

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50366620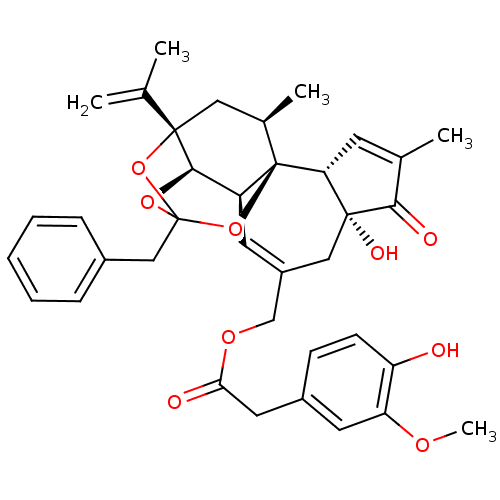 (RESINIFERATOXIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134709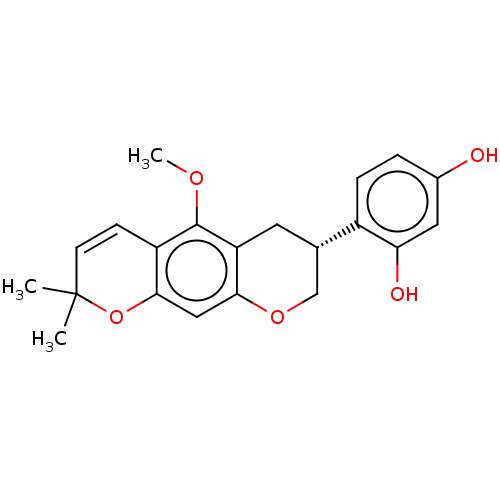 (CHEMBL3747057) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Time dependent inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate measured every 30 secs by Morrison and Walsh... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50432208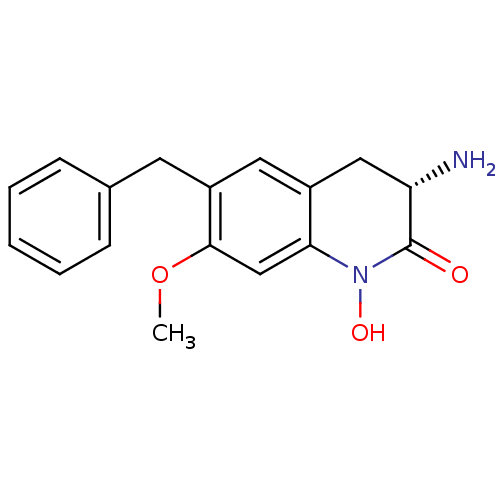 (CHEMBL2347110) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50426340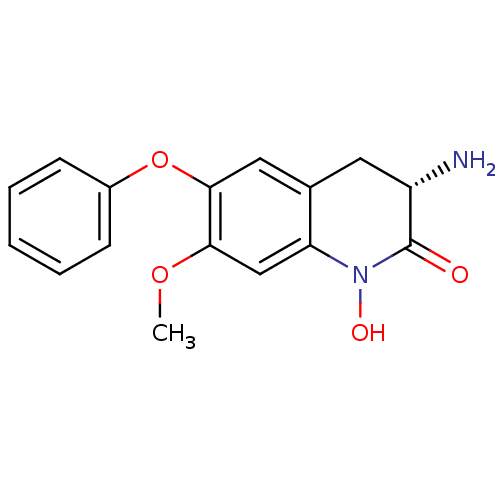 (CHEMBL2321943) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis | ACS Med Chem Lett 4: 37-40 (2013) Article DOI: 10.1021/ml300237v BindingDB Entry DOI: 10.7270/Q2KH0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107730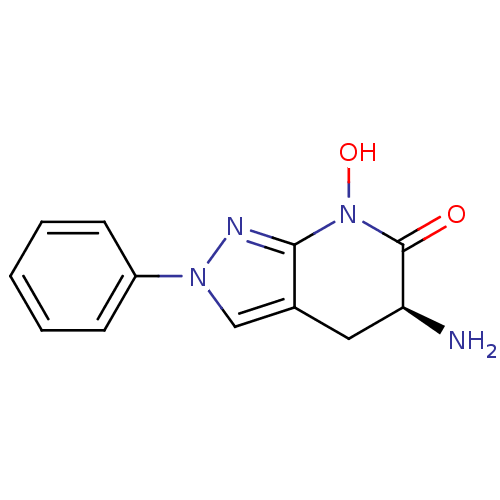 (CHEMBL2347108 | US8933095, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50426341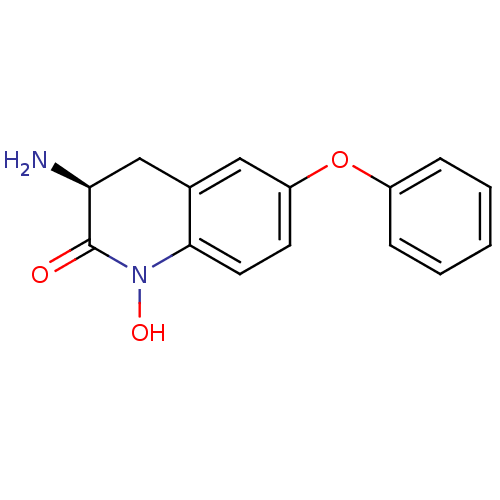 (CHEMBL2321944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis | ACS Med Chem Lett 