 Found 3641 hits with Last Name = 'powers' and Initial = 'j'
Found 3641 hits with Last Name = 'powers' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50527134

(CHEMBL4471306 | US20230295213, Compound a)Show SMILES C[C@H](Nc1cc(Cl)nc2n(ncc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O)c1ccccc1F |r| Show InChI InChI=1S/C20H24ClFN4O9P2/c1-10(11-4-2-3-5-13(11)22)24-14-6-16(21)25-19-12(14)7-23-26(19)20-18(28)17(27)15(35-20)8-34-37(32,33)9-36(29,30)31/h2-7,10,15,17-18,20,27-28H,8-9H2,1H3,(H,24,25)(H,32,33)(H2,29,30,31)/t10-,15+,17+,18+,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive reversible inhibition of human C-terminal His6-tagged CD73 expressed in HEK293 cells using AMP as substrate preincubated with substrate f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00525
BindingDB Entry DOI: 10.7270/Q29W0K29 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50527134

(CHEMBL4471306 | US20230295213, Compound a)Show SMILES C[C@H](Nc1cc(Cl)nc2n(ncc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O)c1ccccc1F |r| Show InChI InChI=1S/C20H24ClFN4O9P2/c1-10(11-4-2-3-5-13(11)22)24-14-6-16(21)25-19-12(14)7-23-26(19)20-18(28)17(27)15(35-20)8-34-37(32,33)9-36(29,30)31/h2-7,10,15,17-18,20,27-28H,8-9H2,1H3,(H,24,25)(H,32,33)(H2,29,30,31)/t10-,15+,17+,18+,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arcus Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His-tagged CD73 (27 to 549 residues) expressed in HEK293 cells using AMP as substrate preincubated for 1 h... |
J Med Chem 63: 3935-3955 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01713
BindingDB Entry DOI: 10.7270/Q2G1648T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327601
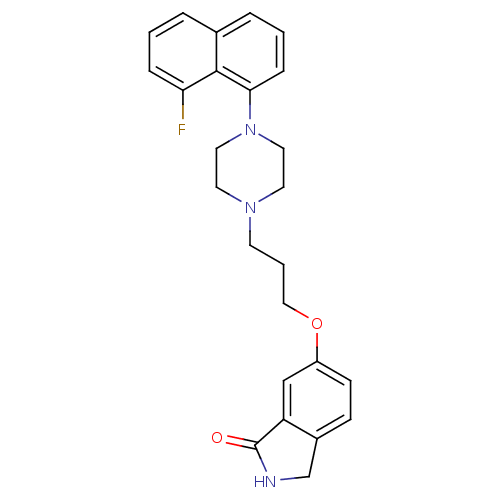
(6-(3-(4-(8-fluoronaphthalen-1-yl)piperazin-1-yl)pr...)Show SMILES Fc1cccc2cccc(N3CCN(CCCOc4ccc5CNC(=O)c5c4)CC3)c12 Show InChI InChI=1S/C25H26FN3O2/c26-22-6-1-4-18-5-2-7-23(24(18)22)29-13-11-28(12-14-29)10-3-15-31-20-9-8-19-17-27-25(30)21(19)16-20/h1-2,4-9,16H,3,10-15,17H2,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HeLa cells |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327602
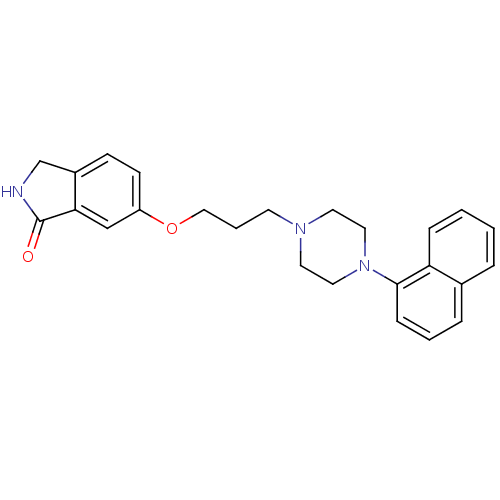
(6-(3-(4-(naphthalen-1-yl)piperazin-1-yl)propoxy)is...)Show SMILES O=C1NCc2ccc(OCCCN3CCN(CC3)c3cccc4ccccc34)cc12 Show InChI InChI=1S/C25H27N3O2/c29-25-23-17-21(10-9-20(23)18-26-25)30-16-4-11-27-12-14-28(15-13-27)24-8-3-6-19-5-1-2-7-22(19)24/h1-3,5-10,17H,4,11-16,18H2,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HeLa cells |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327603
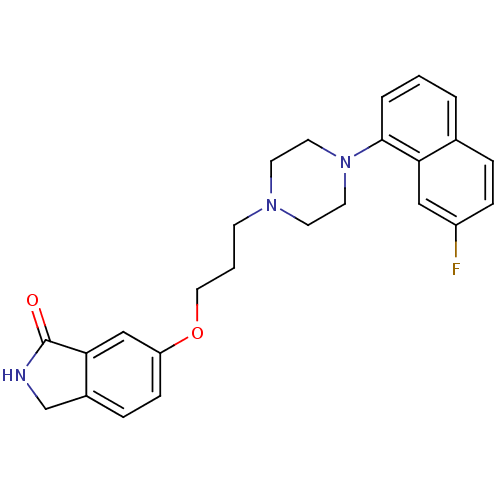
(6-(3-(4-(7-fluoronaphthalen-1-yl)piperazin-1-yl)pr...)Show SMILES Fc1ccc2cccc(N3CCN(CCCOc4ccc5CNC(=O)c5c4)CC3)c2c1 Show InChI InChI=1S/C25H26FN3O2/c26-20-7-5-18-3-1-4-24(22(18)15-20)29-12-10-28(11-13-29)9-2-14-31-21-8-6-19-17-27-25(30)23(19)16-21/h1,3-8,15-16H,2,9-14,17H2,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HeLa cells |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327604
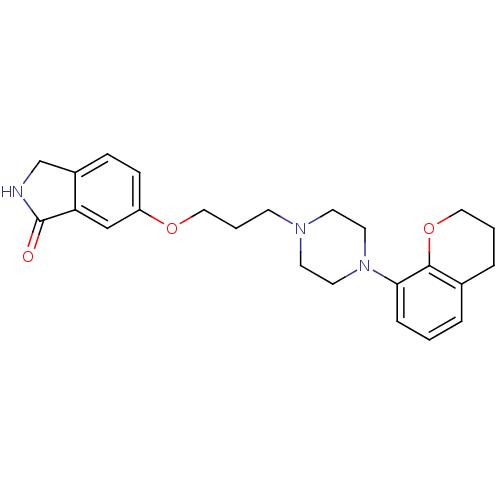
(6-(3-(4-(chroman-8-yl)piperazin-1-yl)propoxy)isoin...)Show SMILES O=C1NCc2ccc(OCCCN3CCN(CC3)c3cccc4CCCOc34)cc12 Show InChI InChI=1S/C24H29N3O3/c28-24-21-16-20(8-7-19(21)17-25-24)29-15-3-9-26-10-12-27(13-11-26)22-6-1-4-18-5-2-14-30-23(18)22/h1,4,6-8,16H,2-3,5,9-15,17H2,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor expressed in HeLa cells by scintillation proximity assay |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327605
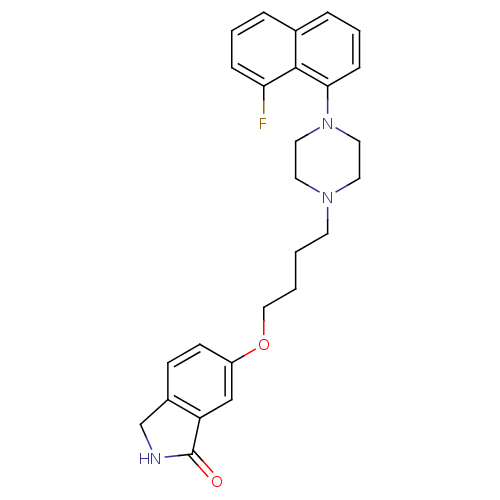
(6-(4-(4-(8-fluoronaphthalen-1-yl)piperazin-1-yl)bu...)Show SMILES Fc1cccc2cccc(N3CCN(CCCCOc4ccc5CNC(=O)c5c4)CC3)c12 Show InChI InChI=1S/C26H28FN3O2/c27-23-7-3-5-19-6-4-8-24(25(19)23)30-14-12-29(13-15-30)11-1-2-16-32-21-10-9-20-18-28-26(31)22(20)17-21/h3-10,17H,1-2,11-16,18H2,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0504 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HeLa cells |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50327606
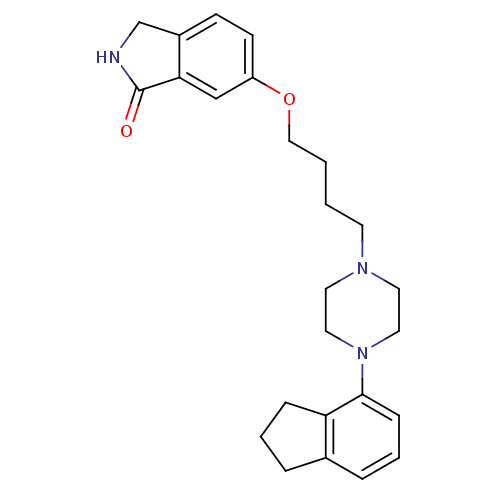
(6-(4-(4-(2,3-dihydro-1H-inden-4-yl)piperazin-1-yl)...)Show SMILES O=C1NCc2ccc(OCCCCN3CCN(CC3)c3cccc4CCCc34)cc12 Show InChI InChI=1S/C25H31N3O2/c29-25-23-17-21(10-9-20(23)18-26-25)30-16-2-1-11-27-12-14-28(15-13-27)24-8-4-6-19-5-3-7-22(19)24/h4,6,8-10,17H,1-3,5,7,11-16,18H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Spiperone from human dopamine D2L receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Bos taurus (bovine)) | BDBM50053851
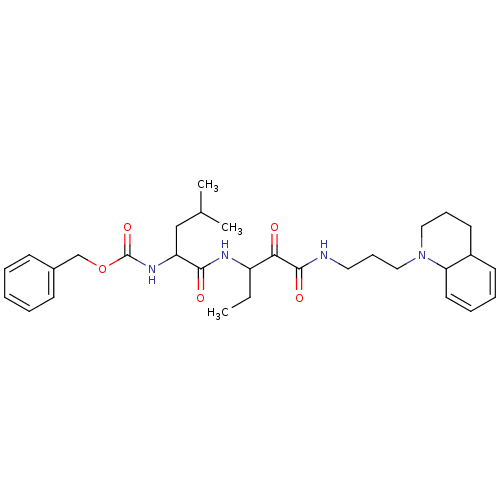
((3-Methyl-1-{1-[3-(3,4,4a,8a-tetrahydro-2H-quinoli...)Show SMILES CCC(NC(=O)C(CC(C)C)NC(=O)OCc1ccccc1)C(=O)C(=O)NCCCN1CCCC2C=CC=CC12 |c:36,38| Show InChI InChI=1S/C31H44N4O5/c1-4-25(28(36)30(38)32-17-11-19-35-18-10-15-24-14-8-9-16-27(24)35)33-29(37)26(20-22(2)3)34-31(39)40-21-23-12-6-5-7-13-23/h5-9,12-14,16,22,24-27H,4,10-11,15,17-21H2,1-3H3,(H,32,38)(H,33,37)(H,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of bovine cathepsin B |
J Med Chem 39: 4089-98 (1996)
Article DOI: 10.1021/jm950541c
BindingDB Entry DOI: 10.7270/Q2TX3DGR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327607
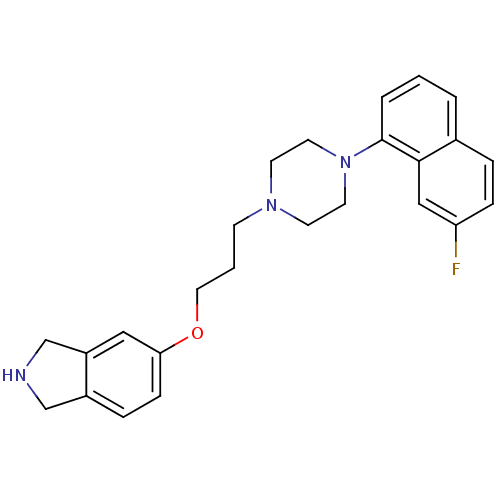
