 Found 16332 hits with Last Name = 'park' and Initial = 'j'
Found 16332 hits with Last Name = 'park' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86041
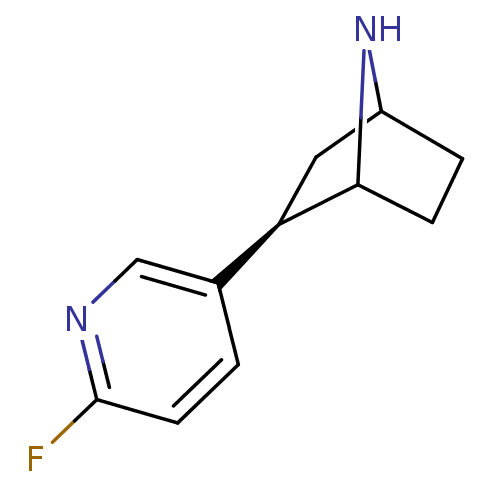
(NFEP)Show SMILES Fc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13FN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1
(Xenopus) | BDBM86042

(NEP)Show InChI InChI=1S/C11H14N2/c1-2-8(7-12-5-1)10-6-9-3-4-11(10)13-9/h1-2,5,7,9-11,13H,3-4,6H2/t9?,10-,11?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1
(Xenopus) | BDBM86041

(NFEP)Show SMILES Fc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13FN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1
(Xenopus) | BDBM86040
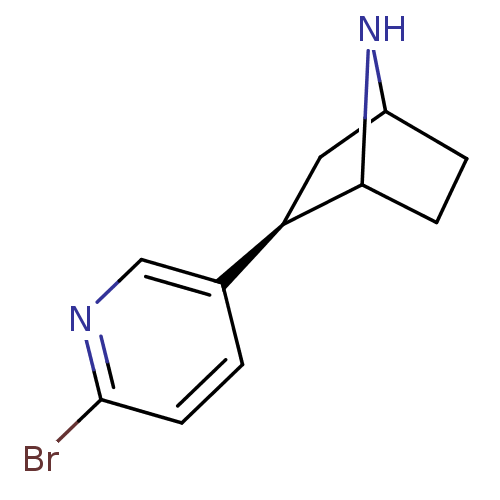
(NBEP)Show SMILES Brc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13BrN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-4
(Xenopus) | BDBM86042

(NEP)Show InChI InChI=1S/C11H14N2/c1-2-8(7-12-5-1)10-6-9-3-4-11(10)13-9/h1-2,5,7,9-11,13H,3-4,6H2/t9?,10-,11?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-4
(Xenopus) | BDBM86041

(NFEP)Show SMILES Fc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13FN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-4
(Xenopus) | BDBM86040

(NBEP)Show SMILES Brc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13BrN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86040

(NBEP)Show SMILES Brc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13BrN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM13061

(4,4 -(1H-1,2,4-triazol-1-ylmethanediyl)dibenzonitr...)Show InChI InChI=1S/C17H11N5/c18-9-13-1-5-15(6-2-13)17(22-12-20-11-21-22)16-7-3-14(10-19)4-8-16/h1-8,11-12,17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... |
Bioorg Med Chem 20: 2427-34 (2012)
Article DOI: 10.1016/j.bmc.2012.01.047
BindingDB Entry DOI: 10.7270/Q2DJ5G3W |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86042

(NEP)Show InChI InChI=1S/C11H14N2/c1-2-8(7-12-5-1)10-6-9-3-4-11(10)13-9/h1-2,5,7,9-11,13H,3-4,6H2/t9?,10-,11?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50288406
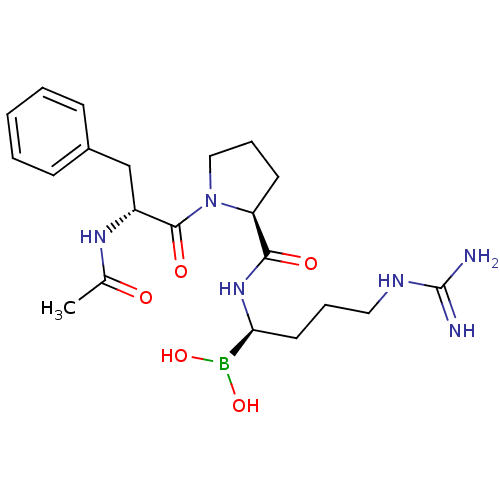
(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)B(O)O Show InChI InChI=1S/C21H33BN6O5/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25)/t16-,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM176555
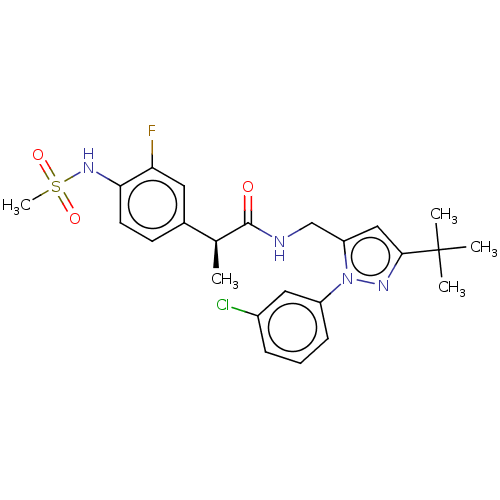
(US9120756, 17)Show SMILES C[C@H](C(=O)NCc1cc(nn1-c1cccc(Cl)c1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C24H28ClFN4O3S/c1-15(16-9-10-21(20(26)11-16)29-34(5,32)33)23(31)27-14-19-13-22(24(2,3)4)28-30(19)18-8-6-7-17(25)12-18/h6-13,15,29H,14H2,1-5H3,(H,27,31)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of NADA-induced intracellular calcium level preincubated with cells ... |
Bioorg Med Chem Lett 27: 4383-4388 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.020
BindingDB Entry DOI: 10.7270/Q2CF9SPD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50451005

