 Found 64 hits with Last Name = 'landro' and Initial = 'j'
Found 64 hits with Last Name = 'landro' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135430

(CHEMBL434033 | Naphthalene-2-carboxylic acid (3R,5...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@@H](NC(=O)[C@H](NC(=O)CCCCC(O)=O)C(C)C)C(C)C)OC(=O)c1ccc2ccccc2c1)C=O Show InChI InChI=1S/C36H48N4O9/c1-6-26(20-41)37-33(45)28-18-27(49-36(48)25-16-15-23-11-7-8-12-24(23)17-25)19-40(28)35(47)32(22(4)5)39-34(46)31(21(2)3)38-29(42)13-9-10-14-30(43)44/h7-8,11-12,15-17,20-22,26-28,31-32H,6,9-10,13-14,18-19H2,1-5H3,(H,37,45)(H,38,42)(H,39,46)(H,43,44)/t26-,27+,28-,31+,32-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135432

(CHEMBL340890 | Naphthalene-2-carboxylic acid (3R,5...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)c1ccc2ccccc2c1)C=O Show InChI InChI=1S/C35H42N6O7/c1-6-25(19-42)38-32(44)28-16-26(48-35(47)24-12-11-22-9-7-8-10-23(22)15-24)18-41(28)34(46)30(21(4)5)40-33(45)29(20(2)3)39-31(43)27-17-36-13-14-37-27/h7-15,17,19-21,25-26,28-30H,6,16,18H2,1-5H3,(H,38,44)(H,39,43)(H,40,45)/t25-,26+,28-,29+,30+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135442
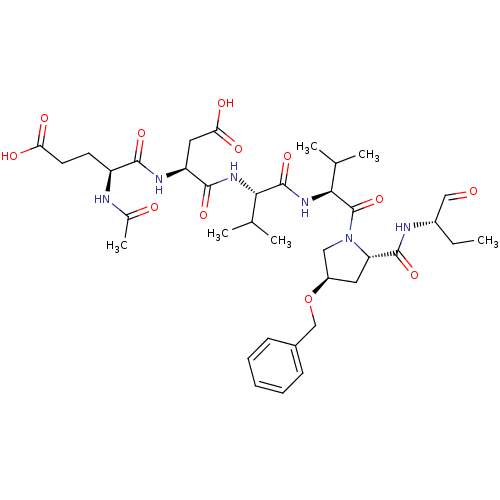
((S)-4-Acetylamino-4-[(S)-1-((S)-1-{(S)-1-[(2S,4R)-...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(C)=O)C(C)C)C(C)C)OCc1ccccc1)C=O Show InChI InChI=1S/C37H54N6O12/c1-7-24(18-44)39-35(52)28-15-25(55-19-23-11-9-8-10-12-23)17-43(28)37(54)32(21(4)5)42-36(53)31(20(2)3)41-34(51)27(16-30(48)49)40-33(50)26(38-22(6)45)13-14-29(46)47/h8-12,18,20-21,24-28,31-32H,7,13-17,19H2,1-6H3,(H,38,45)(H,39,52)(H,40,50)(H,41,51)(H,42,53)(H,46,47)(H,48,49)/t24-,25+,26-,27-,28-,31-,32-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135437

(3,4-Dihydro-2H-quinoline-1-carboxylic acid (3R,5S)...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)N1CCCc2ccccc12)C=O Show InChI InChI=1S/C34H45N7O7/c1-6-23(19-42)37-31(44)27-16-24(48-34(47)40-15-9-11-22-10-7-8-12-26(22)40)18-41(27)33(46)29(21(4)5)39-32(45)28(20(2)3)38-30(43)25-17-35-13-14-36-25/h7-8,10,12-14,17,19-21,23-24,27-29H,6,9,11,15-16,18H2,1-5H3,(H,37,44)(H,38,43)(H,39,45)/t23-,24+,27-,28+,29+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135429
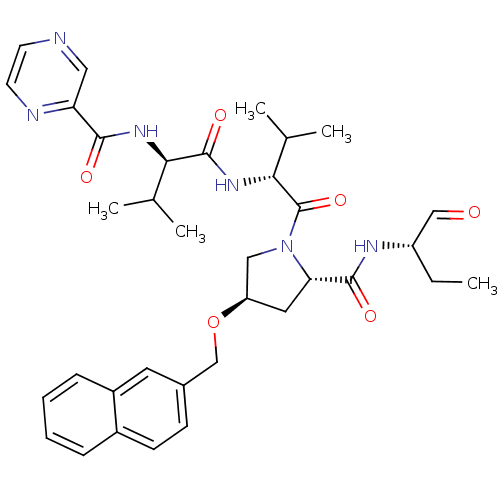
(CHEMBL335769 | Pyrazine-2-carboxylic acid ((R)-1-{...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OCc1ccc2ccccc2c1)C=O Show InChI InChI=1S/C35H44N6O6/c1-6-26(19-42)38-33(44)29-16-27(47-20-23-11-12-24-9-7-8-10-25(24)15-23)18-41(29)35(46)31(22(4)5)40-34(45)30(21(2)3)39-32(43)28-17-36-13-14-37-28/h7-15,17,19,21-22,26-27,29-31H,6,16,18,20H2,1-5H3,(H,38,44)(H,39,43)(H,40,45)/t26-,27+,29-,30+,31+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135439

(CHEMBL128918 | Naphthalene-1-carboxylic acid (3R,5...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)c1cccc2ccccc12)C=O Show InChI InChI=1S/C35H42N6O7/c1-6-23(19-42)38-32(44)28-16-24(48-35(47)26-13-9-11-22-10-7-8-12-25(22)26)18-41(28)34(46)30(21(4)5)40-33(45)29(20(2)3)39-31(43)27-17-36-14-15-37-27/h7-15,17,19-21,23-24,28-30H,6,16,18H2,1-5H3,(H,38,44)(H,39,43)(H,40,45)/t23-,24+,28-,29+,30+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135434

