,(H,16,18)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.430 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee Health Science Center
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem 14: 6525-38 (2006)
Article DOI: 10.1016/j.bmc.2006.06.019
BindingDB Entry DOI: 10.7270/Q2WQ022N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50215713
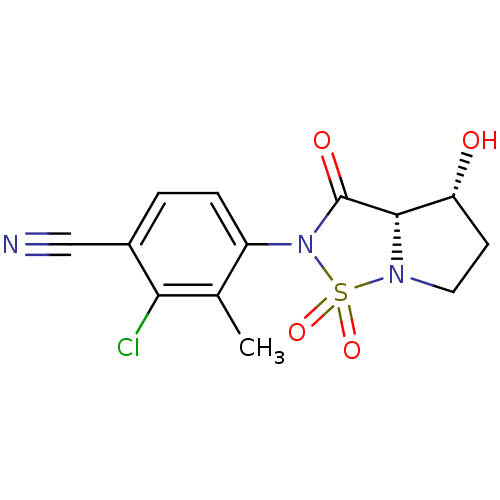
(2-Chloro-4-((3aS,4R)-4-hydroxy-1,1,3-trioxo-tetrah...)Show SMILES Cc1c(Cl)c(ccc1N1C(=O)[C@@H]2[C@H](O)CCN2S1(=O)=O)C#N |r| Show InChI InChI=1S/C13H12ClN3O4S/c1-7-9(3-2-8(6-15)11(7)14)17-13(19)12-10(18)4-5-16(12)22(17,20)21/h2-3,10,12,18H,4-5H2,1H3/t10-,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50528793
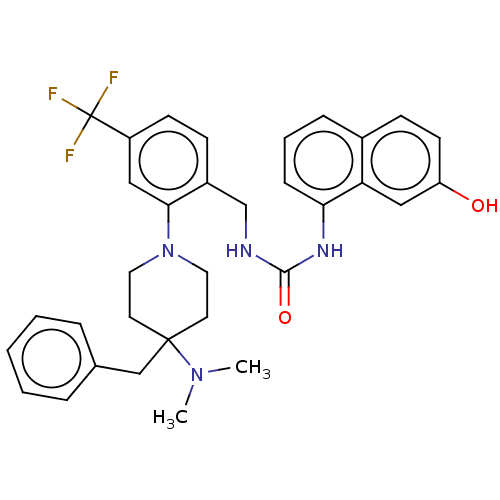
(CHEMBL4471708)Show SMILES CN(C)C1(Cc2ccccc2)CCN(CC1)c1cc(ccc1CNC(=O)Nc1cccc2ccc(O)cc12)C(F)(F)F Show InChI InChI=1S/C33H35F3N4O2/c1-39(2)32(21-23-7-4-3-5-8-23)15-17-40(18-16-32)30-19-26(33(34,35)36)13-11-25(30)22-37-31(42)38-29-10-6-9-24-12-14-27(41)20-28(24)29/h3-14,19-20,41H,15-18,21-22H2,1-2H3,(H2,37,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111634
BindingDB Entry DOI: 10.7270/Q2765JR7 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM26260
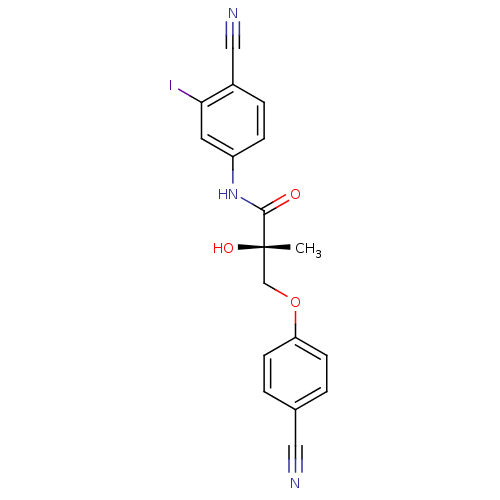
((2S)-N-(4-cyano-3-iodophenyl)-3-(4-cyanophenoxy)-2...)Show SMILES C[C@](O)(COc1ccc(cc1)C#N)C(=O)Nc1ccc(C#N)c(I)c1 |r| Show InChI InChI=1S/C18H14IN3O3/c1-18(24,11-25-15-6-2-12(9-20)3-7-15)17(23)22-14-5-4-13(10-21)16(19)8-14/h2-8,24H,11H2,1H3,(H,22,23)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| 0.540 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem Lett 18: 5567-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.002
BindingDB Entry DOI: 10.7270/Q2W0948B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18681

((2R)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydro...)Show SMILES C[C@](O)(CSc1ccc(cc1)N=C=S)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C19H14F3N3O2S2/c1-18(27,10-29-15-6-4-13(5-7-15)24-11-28)17(26)25-14-3-2-12(9-23)16(8-14)19(20,21)22/h2-8,27H,10H2,1H3,(H,25,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | -48.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee Health Science Center
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem 14: 6525-38 (2006)
Article DOI: 10.1016/j.bmc.2006.06.019
BindingDB Entry DOI: 10.7270/Q2WQ022N |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50528804
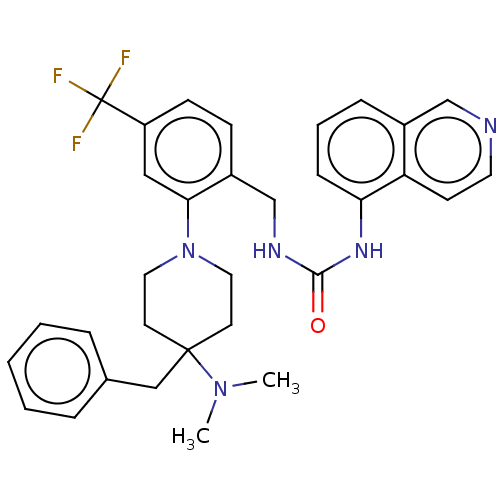
(CHEMBL4553702)Show SMILES CN(C)C1(Cc2ccccc2)CCN(CC1)c1cc(ccc1CNC(=O)Nc1cccc2cnccc12)C(F)(F)F Show InChI InChI=1S/C32H34F3N5O/c1-39(2)31(20-23-7-4-3-5-8-23)14-17-40(18-15-31)29-19-26(32(33,34)35)12-11-25(29)22-37-30(41)38-28-10-6-9-24-21-36-16-13-27(24)28/h3-13,16,19,21H,14-15,17-18,20,22H2,1-2H3,(H2,37,38,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111634
BindingDB Entry DOI: 10.7270/Q2765JR7 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18177
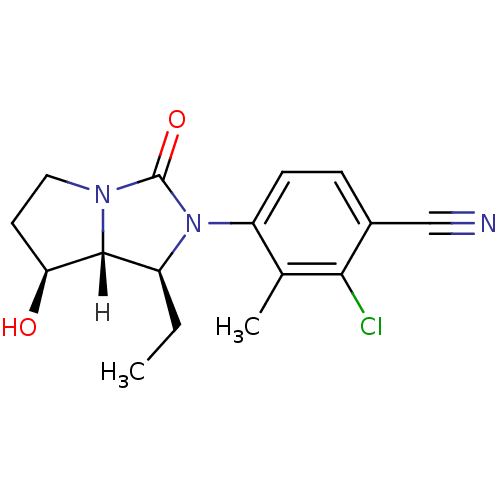