(CHEMBL290376 | DuP-714)Show SMILES CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)B(O)O |r| Show InChI InChI=1S/C21H33BN6O5/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25)/t16-,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor II |
Bioorg Med Chem Lett 9: 925-30 (1999)
BindingDB Entry DOI: 10.7270/Q2KS6QQ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinergic receptor, nicotinic alpha 1
(Xenopus) | BDBM86040

(NBEP)Show SMILES Brc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13BrN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50288406

(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)B(O)O Show InChI InChI=1S/C21H33BN6O5/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25)/t16-,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8611

(4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...)Show InChI InChI=1S/C14H13N3/c15-8-11-4-6-12(7-5-11)14-3-1-2-13-9-16-10-17(13)14/h4-7,9-10,14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... |
Bioorg Med Chem 20: 2427-34 (2012)
Article DOI: 10.1016/j.bmc.2012.01.047
BindingDB Entry DOI: 10.7270/Q2DJ5G3W |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86040

(NBEP)Show SMILES Brc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13BrN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50366125
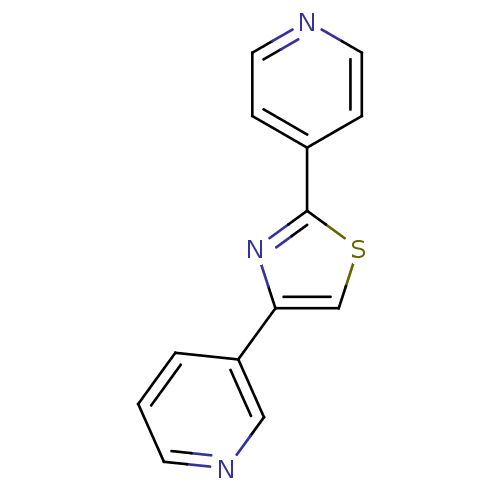
(CHEMBL1957214)Show InChI InChI=1S/C13H9N3S/c1-2-11(8-15-5-1)12-9-17-13(16-12)10-3-6-14-7-4-10/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... |
Bioorg Med Chem 20: 2427-34 (2012)
Article DOI: 10.1016/j.bmc.2012.01.047
BindingDB Entry DOI: 10.7270/Q2DJ5G3W |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-2
(Xenopus) | BDBM86045
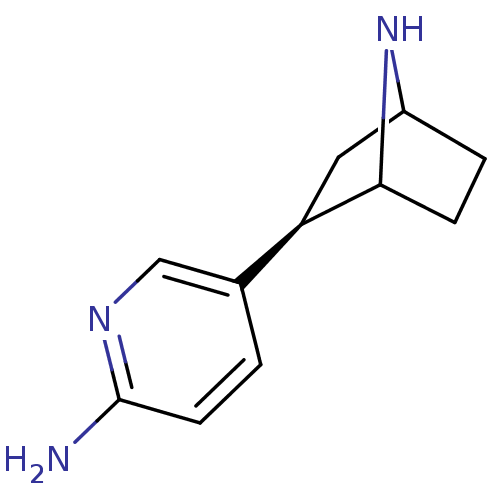
(NNEP)Show SMILES Nc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H15N3/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2,(H2,12,13)/t8?,9-,10?/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
cholinergic receptor, nicotinic, alpha 1 (Muscle) isoform X1
(Xenopus) | BDBM86045