(5-(3-(4-(7-fluoronaphthalen-1-yl)piperazin-1-yl)pr...)Show SMILES Fc1ccc2cccc(N3CCN(CCCOc4ccc5CNCc5c4)CC3)c2c1 Show InChI InChI=1S/C25H28FN3O/c26-22-7-5-19-3-1-4-25(24(19)16-22)29-12-10-28(11-13-29)9-2-14-30-23-8-6-20-17-27-18-21(20)15-23/h1,3-8,15-16,27H,2,9-14,17-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HeLa cells |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327608
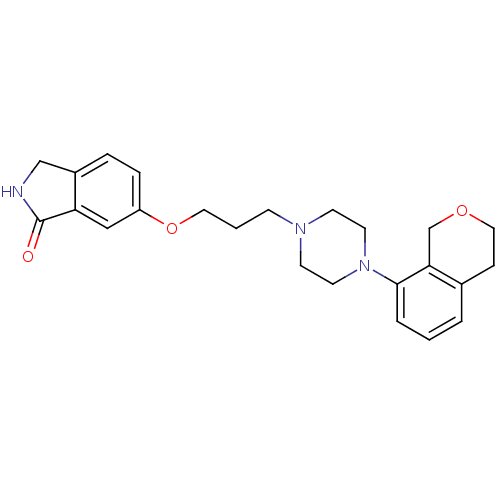
(6-(3-(4-(isochroman-8-yl)piperazin-1-yl)propoxy)is...)Show SMILES O=C1NCc2ccc(OCCCN3CCN(CC3)c3cccc4CCOCc34)cc12 Show InChI InChI=1S/C24H29N3O3/c28-24-21-15-20(6-5-19(21)16-25-24)30-13-2-8-26-9-11-27(12-10-26)23-4-1-3-18-7-14-29-17-22(18)23/h1,3-6,15H,2,7-14,16-17H2,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HeLa cells |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327593
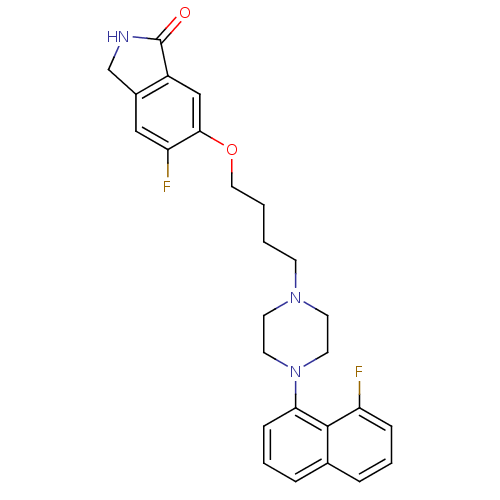
(5-fluoro-6-(4-(4-(8-fluoronaphthalen-1-yl)piperazi...)Show SMILES Fc1cc2CNC(=O)c2cc1OCCCCN1CCN(CC1)c1cccc2cccc(F)c12 Show InChI InChI=1S/C26H27F2N3O2/c27-21-7-3-5-18-6-4-8-23(25(18)21)31-12-10-30(11-13-31)9-1-2-14-33-24-16-20-19(15-22(24)28)17-29-26(20)32/h3-8,15-16H,1-2,9-14,17H2,(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor expressed in HeLa cells by scintillation proximity assay |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327609
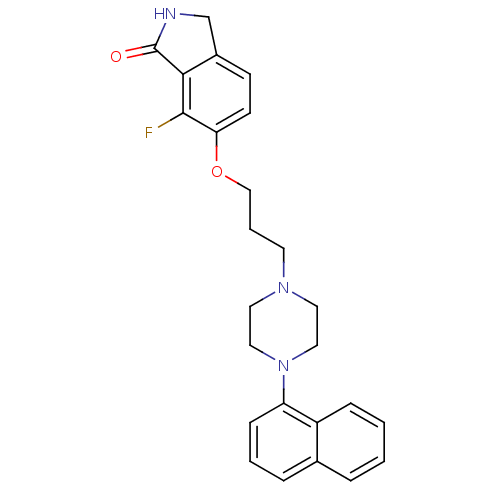
(7-fluoro-6-(3-(4-(naphthalen-1-yl)piperazin-1-yl)p...)Show SMILES Fc1c2C(=O)NCc2ccc1OCCCN1CCN(CC1)c1cccc2ccccc12 Show InChI InChI=1S/C25H26FN3O2/c26-24-22(10-9-19-17-27-25(30)23(19)24)31-16-4-11-28-12-14-29(15-13-28)21-8-3-6-18-5-1-2-7-20(18)21/h1-3,5-10H,4,11-17H2,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor expressed in HeLa cells by scintillation proximity assay |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Bos taurus (bovine)) | BDBM50053800
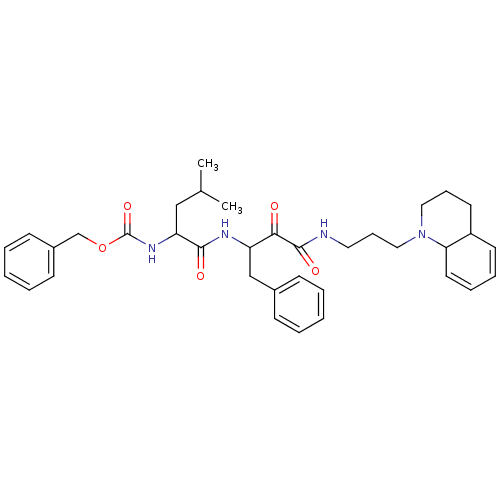
((1-{1-Benzyl-2-oxo-2-[3-(3,4,4a,8a-tetrahydro-2H-q...)Show SMILES CC(C)CC(NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C(=O)C(=O)NCCCN1CCCC2C=CC=CC12 |c:42,44| Show InChI InChI=1S/C36H46N4O5/c1-26(2)23-31(39-36(44)45-25-28-15-7-4-8-16-28)34(42)38-30(24-27-13-5-3-6-14-27)33(41)35(43)37-20-12-22-40-21-11-18-29-17-9-10-19-32(29)40/h3-10,13-17,19,26,29-32H,11-12,18,20-25H2,1-2H3,(H,37,43)(H,38,42)(H,39,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of bovine cathepsin B |
J Med Chem 39: 4089-98 (1996)
Article DOI: 10.1021/jm950541c
BindingDB Entry DOI: 10.7270/Q2TX3DGR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327588
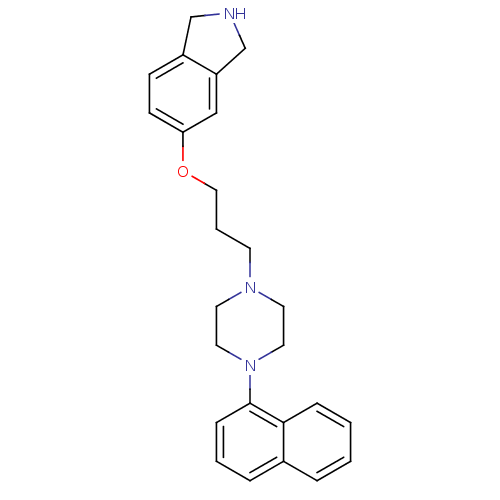
(5-(3-(4-(naphthalen-1-yl)piperazin-1-yl)propoxy)is...)Show SMILES C(COc1ccc2CNCc2c1)CN1CCN(CC1)c1cccc2ccccc12 Show InChI InChI=1S/C25H29N3O/c1-2-7-24-20(5-1)6-3-8-25(24)28-14-12-27(13-15-28)11-4-16-29-23-10-9-21-18-26-19-22(21)17-23/h1-3,5-10,17,26H,4,11-16,18-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HeLa cells |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327582
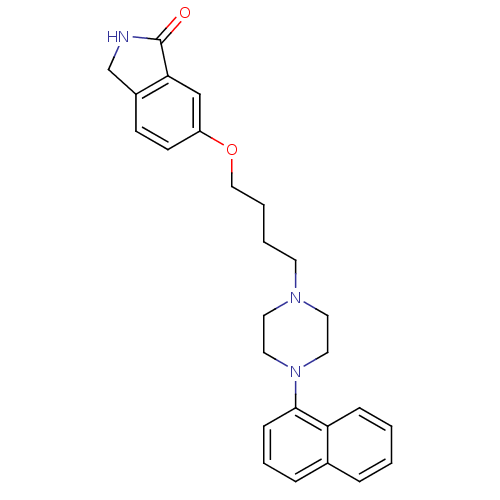
(6-(4-(4-(naphthalen-1-yl)piperazin-1-yl)butoxy)iso...)Show SMILES O=C1NCc2ccc(OCCCCN3CCN(CC3)c3cccc4ccccc34)cc12 Show InChI InChI=1S/C26H29N3O2/c30-26-24-18-22(11-10-21(24)19-27-26)31-17-4-3-12-28-13-15-29(16-14-28)25-9-5-7-20-6-1-2-8-23(20)25/h1-2,5-11,18H,3-4,12-17,19H2,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HeLa cells |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327610
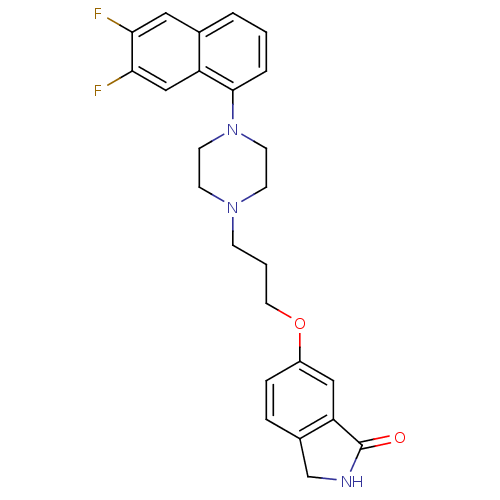
(6-(3-(4-(6,7-difluoronaphthalen-1-yl)piperazin-1-y...)Show SMILES Fc1cc2cccc(N3CCN(CCCOc4ccc5CNC(=O)c5c4)CC3)c2cc1F Show InChI InChI=1S/C25H25F2N3O2/c26-22-13-17-3-1-4-24(20(17)15-23(22)27)30-10-8-29(9-11-30)7-2-12-32-19-6-5-18-16-28-25(31)21(18)14-19/h1,3-6,13-15H,2,7-12,16H2,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.179 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HeLa cells |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
Calpain-2 catalytic subunit
(Homo sapiens (Human)) | BDBM50053865
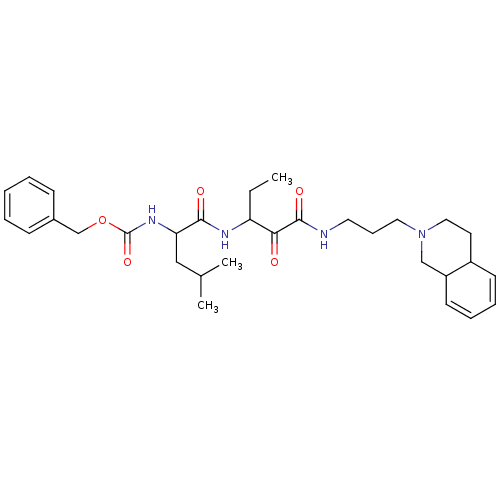
((3-Methyl-1-{1-[3-(3,4,4a,8a-tetrahydro-1H-isoquin...)Show SMILES CCC(NC(=O)C(CC(C)C)NC(=O)OCc1ccccc1)C(=O)C(=O)NCCCN1CCC2C=CC=CC2C1 |c:35,37| Show InChI InChI=1S/C31H44N4O5/c1-4-26(28(36)30(38)32-16-10-17-35-18-15-24-13-8-9-14-25(24)20-35)33-29(37)27(19-22(2)3)34-31(39)40-21-23-11-6-5-7-12-23/h5-9,11-14,22,24-27H,4,10,15-21H2,1-3H3,(H,32,38)(H,33,37)(H,34,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Cysteine protease Calpain 2 |
J Med Chem 39: 4089-98 (1996)
Article DOI: 10.1021/jm950541c
BindingDB Entry DOI: 10.7270/Q2TX3DGR |
More data for this
Ligand-Target Pair | |
Calpain-2 catalytic subunit
(Homo sapiens (Human)) | BDBM50053813
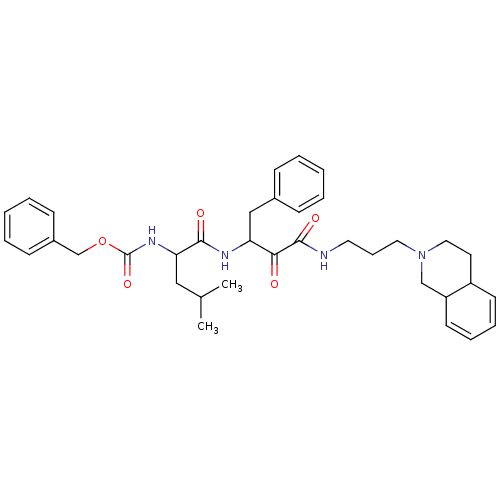
((1-{1-Benzyl-2-oxo-2-[3-(3,4,4a,8a-tetrahydro-1H-i...)Show SMILES CC(C)CC(NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C(=O)C(=O)NCCCN1CCC2C=CC=CC2C1 |c:41,43| Show InChI InChI=1S/C36H46N4O5/c1-26(2)22-32(39-36(44)45-25-28-14-7-4-8-15-28)34(42)38-31(23-27-12-5-3-6-13-27)33(41)35(43)37-19-11-20-40-21-18-29-16-9-10-17-30(29)24-40/h3-10,12-17,26,29-32H,11,18-25H2,1-2H3,(H,37,43)(H,38,42)(H,39,44) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Cysteine protease Calpain 2 |
J Med Chem 39: 4089-98 (1996)
Article DOI: 10.1021/jm950541c
BindingDB Entry DOI: 10.7270/Q2TX3DGR |
More data for this
Ligand-Target Pair | |
Calpain-2 catalytic subunit
(Homo sapiens (Human)) | BDBM50053851