(4-[(1S,7S,7aR)-1-ethyl-7-hydroxy-3-oxo-hexahydro-1...)Show SMILES [H][C@@]12[C@@H](O)CCN1C(=O)N([C@H]2CC)c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C16H18ClN3O2/c1-3-11-15-13(21)6-7-19(15)16(22)20(11)12-5-4-10(8-18)14(17)9(12)2/h4-5,11,13,15,21H,3,6-7H2,1-2H3/t11-,13-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50528797
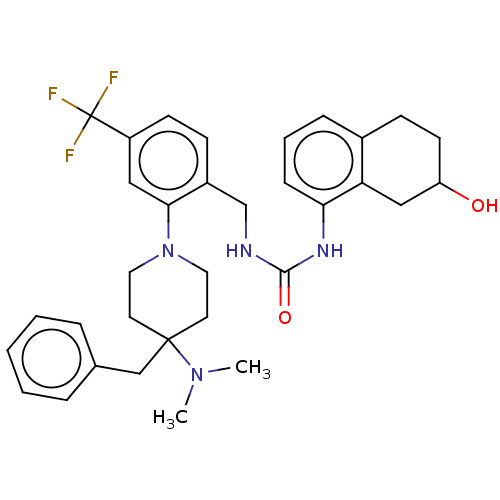
(CHEMBL4438643)Show SMILES CN(C)C1(Cc2ccccc2)CCN(CC1)c1cc(ccc1CNC(=O)Nc1cccc2CCC(O)Cc12)C(F)(F)F Show InChI InChI=1S/C33H39F3N4O2/c1-39(2)32(21-23-7-4-3-5-8-23)15-17-40(18-16-32)30-19-26(33(34,35)36)13-11-25(30)22-37-31(42)38-29-10-6-9-24-12-14-27(41)20-28(24)29/h3-11,13,19,27,41H,12,14-18,20-22H2,1-2H3,(H2,37,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111634
BindingDB Entry DOI: 10.7270/Q2765JR7 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50258751

((R)-2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)-1,1,...)Show SMILES O[C@](C=C)(c1nc2cc(Cl)c(Cl)cc2[nH]1)C(F)(F)F |r| Show InChI InChI=1S/C11H7Cl2F3N2O/c1-2-10(19,11(14,15)16)9-17-7-3-5(12)6(13)4-8(7)18-9/h2-4,19H,1H2,(H,17,18)/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM26262
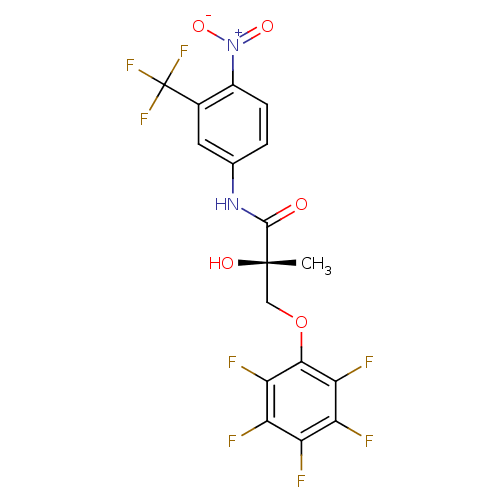
((2S)-2-hydroxy-2-methyl-N-[4-nitro-3-(trifluoromet...)Show SMILES C[C@](O)(COc1c(F)c(F)c(F)c(F)c1F)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C17H10F8N2O5/c1-16(29,5-32-14-12(21)10(19)9(18)11(20)13(14)22)15(28)26-6-2-3-8(27(30)31)7(4-6)17(23,24)25/h2-4,29H,5H2,1H3,(H,26,28)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.40 | -47.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem Lett 18: 5567-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.002
BindingDB Entry DOI: 10.7270/Q2W0948B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18524
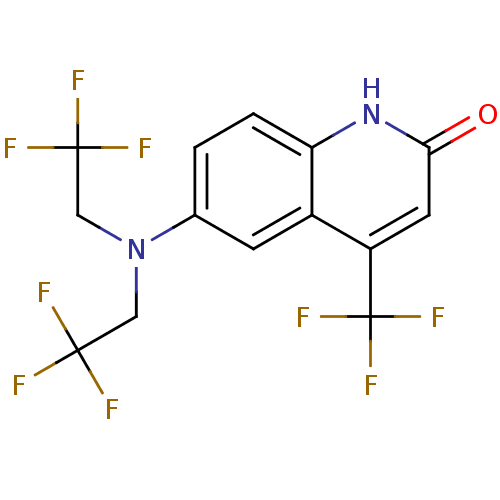
(6-[bis(2,2,2-trifluoroethyl)amino]-4-(trifluoromet...)Show SMILES FC(F)(F)CN(CC(F)(F)F)c1ccc2[nH]c(=O)cc(c2c1)C(F)(F)F Show InChI InChI=1S/C14H9F9N2O/c15-12(16,17)5-25(6-13(18,19)20)7-1-2-10-8(3-7)9(14(21,22)23)4-11(26)24-10/h1-4H,5-6H2,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18522
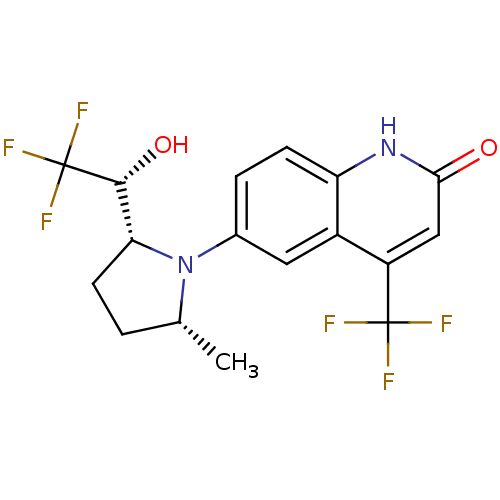
(6-(1-Pyrrolidine)quinolin-2(1H)-one, 6a | 6-[(2R,5...)Show SMILES C[C@@H]1CC[C@H]([C@@H](O)C(F)(F)F)N1c1ccc2[nH]c(=O)cc(c2c1)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O2/c1-8-2-5-13(15(27)17(21,22)23)25(8)9-3-4-12-10(6-9)11(16(18,19)20)7-14(26)24-12/h3-4,6-8,13,15,27H,2,5H2,1H3,(H,24,26)/t8-,13-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18685
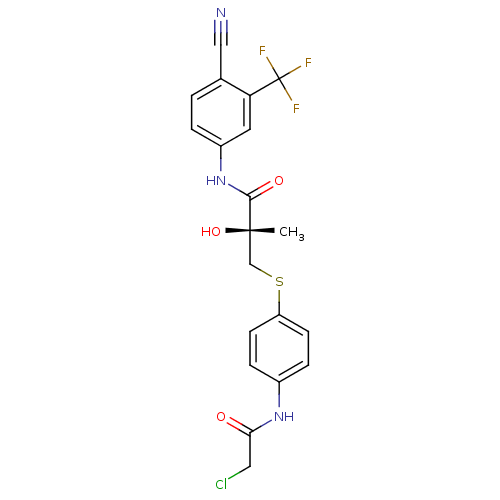
((2R)-3-{[4-(2-chloroacetamido)phenyl]sulfanyl}-N-[...)Show SMILES C[C@](O)(CSc1ccc(NC(=O)CCl)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C20H17ClF3N3O3S/c1-19(30,11-31-15-6-4-13(5-7-15)26-17(28)9-21)18(29)27-14-3-2-12(10-25)16(8-14)20(22,23)24/h2-8,30H,9,11H2,1H3,(H,26,28)(H,27,29)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM26261
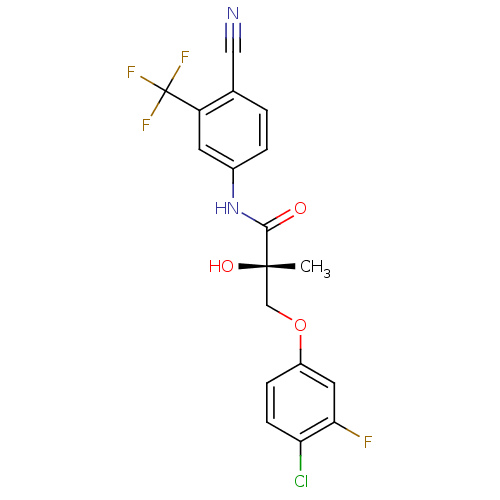