(NNEP)Show SMILES Nc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H15N3/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2,(H2,12,13)/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM142712
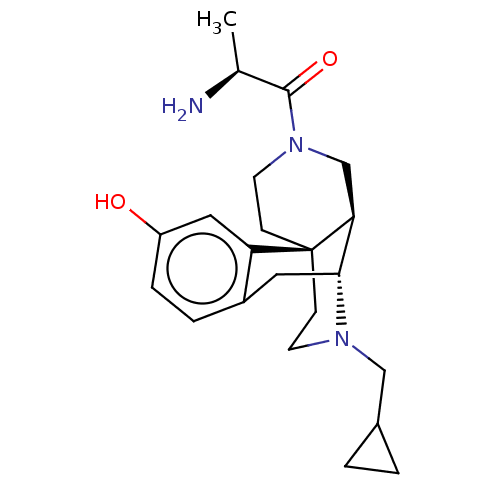
(US8937084, 14)Show SMILES C[C@H](N)C(=O)N1CC[C@]23CCN(CC4CC4)[C@H](Cc4ccc(O)cc24)[C@@H]3C1 |r| Show InChI InChI=1S/C22H31N3O2/c1-14(23)21(27)25-9-7-22-6-8-24(12-15-2-3-15)20(19(22)13-25)10-16-4-5-17(26)11-18(16)22/h4-5,11,14-15,19-20,26H,2-3,6-10,12-13,23H2,1H3/t14-,19-,20+,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0900 | -62.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma L.P.
US Patent
| Assay Description
Radioligand dose-displacement binding assays for u-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane... |
US Patent US8937084 (2015)
BindingDB Entry DOI: 10.7270/Q2CJ8C5C |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50450981
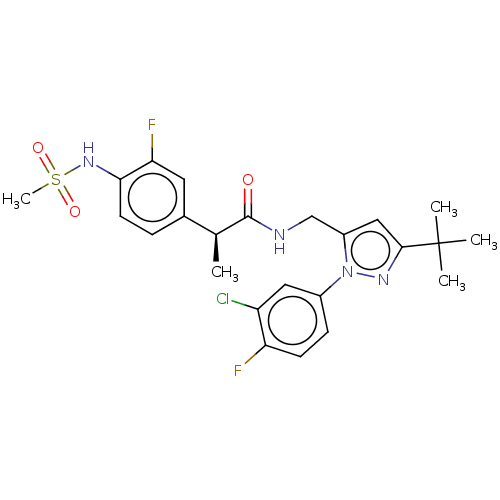
(CHEMBL4215829)Show SMILES C[C@H](C(=O)NCc1cc(nn1-c1ccc(F)c(Cl)c1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C24H27ClF2N4O3S/c1-14(15-6-9-21(20(27)10-15)30-35(5,33)34)23(32)28-13-17-12-22(24(2,3)4)29-31(17)16-7-8-19(26)18(25)11-16/h6-12,14,30H,13H2,1-5H3,(H,28,32)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced intracellular calcium level preincubated for 6 ... |
Bioorg Med Chem Lett 27: 4383-4388 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.020
BindingDB Entry DOI: 10.7270/Q2CF9SPD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50034332
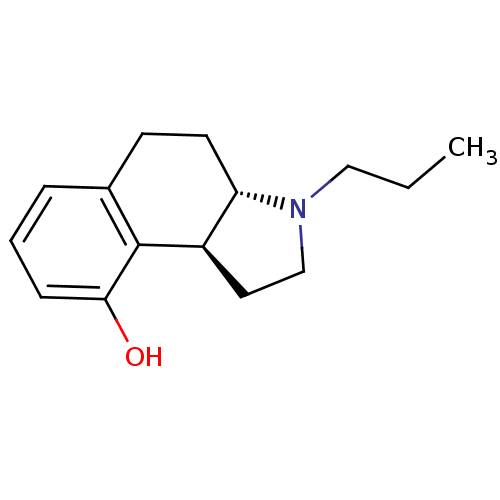
((3aS,9bS)-3-Propyl-2,3,3a,4,5,9b-hexahydro-1H-benz...)Show InChI InChI=1S/C15H21NO/c1-2-9-16-10-8-12-13(16)7-6-11-4-3-5-14(17)15(11)12/h3-5,12-13,17H,2,6-10H2,1H3/t12-,13+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Tested for activity against 5-hydroxytryptamine 1A receptor from bovine hippocampus |
J Med Chem 38: 1295-308 (1995)
BindingDB Entry DOI: 10.7270/Q2CJ8CJ9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM176555

(US9120756, 17)Show SMILES C[C@H](C(=O)NCc1cc(nn1-c1cccc(Cl)c1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C24H28ClFN4O3S/c1-15(16-9-10-21(20(26)11-16)29-34(5,32)33)23(31)27-14-19-13-22(24(2,3)4)28-30(19)18-8-6-7-17(25)12-18/h6-13,15,29H,14H2,1-5H3,(H,27,31)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced intracellular calcium level preincubated for 6 ... |
Bioorg Med Chem Lett 27: 4383-4388 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.020
BindingDB Entry DOI: 10.7270/Q2CF9SPD |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM176555

(US9120756, 17)Show SMILES C[C@H](C(=O)NCc1cc(nn1-c1cccc(Cl)c1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C24H28ClFN4O3S/c1-15(16-9-10-21(20(26)11-16)29-34(5,32)33)23(31)27-14-19-13-22(24(2,3)4)28-30(19)18-8-6-7-17(25)12-18/h6-13,15,29H,14H2,1-5H3,(H,27,31)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced intracellular calcium level preincubated for 6 mi... |
Bioorg Med Chem Lett 27: 4383-4388 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.020
BindingDB Entry DOI: 10.7270/Q2CF9SPD |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM176564
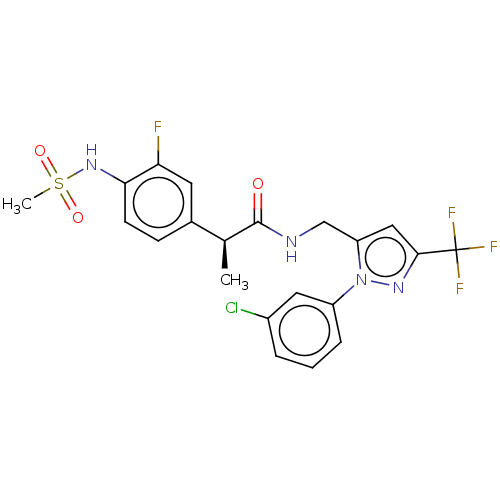
(US9120756, 26)Show SMILES C[C@H](C(=O)NCc1cc(nn1-c1cccc(Cl)c1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C21H19ClF4N4O3S/c1-12(13-6-7-18(17(23)8-13)29-34(2,32)33)20(31)27-11-16-10-19(21(24,25)26)28-30(16)15-5-3-4-14(22)9-15/h3-10,12,29H,11H2,1-2H3,(H,27,31)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced intracellular calcium level preincubated for 6 ... |
Bioorg Med Chem Lett 27: 4383-4388 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.020
BindingDB Entry DOI: 10.7270/Q2CF9SPD |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM176564