((3-Methyl-1-{1-[3-(3,4,4a,8a-tetrahydro-2H-quinoli...)Show SMILES CCC(NC(=O)C(CC(C)C)NC(=O)OCc1ccccc1)C(=O)C(=O)NCCCN1CCCC2C=CC=CC12 |c:36,38| Show InChI InChI=1S/C31H44N4O5/c1-4-25(28(36)30(38)32-17-11-19-35-18-10-15-24-14-8-9-16-27(24)35)33-29(37)26(20-22(2)3)34-31(39)40-21-23-12-6-5-7-13-23/h5-9,12-14,16,22,24-27H,4,10-11,15,17-21H2,1-3H3,(H,32,38)(H,33,37)(H,34,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Cysteine protease Calpain 2 |
J Med Chem 39: 4089-98 (1996)
Article DOI: 10.1021/jm950541c
BindingDB Entry DOI: 10.7270/Q2TX3DGR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327573
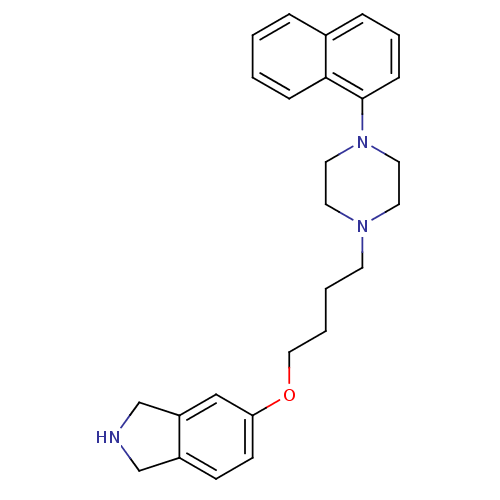
(5-(4-(4-(naphthalen-1-yl)piperazin-1-yl)butoxy)iso...)Show SMILES C(CCN1CCN(CC1)c1cccc2ccccc12)COc1ccc2CNCc2c1 Show InChI InChI=1S/C26H31N3O/c1-2-8-25-21(6-1)7-5-9-26(25)29-15-13-28(14-16-29)12-3-4-17-30-24-11-10-22-19-27-20-23(22)18-24/h1-2,5-11,18,27H,3-4,12-17,19-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HeLa cells |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50053813

