 Found 1583 hits with Last Name = 'han' and Initial = 'jw'
Found 1583 hits with Last Name = 'han' and Initial = 'jw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213665
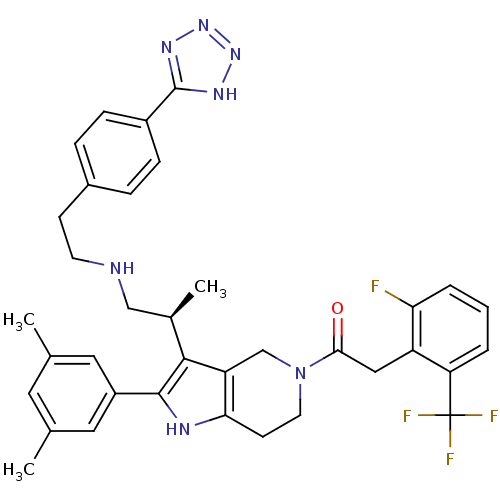
((S)-1-(3-(1-(4-(1H-tetrazol-5-yl)phenethylamino)pr...)Show SMILES C[C@H](CNCCc1ccc(cc1)-c1nnn[nH]1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1c(F)cccc1C(F)(F)F |r| Show InChI InChI=1S/C36H37F4N7O/c1-21-15-22(2)17-26(16-21)34-33(23(3)19-41-13-11-24-7-9-25(10-8-24)35-43-45-46-44-35)28-20-47(14-12-31(28)42-34)32(48)18-27-29(36(38,39)40)5-4-6-30(27)37/h4-10,15-17,23,41-42H,11-14,18-20H2,1-3H3,(H,43,44,45,46)/t23-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213671
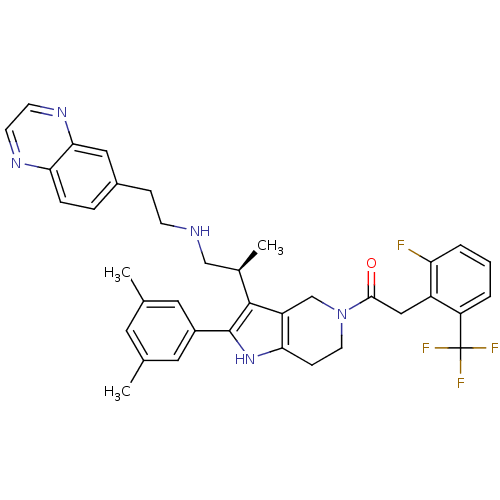
((S)-1-(2-(3,5-dimethylphenyl)-3-(1-(2-(quinoxalin-...)Show SMILES C[C@H](CNCCc1ccc2nccnc2c1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1c(F)cccc1C(F)(F)F Show InChI InChI=1S/C37H37F4N5O/c1-22-15-23(2)17-26(16-22)36-35(24(3)20-42-11-9-25-7-8-32-33(18-25)44-13-12-43-32)28-21-46(14-10-31(28)45-36)34(47)19-27-29(37(39,40)41)5-4-6-30(27)38/h4-8,12-13,15-18,24,42,45H,9-11,14,19-21H2,1-3H3/t24-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213664
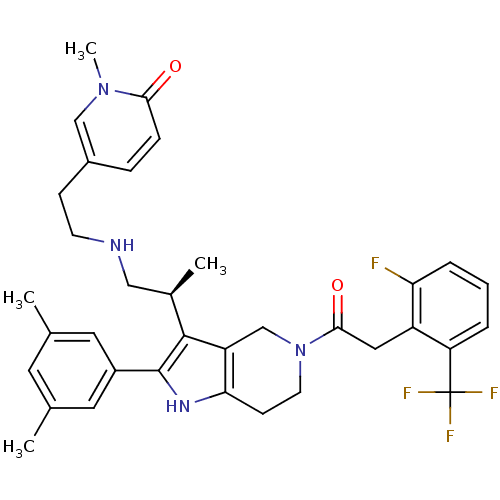
((S)-5-(2-(2-(2-(3,5-dimethylphenyl)-5-(2-(2-fluoro...)Show SMILES C[C@H](CNCCc1ccc(=O)n(C)c1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1c(F)cccc1C(F)(F)F Show InChI InChI=1S/C35H38F4N4O2/c1-21-14-22(2)16-25(15-21)34-33(23(3)18-40-12-10-24-8-9-31(44)42(4)19-24)27-20-43(13-11-30(27)41-34)32(45)17-26-28(35(37,38)39)6-5-7-29(26)36/h5-9,14-16,19,23,40-41H,10-13,17-18,20H2,1-4H3/t23-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213659
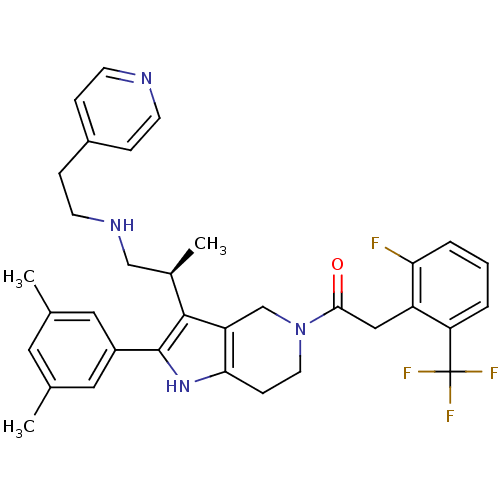