(US9120756, 26)Show SMILES C[C@H](C(=O)NCc1cc(nn1-c1cccc(Cl)c1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C21H19ClF4N4O3S/c1-12(13-6-7-18(17(23)8-13)29-34(2,32)33)20(31)27-11-16-10-19(21(24,25)26)28-30(16)15-5-3-4-14(22)9-15/h3-10,12,29H,11H2,1-2H3,(H,27,31)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of NADA-induced intracellular calcium level preincubated with cells ... |
Bioorg Med Chem Lett 27: 4383-4388 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.020
BindingDB Entry DOI: 10.7270/Q2CF9SPD |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM176564

(US9120756, 26)Show SMILES C[C@H](C(=O)NCc1cc(nn1-c1cccc(Cl)c1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C21H19ClF4N4O3S/c1-12(13-6-7-18(17(23)8-13)29-34(2,32)33)20(31)27-11-16-10-19(21(24,25)26)28-30(16)15-5-3-4-14(22)9-15/h3-10,12,29H,11H2,1-2H3,(H,27,31)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced intracellular calcium level preincubated for 6 mi... |
Bioorg Med Chem Lett 27: 4383-4388 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.020
BindingDB Entry DOI: 10.7270/Q2CF9SPD |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50289586

(3-Methyl-2'-sulfamoyl-biphenyl-4-carboxylic acid [...)Show SMILES Cc1cc(ccc1C(=O)N[C@@H](CCCNC(N)=N)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1)-c1ccccc1S(N)(=O)=O |TLB:30:28:25:23| Show InChI InChI=1S/C29H40BN5O5S/c1-17-14-18(21-8-5-6-9-22(21)41(33,37)38)11-12-20(17)26(36)35-25(10-7-13-34-27(31)32)30-39-24-16-19-15-23(28(19,2)3)29(24,4)40-30/h5-6,8-9,11-12,14,19,23-25H,7,10,13,15-16H2,1-4H3,(H,35,36)(H4,31,32,34)(H2,33,37,38)/t19?,23?,24-,25+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10015
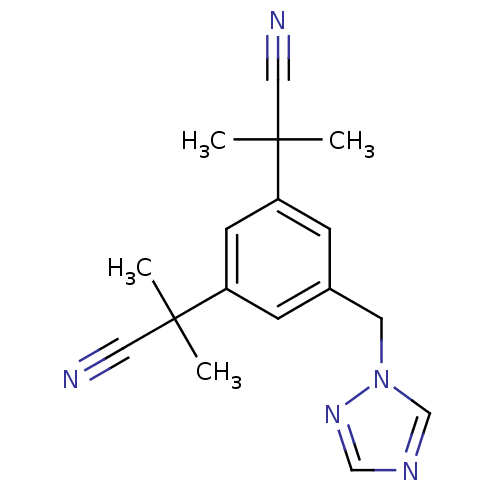
(2-[3-(1-cyano-1-methylethyl)-5-(1H-1,2,4-triazol-1...)Show InChI InChI=1S/C17H19N5/c1-16(2,9-18)14-5-13(8-22-12-20-11-21-22)6-15(7-14)17(3,4)10-19/h5-7,11-12H,8H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human aromatase using dibenzylfluorescein substrate after 10 mins preincubation measured every 10 sec for 5 mins by Michael... |
Bioorg Med Chem 20: 2427-34 (2012)
Article DOI: 10.1016/j.bmc.2012.01.047
BindingDB Entry DOI: 10.7270/Q2DJ5G3W |
More data for this
Ligand-Target Pair | |
Enteropeptidase
(Homo sapiens (Human)) | BDBM571836

((Z)-3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbon...)Show SMILES CCn1c2ccc(cc2s\c1=N/CC(C)(C)C(O)=O)C(=O)Oc1ccc(cc1F)C(N)=N | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2WD43TB |
More data for this
Ligand-Target Pair | |
Enteropeptidase
(Homo sapiens (Human)) | BDBM571836

