

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50440252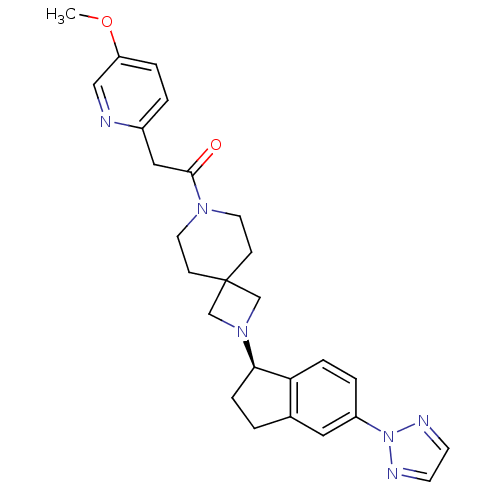 (CHEMBL2426686) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM335698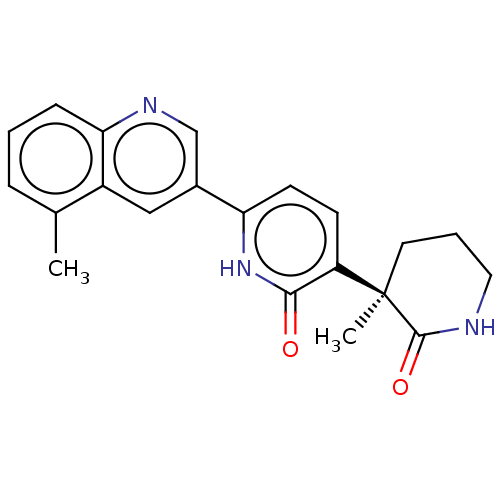 ((R)-3-(3-Methyl-2-oxopiperidin-3-yl)-6-(5-methylqu...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P... | US Patent US9738626 (2017) BindingDB Entry DOI: 10.7270/Q2CF9S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50440255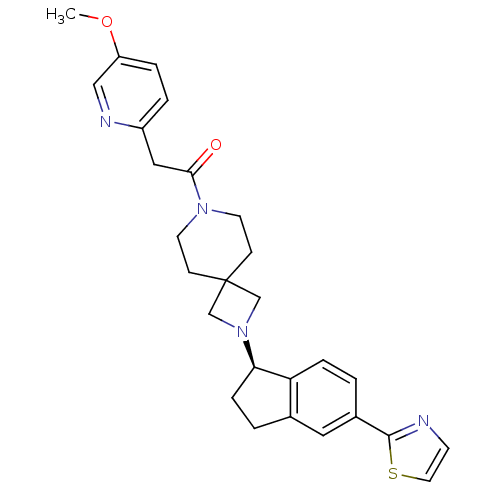 (CHEMBL2426683) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM335700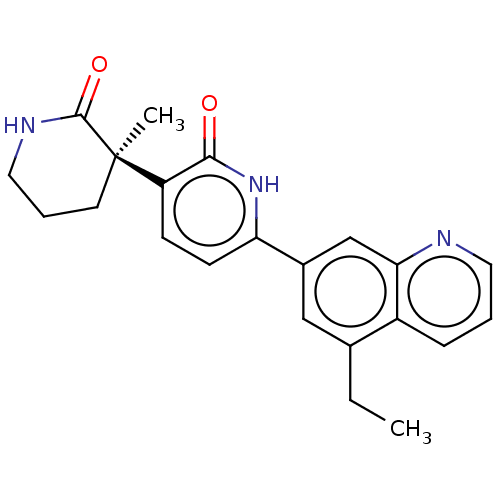 ((R)-6-(5-Ethylquinolin-7-yl)-3-(3-methyl-2-oxopipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P... | US Patent US9738626 (2017) BindingDB Entry DOI: 10.7270/Q2CF9S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50386955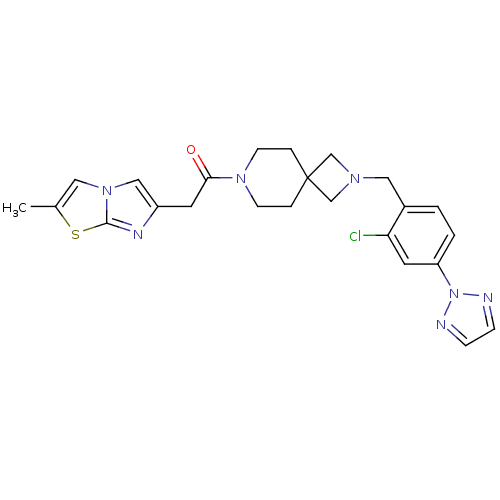 (CHEMBL2048820) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50440261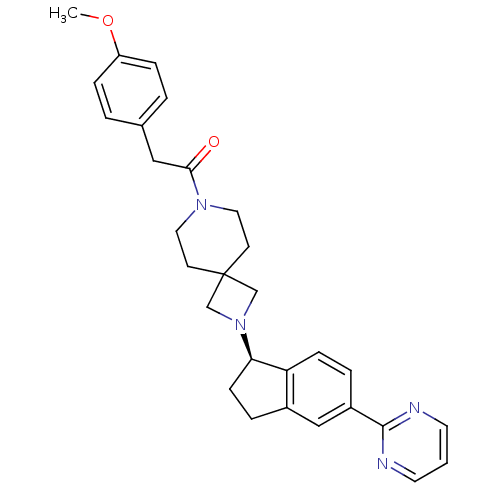 (CHEMBL2426677) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM335699 ((R)-3-(3-Methyl-2-oxopiperidin-3-yl)-6-(5-methylqu...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P... | US Patent US9738626 (2017) BindingDB Entry DOI: 10.7270/Q2CF9S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM335702 ((R)-6-(5-Cyclopropylquinolin-7-yl)-3-(3-methyl-2-o...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P... | US Patent US9738626 (2017) BindingDB Entry DOI: 10.7270/Q2CF9S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50440254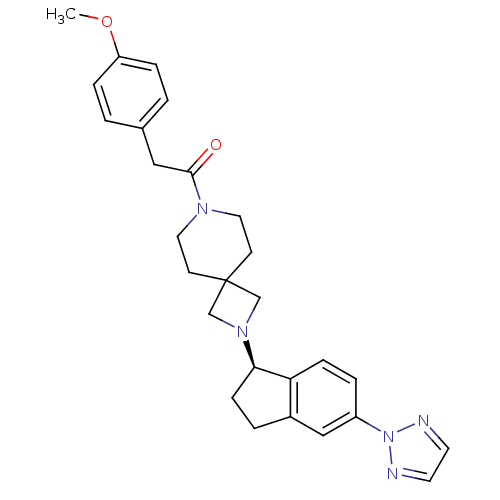 (CHEMBL2426684) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM335701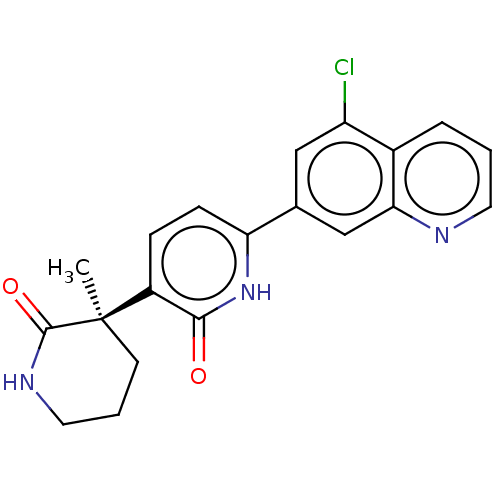 ((R)-6-(5-Chloroquinolin-7-yl)-3-(3-methyl-2-oxopip...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P... | US Patent US9738626 (2017) BindingDB Entry DOI: 