(CHEMBL339075 | Pyrazine-2-carboxylic acid ((R)-1-{...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)Oc1cccc2ccccc12)C=O Show InChI InChI=1S/C34H42N6O6/c1-6-23(19-41)37-32(43)27-16-24(46-28-13-9-11-22-10-7-8-12-25(22)28)18-40(27)34(45)30(21(4)5)39-33(44)29(20(2)3)38-31(42)26-17-35-14-15-36-26/h7-15,17,19-21,23-24,27,29-30H,6,16,18H2,1-5H3,(H,37,43)(H,38,42)(H,39,44)/t23-,24+,27-,29+,30+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135441
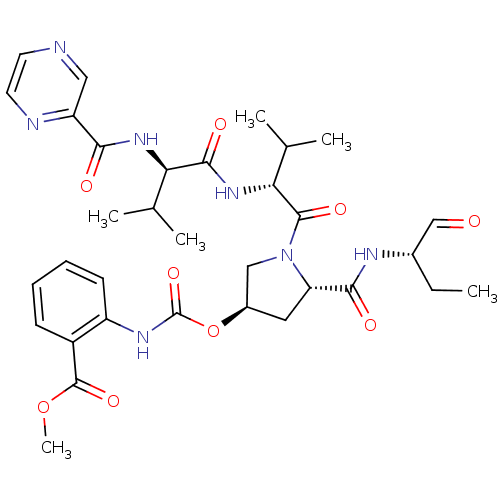
(2-[(3R,5S)-5-((S)-1-Formyl-propylcarbamoyl)-1-((R)...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)Nc1ccccc1C(=O)OC)C=O Show InChI InChI=1S/C33H43N7O9/c1-7-20(17-41)36-29(43)25-14-21(49-33(47)37-23-11-9-8-10-22(23)32(46)48-6)16-40(25)31(45)27(19(4)5)39-30(44)26(18(2)3)38-28(42)24-15-34-12-13-35-24/h8-13,15,17-21,25-27H,7,14,16H2,1-6H3,(H,36,43)(H,37,47)(H,38,42)(H,39,44)/t20-,21+,25-,26+,27+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135440
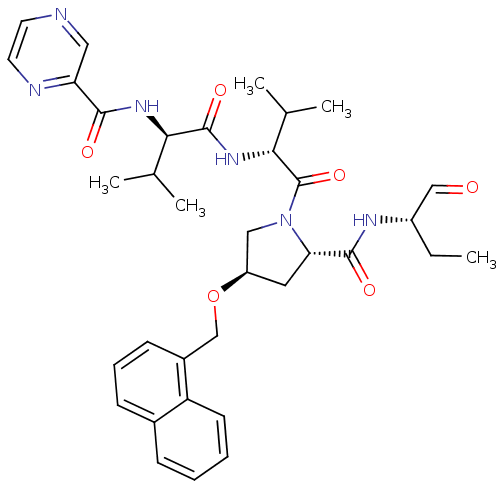
(CHEMBL334814 | Pyrazine-2-carboxylic acid ((R)-1-{...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OCc1cccc2ccccc12)C=O Show InChI InChI=1S/C35H44N6O6/c1-6-25(19-42)38-33(44)29-16-26(47-20-24-12-9-11-23-10-7-8-13-27(23)24)18-41(29)35(46)31(22(4)5)40-34(45)30(21(2)3)39-32(43)28-17-36-14-15-37-28/h7-15,17,19,21-22,25-26,29-31H,6,16,18,20H2,1-5H3,(H,38,44)(H,39,43)(H,40,45)/t25-,26+,29-,30+,31+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135438
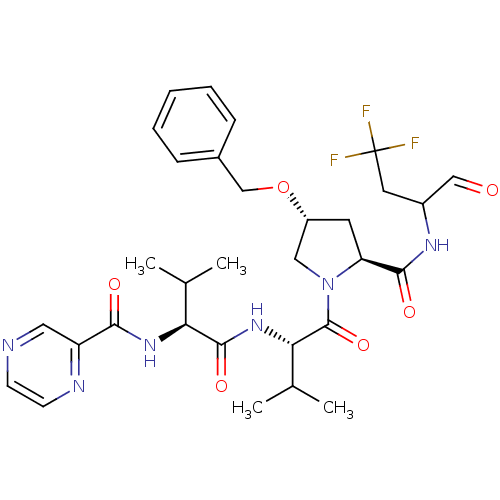
(CHEMBL339012 | Pyrazine-2-carboxylic acid ((S)-1-{...)Show SMILES CC(C)[C@H](NC(=O)c1cnccn1)C(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C[C@H]1C(=O)NC(CC(F)(F)F)C=O)OCc1ccccc1 Show InChI InChI=1S/C31H39F3N6O6/c1-18(2)25(38-27(42)23-14-35-10-11-36-23)29(44)39-26(19(3)4)30(45)40-15-22(46-17-20-8-6-5-7-9-20)12-24(40)28(43)37-21(16-41)13-31(32,33)34/h5-11,14,16,18-19,21-22,24-26H,12-13,15,17H2,1-4H3,(H,37,43)(H,38,42)(H,39,44)/t21?,22-,24+,25+,26+/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135443