((S)-1-(2-(3,5-dimethylphenyl)-3-(1-(2-(pyridin-4-y...)Show SMILES C[C@H](CNCCc1ccncc1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1c(F)cccc1C(F)(F)F Show InChI InChI=1S/C34H36F4N4O/c1-21-15-22(2)17-25(16-21)33-32(23(3)19-40-13-9-24-7-11-39-12-8-24)27-20-42(14-10-30(27)41-33)31(43)18-26-28(34(36,37)38)5-4-6-29(26)35/h4-8,11-12,15-17,23,40-41H,9-10,13-14,18-20H2,1-3H3/t23-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213658
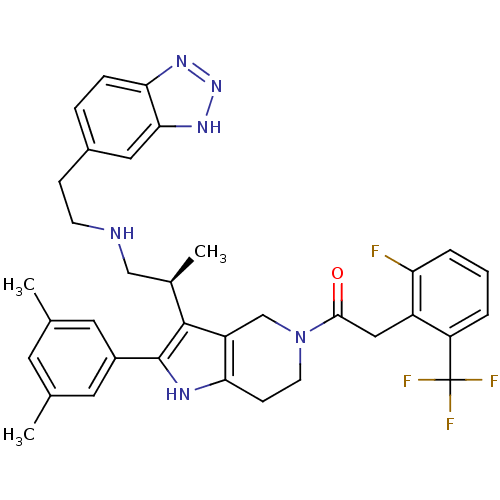
((S)-1-(3-(1-(2-(1H-benzo[d][1,2,3]triazol-5-yl)eth...)Show SMILES C[C@H](CNCCc1ccc2nn[nH]c2c1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1c(F)cccc1C(F)(F)F Show InChI InChI=1S/C35H36F4N6O/c1-20-13-21(2)15-24(14-20)34-33(22(3)18-40-11-9-23-7-8-30-31(16-23)43-44-42-30)26-19-45(12-10-29(26)41-34)32(46)17-25-27(35(37,38)39)5-4-6-28(25)36/h4-8,13-16,22,40-41H,9-12,17-19H2,1-3H3,(H,42,43,44)/t22-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213663
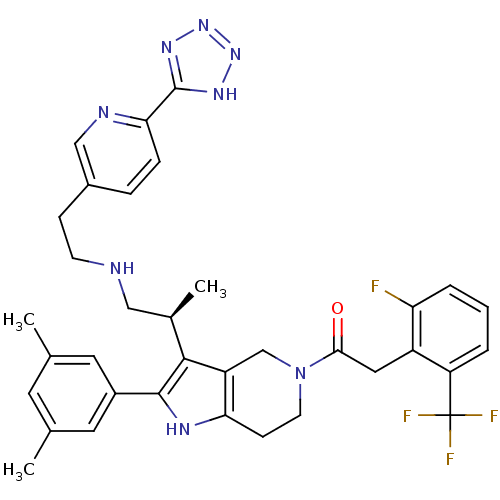
(1-(3-((S)-1-(2-(6-(1H-tetrazol-5-yl)pyridin-3-yl)e...)Show SMILES C[C@H](CNCCc1ccc(nc1)-c1nnn[nH]1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1c(F)cccc1C(F)(F)F Show InChI InChI=1S/C35H36F4N8O/c1-20-13-21(2)15-24(14-20)33-32(22(3)17-40-11-9-23-7-8-30(41-18-23)34-43-45-46-44-34)26-19-47(12-10-29(26)42-33)31(48)16-25-27(35(37,38)39)5-4-6-28(25)36/h4-8,13-15,18,22,40,42H,9-12,16-17,19H2,1-3H3,(H,43,44,45,46)/t22-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213652
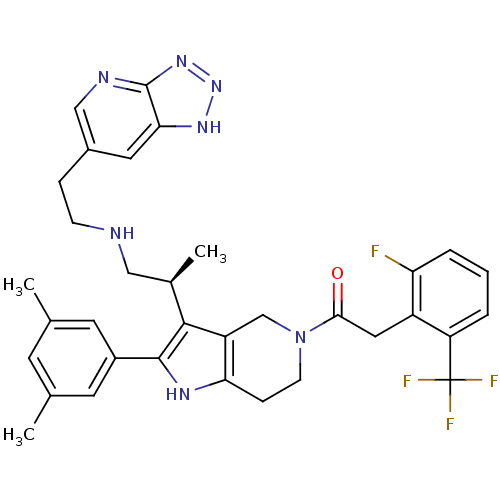
((S)-1-(3-(1-(2-(3H-[1,2,3]triazolo[4,5-b]pyridin-6...)Show SMILES C[C@H](CNCCc1cnc2nn[nH]c2c1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1c(F)cccc1C(F)(F)F Show InChI InChI=1S/C34H35F4N7O/c1-19-11-20(2)13-23(12-19)32-31(21(3)16-39-9-7-22-14-29-33(40-17-22)43-44-42-29)25-18-45(10-8-28(25)41-32)30(46)15-24-26(34(36,37)38)5-4-6-27(24)35/h4-6,11-14,17,21,39,41H,7-10,15-16,18H2,1-3H3,(H,40,42,43,44)/t21-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC6 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50508937