10.7270/Q2CF9S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50440253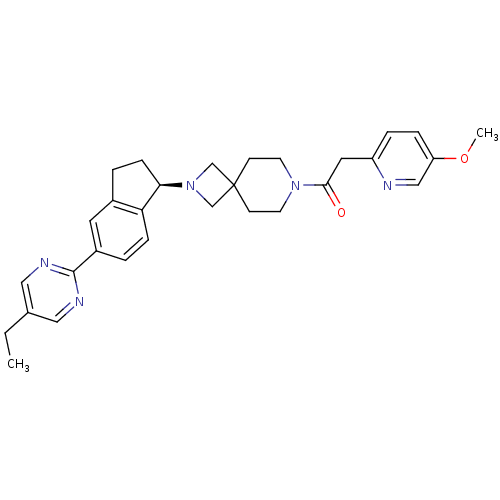 (CHEMBL2426685) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50440251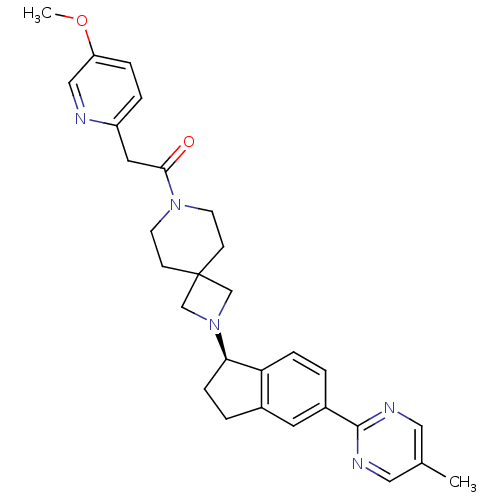 (CHEMBL2426687) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50440249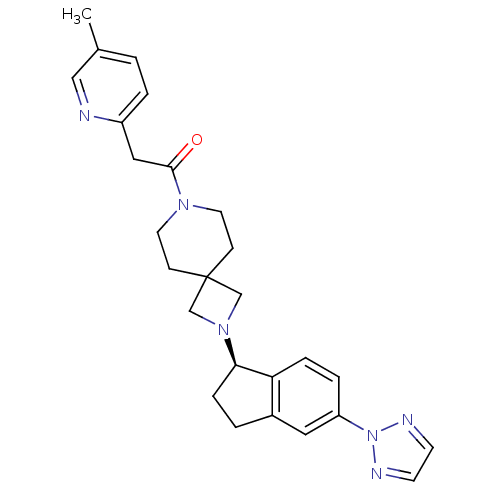 (CHEMBL2426689) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50440250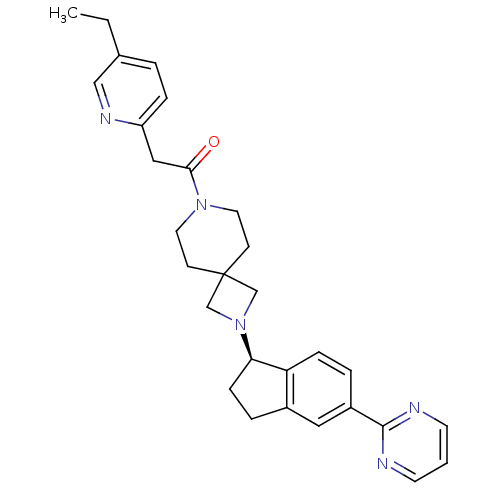 (CHEMBL2426688) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50440247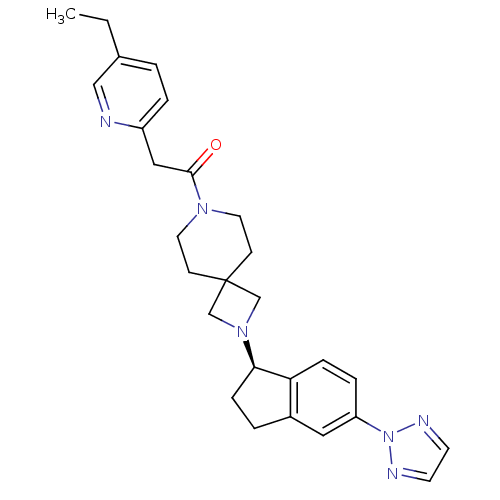 (CHEMBL2426666) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM335704 ((R)-3-(3-Methyl-2-oxopyrrolidin-3-yl)-6-(5-methylq...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P... | US Patent US9738626 (2017) BindingDB Entry DOI: 10.7270/Q2CF9S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50440248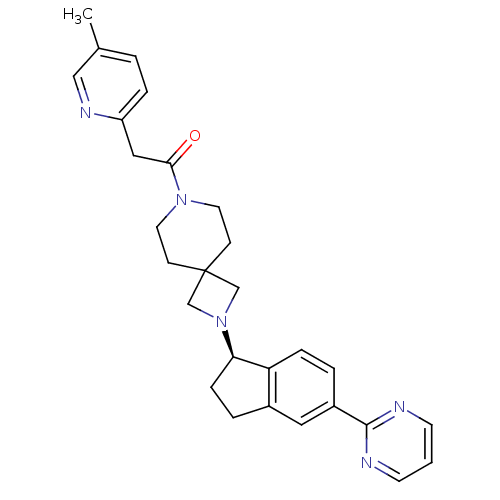 (CHEMBL2426667) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50440259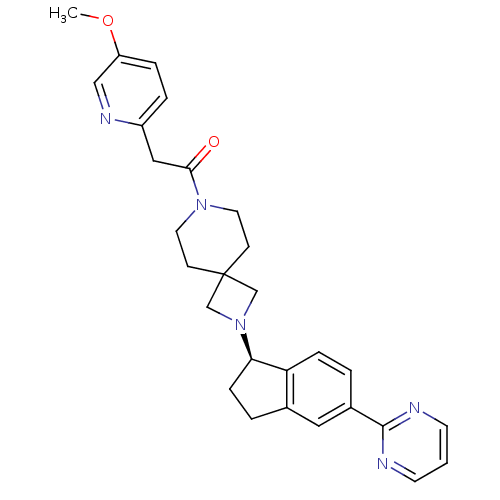 (CHEMBL2426680) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50005094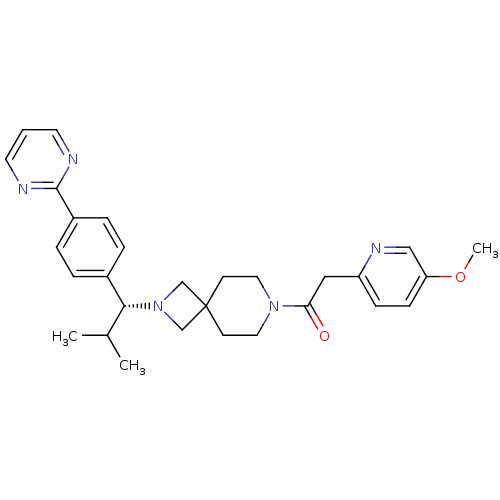 (CHEMBL2424680) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM335703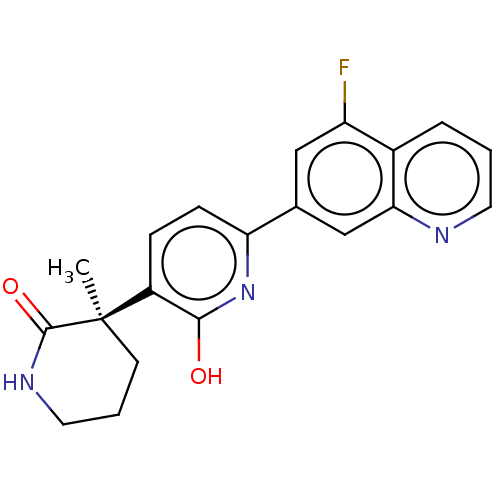 ((R)-3-(6-(5-fluoroquinolin-7-yl)-2-hydroxypyridin-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 48.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P... | US Patent US9738626 (2017) BindingDB Entry DOI: 10.7270/Q2CF9S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50005095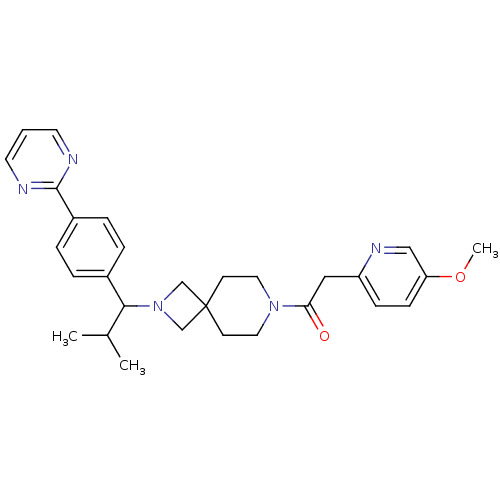 (CHEMBL2426672) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50386955 (CHEMBL2048820) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 269 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to muscarinic acetylcholine receptor M2 (unknown origin) | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50440260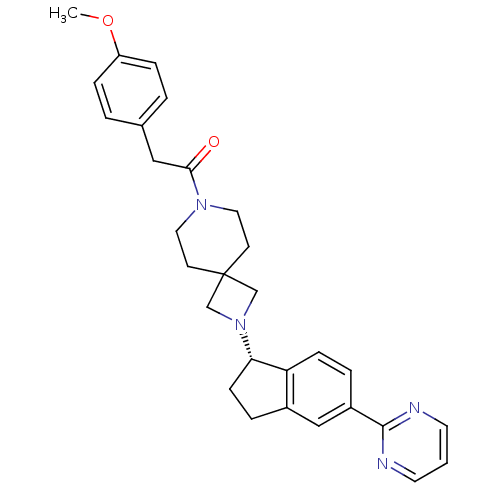 (CHEMBL2426676) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50440263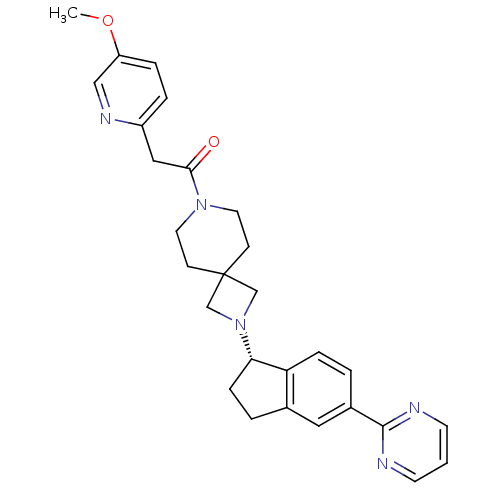 (CHEMBL2426679) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50005339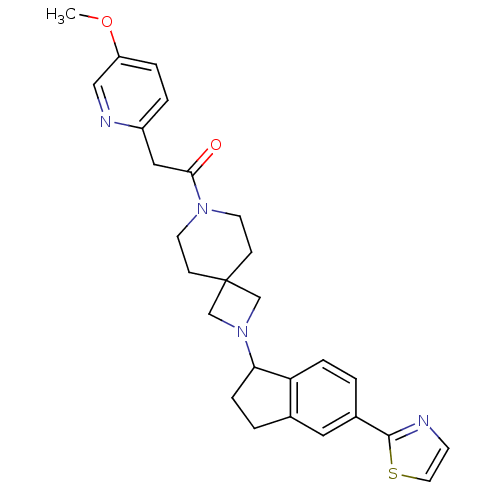 (CHEMBL2426681) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50005342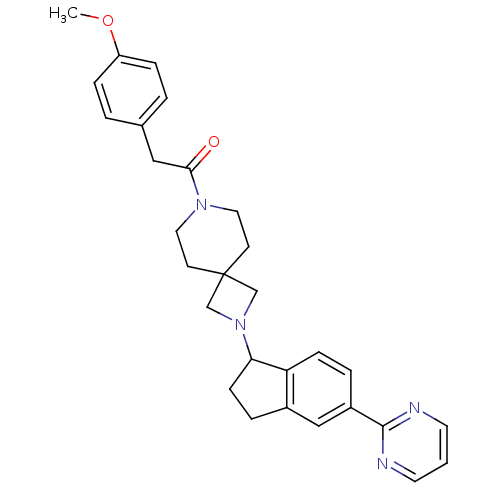 (CHEMBL2426675) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50005096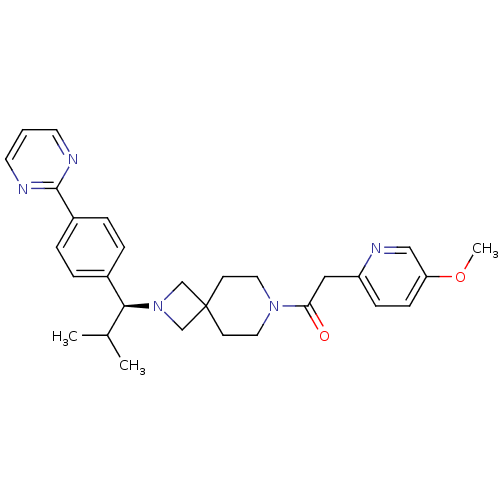 (CHEMBL2426673) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50440257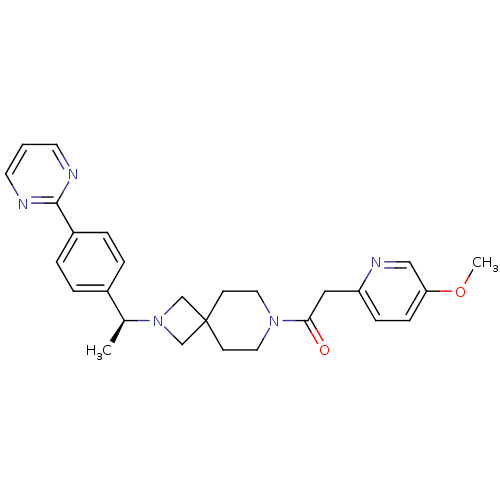 (CHEMBL2426669) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50440260 (CHEMBL2426676) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50440264 (CHEMBL2426682) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50440264 (CHEMBL2426682) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50005341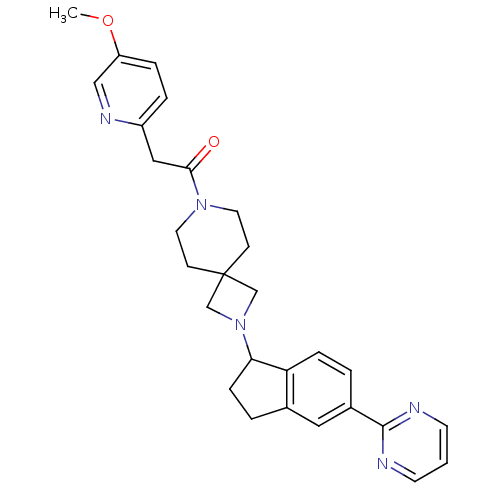 (CHEMBL2426678) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50005343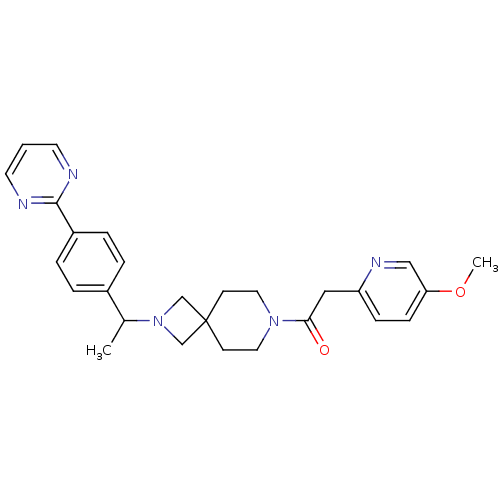 (CHEMBL2426671) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50440262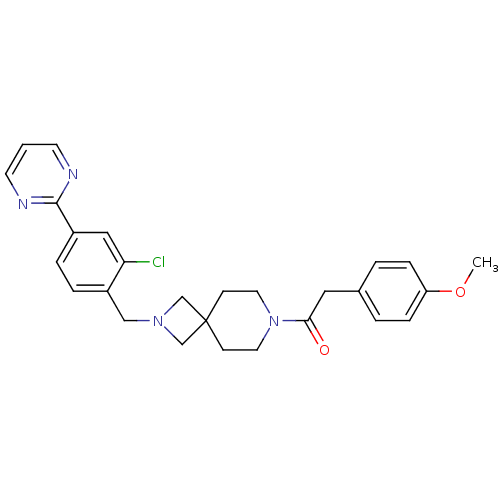 (CHEMBL2426674) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50440257 (CHEMBL2426669) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50005343 (CHEMBL2426671) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50440256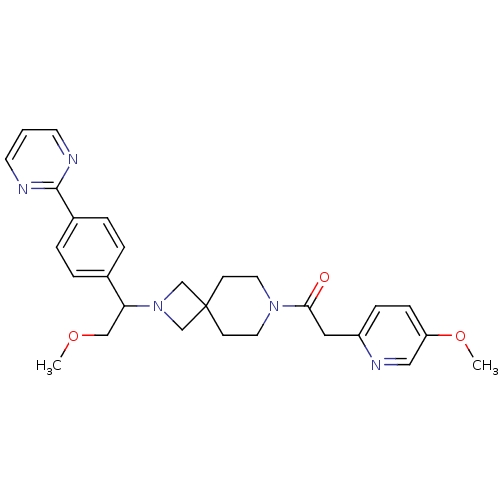 (CHEMBL2426668) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50440256 (CHEMBL2426668) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50440261 (CHEMBL2426677) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50440259 (CHEMBL2426680) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50440258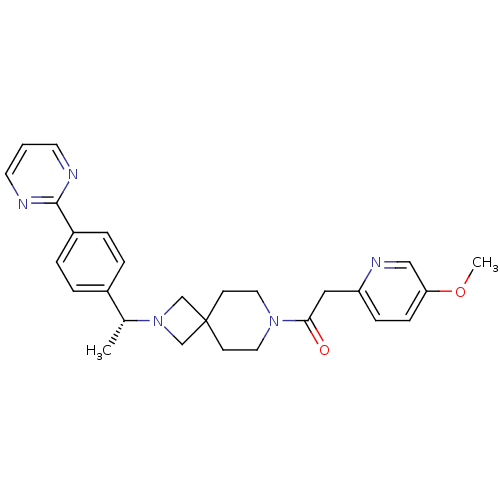 (CHEMBL2426670) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50005096 (CHEMBL2426673) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50440249 (CHEMBL2426689) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50440258 (CHEMBL2426670) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50440247 (CHEMBL2426666) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50440248 (CHEMBL2426667) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50440250 (CHEMBL2426688) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50005094 (CHEMBL2424680) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50005095 (CHEMBL2426672) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50440252 (CHEMBL2426686) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of N-methyl [3H]-scopolamine from recombinant human muscarinic acetylcholine receptor M2 expressed in CHO cells after 2 hrs by liquid sc... | Bioorg Med Chem Lett 23: 5410-4 (2013) Article DOI: 10.1016/j.bmcl.2013.07.044 BindingDB Entry DOI: 10.7270/Q25H7HP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 592 total ) | Next | Last >> |