((2,5-Dichloro-phenyl)-carbamic acid (3R,5S)-5-((S)...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)Nc1cc(Cl)ccc1Cl)C=O Show InChI InChI=1S/C31H39Cl2N7O7/c1-6-19(15-41)36-28(43)24-12-20(47-31(46)37-22-11-18(32)7-8-21(22)33)14-40(24)30(45)26(17(4)5)39-29(44)25(16(2)3)38-27(42)23-13-34-9-10-35-23/h7-11,13,15-17,19-20,24-26H,6,12,14H2,1-5H3,(H,36,43)(H,37,46)(H,38,42)(H,39,44)/t19-,20+,24-,25+,26+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135447
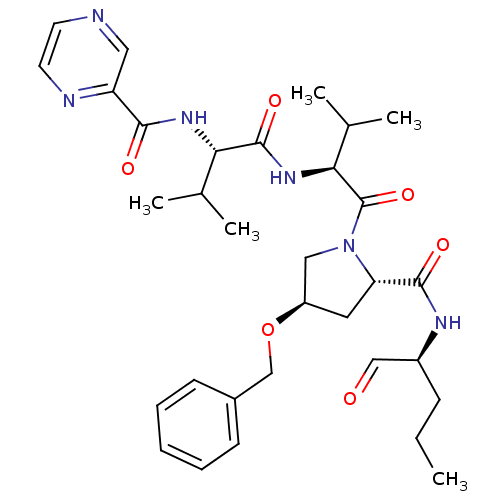
(CHEMBL408494 | Pyrazine-2-carboxylic acid ((S)-1-{...)Show SMILES CCC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OCc1ccccc1)C=O Show InChI InChI=1S/C32H44N6O6/c1-6-10-23(18-39)35-30(41)26-15-24(44-19-22-11-8-7-9-12-22)17-38(26)32(43)28(21(4)5)37-31(42)27(20(2)3)36-29(40)25-16-33-13-14-34-25/h7-9,11-14,16,18,20-21,23-24,26-28H,6,10,15,17,19H2,1-5H3,(H,35,41)(H,36,40)(H,37,42)/t23-,24+,26-,27-,28-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135449
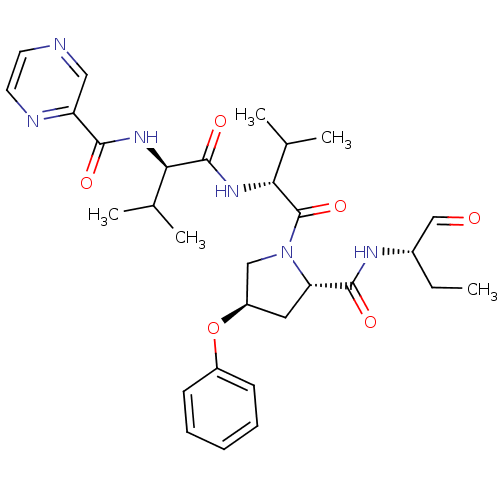
(CHEMBL130397 | Pyrazine-2-carboxylic acid ((R)-1-{...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)Oc1ccccc1)C=O Show InChI InChI=1S/C30H40N6O6/c1-6-20(17-37)33-28(39)24-14-22(42-21-10-8-7-9-11-21)16-36(24)30(41)26(19(4)5)35-29(40)25(18(2)3)34-27(38)23-15-31-12-13-32-23/h7-13,15,17-20,22,24-26H,6,14,16H2,1-5H3,(H,33,39)(H,34,38)(H,35,40)/t20-,22+,24-,25+,26+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135427
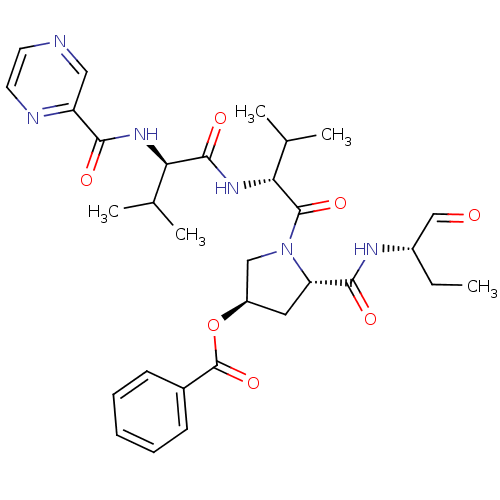
(Benzoic acid (3R,5S)-5-((S)-1-formyl-propylcarbamo...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)c1ccccc1)C=O Show InChI InChI=1S/C31H40N6O7/c1-6-21(17-38)34-28(40)24-14-22(44-31(43)20-10-8-7-9-11-20)16-37(24)30(42)26(19(4)5)36-29(41)25(18(2)3)35-27(39)23-15-32-12-13-33-23/h7-13,15,17-19,21-22,24-26H,6,14,16H2,1-5H3,(H,34,40)(H,35,39)(H,36,41)/t21-,22+,24-,25+,26+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135446
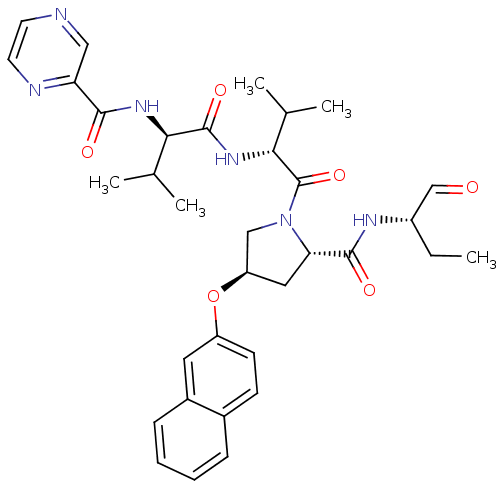
(CHEMBL334570 | Pyrazine-2-carboxylic acid ((R)-1-{...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)Oc1ccc2ccccc2c1)C=O Show InChI InChI=1S/C34H42N6O6/c1-6-24(19-41)37-32(43)28-16-26(46-25-12-11-22-9-7-8-10-23(22)15-25)18-40(28)34(45)30(21(4)5)39-33(44)29(20(2)3)38-31(42)27-17-35-13-14-36-27/h7-15,17,19-21,24,26,28-30H,6,16,18H2,1-5H3,(H,37,43)(H,38,42)(H,39,44)/t24-,26+,28-,29+,30+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135445
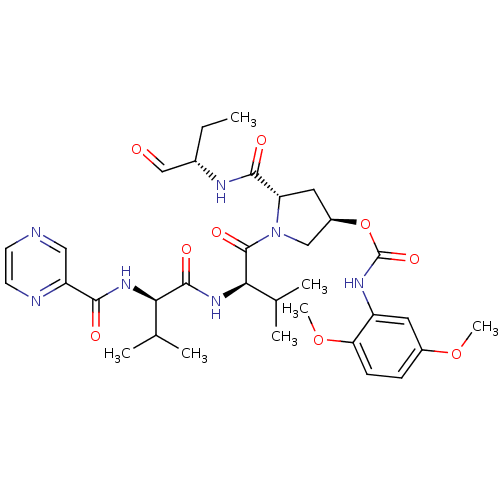
((2,5-Dimethoxy-phenyl)-carbamic acid (3R,5S)-5-((S...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)Nc1cc(OC)ccc1OC)C=O Show InChI InChI=1S/C33H45N7O9/c1-8-20(17-41)36-30(43)25-14-22(49-33(46)37-23-13-21(47-6)9-10-26(23)48-7)16-40(25)32(45)28(19(4)5)39-31(44)27(18(2)3)38-29(42)24-15-34-11-12-35-24/h9-13,15,17-20,22,25,27-28H,8,14,16H2,1-7H3,(H,36,43)(H,37,46)(H,38,42)(H,39,44)/t20-,22+,25-,27+,28+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135428
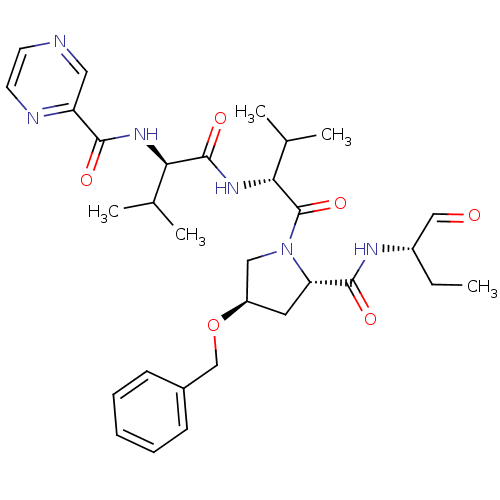
(CHEMBL407674 | Pyrazine-2-carboxylic acid ((R)-1-{...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OCc1ccccc1)C=O Show InChI InChI=1S/C31H42N6O6/c1-6-22(17-38)34-29(40)25-14-23(43-18-21-10-8-7-9-11-21)16-37(25)31(42)27(20(4)5)36-30(41)26(19(2)3)35-28(39)24-15-32-12-13-33-24/h7-13,15,17,19-20,22-23,25-27H,6,14,16,18H2,1-5H3,(H,34,40)(H,35,39)(H,36,41)/t22-,23+,25-,26+,27+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135444
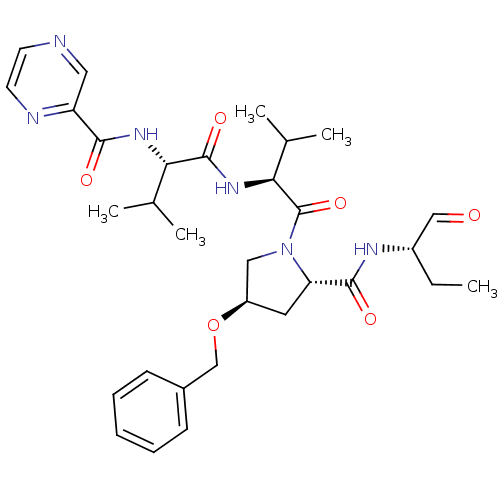