(CHEMBL4448046)Show SMILES Cn1nc2CSCc3nn(C)c(Cl)c3-c3c(Cl)ccc4c(CCCOc5cc(SCc1c2)cc1ccccc51)c(C(O)=O)n(C)c34 Show InChI InChI=1S/C34H31Cl2N5O3S2/c1-39-31-25-10-11-26(35)29(31)30-27(38-41(3)33(30)36)18-45-16-20-14-21(40(2)37-20)17-46-22-13-19-7-4-5-8-23(19)28(15-22)44-12-6-9-24(25)32(39)34(42)43/h4-5,7-8,10-11,13-15H,6,9,12,16-18H2,1-3H3,(H,42,43) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Mcl1 (unknown origin) by TR-FRET assay |
J Med Chem 62: 9418-9437 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00716
BindingDB Entry DOI: 10.7270/Q2PV6PNH |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213657
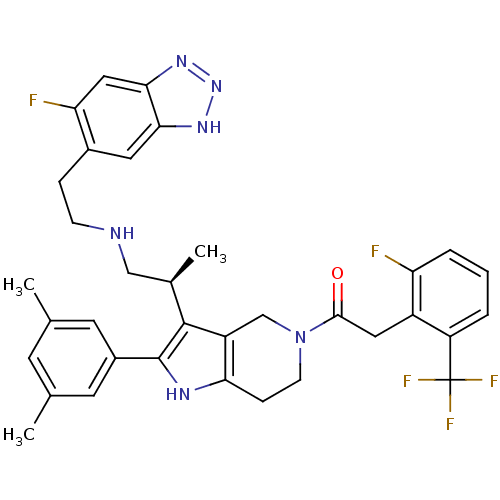
((S)-1-(2-(3,5-dimethylphenyl)-3-(1-(2-(6-fluoro-1H...)Show SMILES C[C@H](CNCCc1cc2[nH]nnc2cc1F)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1c(F)cccc1C(F)(F)F Show InChI InChI=1S/C35H35F5N6O/c1-19-11-20(2)13-23(12-19)34-33(21(3)17-41-9-7-22-14-30-31(16-28(22)37)44-45-43-30)25-18-46(10-8-29(25)42-34)32(47)15-24-26(35(38,39)40)5-4-6-27(24)36/h4-6,11-14,16,21,41-42H,7-10,15,17-18H2,1-3H3,(H,43,44,45)/t21-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213662
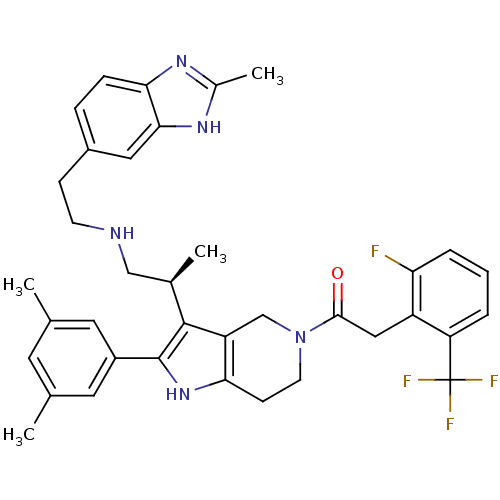
((S)-1-(2-(3,5-dimethylphenyl)-3-(1-(2-(2-methyl-1H...)Show SMILES C[C@H](CNCCc1ccc2nc(C)[nH]c2c1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1c(F)cccc1C(F)(F)F Show InChI InChI=1S/C37H39F4N5O/c1-21-14-22(2)16-26(15-21)36-35(23(3)19-42-12-10-25-8-9-32-33(17-25)44-24(4)43-32)28-20-46(13-11-31(28)45-36)34(47)18-27-29(37(39,40)41)6-5-7-30(27)38/h5-9,14-17,23,42,45H,10-13,18-20H2,1-4H3,(H,43,44)/t23-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC9 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213653
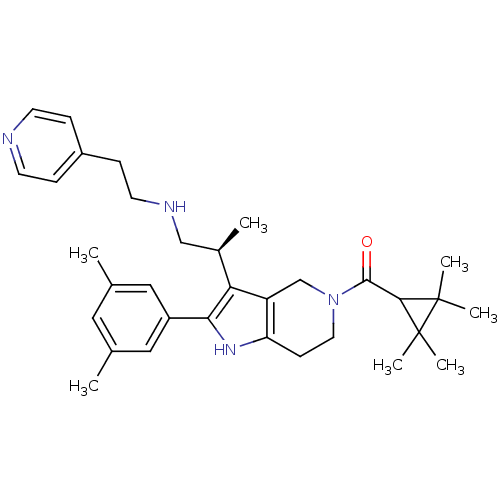
((S)-(2-(3,5-dimethylphenyl)-3-(1-(2-(pyridin-4-yl)...)Show SMILES C[C@H](CNCCc1ccncc1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)C1C(C)(C)C1(C)C Show InChI InChI=1S/C33H44N4O/c1-21-16-22(2)18-25(17-21)29-28(23(3)19-35-14-10-24-8-12-34-13-9-24)26-20-37(15-11-27(26)36-29)31(38)30-32(4,5)33(30,6)7/h8-9,12-13,16-18,23,30,35-36H,10-11,14-15,19-20H2,1-7H3/t23-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213669
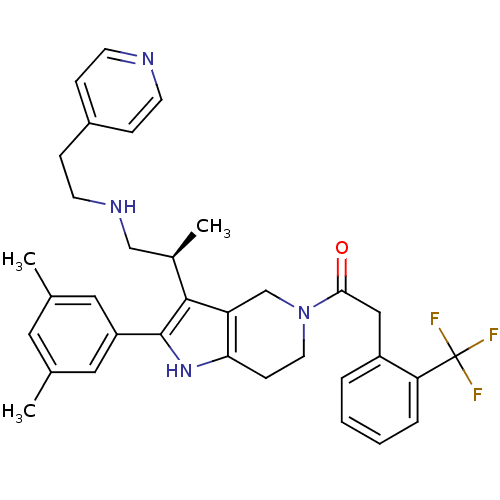
((S)-1-(2-(3,5-dimethylphenyl)-3-(1-(2-(pyridin-4-y...)Show SMILES C[C@H](CNCCc1ccncc1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1ccccc1C(F)(F)F Show InChI InChI=1S/C34H37F3N4O/c1-22-16-23(2)18-27(17-22)33-32(24(3)20-39-14-10-25-8-12-38-13-9-25)28-21-41(15-11-30(28)40-33)31(42)19-26-6-4-5-7-29(26)34(35,36)37/h4-9,12-13,16-18,24,39-40H,10-11,14-15,19-21H2,1-3H3/t24-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213650