((2S)-3-(4-chloro-3-fluorophenoxy)-N-[4-cyano-3-(tr...)Show SMILES C[C@](O)(COc1ccc(Cl)c(F)c1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C18H13ClF4N2O3/c1-17(27,9-28-12-4-5-14(19)15(20)7-12)16(26)25-11-3-2-10(8-24)13(6-11)18(21,22)23/h2-7,27H,9H2,1H3,(H,25,26)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 1.70 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem Lett 18: 5567-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.002
BindingDB Entry DOI: 10.7270/Q2W0948B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50528802
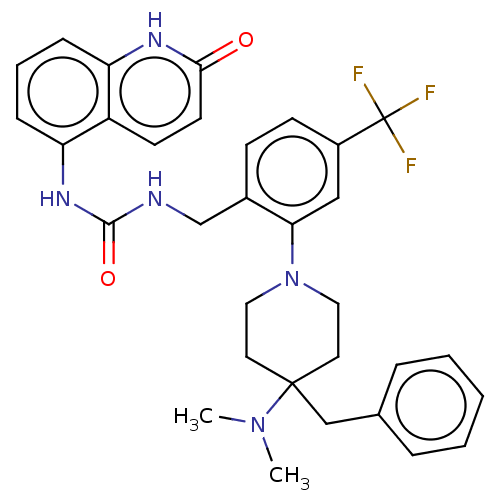
(CHEMBL4562769)Show SMILES CN(C)C1(Cc2ccccc2)CCN(CC1)c1cc(ccc1CNC(=O)Nc1cccc2[nH]c(=O)ccc12)C(F)(F)F Show InChI InChI=1S/C32H34F3N5O2/c1-39(2)31(20-22-7-4-3-5-8-22)15-17-40(18-16-31)28-19-24(32(33,34)35)12-11-23(28)21-36-30(42)38-27-10-6-9-26-25(27)13-14-29(41)37-26/h3-14,19H,15-18,20-21H2,1-2H3,(H,37,41)(H2,36,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111634
BindingDB Entry DOI: 10.7270/Q2765JR7 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM26261

((2S)-3-(4-chloro-3-fluorophenoxy)-N-[4-cyano-3-(tr...)Show SMILES C[C@](O)(COc1ccc(Cl)c(F)c1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C18H13ClF4N2O3/c1-17(27,9-28-12-4-5-14(19)15(20)7-12)16(26)25-11-3-2-10(8-24)13(6-11)18(21,22)23/h2-7,27H,9H2,1H3,(H,25,26)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18173
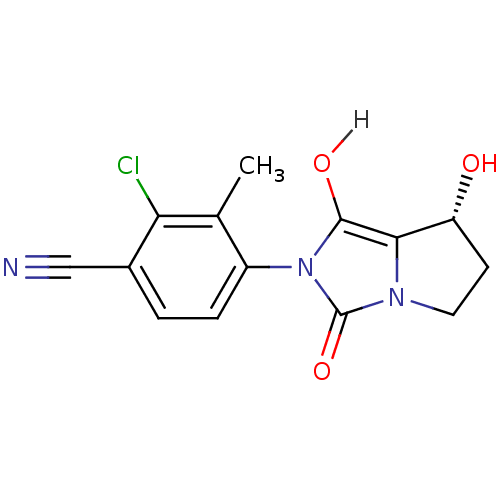
(4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrro...)Show SMILES Cc1c(Cl)c(ccc1-n1c(O)c2[C@H](O)CCn2c1=O)C#N |r,wU:12.13,(.01,.36,;.81,1.67,;2.35,1.63,;3.09,.28,;3.16,2.94,;2.42,4.3,;.88,4.34,;.08,3.02,;-1.46,3.02,;-1.94,4.49,;-1.03,5.73,;-3.48,4.49,;-4.72,5.39,;-4.72,6.93,;-5.97,4.49,;-5.5,3.02,;-3.95,3.02,;-2.71,2.12,;-2.71,.58,;4.7,2.9,;6.24,2.9,)| Show InChI InChI=1S/C14H12ClN3O3/c1-7-9(3-2-8(6-16)11(7)15)18-13(20)12-10(19)4-5-17(12)14(18)21/h2-3,10,19-20H,4-5H2,1H3/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM26259
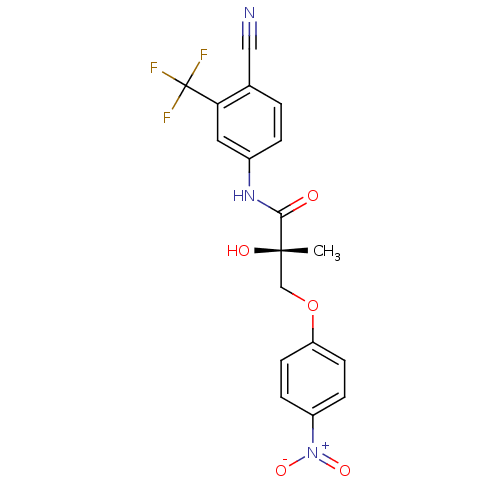
((2S)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydro...)Show SMILES C[C@](O)(COc1ccc(cc1)[N+]([O-])=O)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C18H14F3N3O5/c1-17(26,10-29-14-6-4-13(5-7-14)24(27)28)16(25)23-12-3-2-11(9-22)15(8-12)18(19,20)21/h2-8,26H,10H2,1H3,(H,23,25)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| 2.5 | -45.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem Lett 18: 5567-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.002
BindingDB Entry DOI: 10.7270/Q2W0948B |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20330
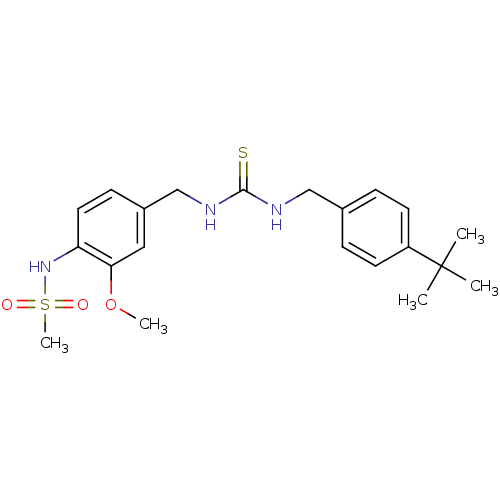
(3-[(4-tert-butylphenyl)methyl]-1-[(4-methanesulfon...)Show SMILES COc1cc(CNC(=S)NCc2ccc(cc2)C(C)(C)C)ccc1NS(C)(=O)=O Show InChI InChI=1S/C21H29N3O3S2/c1-21(2,3)17-9-6-15(7-10-17)13-22-20(28)23-14-16-8-11-18(19(12-16)27-4)24-29(5,25)26/h6-12,24H,13-14H2,1-5H3,(H2,22,23,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity towards rat TRPV1 expressed in CHO cells |
Bioorg Med Chem Lett 15: 4143-50 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.006
BindingDB Entry DOI: 10.7270/Q2JH3KQB |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50529668