((Z)-3-((6-((4-Carbamimidoyl-2-fluorophenoxy)carbon...)Show SMILES CCn1c2ccc(cc2s\c1=N/CC(C)(C)C(O)=O)C(=O)Oc1ccc(cc1F)C(N)=N | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity of the enteropeptidase inhibitor synthesized using the purified Recombinant Human Enteropeptidase and the substrate Acetyl-As... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2WD43TB |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM142705
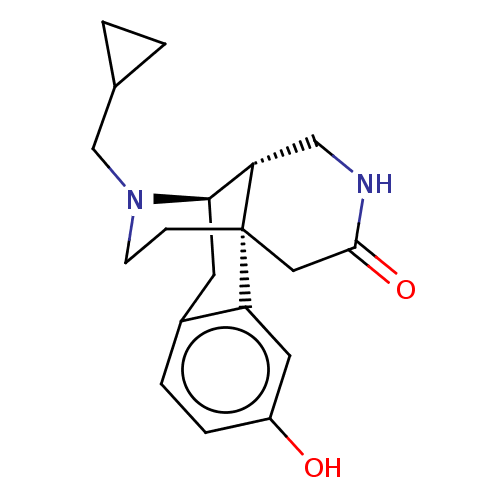
(US8937084, 7)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CNC(=O)C[C@]4(CCN3CC3CC3)c2c1 |r| Show InChI InChI=1S/C19H24N2O2/c22-14-4-3-13-7-17-16-10-20-18(23)9-19(16,15(13)8-14)5-6-21(17)11-12-1-2-12/h3-4,8,12,16-17,22H,1-2,5-7,9-11H2,(H,20,23)/t16-,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.180 | -60.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma L.P.
US Patent
| Assay Description
Radioligand dose-displacement binding assays for u-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane... |
US Patent US8937084 (2015)
BindingDB Entry DOI: 10.7270/Q2CJ8C5C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50034331
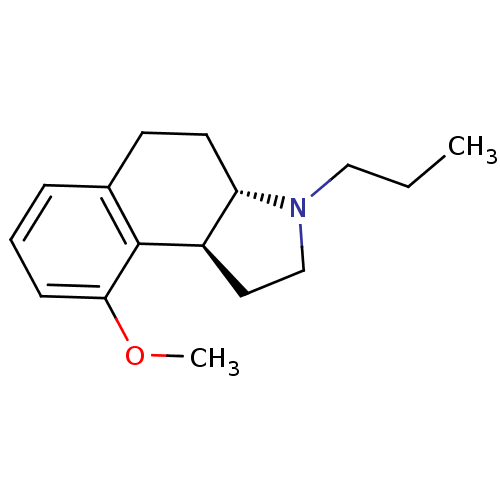
((3aS,9bS)-9-Methoxy-3-propyl-2,3,3a,4,5,9b-hexahyd...)Show InChI InChI=1S/C16H23NO/c1-3-10-17-11-9-13-14(17)8-7-12-5-4-6-15(18-2)16(12)13/h4-6,13-14H,3,7-11H2,1-2H3/t13-,14+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Tested for activity against 5-hydroxytryptamine 1A receptor from bovine hippocampus |
J Med Chem 38: 1295-308 (1995)
BindingDB Entry DOI: 10.7270/Q2CJ8CJ9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50034322
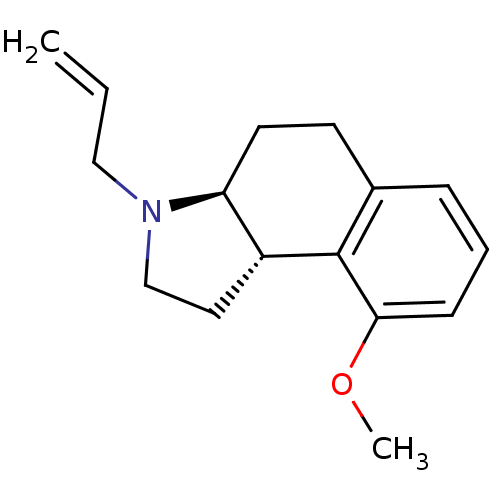
((3aS,9bS)-3-Allyl-9-methoxy-2,3,3a,4,5,9b-hexahydr...)Show InChI InChI=1S/C16H21NO/c1-3-10-17-11-9-13-14(17)8-7-12-5-4-6-15(18-2)16(12)13/h3-6,13-14H,1,7-11H2,2H3/t13-,14+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Tested for activity against 5-hydroxytryptamine 1A receptor from bovine hippocampus |
J Med Chem 38: 1295-308 (1995)
BindingDB Entry DOI: 10.7270/Q2CJ8CJ9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM142711
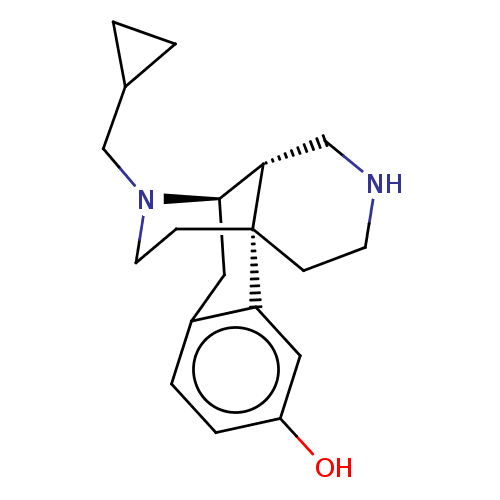
(US8937084, 13)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CNCC[C@]4(CCN3CC3CC3)c2c1 |r| Show InChI InChI=1S/C19H26N2O/c22-15-4-3-14-9-18-17-11-20-7-5-19(17,16(14)10-15)6-8-21(18)12-13-1-2-13/h3-4,10,13,17-18,20,22H,1-2,5-9,11-12H2/t17-,18+,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.210 | -59.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma L.P.
US Patent
| Assay Description
Radioligand dose-displacement binding assays for u-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane... |
US Patent US8937084 (2015)
BindingDB Entry DOI: 10.7270/Q2CJ8C5C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50084682

(1-[3-(4-Carbamimidoyl-phenyl)-2-(2-methyl-1,2,3,4-...)Show SMILES Cc1cc(ccc1C(=O)N[C@@H](CCCNC(N)=N)B1O[C@@H]2CC3CC(C3(C)C)[C@]2(C)O1)-c1ccccc1S(=O)(=O)NC(C)(C)C |TLB:30:28:25:23| Show InChI InChI=1S/C33H48BN5O5S/c1-20-17-21(24-11-8-9-12-25(24)45(41,42)39-31(2,3)4)14-15-23(20)29(40)38-28(13-10-16-37-30(35)36)34-43-27-19-22-18-26(32(22,5)6)33(27,7)44-34/h8-9,11-12,14-15,17,22,26-28,39H,10,13,16,18-19H2,1-7H3,(H,38,40)(H4,35,36,37)/t22?,26?,27-,28+,33+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Cholinergic receptor nicotinic alpha 5 subunit
(Xenopus) | BDBM86040