(CHEMBL338447 | N-((S)-1-((S)-1-((2S,4R)-2-(((S)-1-...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OCc1ccccc1)C=O Show InChI InChI=1S/C31H42N6O6/c1-6-22(17-38)34-29(40)25-14-23(43-18-21-10-8-7-9-11-21)16-37(25)31(42)27(20(4)5)36-30(41)26(19(2)3)35-28(39)24-15-32-12-13-33-24/h7-13,15,17,19-20,22-23,25-27H,6,14,16,18H2,1-5H3,(H,34,40)(H,35,39)(H,36,41)/t22-,23+,25-,26-,27-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135435
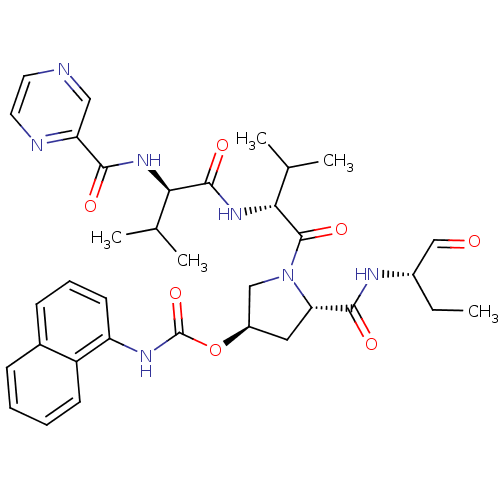
(CHEMBL446617 | Naphthalen-1-yl-carbamic acid (3R,5...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)Nc1cccc2ccccc12)C=O Show InChI InChI=1S/C35H43N7O7/c1-6-23(19-43)38-32(45)28-16-24(49-35(48)39-26-13-9-11-22-10-7-8-12-25(22)26)18-42(28)34(47)30(21(4)5)41-33(46)29(20(2)3)40-31(44)27-17-36-14-15-37-27/h7-15,17,19-21,23-24,28-30H,6,16,18H2,1-5H3,(H,38,45)(H,39,48)(H,40,44)(H,41,46)/t23-,24+,28-,29+,30+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135436
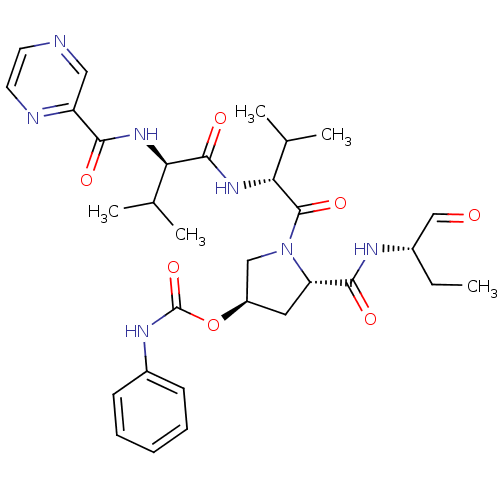
(CHEMBL130439 | Phenyl-carbamic acid (3R,5S)-5-((S)...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OC(=O)Nc1ccccc1)C=O Show InChI InChI=1S/C31H41N7O7/c1-6-20(17-39)34-28(41)24-14-22(45-31(44)35-21-10-8-7-9-11-21)16-38(24)30(43)26(19(4)5)37-29(42)25(18(2)3)36-27(40)23-15-32-12-13-33-23/h7-13,15,17-20,22,24-26H,6,14,16H2,1-5H3,(H,34,41)(H,35,44)(H,36,40)(H,37,42)/t20-,22+,24-,25+,26+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135448
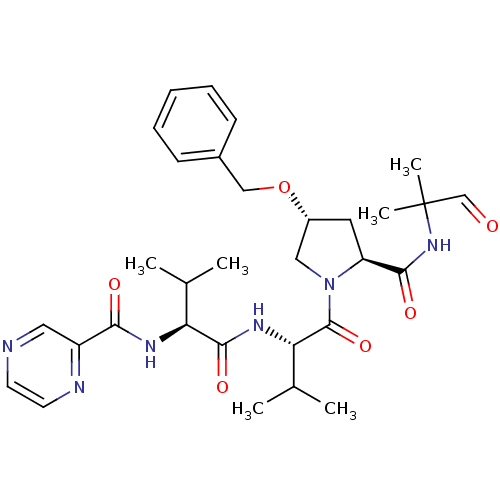
(CHEMBL130430 | Pyrazine-2-carboxylic acid ((S)-1-{...)Show SMILES CC(C)[C@H](NC(=O)c1cnccn1)C(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H](C[C@H]1C(=O)NC(C)(C)C=O)OCc1ccccc1 Show InChI InChI=1S/C31H42N6O6/c1-19(2)25(34-27(39)23-15-32-12-13-33-23)29(41)35-26(20(3)4)30(42)37-16-22(43-17-21-10-8-7-9-11-21)14-24(37)28(40)36-31(5,6)18-38/h7-13,15,18-20,22,24-26H,14,16-17H2,1-6H3,(H,34,39)(H,35,41)(H,36,40)/t22-,24+,25+,26+/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135433
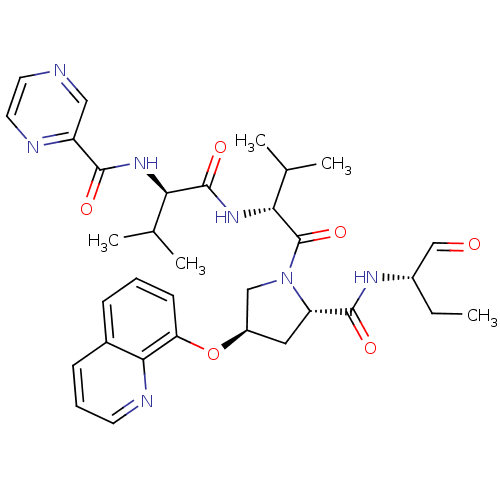
(CHEMBL131367 | Pyrazine-2-carboxylic acid ((R)-1-{...)Show SMILES CC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@H](NC(=O)[C@H](NC(=O)c1cnccn1)C(C)C)C(C)C)Oc1cccc2cccnc12)C=O Show InChI InChI=1S/C33H41N7O6/c1-6-22(18-41)37-31(43)25-15-23(46-26-11-7-9-21-10-8-12-36-29(21)26)17-40(25)33(45)28(20(4)5)39-32(44)27(19(2)3)38-30(42)24-16-34-13-14-35-24/h7-14,16,18-20,22-23,25,27-28H,6,15,17H2,1-5H3,(H,37,43)(H,38,42)(H,39,44)/t22-,23+,25-,27+,28+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50135431
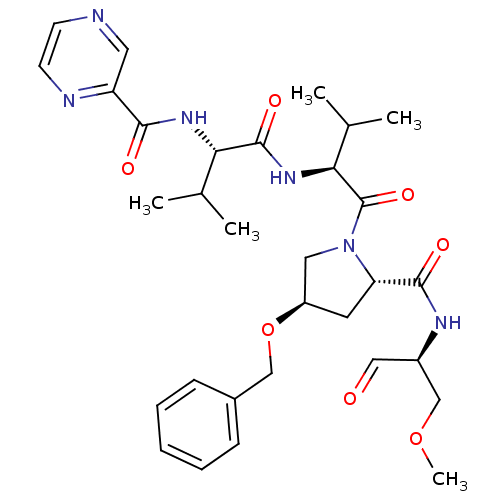
(CHEMBL335770 | Pyrazine-2-carboxylic acid ((S)-1-{...)Show SMILES COC[C@H](NC(=O)[C@@H]1C[C@H](CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)OCc1ccccc1)C=O Show InChI InChI=1S/C31H42N6O7/c1-19(2)26(35-28(39)24-14-32-11-12-33-24)30(41)36-27(20(3)4)31(42)37-15-23(44-17-21-9-7-6-8-10-21)13-25(37)29(40)34-22(16-38)18-43-5/h6-12,14,16,19-20,22-23,25-27H,13,15,17-18H2,1-5H3,(H,34,40)(H,35,39)(H,36,41)/t22-,23-,25+,26+,27+/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HCV (Hepatitis C Virus) NS3-4A protease. |
Bioorg Med Chem Lett 13: 4059-63 (2003)
BindingDB Entry DOI: 10.7270/Q2028QX4 |
More data for this
Ligand-Target Pair | |
cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A
(Homo sapiens (Human)) | BDBM237920
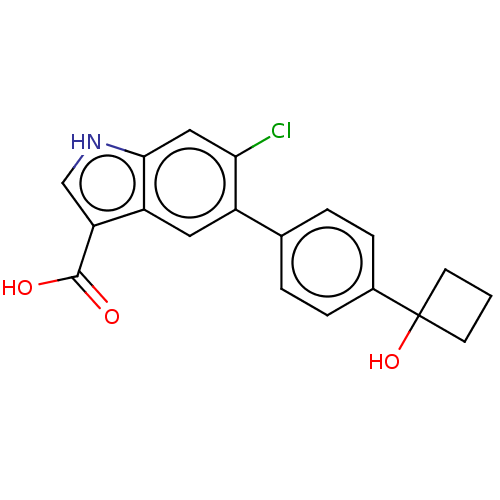
(US9394285, 1)Show SMILES OC(=O)c1c[nH]c2cc(Cl)c(cc12)-c1ccc(cc1)C1(O)CCC1 Show InChI InChI=1S/C19H16ClNO3/c20-16-9-17-14(15(10-21-17)18(22)23)8-13(16)11-2-4-12(5-3-11)19(24)6-1-7-19/h2-5,8-10,21,24H,1,6-7H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE3A (unknown origin) |
J Med Chem 59: 8068-81 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00866
BindingDB Entry DOI: 10.7270/Q2PZ5BRC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50394682