(Enobosarm | Gtx-024 | MK-2866 | Ostarine | US10806...)Show SMILES C[C@](O)(COc1ccc(cc1)C#N)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C19H14F3N3O3/c1-18(27,11-28-15-6-2-12(9-23)3-7-15)17(26)25-14-5-4-13(10-24)16(8-14)19(20,21)22/h2-8,27H,11H2,1H3,(H,25,26)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mibolerone from recombinant wild-type GST-tagged androgen receptor LBD (unknown origin) after 16 hrs by scintillation counting a... |
J Med Chem 62: 491-511 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00973
BindingDB Entry DOI: 10.7270/Q2GF0XZ5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50529668

(Enobosarm | Gtx-024 | MK-2866 | Ostarine | US10806...)Show SMILES C[C@](O)(COc1ccc(cc1)C#N)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C19H14F3N3O3/c1-18(27,11-28-15-6-2-12(9-23)3-7-15)17(26)25-14-5-4-13(10-24)16(8-14)19(20,21)22/h2-8,27H,11H2,1H3,(H,25,26)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mibolerone from recombinant wild-type GST-tagged androgen receptor LBD (unknown origin) after 16 hrs by scintillation counting a... |
J Med Chem 62: 491-511 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00973
BindingDB Entry DOI: 10.7270/Q2GF0XZ5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50528800
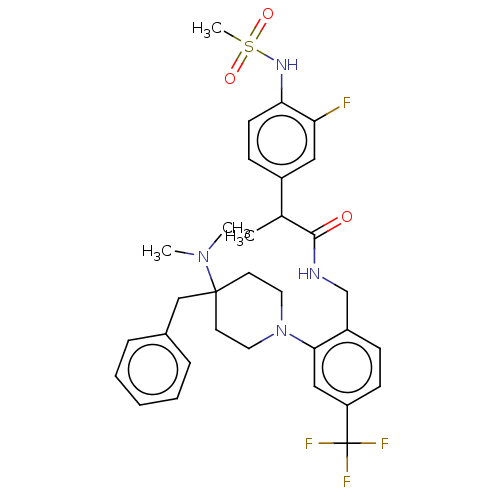
(CHEMBL4586277)Show SMILES CC(C(=O)NCc1ccc(cc1N1CCC(Cc2ccccc2)(CC1)N(C)C)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C32H38F4N4O3S/c1-22(24-11-13-28(27(33)18-24)38-44(4,42)43)30(41)37-21-25-10-12-26(32(34,35)36)19-29(25)40-16-14-31(15-17-40,39(2)3)20-23-8-6-5-7-9-23/h5-13,18-19,22,38H,14-17,20-21H2,1-4H3,(H,37,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111634
BindingDB Entry DOI: 10.7270/Q2765JR7 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18665
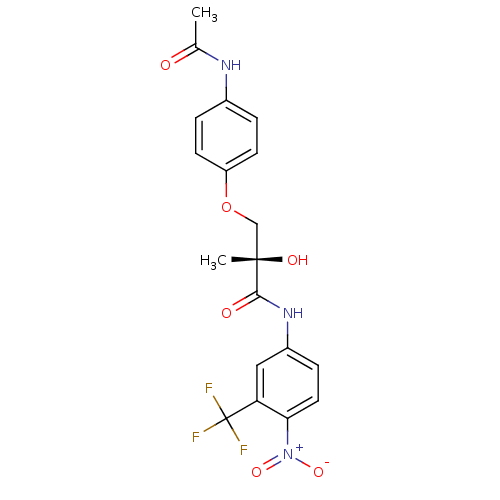
((2S)-3-(4-acetamidophenoxy)-2-hydroxy-2-methyl-N-[...)Show SMILES CC(=O)Nc1ccc(OC[C@](C)(O)C(=O)Nc2ccc(c(c2)C(F)(F)F)[N+]([O-])=O)cc1 |r| Show InChI InChI=1S/C19H18F3N3O6/c1-11(26)23-12-3-6-14(7-4-12)31-10-18(2,28)17(27)24-13-5-8-16(25(29)30)15(9-13)19(20,21)22/h3-9,28H,10H2,1-2H3,(H,23,26)(H,24,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 4 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem Lett 18: 5567-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.002
BindingDB Entry DOI: 10.7270/Q2W0948B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18665

((2S)-3-(4-acetamidophenoxy)-2-hydroxy-2-methyl-N-[...)Show SMILES CC(=O)Nc1ccc(OC[C@](C)(O)C(=O)Nc2ccc(c(c2)C(F)(F)F)[N+]([O-])=O)cc1 |r| Show InChI InChI=1S/C19H18F3N3O6/c1-11(26)23-12-3-6-14(7-4-12)31-10-18(2,28)17(27)24-13-5-8-16(25(29)30)15(9-13)19(20,21)22/h3-9,28H,10H2,1-2H3,(H,23,26)(H,24,27)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18675

((2S)-2-hydroxy-3-(4-isothiocyanatophenoxy)-2-methy...)Show SMILES CC(O)(COc1ccc([N-]C#[S+])cc1)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C18H14F3N3O5S/c1-17(26,9-29-13-5-2-11(3-6-13)22-10-30)16(25)23-12-4-7-15(24(27)28)14(8-12)18(19,20)21/h2-8,26H,9H2,1H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.60 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee Health Science Center