(NBEP)Show SMILES Brc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H13BrN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2/t8?,9-,10?/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50252829
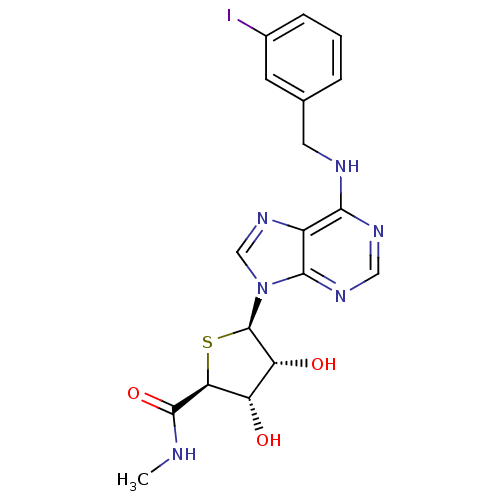
((2S,3S,4R,5R)-5-(6-(3-iodobenzylamino)-9H-purin-9-...)Show SMILES CNC(=O)[C@H]1S[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)ncnc12 |r| Show InChI InChI=1S/C18H19IN6O3S/c1-20-17(28)14-12(26)13(27)18(29-14)25-8-24-11-15(22-7-23-16(11)25)21-6-9-3-2-4-10(19)5-9/h2-5,7-8,12-14,18,26-27H,6H2,1H3,(H,20,28)(H,21,22,23)/t12-,13+,14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counting analysis |
J Med Chem 60: 3422-3437 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00241
BindingDB Entry DOI: 10.7270/Q2J105FT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 17B
(Homo sapiens (Human)) | BDBM50166121
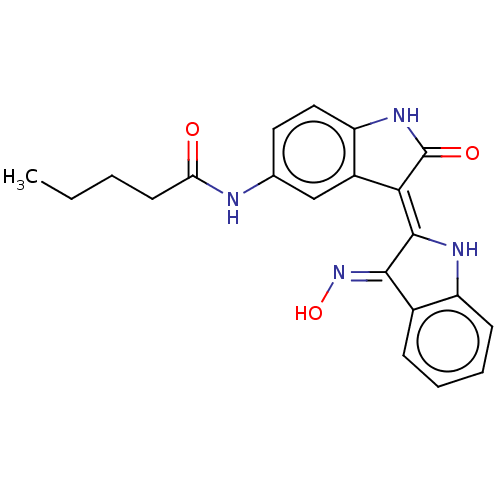
(CHEMBL3797480)Show SMILES CCCCC(=O)Nc1ccc2NC(=O)\C(=C3/Nc4ccccc4/C/3=N\O)c2c1 Show InChI InChI=1S/C21H20N4O3/c1-2-3-8-17(26)22-12-9-10-16-14(11-12)18(21(27)24-16)20-19(25-28)13-6-4-5-7-15(13)23-20/h4-7,9-11,23,28H,2-3,8H2,1H3,(H,22,26)(H,24,27)/b20-18-,25-19+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Competitive inhibition of DRAK2 (unknown origin) using MRLC3 peptide as substrate incubated for 2 hrs by Lineweaver-Burk plot analysis in presence of... |
Bioorg Med Chem Lett 26: 2719-23 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.111
BindingDB Entry DOI: 10.7270/Q2N29ZTZ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM142709
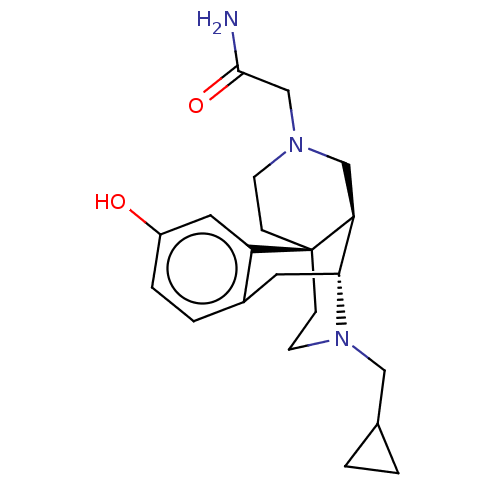
(US8937084, 11)Show SMILES NC(=O)CN1CC[C@]23CCN(CC4CC4)[C@H](Cc4ccc(O)cc24)[C@@H]3C1 |r| Show InChI InChI=1S/C21H29N3O2/c22-20(26)13-23-7-5-21-6-8-24(11-14-1-2-14)19(18(21)12-23)9-15-3-4-16(25)10-17(15)21/h3-4,10,14,18-19,25H,1-2,5-9,11-13H2,(H2,22,26)/t18-,19+,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.260 | -59.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma L.P.
US Patent
| Assay Description
Radioligand dose-displacement binding assays for u-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane... |
US Patent US8937084 (2015)
BindingDB Entry DOI: 10.7270/Q2CJ8C5C |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM176554
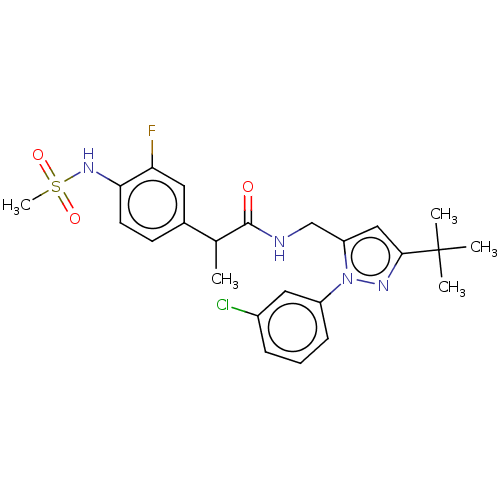
(US9120756, 16)Show SMILES CC(C(=O)NCc1cc(nn1-c1cccc(Cl)c1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C24H28ClFN4O3S/c1-15(16-9-10-21(20(26)11-16)29-34(5,32)33)23(31)27-14-19-13-22(24(2,3)4)28-30(19)18-8-6-7-17(25)12-18/h6-13,15,29H,14H2,1-5H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced intracellular calcium level preincubated for 6 ... |
Bioorg Med Chem Lett 27: 4383-4388 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.020
BindingDB Entry DOI: 10.7270/Q2CF9SPD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50034348
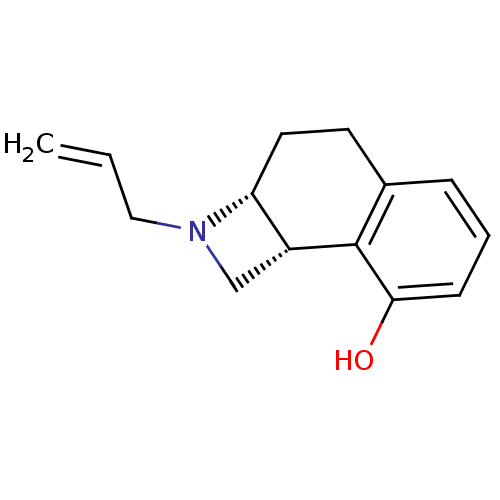
((2aR,8bR)-2-Allyl-1,2,2a,3,4,8b-hexahydro-naphtho[...)Show InChI InChI=1S/C14H17NO/c1-2-8-15-9-11-12(15)7-6-10-4-3-5-13(16)14(10)11/h2-5,11-12,16H,1,6-9H2/t11-,12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Tested for activity against 5-hydroxytryptamine 1A receptor from bovine hippocampus |
J Med Chem 38: 1295-308 (1995)
BindingDB Entry DOI: 10.7270/Q2CJ8CJ9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM176561