(CHEMBL233341 | adamantan-1-yl-{2-(3,5-dimethyl-phe...)Show SMILES C[C@H](CNCCc1ccncc1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)C12CC3CC(CC(C3)C1)C2 |TLB:40:31:38:34.35.36,THB:40:35:38:31.32.39,29:31:38:34.35.36,39:31:34:38.37.36,39:37:34:40.31.32| Show InChI InChI=1S/C36H46N4O/c1-23-12-24(2)14-30(13-23)34-33(25(3)21-38-10-6-26-4-8-37-9-5-26)31-22-40(11-7-32(31)39-34)35(41)36-18-27-15-28(19-36)17-29(16-27)20-36/h4-5,8-9,12-14,25,27-29,38-39H,6-7,10-11,15-22H2,1-3H3/t25-,27?,28?,29?,36?/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213670
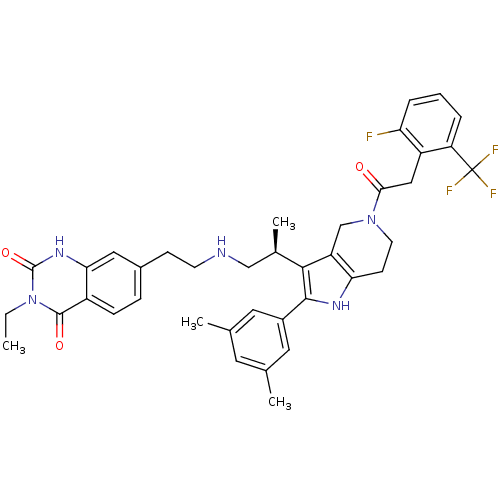
((S)-7-(2-(2-(2-(3,5-dimethylphenyl)-5-(2-(2-fluoro...)Show SMILES CCn1c(=O)[nH]c2cc(CCNC[C@@H](C)c3c4CN(CCc4[nH]c3-c3cc(C)cc(C)c3)C(=O)Cc3c(F)cccc3C(F)(F)F)ccc2c1=O Show InChI InChI=1S/C39H41F4N5O3/c1-5-48-37(50)27-10-9-25(18-33(27)46-38(48)51)11-13-44-20-24(4)35-29-21-47(34(49)19-28-30(39(41,42)43)7-6-8-31(28)40)14-12-32(29)45-36(35)26-16-22(2)15-23(3)17-26/h6-10,15-18,24,44-45H,5,11-14,19-21H2,1-4H3,(H,46,51)/t24-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213666
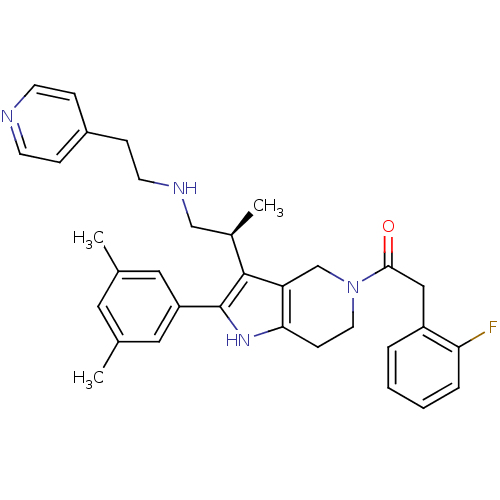
((S)-1-(2-(3,5-dimethylphenyl)-3-(1-(2-(pyridin-4-y...)Show SMILES C[C@H](CNCCc1ccncc1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1ccccc1F Show InChI InChI=1S/C33H37FN4O/c1-22-16-23(2)18-27(17-22)33-32(24(3)20-36-14-10-25-8-12-35-13-9-25)28-21-38(15-11-30(28)37-33)31(39)19-26-6-4-5-7-29(26)34/h4-9,12-13,16-18,24,36-37H,10-11,14-15,19-21H2,1-3H3/t24-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC2 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213656
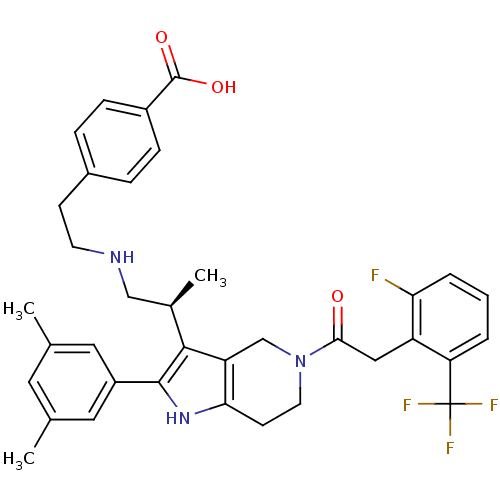
((S)-4-(2-(2-(2-(3,5-dimethylphenyl)-5-(2-(2-fluoro...)Show SMILES C[C@H](CNCCc1ccc(cc1)C(O)=O)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1c(F)cccc1C(F)(F)F Show InChI InChI=1S/C36H37F4N3O3/c1-21-15-22(2)17-26(16-21)34-33(23(3)19-41-13-11-24-7-9-25(10-8-24)35(45)46)28-20-43(14-12-31(28)42-34)32(44)18-27-29(36(38,39)40)5-4-6-30(27)37/h4-10,15-17,23,41-42H,11-14,18-20H2,1-3H3,(H,45,46)/t23-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC7 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC3 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50353233
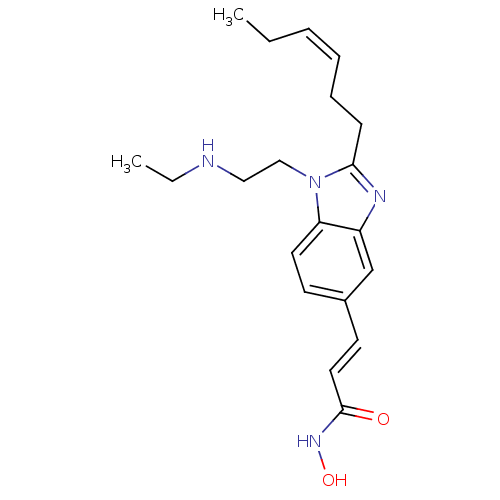
(CHEMBL1830536)Show SMILES CCNCCn1c(CC\C=C/CC)nc2cc(\C=C\C(=O)NO)ccc12 Show InChI InChI=1S/C20H28N4O2/c1-3-5-6-7-8-19-22-17-15-16(10-12-20(25)23-26)9-11-18(17)24(19)14-13-21-4-2/h5-6,9-12,15,21,26H,3-4,7-8,13-14H2,1-2H3,(H,23,25)/b6-5-,12-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC1 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213667
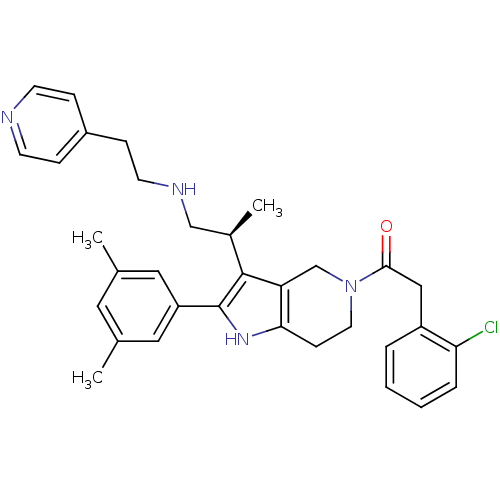
((S)-2-(2-chlorophenyl)-1-(2-(3,5-dimethylphenyl)-3...)Show SMILES C[C@H](CNCCc1ccncc1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1ccccc1Cl Show InChI InChI=1S/C33H37ClN4O/c1-22-16-23(2)18-27(17-22)33-32(24(3)20-36-14-10-25-8-12-35-13-9-25)28-21-38(15-11-30(28)37-33)31(39)19-26-6-4-5-7-29(26)34/h4-9,12-13,16-18,24,36-37H,10-11,14-15,19-21H2,1-3H3/t24-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50353233