(CHEMBL2165619)Show SMILES Cc1ccc(NC(=O)[C@H](CC2CCCC2)n2cnc(c2)C(F)(F)F)nc1 |r| Show InChI InChI=1S/C18H21F3N4O/c1-12-6-7-16(22-9-12)24-17(26)14(8-13-4-2-3-5-13)25-10-15(23-11-25)18(19,20)21/h6-7,9-11,13-14H,2-5,8H2,1H3,(H,22,24,26)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 153 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of glucokinase in rat INS-1 cells assessed as stimulation of glucose-induced insulin secretion |
J Med Chem 55: 1318-33 (2012)
Article DOI: 10.1021/jm2014887
BindingDB Entry DOI: 10.7270/Q2NG4RRT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50394681

(CHEMBL2165620)Show SMILES OC(=O)c1ccc(NC(=O)[C@H](CC2CCCC2)n2cnc(c2)C(F)(F)F)nc1 |r| Show InChI InChI=1S/C18H19F3N4O3/c19-18(20,21)14-9-25(10-23-14)13(7-11-3-1-2-4-11)16(26)24-15-6-5-12(8-22-15)17(27)28/h5-6,8-11,13H,1-4,7H2,(H,27,28)(H,22,24,26)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of glucokinase in rat INS-1 cells assessed as stimulation of glucose-induced insulin secretion |
J Med Chem 55: 1318-33 (2012)
Article DOI: 10.1021/jm2014887
BindingDB Entry DOI: 10.7270/Q2NG4RRT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50394684

(CHEMBL2165615)Show SMILES CN(C)C(=O)c1ncc(Oc2cc(cc3oc(C)cc23)C(=O)Nc2cnc(C)cn2)cn1 Show InChI InChI=1S/C22H20N6O4/c1-12-8-24-19(11-23-12)27-21(29)14-6-17-16(5-13(2)31-17)18(7-14)32-15-9-25-20(26-10-15)22(30)28(3)4/h5-11H,1-4H3,(H,24,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 188 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of human recombinant glucokinase using 6.5 mM glucose by spectrophotometric analysis |
J Med Chem 55: 1318-33 (2012)
Article DOI: 10.1021/jm2014887
BindingDB Entry DOI: 10.7270/Q2NG4RRT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50394685

(CHEMBL2165618)Show SMILES OC(=O)c1cnc(NC(=O)[C@H](CC2CCCC2)n2cnc(c2)C(F)(F)F)cn1 |r| Show InChI InChI=1S/C17H18F3N5O3/c18-17(19,20)13-8-25(9-23-13)12(5-10-3-1-2-4-10)15(26)24-14-7-21-11(6-22-14)16(27)28/h6-10,12H,1-5H2,(H,27,28)(H,22,24,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of human recombinant glucokinase using 6.5 mM glucose by spectrophotometric analysis |
J Med Chem 55: 1318-33 (2012)
Article DOI: 10.1021/jm2014887
BindingDB Entry DOI: 10.7270/Q2NG4RRT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50394686
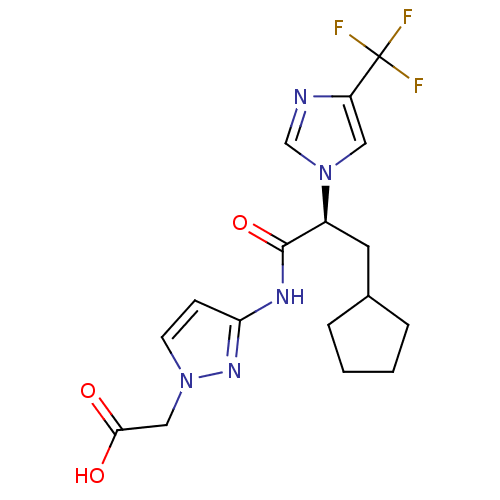
(CHEMBL2165617)Show SMILES OC(=O)Cn1ccc(NC(=O)[C@H](CC2CCCC2)n2cnc(c2)C(F)(F)F)n1 |r| Show InChI InChI=1S/C17H20F3N5O3/c18-17(19,20)13-8-24(10-21-13)12(7-11-3-1-2-4-11)16(28)22-14-5-6-25(23-14)9-15(26)27/h5-6,8,10-12H,1-4,7,9H2,(H,26,27)(H,22,23,28)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of human recombinant glucokinase using 6.5 mM glucose by spectrophotometric analysis |
J Med Chem 55: 1318-33 (2012)
Article DOI: 10.1021/jm2014887
BindingDB Entry DOI: 10.7270/Q2NG4RRT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50394687