((1-{1-Benzyl-2-oxo-2-[3-(3,4,4a,8a-tetrahydro-1H-i...)Show SMILES CC(C)CC(NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C(=O)C(=O)NCCCN1CCC2C=CC=CC2C1 |c:41,43| Show InChI InChI=1S/C36H46N4O5/c1-26(2)22-32(39-36(44)45-25-28-14-7-4-8-15-28)34(42)38-31(23-27-12-5-3-6-13-27)33(41)35(43)37-19-11-20-40-21-18-29-16-9-10-17-30(29)24-40/h3-10,12-17,26,29-32H,11,18-25H2,1-2H3,(H,37,43)(H,38,42)(H,39,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of the cysteine protease human Calpain 1 |
J Med Chem 39: 4089-98 (1996)
Article DOI: 10.1021/jm950541c
BindingDB Entry DOI: 10.7270/Q2TX3DGR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327611

(5-(4-(4-(7-fluoronaphthalen-1-yl)piperazin-1-yl)bu...)Show SMILES Fc1ccc2cccc(N3CCN(CCCCOc4ccc5CNCc5c4)CC3)c2c1 Show InChI InChI=1S/C26H30FN3O/c27-23-8-6-20-4-3-5-26(25(20)17-23)30-13-11-29(12-14-30)10-1-2-15-31-24-9-7-21-18-28-19-22(21)16-24/h3-9,16-17,28H,1-2,10-15,18-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HeLa cells |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
Calpain-2 catalytic subunit
(Homo sapiens (Human)) | BDBM50053800