(US9120756, 23)Show SMILES C[C@H](C(=O)NCc1cc(nn1-c1ccc(Cl)cc1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C21H19ClF4N4O3S/c1-12(13-3-8-18(17(23)9-13)29-34(2,32)33)20(31)27-11-16-10-19(21(24,25)26)28-30(16)15-6-4-14(22)5-7-15/h3-10,12,29H,11H2,1-2H3,(H,27,31)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced intracellular calcium level preincubated for 6 ... |
Bioorg Med Chem Lett 27: 4383-4388 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.020
BindingDB Entry DOI: 10.7270/Q2CF9SPD |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM176562
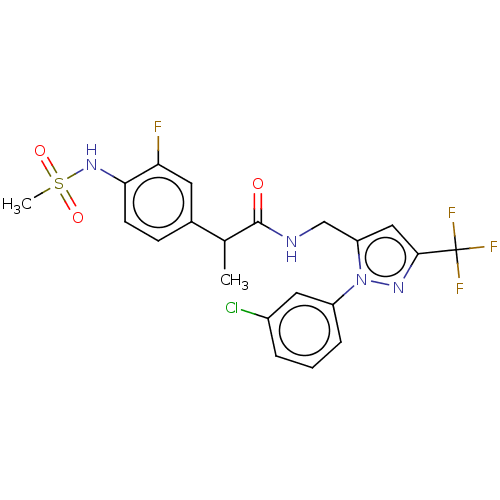
(US9120756, 24)Show SMILES CC(C(=O)NCc1cc(nn1-c1cccc(Cl)c1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C21H19ClF4N4O3S/c1-12(13-6-7-18(17(23)8-13)29-34(2,32)33)20(31)27-11-16-10-19(21(24,25)26)28-30(16)15-5-3-4-14(22)9-15/h3-10,12,29H,11H2,1-2H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced intracellular calcium level preincubated for 6 ... |
Bioorg Med Chem Lett 27: 4383-4388 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.020
BindingDB Entry DOI: 10.7270/Q2CF9SPD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50034360
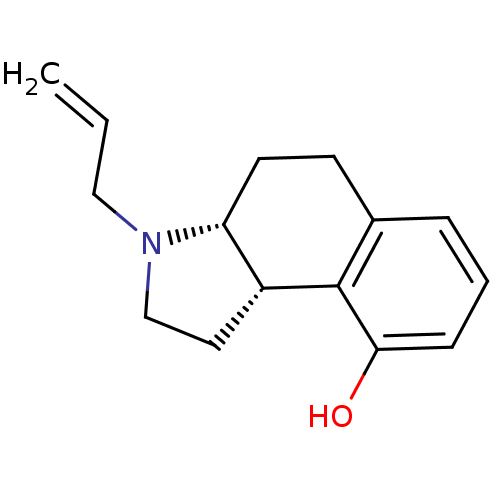
((3aR,9bS)-3-Allyl-2,3,3a,4,5,9b-hexahydro-1H-benzo...)Show InChI InChI=1S/C15H19NO/c1-2-9-16-10-8-12-13(16)7-6-11-4-3-5-14(17)15(11)12/h2-5,12-13,17H,1,6-10H2/t12-,13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arris Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Tested for activity against 5-hydroxytryptamine 1A receptor from bovine hippocampus |
J Med Chem 38: 1295-308 (1995)
BindingDB Entry DOI: 10.7270/Q2CJ8CJ9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50094092
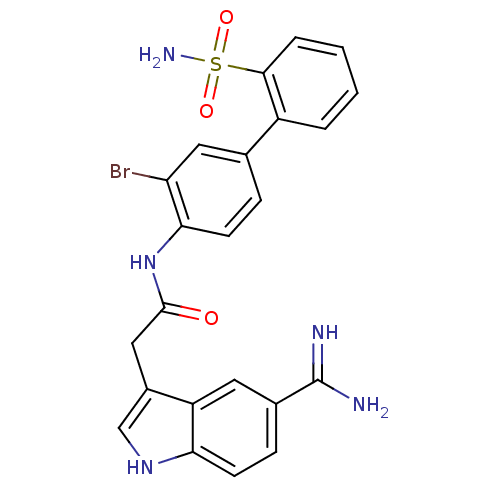
(CHEMBL336661 | N-(3-Bromo-2'-sulfamoyl-biphenyl-4-...)Show SMILES NC(=N)c1ccc2[nH]cc(CC(=O)Nc3ccc(cc3Br)-c3ccccc3S(N)(=O)=O)c2c1 Show InChI InChI=1S/C23H20BrN5O3S/c24-18-10-13(16-3-1-2-4-21(16)33(27,31)32)5-8-20(18)29-22(30)11-15-12-28-19-7-6-14(23(25)26)9-17(15)19/h1-10,12,28H,11H2,(H3,25,26)(H,29,30)(H2,27,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 43: 4398-415 (2000)
BindingDB Entry DOI: 10.7270/Q2JS9PP5 |
More data for this
Ligand-Target Pair | |
neuronal acetylcholine receptor subunit alpha-4
(Xenopus) | BDBM86045