(CHEMBL2165627)Show SMILES OC(=O)C(=O)Nc1ccc(NC(=O)[C@H](CC2CCCC2)n2cnc(c2)C(F)(F)F)nc1 |r| Show InChI InChI=1S/C19H20F3N5O4/c20-19(21,22)14-9-27(10-24-14)13(7-11-3-1-2-4-11)16(28)26-15-6-5-12(8-23-15)25-17(29)18(30)31/h5-6,8-11,13H,1-4,7H2,(H,25,29)(H,30,31)(H,23,26,28)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 456 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of human recombinant glucokinase using 6.5 mM glucose by spectrophotometric analysis |
J Med Chem 55: 1318-33 (2012)
Article DOI: 10.1021/jm2014887
BindingDB Entry DOI: 10.7270/Q2NG4RRT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50394688
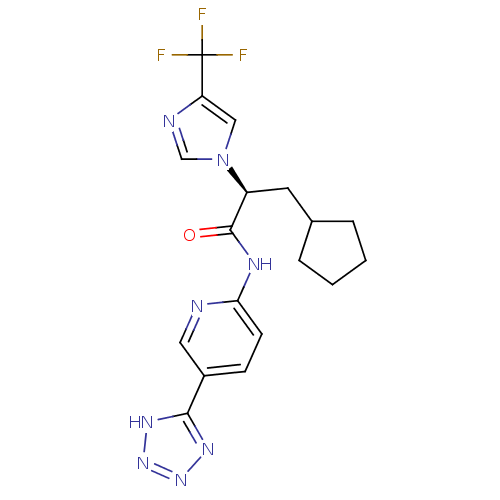
(CHEMBL2165626)Show SMILES FC(F)(F)c1cn(cn1)[C@@H](CC1CCCC1)C(=O)Nc1ccc(cn1)-c1nnn[nH]1 |r| Show InChI InChI=1S/C18H19F3N8O/c19-18(20,21)14-9-29(10-23-14)13(7-11-3-1-2-4-11)17(30)24-15-6-5-12(8-22-15)16-25-27-28-26-16/h5-6,8-11,13H,1-4,7H2,(H,22,24,30)(H,25,26,27,28)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 284 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of human recombinant glucokinase using 6.5 mM glucose by spectrophotometric analysis |
J Med Chem 55: 1318-33 (2012)
Article DOI: 10.1021/jm2014887
BindingDB Entry DOI: 10.7270/Q2NG4RRT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50394689
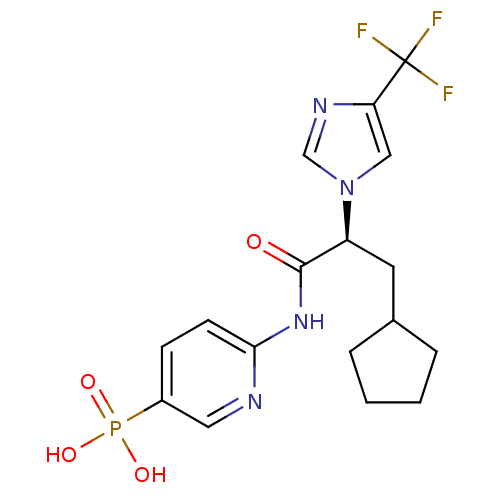
(CHEMBL2165625)Show SMILES OP(O)(=O)c1ccc(NC(=O)[C@H](CC2CCCC2)n2cnc(c2)C(F)(F)F)nc1 |r| Show InChI InChI=1S/C17H20F3N4O4P/c18-17(19,20)14-9-24(10-22-14)13(7-11-3-1-2-4-11)16(25)23-15-6-5-12(8-21-15)29(26,27)28/h5-6,8-11,13H,1-4,7H2,(H,21,23,25)(H2,26,27,28)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of human recombinant glucokinase using 6.5 mM glucose by spectrophotometric analysis |
J Med Chem 55: 1318-33 (2012)
Article DOI: 10.1021/jm2014887
BindingDB Entry DOI: 10.7270/Q2NG4RRT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50394690
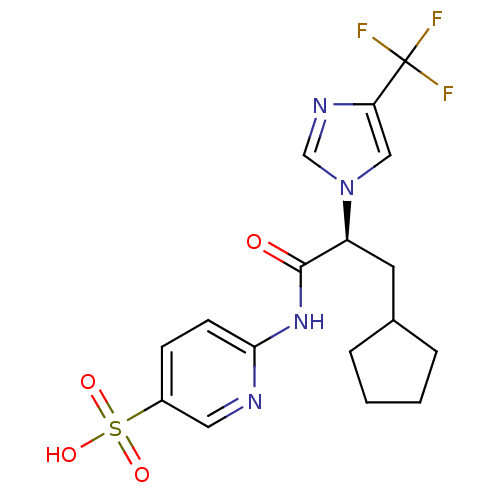
(CHEMBL2165624)Show SMILES OS(=O)(=O)c1ccc(NC(=O)[C@H](CC2CCCC2)n2cnc(c2)C(F)(F)F)nc1 |r| Show InChI InChI=1S/C17H19F3N4O4S/c18-17(19,20)14-9-24(10-22-14)13(7-11-3-1-2-4-11)16(25)23-15-6-5-12(8-21-15)29(26,27)28/h5-6,8-11,13H,1-4,7H2,(H,21,23,25)(H,26,27,28)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.49E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of human recombinant glucokinase using 6.5 mM glucose by spectrophotometric analysis |
J Med Chem 55: 1318-33 (2012)
Article DOI: 10.1021/jm2014887
BindingDB Entry DOI: 10.7270/Q2NG4RRT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50394691

(CHEMBL2165623)Show SMILES OC(=O)Cc1ccc(NC(=O)[C@H](CC2CCCC2)n2cnc(c2)C(F)(F)F)nc1 |r| Show InChI InChI=1S/C19H21F3N4O3/c20-19(21,22)15-10-26(11-24-15)14(7-12-3-1-2-4-12)18(29)25-16-6-5-13(9-23-16)8-17(27)28/h5-6,9-12,14H,1-4,7-8H2,(H,27,28)(H,23,25,29)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 565 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of human recombinant glucokinase using 6.5 mM glucose by spectrophotometric analysis |
J Med Chem 55: 1318-33 (2012)
Article DOI: 10.1021/jm2014887
BindingDB Entry DOI: 10.7270/Q2NG4RRT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50394692