((1-{1-Benzyl-2-oxo-2-[3-(3,4,4a,8a-tetrahydro-2H-q...)Show SMILES CC(C)CC(NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C(=O)C(=O)NCCCN1CCCC2C=CC=CC12 |c:42,44| Show InChI InChI=1S/C36H46N4O5/c1-26(2)23-31(39-36(44)45-25-28-15-7-4-8-16-28)34(42)38-30(24-27-13-5-3-6-14-27)33(41)35(43)37-20-12-22-40-21-11-18-29-17-9-10-19-32(29)40/h3-10,13-17,19,26,29-32H,11-12,18,20-25H2,1-2H3,(H,37,43)(H,38,42)(H,39,44) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Cysteine protease Calpain 2 |
J Med Chem 39: 4089-98 (1996)
Article DOI: 10.1021/jm950541c
BindingDB Entry DOI: 10.7270/Q2TX3DGR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327612
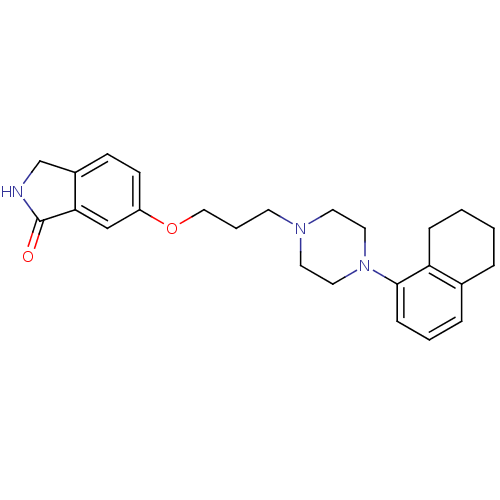
(6-(3-(4-(5,6,7,8-tetrahydronaphthalen-1-yl)piperaz...)Show SMILES O=C1NCc2ccc(OCCCN3CCN(CC3)c3cccc4CCCCc34)cc12 Show InChI InChI=1S/C25H31N3O2/c29-25-23-17-21(10-9-20(23)18-26-25)30-16-4-11-27-12-14-28(15-13-27)24-8-3-6-19-5-1-2-7-22(19)24/h3,6,8-10,17H,1-2,4-5,7,11-16,18H2,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.269 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor expressed in HeLa cells by scintillation proximity assay |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327575
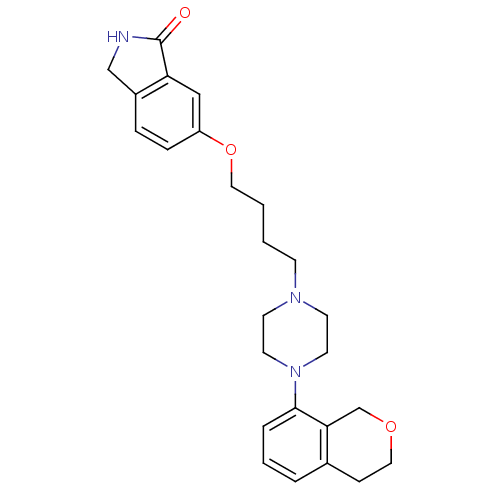
(6-(4-(4-(isochroman-8-yl)piperazin-1-yl)butoxy)iso...)Show SMILES O=C1NCc2ccc(OCCCCN3CCN(CC3)c3cccc4CCOCc34)cc12 Show InChI InChI=1S/C25H31N3O3/c29-25-22-16-21(7-6-20(22)17-26-25)31-14-2-1-9-27-10-12-28(13-11-27)24-5-3-4-19-8-15-30-18-23(19)24/h3-7,16H,1-2,8-15,17-18H2,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.279 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in HeLa cells |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50327613
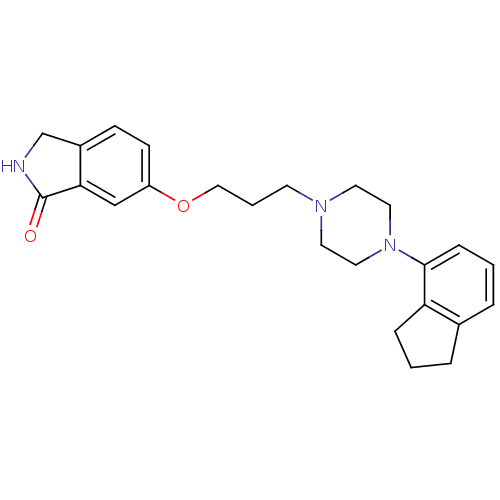
(6-(3-(4-(2,3-dihydro-1H-inden-4-yl)piperazin-1-yl)...)Show SMILES O=C1NCc2ccc(OCCCN3CCN(CC3)c3cccc4CCCc34)cc12 Show InChI InChI=1S/C24H29N3O2/c28-24-22-16-20(9-8-19(22)17-25-24)29-15-3-10-26-11-13-27(14-12-26)23-7-2-5-18-4-1-6-21(18)23/h2,5,7-9,16H,1,3-4,6,10-15,17H2,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Spiperone from human dopamine D2L receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50053865

((3-Methyl-1-{1-[3-(3,4,4a,8a-tetrahydro-1H-isoquin...)Show SMILES CCC(NC(=O)C(CC(C)C)NC(=O)OCc1ccccc1)C(=O)C(=O)NCCCN1CCC2C=CC=CC2C1 |c:35,37| Show InChI InChI=1S/C31H44N4O5/c1-4-26(28(36)30(38)32-16-10-17-35-18-15-24-13-8-9-14-25(24)20-35)33-29(37)27(19-22(2)3)34-31(39)40-21-23-11-6-5-7-12-23/h5-9,11-14,22,24-27H,4,10,15-21H2,1-3H3,(H,32,38)(H,33,37)(H,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of the cysteine protease human Calpain 1 |
J Med Chem 39: 4089-98 (1996)
Article DOI: 10.1021/jm950541c
BindingDB Entry DOI: 10.7270/Q2TX3DGR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327587
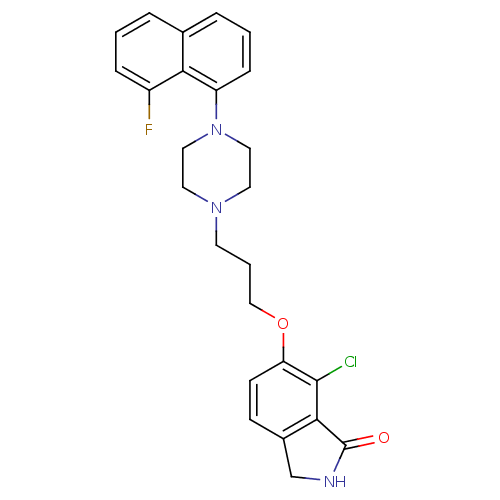
(7-chloro-6-(3-(4-(8-fluoronaphthalen-1-yl)piperazi...)Show SMILES Fc1cccc2cccc(N3CCN(CCCOc4ccc5CNC(=O)c5c4Cl)CC3)c12 Show InChI InChI=1S/C25H25ClFN3O2/c26-24-21(9-8-18-16-28-25(31)23(18)24)32-15-3-10-29-11-13-30(14-12-29)20-7-2-5-17-4-1-6-19(27)22(17)20/h1-2,4-9H,3,10-16H2,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.312 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor expressed in HeLa cells by scintillation proximity assay |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499195