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem 14: 6525-38 (2006)
Article DOI: 10.1016/j.bmc.2006.06.019
BindingDB Entry DOI: 10.7270/Q2WQ022N |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50258791
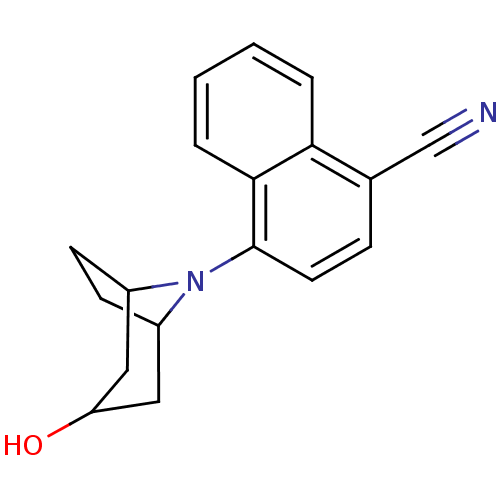
(4-(3-exo-Hydroxy-8-azabicyclo[3.2.1]oct-8-yl)napht...)Show SMILES OC1CC2CCC(C1)N2c1ccc(C#N)c2ccccc12 |TLB:0:1:8:4.5,9:8:7.1.2:4.5| Show InChI InChI=1S/C18H18N2O/c19-11-12-5-8-18(17-4-2-1-3-16(12)17)20-13-6-7-14(20)10-15(21)9-13/h1-5,8,13-15,21H,6-7,9-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18663
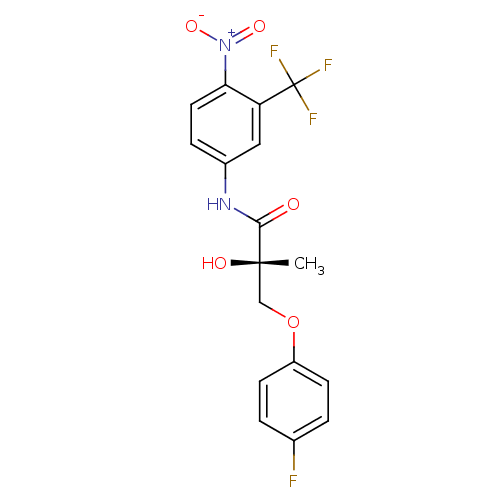
((2S)-3-(4-fluorophenoxy)-2-hydroxy-2-methyl-N-[4-n...)Show SMILES C[C@](O)(COc1ccc(F)cc1)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C17H14F4N2O5/c1-16(25,9-28-12-5-2-10(18)3-6-12)15(24)22-11-4-7-14(23(26)27)13(8-11)17(19,20)21/h2-8,25H,9H2,1H3,(H,22,24)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 6.10 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem Lett 18: 5567-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.002
BindingDB Entry DOI: 10.7270/Q2W0948B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM18663

((2S)-3-(4-fluorophenoxy)-2-hydroxy-2-methyl-N-[4-n...)Show SMILES C[C@](O)(COc1ccc(F)cc1)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C17H14F4N2O5/c1-16(25,9-28-12-5-2-10(18)3-6-12)15(24)22-11-4-7-14(23(26)27)13(8-11)17(19,20)21/h2-8,25H,9H2,1H3,(H,22,24)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DAMGO from full length human MOR expressed in chem5 cells incubated for 60 mins by liquid scintillation counter |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111634
BindingDB Entry DOI: 10.7270/Q2765JR7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM19242
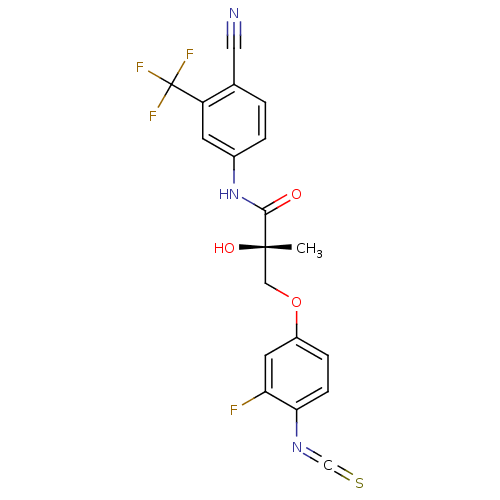
((2S)-N-[4-cyano-3-(trifluoromethyl)phenyl]-3-(3-fl...)Show SMILES C[C@](O)(COc1ccc(N=C=S)c(F)c1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C19H13F4N3O3S/c1-18(28,9-29-13-4-5-16(25-10-30)15(20)7-13)17(27)26-12-3-2-11(8-24)14(6-12)19(21,22)23/h2-7,28H,9H2,1H3,(H,26,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee Health Science Center
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem 14: 6525-38 (2006)
Article DOI: 10.1016/j.bmc.2006.06.019
BindingDB Entry DOI: 10.7270/Q2WQ022N |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18216
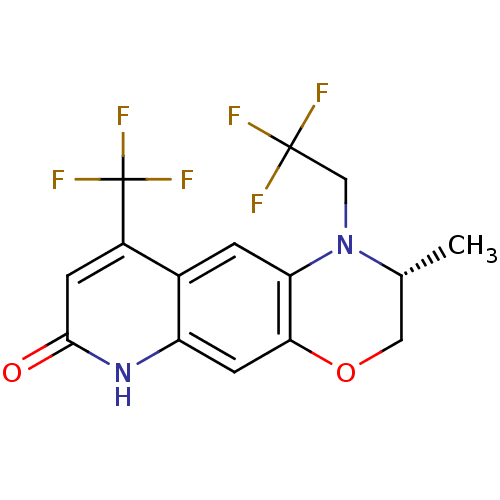
((2R)-2-methyl-1-(2,2,2-trifluoroethyl)-9-(trifluor...)Show SMILES C[C@@H]1COc2cc3[nH]c(=O)cc(c3cc2N1CC(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C15H12F6N2O2/c1-7-5-25-12-4-10-8(2-11(12)23(7)6-14(16,17)18)9(15(19,20)21)3-13(24)22-10/h2-4,7H,5-6H2,1H3,(H,22,24)/t7-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50528807
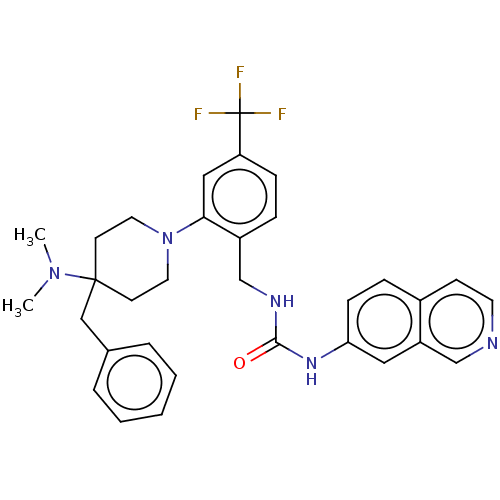
(CHEMBL4453980)Show SMILES CN(C)C1(Cc2ccccc2)CCN(CC1)c1cc(ccc1CNC(=O)Nc1ccc2ccncc2c1)C(F)(F)F Show InChI InChI=1S/C32H34F3N5O/c1-39(2)31(20-23-6-4-3-5-7-23)13-16-40(17-14-31)29-19-27(32(33,34)35)10-8-25(29)22-37-30(41)38-28-11-9-24-12-15-36-21-26(24)18-28/h3-12,15,18-19,21H,13-14,16-17,20,22H2,1-2H3,(H2,37,38,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111634