(CHEMBL1830536)Show SMILES CCNCCn1c(CC\C=C/CC)nc2cc(\C=C\C(=O)NO)ccc12 Show InChI InChI=1S/C20H28N4O2/c1-3-5-6-7-8-19-22-17-15-16(10-12-20(25)23-26)9-11-18(17)24(19)14-13-21-4-2/h5-6,9-12,15,21,26H,3-4,7-8,13-14H2,1-2H3,(H,23,25)/b6-5-,12-10+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM29589

(Faridak | LBH-589 | LBH-589B | Panobinostat | US10...)Show SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C21H23N3O2/c1-15-18(19-4-2-3-5-20(19)23-15)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)24-26/h2-11,22-23,26H,12-14H2,1H3,(H,24,25)/b11-10+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC11 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50353234
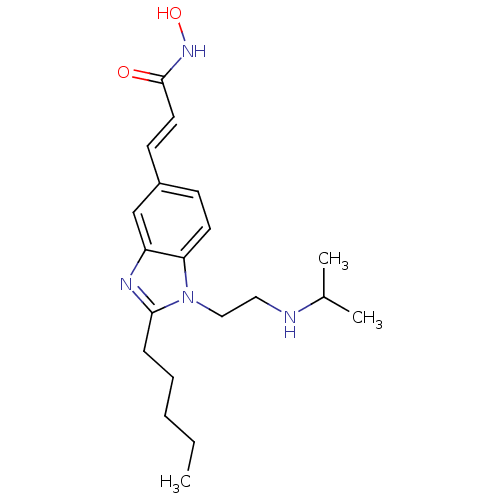
(CHEMBL1830537)Show InChI InChI=1S/C20H30N4O2/c1-4-5-6-7-19-22-17-14-16(9-11-20(25)23-26)8-10-18(17)24(19)13-12-21-15(2)3/h8-11,14-15,21,26H,4-7,12-13H2,1-3H3,(H,23,25)/b11-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50353233

(CHEMBL1830536)Show SMILES CCNCCn1c(CC\C=C/CC)nc2cc(\C=C\C(=O)NO)ccc12 Show InChI InChI=1S/C20H28N4O2/c1-3-5-6-7-8-19-22-17-15-16(10-12-20(25)23-26)9-11-18(17)24(19)14-13-21-4-2/h5-6,9-12,15,21,26H,3-4,7-8,13-14H2,1-2H3,(H,23,25)/b6-5-,12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC6 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50353234