(CHEMBL2165622)Show SMILES OC(=O)c1ccnc(NC(=O)[C@H](CC2CCCC2)n2cnc(c2)C(F)(F)F)c1 |r| Show InChI InChI=1S/C18H19F3N4O3/c19-18(20,21)14-9-25(10-23-14)13(7-11-3-1-2-4-11)16(26)24-15-8-12(17(27)28)5-6-22-15/h5-6,8-11,13H,1-4,7H2,(H,27,28)(H,22,24,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of human recombinant glucokinase using 6.5 mM glucose by spectrophotometric analysis |
J Med Chem 55: 1318-33 (2012)
Article DOI: 10.1021/jm2014887
BindingDB Entry DOI: 10.7270/Q2NG4RRT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50394693

(CHEMBL2165621)Show SMILES OC(=O)c1cccc(NC(=O)[C@H](CC2CCCC2)n2cnc(c2)C(F)(F)F)n1 |r| Show InChI InChI=1S/C18H19F3N4O3/c19-18(20,21)14-9-25(10-22-14)13(8-11-4-1-2-5-11)16(26)24-15-7-3-6-12(23-15)17(27)28/h3,6-7,9-11,13H,1-2,4-5,8H2,(H,27,28)(H,23,24,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of human recombinant glucokinase using 6.5 mM glucose by spectrophotometric analysis |
J Med Chem 55: 1318-33 (2012)
Article DOI: 10.1021/jm2014887
BindingDB Entry DOI: 10.7270/Q2NG4RRT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50394681

(CHEMBL2165620)Show SMILES OC(=O)c1ccc(NC(=O)[C@H](CC2CCCC2)n2cnc(c2)C(F)(F)F)nc1 |r| Show InChI InChI=1S/C18H19F3N4O3/c19-18(20,21)14-9-25(10-23-14)13(7-11-3-1-2-4-11)16(26)24-15-6-5-12(8-22-15)17(27)28/h5-6,8-11,13H,1-4,7H2,(H,27,28)(H,22,24,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of human recombinant glucokinase using 6.5 mM glucose by spectrophotometric analysis |
J Med Chem 55: 1318-33 (2012)
Article DOI: 10.1021/jm2014887
BindingDB Entry DOI: 10.7270/Q2NG4RRT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hexokinase-4
(Homo sapiens (Human)) | BDBM50394682

(CHEMBL2165619)Show SMILES Cc1ccc(NC(=O)[C@H](CC2CCCC2)n2cnc(c2)C(F)(F)F)nc1 |r| Show InChI InChI=1S/C18H21F3N4O/c1-12-6-7-16(22-9-12)24-17(26)14(8-13-4-2-3-5-13)25-10-15(23-11-25)18(19,20)21/h6-7,9-11,13-14H,2-5,8H2,1H3,(H,22,24,26)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 114 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of human recombinant glucokinase using 6.5 mM glucose by spectrophotometric analysis |
J Med Chem 55: 1318-33 (2012)
Article DOI: 10.1021/jm2014887
BindingDB Entry DOI: 10.7270/Q2NG4RRT |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50394684

(CHEMBL2165615)Show SMILES CN(C)C(=O)c1ncc(Oc2cc(cc3oc(C)cc23)C(=O)Nc2cnc(C)cn2)cn1 Show InChI InChI=1S/C22H20N6O4/c1-12-8-24-19(11-23-12)27-21(29)14-6-17-16(5-13(2)31-17)18(7-14)32-15-9-25-20(26-10-15)22(30)28(3)4/h5-11H,1-4H3,(H,24,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Induction of glucokinase translocation from nucleus to cytoplasm in cryopreserved rat hepatocytes after 1 hr by Hoechst staining-based fluorescence m... |
J Med Chem 55: 1318-33 (2012)
Article DOI: 10.1021/jm2014887
BindingDB Entry DOI: 10.7270/Q2NG4RRT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hexokinase-4
(Rattus norvegicus) | BDBM50394681

(CHEMBL2165620)Show SMILES OC(=O)c1ccc(NC(=O)[C@H](CC2CCCC2)n2cnc(c2)C(F)(F)F)nc1 |r| Show InChI InChI=1S/C18H19F3N4O3/c19-18(20,21)14-9-25(10-23-14)13(7-11-3-1-2-4-11)16(26)24-15-6-5-12(8-22-15)17(27)28/h5-6,8-11,13H,1-4,7H2,(H,27,28)(H,22,24,26)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Induction of glucokinase translocation from nucleus to cytoplasm in cryopreserved rat hepatocytes after 1 hr by Hoechst staining-based fluorescence m... |
J Med Chem 55: 1318-33 (2012)
Article DOI: 10.1021/jm2014887
BindingDB Entry DOI: 10.7270/Q2NG4RRT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5'-AMP-activated protein kinase catalytic subunit alpha-1/subunit beta-1/subunit gamma-1
(Homo sapiens (Human)) | BDBM18137

(AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H14N5O7P/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(22-10)1-21-23(18,19)20/h2-4,6-7,10,16-17H,1H2,(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of recombinant human AMPK alpha1/beta1/gamma1 using Cy5-labelled SAMS as substrate assessed as protection from Thr172 residue dephosphoryl... |
J Med Chem 59: 8068-81 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00866
BindingDB Entry DOI: 10.7270/Q2PZ5BRC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5'-AMP-activated protein kinase catalytic subunit alpha-1/subunit beta-1/subunit gamma-1
(Homo sapiens (Human)) | BDBM237920

(US9394285, 1)Show SMILES OC(=O)c1c[nH]c2cc(Cl)c(cc12)-c1ccc(cc1)C1(O)CCC1 Show InChI InChI=1S/C19H16ClNO3/c20-16-9-17-14(15(10-21-17)18(22)23)8-13(16)11-2-4-12(5-3-11)19(24)6-1-7-19/h2-5,8-10,21,24H,1,6-7H2,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of recombinant human AMPK alpha1/beta1/gamma1 using Cy5-labelled SAMS as substrate assessed as protection from Thr172 residue dephosphoryl... |
J Med Chem 59: 8068-81 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00866
BindingDB Entry DOI: 10.7270/Q2PZ5BRC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5'-AMP-activated protein kinase catalytic subunit alpha-1/subunit beta-1/subunit gamma-1
(Homo sapiens (Human)) | BDBM237921
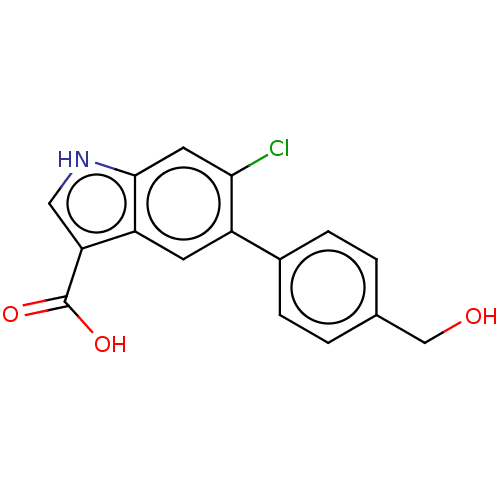
(US9394285, 2)Show InChI InChI=1S/C16H12ClNO3/c17-14-6-15-12(13(7-18-15)16(20)21)5-11(14)10-3-1-9(8-19)2-4-10/h1-7,18-19H,8H2,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human AMPK alpha1/beta1/gamma1 by SPR binding assay |
J Med Chem 59: 8068-81 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00866
BindingDB Entry DOI: 10.7270/Q2PZ5BRC |
More data for this
Ligand-Target Pair | |
5'-AMP-activated protein kinase subunit gamma-1
(Rattus norvegicus) | BDBM237920