BindingDB Entry DOI: 10.7270/Q2765JR7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398494

(CHEMBL2177429)Show SMILES C[C@H](C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111634
BindingDB Entry DOI: 10.7270/Q2765JR7 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM19240

((2S)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydro...)Show SMILES C[C@](O)(COc1ccc(cc1)N=C=S)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C19H14F3N3O3S/c1-18(27,10-28-15-6-4-13(5-7-15)24-11-29)17(26)25-14-3-2-12(9-23)16(8-14)19(20,21)22/h2-8,27H,10H2,1H3,(H,25,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee Health Science Center
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem 14: 6525-38 (2006)
Article DOI: 10.1016/j.bmc.2006.06.019
BindingDB Entry DOI: 10.7270/Q2WQ022N |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM19244

((2S)-3-(3-chloro-4-isothiocyanatophenoxy)-N-[4-cya...)Show SMILES C[C@](O)(COc1ccc(N=C=S)c(Cl)c1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C19H13ClF3N3O3S/c1-18(28,9-29-13-4-5-16(25-10-30)15(20)7-13)17(27)26-12-3-2-11(8-24)14(6-12)19(21,22)23/h2-7,28H,9H2,1H3,(H,26,27)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60 | -42.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee Health Science Center
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem 14: 6525-38 (2006)
Article DOI: 10.1016/j.bmc.2006.06.019
BindingDB Entry DOI: 10.7270/Q2WQ022N |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20321
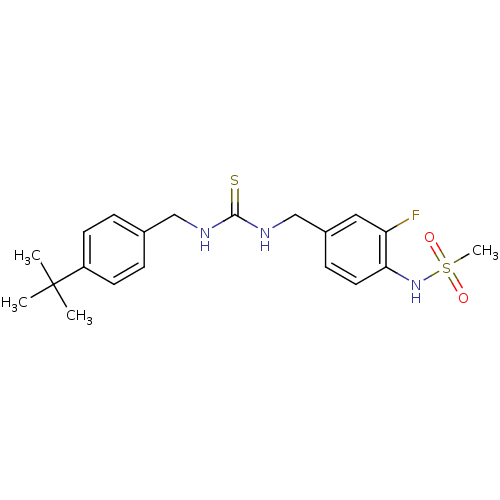
(3-[(4-tert-butylphenyl)methyl]-1-[(3-fluoro-4-meth...)Show SMILES CC(C)(C)c1ccc(CNC(=S)NCc2ccc(NS(C)(=O)=O)c(F)c2)cc1 Show InChI InChI=1S/C20H26FN3O2S2/c1-20(2,3)16-8-5-14(6-9-16)12-22-19(27)23-13-15-7-10-18(17(21)11-15)24-28(4,25)26/h5-11,24H,12-13H2,1-4H3,(H2,22,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity towards rat TRPV1 expressed in CHO cells |
Bioorg Med Chem Lett 15: 4143-50 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.006
BindingDB Entry DOI: 10.7270/Q2JH3KQB |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50528792
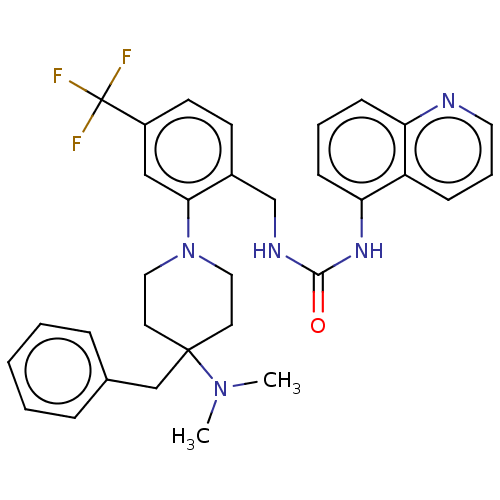
(CHEMBL4438974)Show SMILES CN(C)C1(Cc2ccccc2)CCN(CC1)c1cc(ccc1CNC(=O)Nc1cccc2ncccc12)C(F)(F)F Show InChI InChI=1S/C32H34F3N5O/c1-39(2)31(21-23-8-4-3-5-9-23)15-18-40(19-16-31)29-20-25(32(33,34)35)14-13-24(29)22-37-30(41)38-28-12-6-11-27-26(28)10-7-17-36-27/h3-14,17,20H,15-16,18-19,21-22H2,1-2H3,(H2,37,38,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111634
BindingDB Entry DOI: 10.7270/Q2765JR7 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-MIB from wild-type rat AR LBD measured after 16 hrs by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00943
BindingDB Entry DOI: 10.7270/Q2DV1PHB |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18678
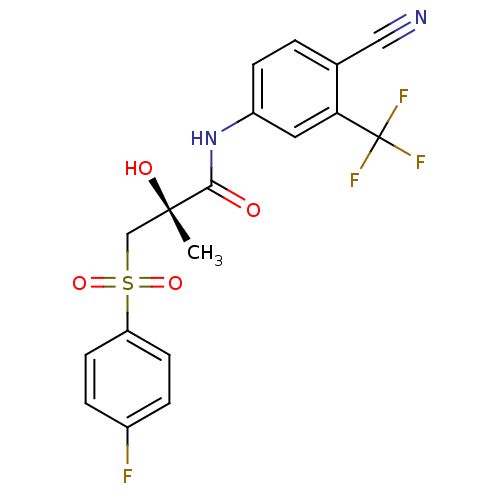