(CHEMBL3735504)Show SMILES CC(C)(C)C(=O)Nc1ccc2n([C@@H]3CC[C@H](CO)CC3)c(NC(=O)c3cccc(c3)C(F)(F)F)nc2c1 |r,wD:12.11,15.15,(-7.45,.88,;-6.39,1.5,;-7.46,2.11,;-6.4,2.74,;-5.05,.74,;-5.04,-.49,;-3.72,1.53,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.24,-2.7,;3.72,-3.12,;4.09,-4.62,;2.98,-5.68,;3.36,-7.18,;4.54,-7.52,;1.5,-5.26,;1.13,-3.76,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;9,-2.3,;10.23,-2.29,;1.76,1.24,;.3,.77,;-1.03,1.55,)| Show InChI InChI=1S/C27H31F3N4O3/c1-26(2,3)24(37)31-19-9-12-22-21(14-19)32-25(34(22)20-10-7-16(15-35)8-11-20)33-23(36)17-5-4-6-18(13-17)27(28,29)30/h4-6,9,12-14,16,20,35H,7-8,10-11,15H2,1-3H3,(H,31,37)(H,32,33,36)/t16-,20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327578

(5-fluoro-6-(3-(4-(7-fluoronaphthalen-1-yl)piperazi...)Show SMILES Fc1ccc2cccc(N3CCN(CCCOc4cc5C(=O)NCc5cc4F)CC3)c2c1 Show InChI InChI=1S/C25H25F2N3O2/c26-19-6-5-17-3-1-4-23(20(17)14-19)30-10-8-29(9-11-30)7-2-12-32-24-15-21-18(13-22(24)27)16-28-25(21)31/h1,3-6,13-15H,2,7-12,16H2,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.341 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor expressed in HeLa cells by scintillation proximity assay |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50053851

((3-Methyl-1-{1-[3-(3,4,4a,8a-tetrahydro-2H-quinoli...)Show SMILES CCC(NC(=O)C(CC(C)C)NC(=O)OCc1ccccc1)C(=O)C(=O)NCCCN1CCCC2C=CC=CC12 |c:36,38| Show InChI InChI=1S/C31H44N4O5/c1-4-25(28(36)30(38)32-17-11-19-35-18-10-15-24-14-8-9-16-27(24)35)33-29(37)26(20-22(2)3)34-31(39)40-21-23-12-6-5-7-13-23/h5-9,12-14,16,22,24-27H,4,10-11,15,17-21H2,1-3H3,(H,32,38)(H,33,37)(H,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of the cysteine protease human Calpain 1 |
J Med Chem 39: 4089-98 (1996)
Article DOI: 10.1021/jm950541c
BindingDB Entry DOI: 10.7270/Q2TX3DGR |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50053800

((1-{1-Benzyl-2-oxo-2-[3-(3,4,4a,8a-tetrahydro-2H-q...)Show SMILES CC(C)CC(NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C(=O)C(=O)NCCCN1CCCC2C=CC=CC12 |c:42,44| Show InChI InChI=1S/C36H46N4O5/c1-26(2)23-31(39-36(44)45-25-28-15-7-4-8-16-28)34(42)38-30(24-27-13-5-3-6-14-27)33(41)35(43)37-20-12-22-40-21-11-18-29-17-9-10-19-32(29)40/h3-10,13-17,19,26,29-32H,11-12,18,20-25H2,1-2H3,(H,37,43)(H,38,42)(H,39,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of the cysteine protease human Calpain 1 |
J Med Chem 39: 4089-98 (1996)
Article DOI: 10.1021/jm950541c
BindingDB Entry DOI: 10.7270/Q2TX3DGR |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499203
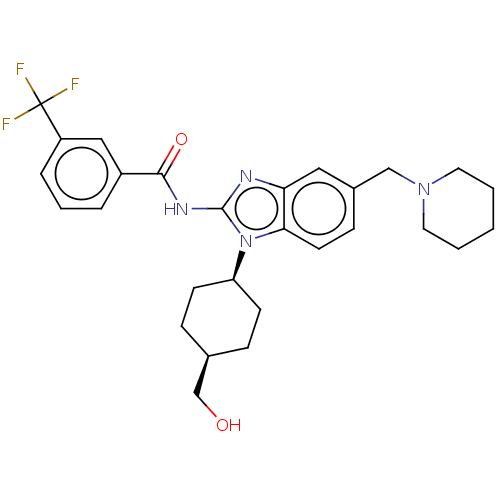
(CHEMBL3736036)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cc(CN3CCCCC3)ccc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;9,-2.3,;10.23,-2.29,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C28H33F3N4O2/c29-28(30,31)22-6-4-5-21(16-22)26(37)33-27-32-24-15-20(17-34-13-2-1-3-14-34)9-12-25(24)35(27)23-10-7-19(18-36)8-11-23/h4-6,9,12,15-16,19,23,36H,1-3,7-8,10-11,13-14,17-18H2,(H,32,33,37)/t19-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499205
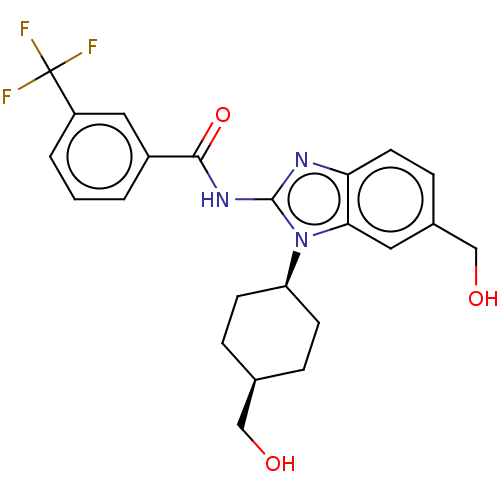
(CHEMBL3734814)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2ccc(CO)cc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.81,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;9,-2.3,;10.23,-2.29,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-3.72,-1.53,;-3.72,-2.76,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C23H24F3N3O3/c24-23(25,26)17-3-1-2-16(11-17)21(32)28-22-27-19-9-6-15(13-31)10-20(19)29(22)18-7-4-14(12-30)5-8-18/h1-3,6,9-11,14,18,30-31H,4-5,7-8,12-13H2,(H,27,28,32)/t14-,18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50327611