(US9394285, 1)Show SMILES OC(=O)c1c[nH]c2cc(Cl)c(cc12)-c1ccc(cc1)C1(O)CCC1 Show InChI InChI=1S/C19H16ClNO3/c20-16-9-17-14(15(10-21-17)18(22)23)8-13(16)11-2-4-12(5-3-11)19(24)6-1-7-19/h2-5,8-10,21,24H,1,6-7H2,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of recombinant rat AMPK alpha1/beta1/gamma1 using Cy5-labelled SAMS as substrate assessed as protection from Thr172 residue dephosphorylat... |
J Med Chem 59: 8068-81 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00866
BindingDB Entry DOI: 10.7270/Q2PZ5BRC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5'-AMP-activated protein kinase subunit gamma-1
(Homo sapiens (Human)) | BDBM237920

(US9394285, 1)Show SMILES OC(=O)c1c[nH]c2cc(Cl)c(cc12)-c1ccc(cc1)C1(O)CCC1 Show InChI InChI=1S/C19H16ClNO3/c20-16-9-17-14(15(10-21-17)18(22)23)8-13(16)11-2-4-12(5-3-11)19(24)6-1-7-19/h2-5,8-10,21,24H,1,6-7H2,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of recombinant human AMPK alpha2/beta2/gamma1 using Cy5-labelled SAMS as substrate in presence of ATP by TR-FRET assay |
J Med Chem 59: 8068-81 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00866
BindingDB Entry DOI: 10.7270/Q2PZ5BRC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5'-AMP-activated protein kinase catalytic subunit alpha-1/subunit beta-1/subunit gamma-1
(Homo sapiens (Human)) | BDBM237920

(US9394285, 1)Show SMILES OC(=O)c1c[nH]c2cc(Cl)c(cc12)-c1ccc(cc1)C1(O)CCC1 Show InChI InChI=1S/C19H16ClNO3/c20-16-9-17-14(15(10-21-17)18(22)23)8-13(16)11-2-4-12(5-3-11)19(24)6-1-7-19/h2-5,8-10,21,24H,1,6-7H2,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of recombinant human AMPK alpha1/beta1/gamma1 using Cy5-labelled SAMS as substrate in presence of ATP by TR-FRET assay |
J Med Chem 59: 8068-81 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00866
BindingDB Entry DOI: 10.7270/Q2PZ5BRC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5'-AMP-activated protein kinase catalytic subunit alpha-1/subunit beta-1/subunit gamma-1
(Homo sapiens (Human)) | BDBM237920

(US9394285, 1)Show SMILES OC(=O)c1c[nH]c2cc(Cl)c(cc12)-c1ccc(cc1)C1(O)CCC1 Show InChI InChI=1S/C19H16ClNO3/c20-16-9-17-14(15(10-21-17)18(22)23)8-13(16)11-2-4-12(5-3-11)19(24)6-1-7-19/h2-5,8-10,21,24H,1,6-7H2,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of recombinant human BAP-tagged AMPK alpha1/beta1/gamma1 assessed as protection from Thr172 residue dephosphorylation preincubated for 15 ... |
J Med Chem 59: 8068-81 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00866
BindingDB Entry DOI: 10.7270/Q2PZ5BRC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5'-AMP-activated protein kinase catalytic subunit alpha-1/subunit beta-2/subunit gamma-1
(Homo sapiens (Human)) | BDBM237920

(US9394285, 1)Show SMILES OC(=O)c1c[nH]c2cc(Cl)c(cc12)-c1ccc(cc1)C1(O)CCC1 Show InChI InChI=1S/C19H16ClNO3/c20-16-9-17-14(15(10-21-17)18(22)23)8-13(16)11-2-4-12(5-3-11)19(24)6-1-7-19/h2-5,8-10,21,24H,1,6-7H2,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of recombinant human AMPK alpha1/beta2/gamma1 using Cy5-labelled SAMS as substrate in presence of ATP by TR-FRET assay |
J Med Chem 59: 8068-81 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00866
BindingDB Entry DOI: 10.7270/Q2PZ5BRC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5'-AMP-activated protein kinase catalytic subunit alpha-1/subunit beta-1/subunit gamma-1
(Homo sapiens (Human)) | BDBM237952
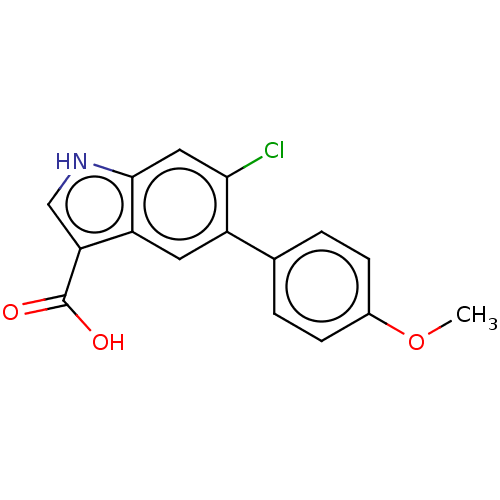
(US9394285, 33)Show InChI InChI=1S/C16H12ClNO3/c1-21-10-4-2-9(3-5-10)11-6-12-13(16(19)20)8-18-15(12)7-14(11)17/h2-8,18H,1H3,(H,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of recombinant human AMPK alpha1/beta1/gamma1 using Cy5-labelled SAMS as substrate assessed as protection from Thr172 residue dephosphoryl... |
J Med Chem 59: 8068-81 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00866
BindingDB Entry DOI: 10.7270/Q2PZ5BRC |
More data for this
Ligand-Target Pair | |
5'-AMP-activated protein kinase catalytic subunit alpha-1/subunit beta-1/subunit gamma-1
(Homo sapiens (Human)) | BDBM237921

(US9394285, 2)Show InChI InChI=1S/C16H12ClNO3/c17-14-6-15-12(13(7-18-15)16(20)21)5-11(14)10-3-1-9(8-19)2-4-10/h1-7,18-19H,8H2,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 53 | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of recombinant human AMPK alpha1/beta1/gamma1 using Cy5-labelled SAMS as substrate assessed as protection from Thr172 residue dephosphoryl... |
J Med Chem 59: 8068-81 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00866
BindingDB Entry DOI: 10.7270/Q2PZ5BRC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data