((2R)-N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-f...)Show SMILES C[C@](O)(CS(=O)(=O)c1ccc(F)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C18H14F4N2O4S/c1-17(26,10-29(27,28)14-6-3-12(19)4-7-14)16(25)24-13-5-2-11(9-23)15(8-13)18(20,21)22/h2-8,26H,10H2,1H3,(H,24,25)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 11 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
The Ohio State University
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem Lett 18: 5567-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.002
BindingDB Entry DOI: 10.7270/Q2W0948B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM18678

((2R)-N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-f...)Show SMILES C[C@](O)(CS(=O)(=O)c1ccc(F)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C18H14F4N2O4S/c1-17(26,10-29(27,28)14-6-3-12(19)4-7-14)16(25)24-13-5-2-11(9-23)15(8-13)18(20,21)22/h2-8,26H,10H2,1H3,(H,24,25)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 11 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee Health Science Center
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem 14: 6525-38 (2006)
Article DOI: 10.1016/j.bmc.2006.06.019
BindingDB Entry DOI: 10.7270/Q2WQ022N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM19243

((2S)-3-(3-chloro-4-isothiocyanatophenoxy)-2-hydrox...)Show SMILES C[C@](O)(COc1ccc(N=C=S)c(Cl)c1)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C18H13ClF3N3O5S/c1-17(27,8-30-11-3-4-14(23-9-31)13(19)7-11)16(26)24-10-2-5-15(25(28)29)12(6-10)18(20,21)22/h2-7,27H,8H2,1H3,(H,24,26)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11.9 | -42.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee Health Science Center
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem 14: 6525-38 (2006)
Article DOI: 10.1016/j.bmc.2006.06.019
BindingDB Entry DOI: 10.7270/Q2WQ022N |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50528795
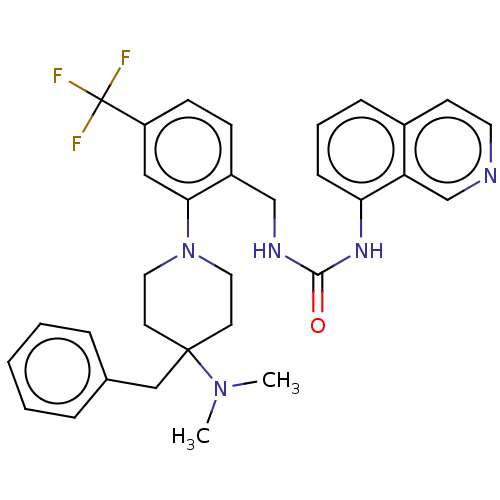
(CHEMBL4442473)Show SMILES CN(C)C1(Cc2ccccc2)CCN(CC1)c1cc(ccc1CNC(=O)Nc1cccc2ccncc12)C(F)(F)F Show InChI InChI=1S/C32H34F3N5O/c1-39(2)31(20-23-7-4-3-5-8-23)14-17-40(18-15-31)29-19-26(32(33,34)35)12-11-25(29)21-37-30(41)38-28-10-6-9-24-13-16-36-22-27(24)28/h3-13,16,19,22H,14-15,17-18,20-21H2,1-2H3,(H2,37,38,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111634
BindingDB Entry DOI: 10.7270/Q2765JR7 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM19241

((2S)-3-(3-fluoro-4-isothiocyanatophenoxy)-2-hydrox...)Show SMILES C[C@](O)(COc1ccc(N=C=S)c(F)c1)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C18H13F4N3O5S/c1-17(27,8-30-11-3-4-14(23-9-31)13(19)7-11)16(26)24-10-2-5-15(25(28)29)12(6-10)18(20,21)22/h2-7,27H,8H2,1H3,(H,24,26)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | -41.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee Health Science Center
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem 14: 6525-38 (2006)
Article DOI: 10.1016/j.bmc.2006.06.019
BindingDB Entry DOI: 10.7270/Q2WQ022N |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50528796
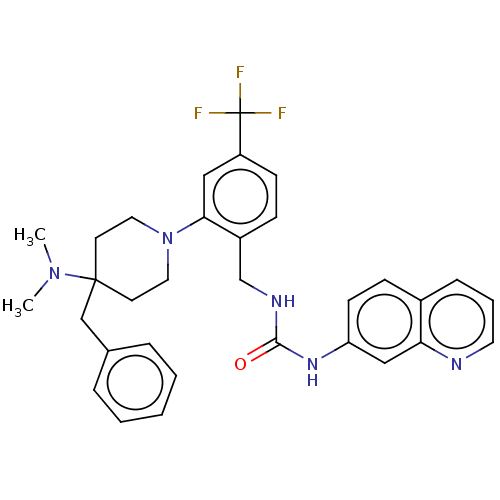