(CHEMBL1830537)Show InChI InChI=1S/C20H30N4O2/c1-4-5-6-7-19-22-17-14-16(9-11-20(25)23-26)8-10-18(17)24(19)13-12-21-15(2)3/h8-11,14-15,21,26H,4-7,12-13H2,1-3H3,(H,23,25)/b11-9+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC11 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50353234

(CHEMBL1830537)Show InChI InChI=1S/C20H30N4O2/c1-4-5-6-7-19-22-17-14-16(9-11-20(25)23-26)8-10-18(17)24(19)13-12-21-15(2)3/h8-11,14-15,21,26H,4-7,12-13H2,1-3H3,(H,23,25)/b11-9+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC9 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213673
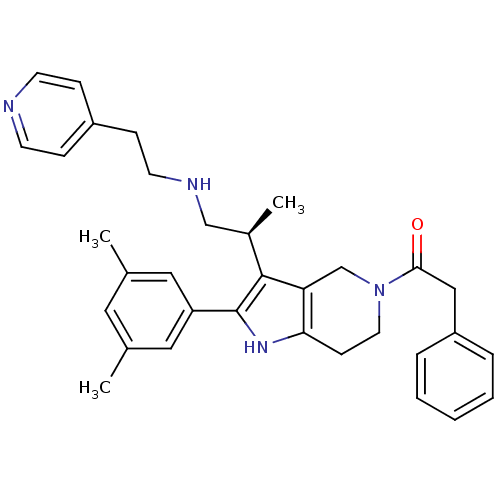
((S)-1-(2-(3,5-dimethylphenyl)-3-(1-(2-(pyridin-4-y...)Show SMILES C[C@H](CNCCc1ccncc1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1ccccc1 Show InChI InChI=1S/C33H38N4O/c1-23-17-24(2)19-28(18-23)33-32(25(3)21-35-15-11-26-9-13-34-14-10-26)29-22-37(16-12-30(29)36-33)31(38)20-27-7-5-4-6-8-27/h4-10,13-14,17-19,25,35-36H,11-12,15-16,20-22H2,1-3H3/t25-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213655
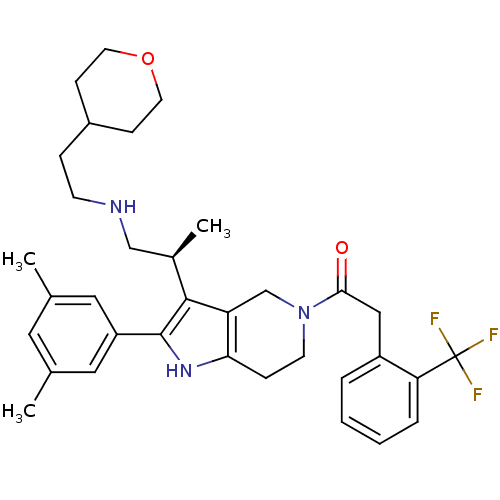
((S)-1-(2-(3,5-dimethylphenyl)-3-(1-(2-(tetrahydro-...)Show SMILES C[C@H](CNCCC1CCOCC1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1ccccc1C(F)(F)F Show InChI InChI=1S/C34H42F3N3O2/c1-22-16-23(2)18-27(17-22)33-32(24(3)20-38-12-8-25-10-14-42-15-11-25)28-21-40(13-9-30(28)39-33)31(41)19-26-6-4-5-7-29(26)34(35,36)37/h4-7,16-18,24-25,38-39H,8-15,19-21H2,1-3H3/t24-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50353232
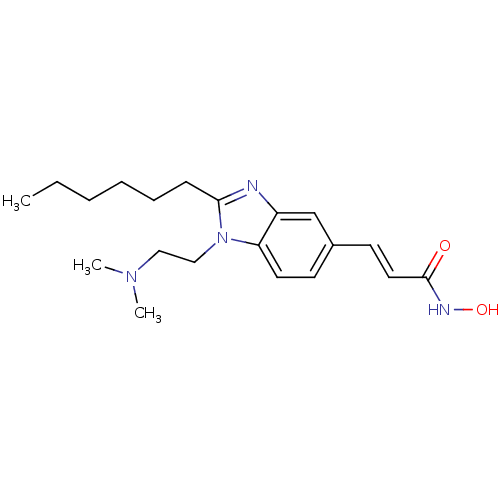
(CHEMBL1830424)Show InChI InChI=1S/C20H30N4O2/c1-4-5-6-7-8-19-21-17-15-16(10-12-20(25)22-26)9-11-18(17)24(19)14-13-23(2)3/h9-12,15,26H,4-8,13-14H2,1-3H3,(H,22,25)/b12-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213661
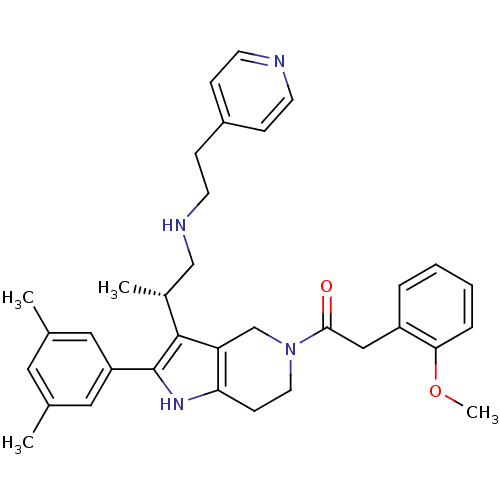
((S)-1-(2-(3,5-dimethylphenyl)-3-(1-(2-(pyridin-4-y...)Show SMILES COc1ccccc1CC(=O)N1CCc2[nH]c(c([C@H](C)CNCCc3ccncc3)c2C1)-c1cc(C)cc(C)c1 Show InChI InChI=1S/C34H40N4O2/c1-23-17-24(2)19-28(18-23)34-33(25(3)21-36-15-11-26-9-13-35-14-10-26)29-22-38(16-12-30(29)37-34)32(39)20-27-7-5-6-8-31(27)40-4/h5-10,13-14,17-19,25,36-37H,11-12,15-16,20-22H2,1-4H3/t25-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50353234