(5-(4-(4-(7-fluoronaphthalen-1-yl)piperazin-1-yl)bu...)Show SMILES Fc1ccc2cccc(N3CCN(CCCCOc4ccc5CNCc5c4)CC3)c2c1 Show InChI InChI=1S/C26H30FN3O/c27-23-8-6-20-4-3-5-26(25(20)17-23)30-13-11-29(12-14-30)10-1-2-15-31-24-9-7-21-18-28-19-22(21)16-24/h3-9,16-17,28H,1-2,10-15,18-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.483 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT2A receptor |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499208
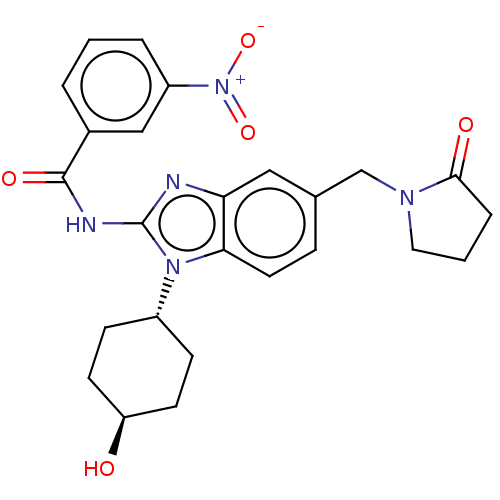
(CHEMBL3734872)Show SMILES O[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCC3=O)ccc12 |r,wU:4.7,wD:1.0,(3.28,-6.88,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-5.17,-.78,;-6.68,-1.11,;-7.46,.21,;-6.44,1.37,;-6.7,2.57,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C25H27N5O5/c31-20-9-7-18(8-10-20)29-22-11-6-16(15-28-12-2-5-23(28)32)13-21(22)26-25(29)27-24(33)17-3-1-4-19(14-17)30(34)35/h1,3-4,6,11,13-14,18,20,31H,2,5,7-10,12,15H2,(H,26,27,33)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
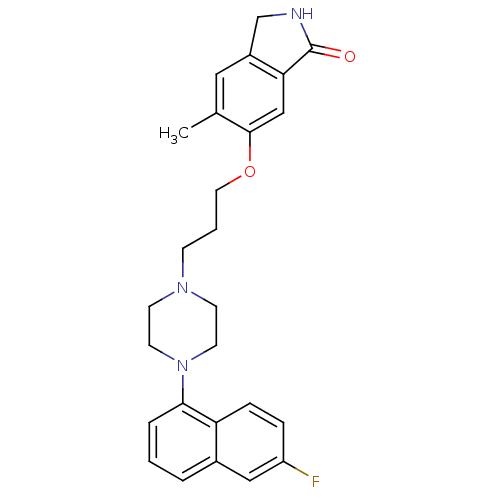
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327595
(6-(3-(4-(6-fluoronaphthalen-1-yl)piperazin-1-yl)pr...)Show SMILES Cc1cc2CNC(=O)c2cc1OCCCN1CCN(CC1)c1cccc2cc(F)ccc12 Show InChI InChI=1S/C26H28FN3O2/c1-18-14-20-17-28-26(31)23(20)16-25(18)32-13-3-8-29-9-11-30(12-10-29)24-5-2-4-19-15-21(27)6-7-22(19)24/h2,4-7,14-16H,3,8-13,17H2,1H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.517 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor expressed in HeLa cells by scintillation proximity assay |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327602

(6-(3-(4-(naphthalen-1-yl)piperazin-1-yl)propoxy)is...)Show SMILES O=C1NCc2ccc(OCCCN3CCN(CC3)c3cccc4ccccc34)cc12 Show InChI InChI=1S/C25H27N3O2/c29-25-23-17-21(10-9-20(23)18-26-25)30-16-4-11-27-12-14-28(15-13-27)24-8-3-6-19-5-1-2-7-22(19)24/h1-3,5-10,17H,4,11-16,18H2,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.541 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor expressed in HeLa cells by scintillation proximity assay |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50327590
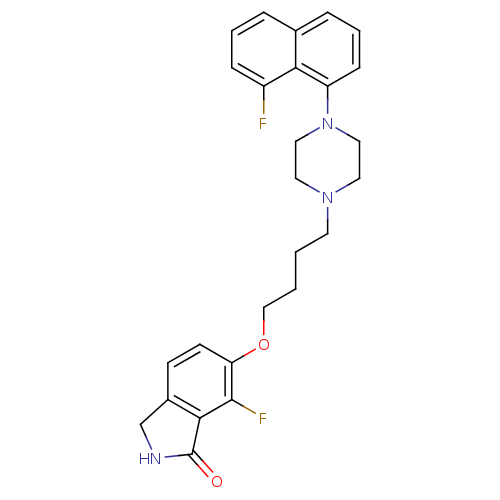
(7-fluoro-6-(4-(4-(8-fluoronaphthalen-1-yl)piperazi...)Show SMILES Fc1cccc2cccc(N3CCN(CCCCOc4ccc5CNC(=O)c5c4F)CC3)c12 Show InChI InChI=1S/C26H27F2N3O2/c27-20-7-3-5-18-6-4-8-21(23(18)20)31-14-12-30(13-15-31)11-1-2-16-33-22-10-9-19-17-29-26(32)24(19)25(22)28/h3-10H,1-2,11-17H2,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.568 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT2A receptor |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50327581
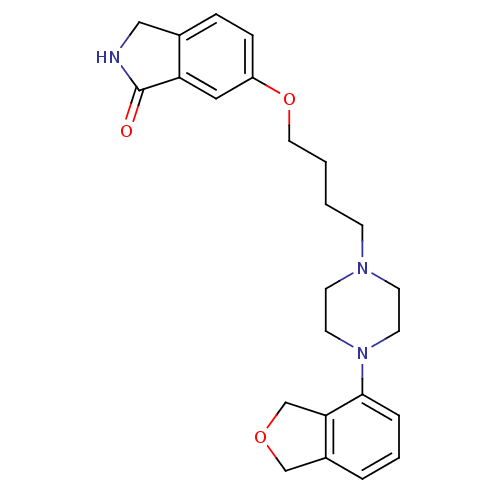
(6-(4-(4-(1,3-dihydroisobenzofuran-4-yl)piperazin-1...)Show SMILES O=C1NCc2ccc(OCCCCN3CCN(CC3)c3cccc4COCc34)cc12 Show InChI InChI=1S/C24H29N3O3/c28-24-21-14-20(7-6-18(21)15-25-24)30-13-2-1-8-26-9-11-27(12-10-26)23-5-3-4-19-16-29-17-22(19)23/h3-7,14H,1-2,8-13,15-17H2,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Spiperone from human dopamine D2L receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50327614
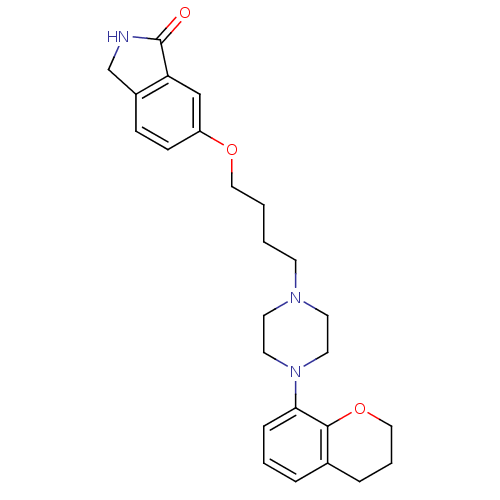
(6-(4-(4-(chroman-8-yl)piperazin-1-yl)butoxy)isoind...)Show SMILES O=C1NCc2ccc(OCCCCN3CCN(CC3)c3cccc4CCCOc34)cc12 Show InChI InChI=1S/C25H31N3O3/c29-25-22-17-21(9-8-20(22)18-26-25)30-15-2-1-10-27-11-13-28(14-12-27)23-7-3-5-19-6-4-16-31-24(19)23/h3,5,7-9,17H,1-2,4,6,10-16,18H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.576 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Spiperone from human dopamine D2L receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327580
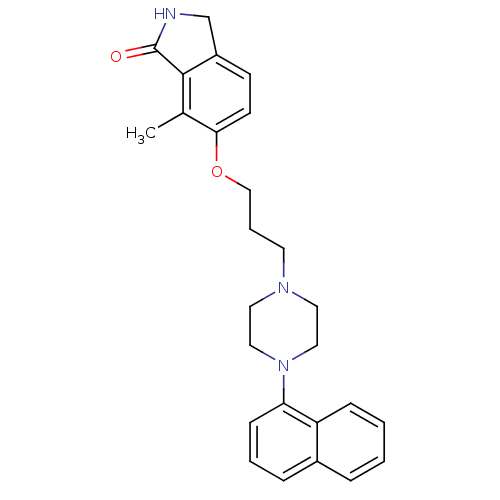
(7-methyl-6-(3-(4-(naphthalen-1-yl)piperazin-1-yl)p...)Show SMILES Cc1c2C(=O)NCc2ccc1OCCCN1CCN(CC1)c1cccc2ccccc12 Show InChI InChI=1S/C26H29N3O2/c1-19-24(11-10-21-18-27-26(30)25(19)21)31-17-5-12-28-13-15-29(16-14-28)23-9-4-7-20-6-2-3-8-22(20)23/h2-4,6-11H,5,12-18H2,1H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.615 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor expressed in HeLa cells by scintillation proximity assay |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327615
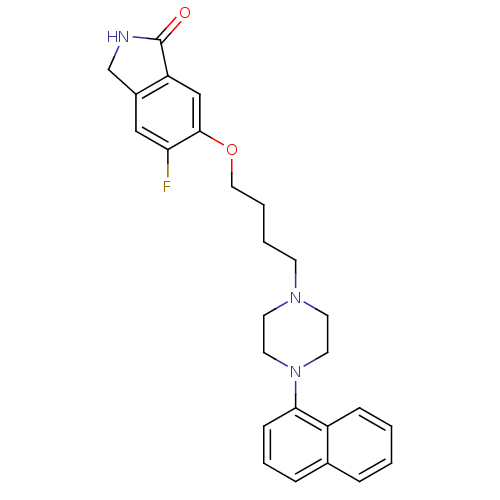
(5-fluoro-6-(4-(4-(naphthalen-1-yl)piperazin-1-yl)b...)Show SMILES Fc1cc2CNC(=O)c2cc1OCCCCN1CCN(CC1)c1cccc2ccccc12 Show InChI InChI=1S/C26H28FN3O2/c27-23-16-20-18-28-26(31)22(20)17-25(23)32-15-4-3-10-29-11-13-30(14-12-29)24-9-5-7-19-6-1-2-8-21(19)24/h1-2,5-9,16-17H,3-4,10-15,18H2,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor expressed in HeLa cells by scintillation proximity assay |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327598