(CHEMBL4459611)Show SMILES CN(C)C1(Cc2ccccc2)CCN(CC1)c1cc(ccc1CNC(=O)Nc1ccc2cccnc2c1)C(F)(F)F Show InChI InChI=1S/C32H34F3N5O/c1-39(2)31(21-23-7-4-3-5-8-23)14-17-40(18-15-31)29-19-26(32(33,34)35)12-10-25(29)22-37-30(41)38-27-13-11-24-9-6-16-36-28(24)20-27/h3-13,16,19-20H,14-15,17-18,21-22H2,1-2H3,(H2,37,38,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111634
BindingDB Entry DOI: 10.7270/Q2765JR7 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM19251
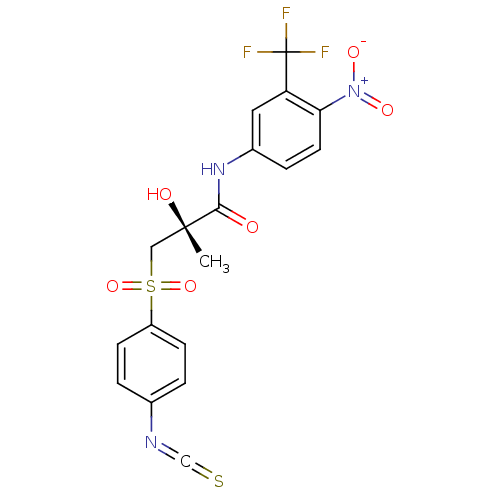
((2R)-2-hydroxy-3-[(4-isothiocyanatobenzene)sulfony...)Show SMILES C[C@](O)(CS(=O)(=O)c1ccc(cc1)N=C=S)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |r| Show InChI InChI=1S/C18H14F3N3O6S2/c1-17(26,9-32(29,30)13-5-2-11(3-6-13)22-10-31)16(25)23-12-4-7-15(24(27)28)14(8-12)18(19,20)21/h2-8,26H,9H2,1H3,(H,23,25)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14.4 | -41.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Tennessee Health Science Center
| Assay Description
The Ki values were determined by the application of the Cheng-Prusoff equation: Ki = (IC50 x Kd)/(Kd+[L]) where [L] is the concentration of [3H]MIB (... |
Bioorg Med Chem 14: 6525-38 (2006)
Article DOI: 10.1016/j.bmc.2006.06.019
BindingDB Entry DOI: 10.7270/Q2WQ022N |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50258752
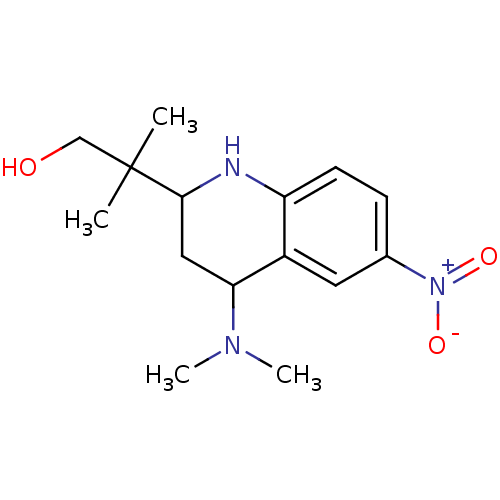
(2-(4-(dimethylamino)-6-nitro-1,2,3,4-tetrahydroqui...)Show SMILES CN(C)C1CC(Nc2ccc(cc12)[N+]([O-])=O)C(C)(C)CO Show InChI InChI=1S/C15H23N3O3/c1-15(2,9-19)14-8-13(17(3)4)11-7-10(18(20)21)5-6-12(11)16-14/h5-7,13-14,16,19H,8-9H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at androgen receptor (unknown origin) |
J Med Chem 52: 3597-617 (2009)
Article DOI: 10.1021/jm900280m
BindingDB Entry DOI: 10.7270/Q2GH9HWD |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50528798
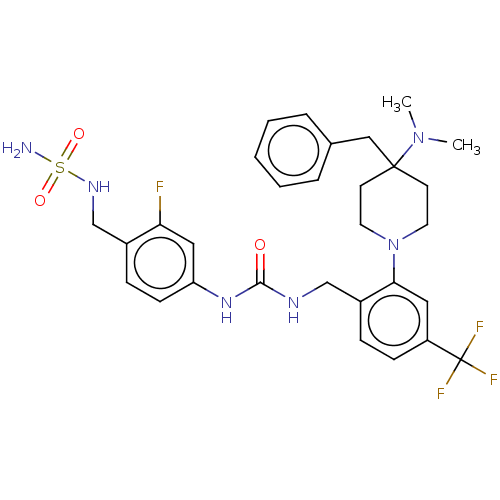
(CHEMBL4536869)Show SMILES CN(C)C1(Cc2ccccc2)CCN(CC1)c1cc(ccc1CNC(=O)Nc1ccc(CNS(N)(=O)=O)c(F)c1)C(F)(F)F Show InChI InChI=1S/C30H36F4N6O3S/c1-39(2)29(18-21-6-4-3-5-7-21)12-14-40(15-13-29)27-16-24(30(32,33)34)10-8-23(27)19-36-28(41)38-25-11-9-22(26(31)17-25)20-37-44(35,42)43/h3-11,16-17,37H,12-15,18-20H2,1-2H3,(H2,35,42,43)(H2,36,38,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111634
BindingDB Entry DOI: 10.7270/Q2765JR7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50528799
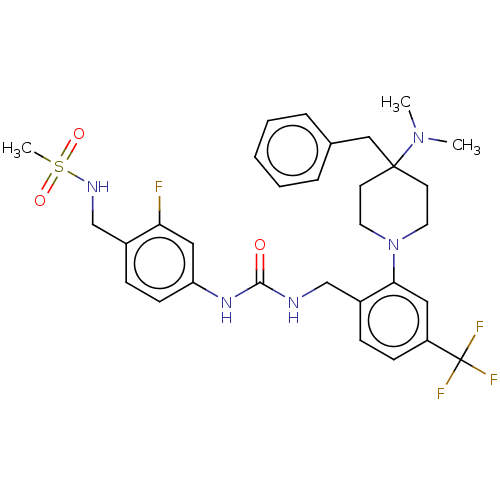
(CHEMBL4571845)Show SMILES CN(C)C1(Cc2ccccc2)CCN(CC1)c1cc(ccc1CNC(=O)Nc1ccc(CNS(C)(=O)=O)c(F)c1)C(F)(F)F Show InChI InChI=1S/C31H37F4N5O3S/c1-39(2)30(19-22-7-5-4-6-8-22)13-15-40(16-14-30)28-17-25(31(33,34)35)11-9-24(28)20-36-29(41)38-26-12-10-23(27(32)18-26)21-37-44(3,42)43/h4-12,17-18,37H,13-16,19-21H2,1-3H3,(H2,36,38,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [3H]RTX from recombinant human TRPV1 expressed in CHO cells incubated for 60 mins by liquid scintillation counter |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111634
BindingDB Entry DOI: 10.7270/Q2765JR7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data