(CHEMBL1830537)Show InChI InChI=1S/C20H30N4O2/c1-4-5-6-7-19-22-17-14-16(9-11-20(25)23-26)8-10-18(17)24(19)13-12-21-15(2)3/h8-11,14-15,21,26H,4-7,12-13H2,1-3H3,(H,23,25)/b11-9+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50353233

(CHEMBL1830536)Show SMILES CCNCCn1c(CC\C=C/CC)nc2cc(\C=C\C(=O)NO)ccc12 Show InChI InChI=1S/C20H28N4O2/c1-3-5-6-7-8-19-22-17-15-16(10-12-20(25)23-26)9-11-18(17)24(19)14-13-21-4-2/h5-6,9-12,15,21,26H,3-4,7-8,13-14H2,1-2H3,(H,23,25)/b6-5-,12-10+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC9 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213660

((S)-1-(3-(1-(2-(4-(1H-tetrazol-5-yl)phenoxy)ethyla...)Show SMILES C[C@H](CNCCOc1ccc(cc1)-c1nnn[nH]1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1c(F)cccc1C(F)(F)F Show InChI InChI=1S/C36H37F4N7O2/c1-21-15-22(2)17-25(16-21)34-33(23(3)19-41-12-14-49-26-9-7-24(8-10-26)35-43-45-46-44-35)28-20-47(13-11-31(28)42-34)32(48)18-27-29(36(38,39)40)5-4-6-30(27)37/h4-10,15-17,23,41-42H,11-14,18-20H2,1-3H3,(H,43,44,45,46)/t23-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50353230
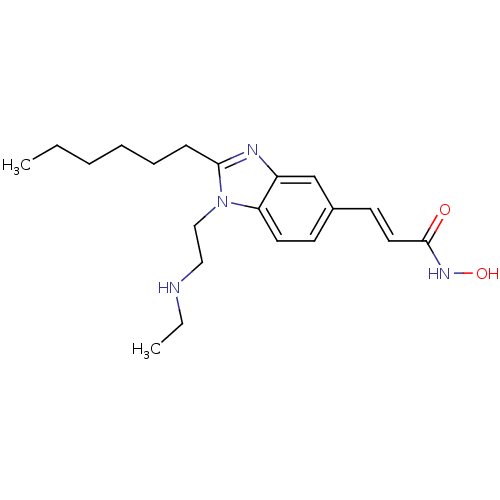
(CHEMBL1830420)Show InChI InChI=1S/C20H30N4O2/c1-3-5-6-7-8-19-22-17-15-16(10-12-20(25)23-26)9-11-18(17)24(19)14-13-21-4-2/h9-12,15,21,26H,3-8,13-14H2,1-2H3,(H,23,25)/b12-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50353231
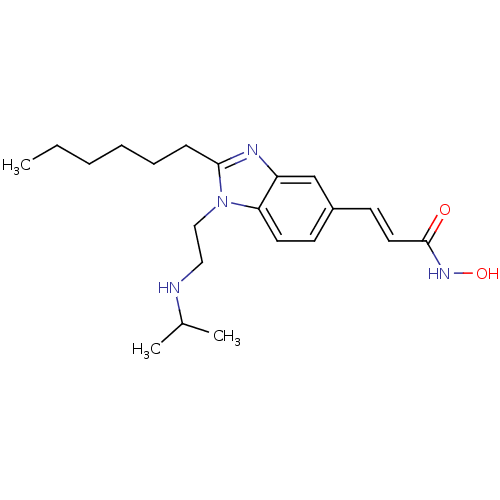
(CHEMBL1830422)Show InChI InChI=1S/C21H32N4O2/c1-4-5-6-7-8-20-23-18-15-17(10-12-21(26)24-27)9-11-19(18)25(20)14-13-22-16(2)3/h9-12,15-16,22,27H,4-8,13-14H2,1-3H3,(H,24,26)/b12-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50353231

(CHEMBL1830422)Show InChI InChI=1S/C21H32N4O2/c1-4-5-6-7-8-20-23-18-15-17(10-12-21(26)24-27)9-11-19(18)25(20)14-13-22-16(2)3/h9-12,15-16,22,27H,4-8,13-14H2,1-3H3,(H,24,26)/b12-10+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50353230

(CHEMBL1830420)Show InChI InChI=1S/C20H30N4O2/c1-3-5-6-7-8-19-22-17-15-16(10-12-20(25)23-26)9-11-18(17)24(19)14-13-21-4-2/h9-12,15,21,26H,3-8,13-14H2,1-2H3,(H,23,25)/b12-10+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50353233