(5-methyl-6-(3-(4-(naphthalen-1-yl)piperazin-1-yl)p...)Show SMILES Cc1cc2CNC(=O)c2cc1OCCCN1CCN(CC1)c1cccc2ccccc12 Show InChI InChI=1S/C26H29N3O2/c1-19-16-21-18-27-26(30)23(21)17-25(19)31-15-5-10-28-11-13-29(14-12-28)24-9-4-7-20-6-2-3-8-22(20)24/h2-4,6-9,16-17H,5,10-15,18H2,1H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor expressed in HeLa cells by scintillation proximity assay |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327586
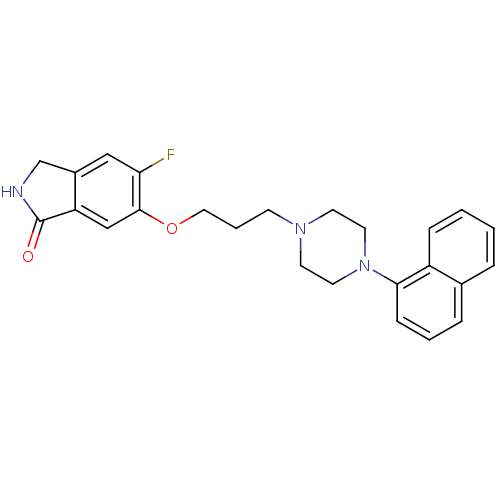
(5-fluoro-6-(3-(4-(naphthalen-1-yl)piperazin-1-yl)p...)Show SMILES Fc1cc2CNC(=O)c2cc1OCCCN1CCN(CC1)c1cccc2ccccc12 Show InChI InChI=1S/C25H26FN3O2/c26-22-15-19-17-27-25(30)21(19)16-24(22)31-14-4-9-28-10-12-29(13-11-28)23-8-3-6-18-5-1-2-7-20(18)23/h1-3,5-8,15-16H,4,9-14,17H2,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.671 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor expressed in HeLa cells by scintillation proximity assay |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499194

(CHEMBL3734854)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCCC3)ccc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C27H33N5O4/c33-18-19-7-10-22(11-8-19)31-25-12-9-20(17-30-13-2-1-3-14-30)15-24(25)28-27(31)29-26(34)21-5-4-6-23(16-21)32(35)36/h4-6,9,12,15-16,19,22,33H,1-3,7-8,10-11,13-14,17-18H2,(H,28,29,34)/t19-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602541
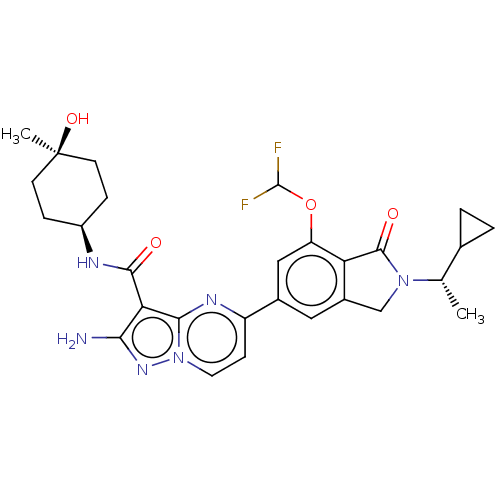
(CHEMBL5209268)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(OC(F)F)c2C1=O)-c1ccn2nc(N)c(C(=O)N[C@H]3CC[C@@](C)(O)CC3)c2n1 |r,wU:30.32,1.1,33.37,(7.66,2.89,;6.89,1.55,;7.66,.22,;7.66,-1.32,;8.99,-.55,;5.35,1.55,;4.44,2.8,;2.98,2.32,;1.65,3.09,;.31,2.32,;.31,.78,;1.65,.01,;1.65,-1.53,;.32,-2.3,;.32,-3.84,;-1.02,-1.53,;2.98,.78,;4.44,.31,;4.84,-1.18,;-1.02,3.09,;-1.02,4.63,;-2.34,5.39,;-3.67,4.63,;-5.14,5.1,;-6.04,3.86,;-7.58,3.86,;-5.14,2.61,;-5.91,1.28,;-7.45,1.28,;-5.14,-.06,;-5.91,-1.39,;-5.14,-2.72,;-5.91,-4.06,;-7.45,-4.06,;-8.22,-5.39,;-8.99,-4.06,;-8.22,-2.72,;-7.45,-1.39,;-3.67,3.09,;-2.34,2.33,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50327607

(5-(3-(4-(7-fluoronaphthalen-1-yl)piperazin-1-yl)pr...)Show SMILES Fc1ccc2cccc(N3CCN(CCCOc4ccc5CNCc5c4)CC3)c2c1 Show InChI InChI=1S/C25H28FN3O/c26-22-7-5-19-3-1-4-25(24(19)16-22)29-12-10-28(11-13-29)9-2-14-30-23-8-6-20-17-27-18-21(20)15-23/h1,3-8,15-16,27H,2,9-14,17-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT2A receptor |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50327606

(6-(4-(4-(2,3-dihydro-1H-inden-4-yl)piperazin-1-yl)...)Show SMILES O=C1NCc2ccc(OCCCCN3CCN(CC3)c3cccc4CCCc34)cc12 Show InChI InChI=1S/C25H31N3O2/c29-25-23-17-21(10-9-20(23)18-26-25)30-16-2-1-11-27-12-14-28(15-13-27)24-8-4-6-19-5-3-7-22(19)24/h4,6,8-10,17H,1-3,5,7,11-16,18H2,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor expressed in HeLa cells by scintillation proximity assay |
Bioorg Med Chem Lett 20: 5666-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.023
BindingDB Entry DOI: 10.7270/Q2HH6K9S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data