(NNEP)Show SMILES Nc1ccc(cn1)[C@H]1CC2CCC1N2 |r,TLB:4:7:10.11:13| Show InChI InChI=1S/C11H15N3/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2,(H2,12,13)/t8?,9-,10?/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 1246-52 (2002)
Article DOI: 10.1124/jpet.102.035899
BindingDB Entry DOI: 10.7270/Q2HQ3XG3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50289591
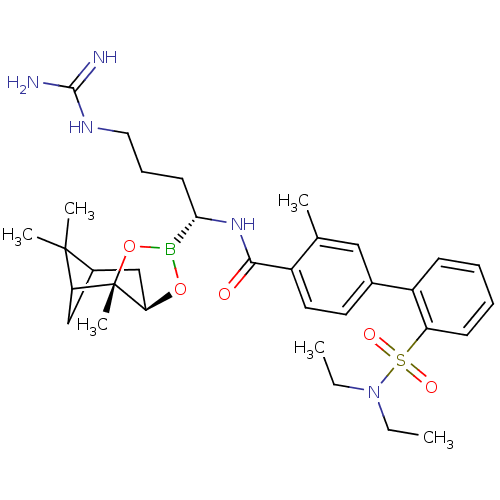
(2'-Diethylsulfamoyl-3-methyl-biphenyl-4-carboxylic...)Show SMILES CCN(CC)S(=O)(=O)c1ccccc1-c1ccc(C(=O)N[C@@H](CCCNC(N)=N)B2O[C@@H]3CC4CC(C4(C)C)[C@]3(C)O2)c(C)c1 |TLB:41:39:36:34| Show InChI InChI=1S/C33H48BN5O5S/c1-7-39(8-2)45(41,42)26-13-10-9-12-25(26)22-15-16-24(21(3)18-22)30(40)38-29(14-11-17-37-31(35)36)34-43-28-20-23-19-27(32(23,4)5)33(28,6)44-34/h9-10,12-13,15-16,18,23,27-29H,7-8,11,14,17,19-20H2,1-6H3,(H,38,40)(H4,35,36,37)/t23?,27?,28-,29+,33+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM176559
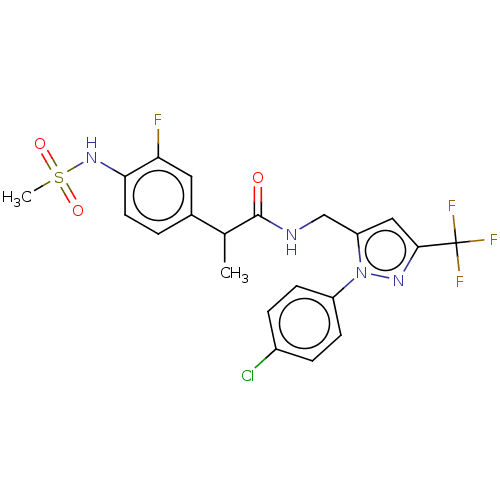
(US9120756, 21)Show SMILES CC(C(=O)NCc1cc(nn1-c1ccc(Cl)cc1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C21H19ClF4N4O3S/c1-12(13-3-8-18(17(23)9-13)29-34(2,32)33)20(31)27-11-16-10-19(21(24,25)26)28-30(16)15-6-4-14(22)5-7-15/h3-10,12,29H,11H2,1-2H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced intracellular calcium level preincubated for 6 ... |
Bioorg Med Chem Lett 27: 4383-4388 (2017)
Article DOI: 10.1016/j.bmcl.2017.08.020
BindingDB Entry DOI: 10.7270/Q2CF9SPD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data