(CHEMBL1830536)Show SMILES CCNCCn1c(CC\C=C/CC)nc2cc(\C=C\C(=O)NO)ccc12 Show InChI InChI=1S/C20H28N4O2/c1-3-5-6-7-8-19-22-17-15-16(10-12-20(25)23-26)9-11-18(17)24(19)14-13-21-4-2/h5-6,9-12,15,21,26H,3-4,7-8,13-14H2,1-2H3,(H,23,25)/b6-5-,12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC1 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50353229
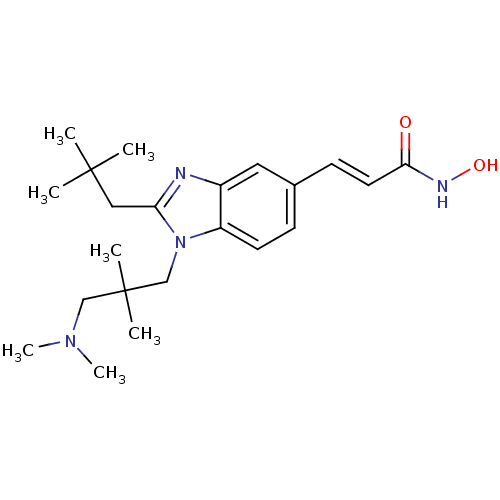
(CHEMBL1830397)Show SMILES CN(C)CC(C)(C)Cn1c(CC(C)(C)C)nc2cc(\C=C\C(=O)NO)ccc12 Show InChI InChI=1S/C22H34N4O2/c1-21(2,3)13-19-23-17-12-16(9-11-20(27)24-28)8-10-18(17)26(19)15-22(4,5)14-25(6)7/h8-12,28H,13-15H2,1-7H3,(H,24,27)/b11-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50248476
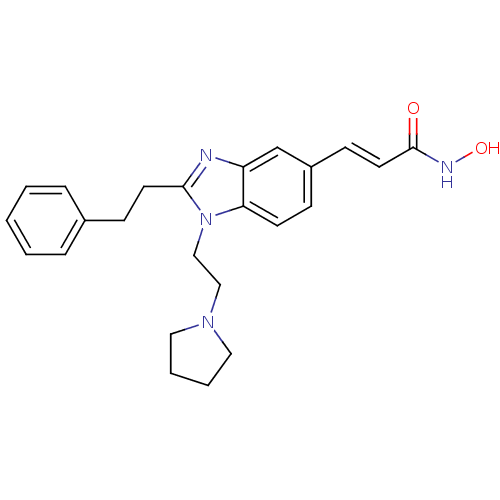
(CHEMBL491316 | N-hydroxy-3-(2-phenethyl-1-(2-(pyrr...)Show SMILES ONC(=O)\C=C\c1ccc2n(CCN3CCCC3)c(CCc3ccccc3)nc2c1 Show InChI InChI=1S/C24H28N4O2/c29-24(26-30)13-10-20-8-11-22-21(18-20)25-23(12-9-19-6-2-1-3-7-19)28(22)17-16-27-14-4-5-15-27/h1-3,6-8,10-11,13,18,30H,4-5,9,12,14-17H2,(H,26,29)/b13-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50353233

(CHEMBL1830536)Show SMILES CCNCCn1c(CC\C=C/CC)nc2cc(\C=C\C(=O)NO)ccc12 Show InChI InChI=1S/C20H28N4O2/c1-3-5-6-7-8-19-22-17-15-16(10-12-20(25)23-26)9-11-18(17)24(19)14-13-21-4-2/h5-6,9-12,15,21,26H,3-4,7-8,13-14H2,1-2H3,(H,23,25)/b6-5-,12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC2 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
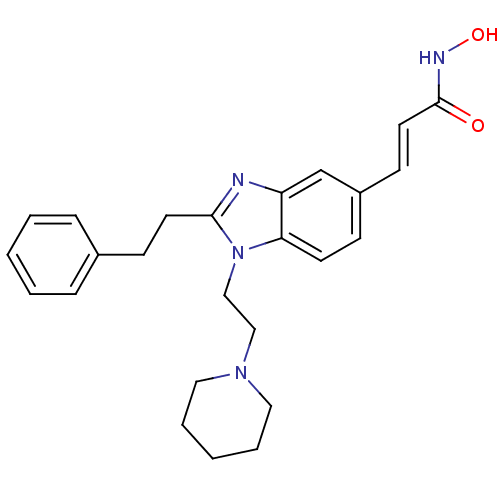
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50248522
(CHEMBL489332 | N-hydroxy-3-(2-phenethyl-1-(2-(pipe...)Show SMILES ONC(=O)\C=C\c1ccc2n(CCN3CCCCC3)c(CCc3ccccc3)nc2c1 Show InChI InChI=1S/C25H30N4O2/c30-25(27-31)14-11-21-9-12-23-22(19-21)26-24(13-10-20-7-3-1-4-8-20)29(23)18-17-28-15-5-2-6-16-28/h1,3-4,7-9,11-12,14,19,31H,2,5-6,10,13,15-18H2,(H,27,30)/b14-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC4 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50353233

(CHEMBL1830536)Show SMILES CCNCCn1c(CC\C=C/CC)nc2cc(\C=C\C(=O)NO)ccc12 Show InChI InChI=1S/C20H28N4O2/c1-3-5-6-7-8-19-22-17-15-16(10-12-20(25)23-26)9-11-18(17)24(19)14-13-21-4-2/h5-6,9-12,15,21,26H,3-4,7-8,13-14H2,1-2H3,(H,23,25)/b6-5-,12-10+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC11 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50353232

(CHEMBL1830424)Show InChI InChI=1S/C20H30N4O2/c1-4-5-6-7-8-19-21-17-15-16(10-12-20(25)22-26)9-11-18(17)24(19)14-13-23(2)3/h9-12,15,26H,4-8,13-14H2,1-3H3,(H,22,25)/b12-10+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S*BIO Pte Ltd.
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC5 using KI-104 as substrate by fluorescence assay |
J Med Chem 54: 4694-720 (2011)
Article DOI: 10.1021/jm2003552
BindingDB Entry DOI: 10.7270/Q29S1RD6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data