4: 37-40 (2013) Article DOI: 10.1021/ml300237v BindingDB Entry DOI: 10.7270/Q2KH0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50386310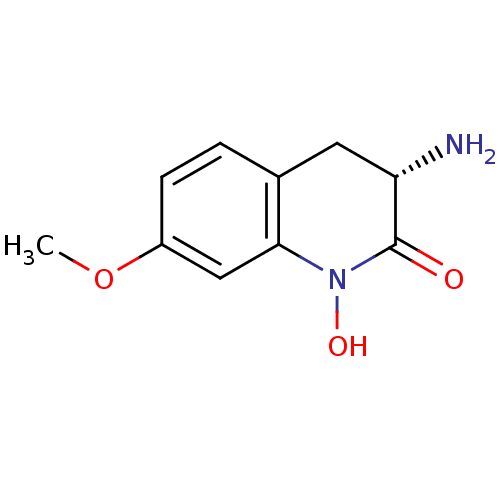 (CHEMBL2049092) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis | ACS Med Chem Lett 4: 37-40 (2013) Article DOI: 10.1021/ml300237v BindingDB Entry DOI: 10.7270/Q2KH0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107747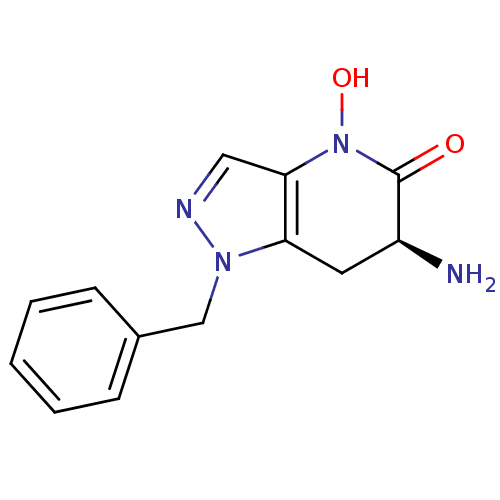 (CHEMBL2347115 | US8933095, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50386292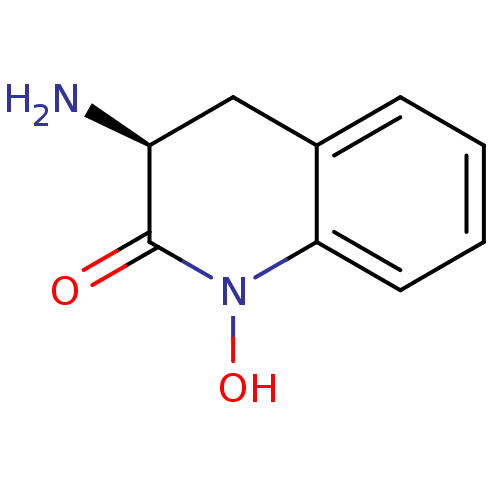 (CHEMBL2047851) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50386292 (CHEMBL2047851) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis | ACS Med Chem Lett 4: 37-40 (2013) Article DOI: 10.1021/ml300237v BindingDB Entry DOI: 10.7270/Q2KH0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134709 (CHEMBL3747057) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate preincubated for 5 to 60 mins by Lineweaver-Bur... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107738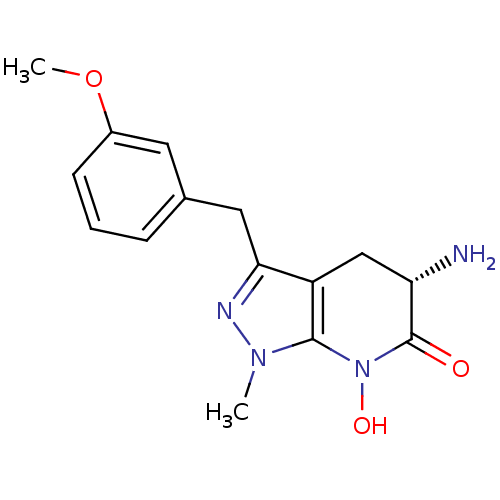 (CHEMBL2347113 | US8933095, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107746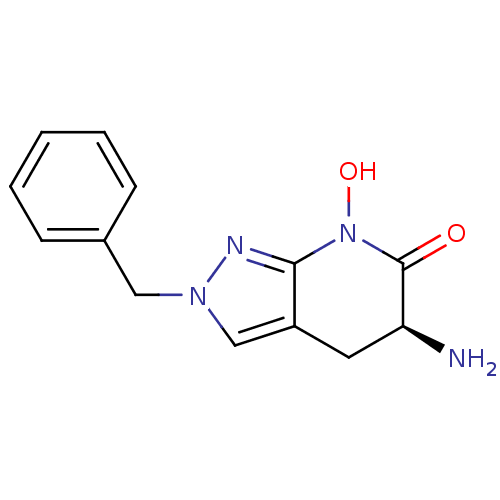 (CHEMBL2347107 | US8933095, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107720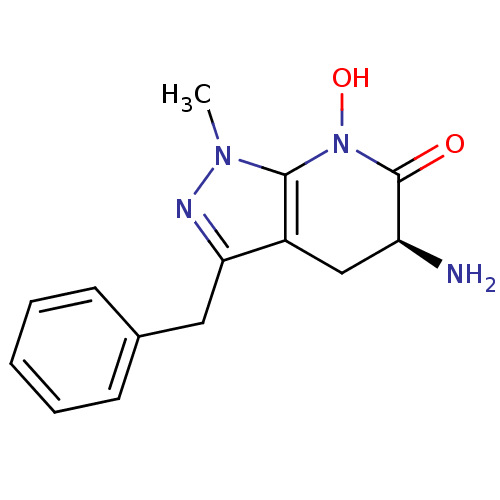 (CHEMBL2347112 | US8598200, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50432200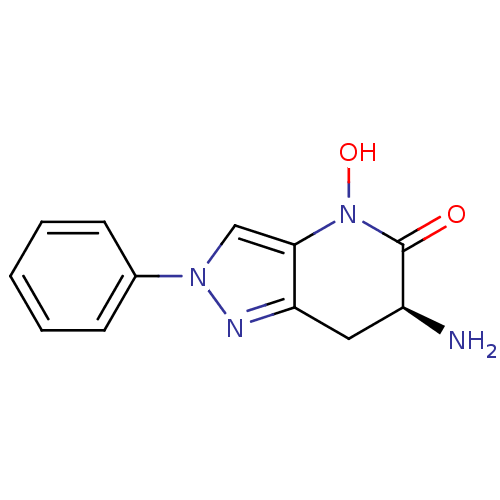 (CHEMBL2347109 | US8933095, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107722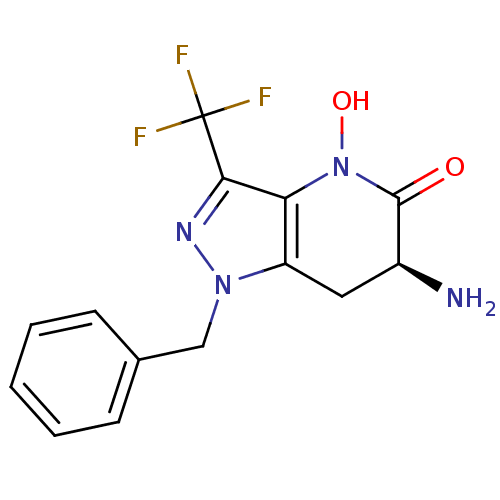 (CHEMBL2347114 | US8933095, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50278443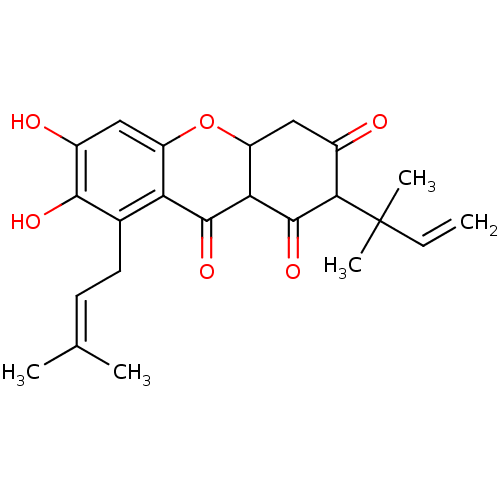 (1,3,6,7-tetrahydroxy-2-(3-methylbut-2-enyl)-8-(2-m...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium perfringens neuraminidase by Lineweaver-Burke plot and Dixon plot | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50175018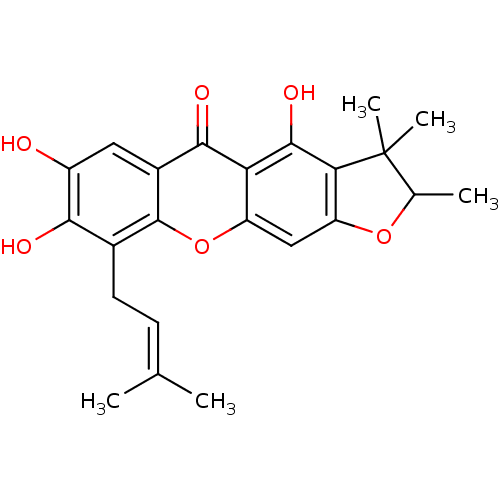 (4,7,8-trihydroxy-2,3,3-trimethyl-9-(3-methylbut-2-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium perfringens neuraminidase by Lineweaver-Burke plot and Dixon plot | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50378020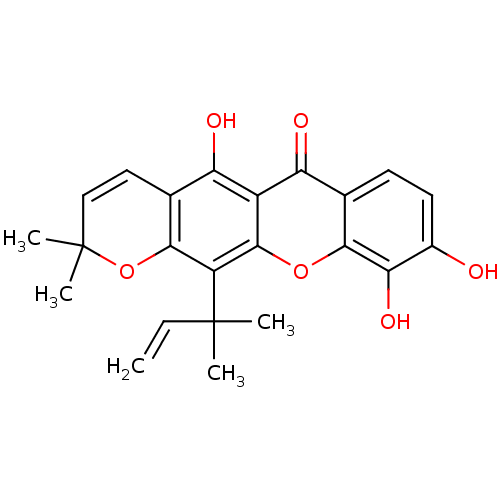 (MACLURAXANTHONE) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium perfringens neuraminidase by Lineweaver-Burke plot and Dixon plot | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50355214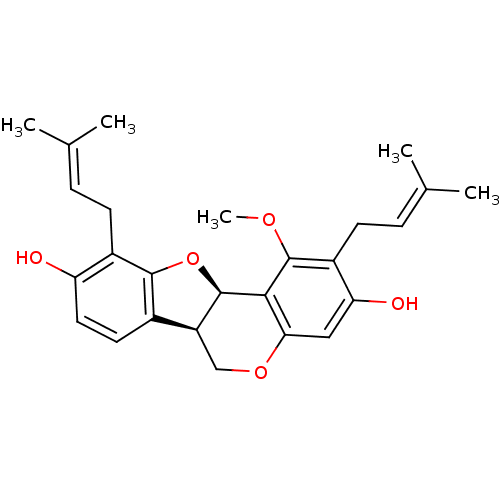 (CHEMBL401566) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate by Linewe... | Bioorg Med Chem Lett 21: 6100-3 (2011) Article DOI: 10.1016/j.bmcl.2011.08.046 BindingDB Entry DOI: 10.7270/Q2C53M8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50278443 (1,3,6,7-tetrahydroxy-2-(3-methylbut-2-enyl)-8-(2-m...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Apparent binding affinity at Clostridium perfringens neuraminidase by fluorimetry | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134709 (CHEMBL3747057) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of diphenolase activity of mushroom tyrosinase using L-DOPA as substrate preincubated for 5 to 60 mins by Lineweaver-Burk and ... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50278346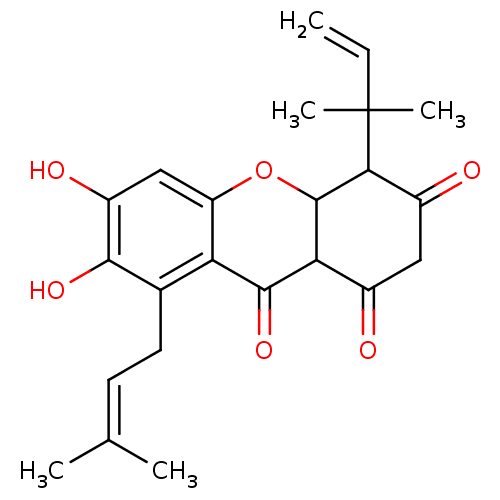 (CHEMBL470844 | Cudratricusxanthone) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium perfringens neuraminidase by Lineweaver-Burke plot and Dixon plot | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50175013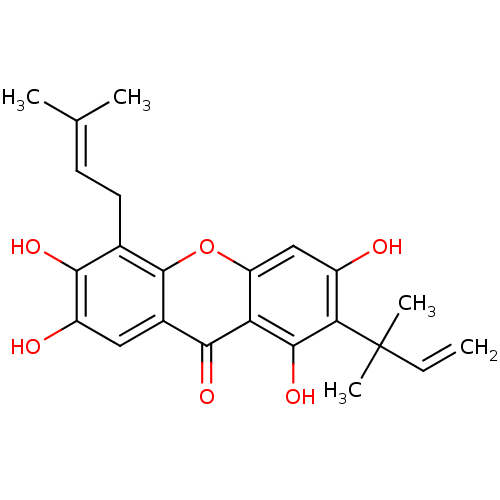 (1,3,6,7-tetrahydroxy-5-(3-methylbut-2-enyl)-2-(2-m...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium perfringens neuraminidase by Lineweaver-Burke plot and Dixon plot | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50175019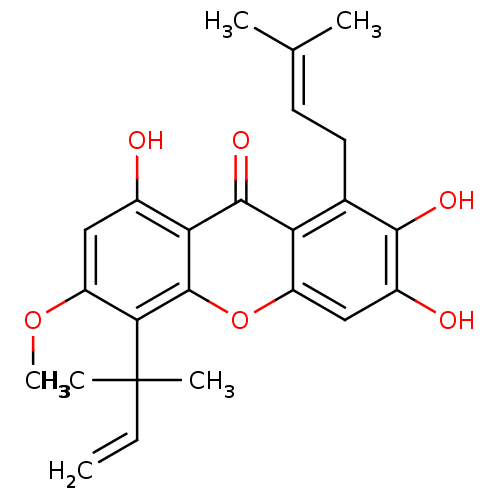 (2,3,8-trihydroxy-6-methoxy-1-(3-methylbut-2-enyl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium perfringens neuraminidase by Lineweaver-Burke plot and Dixon plot | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50134710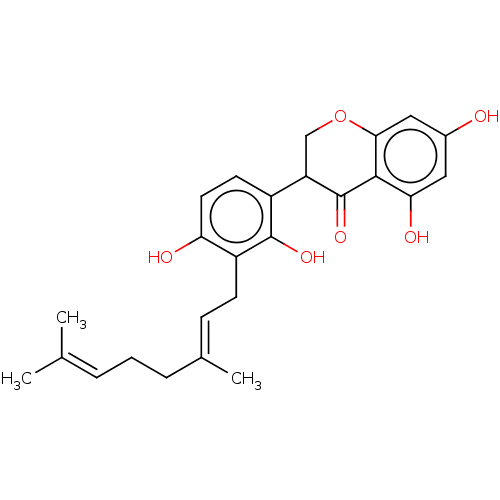 (CHEMBL3745886) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Time dependent inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate measured every 30 secs by Morrison and Walsh... | Bioorg Med Chem 24: 153-9 (2016) Article DOI: 10.1016/j.bmc.2015.11.040 BindingDB Entry DOI: 10.7270/Q2RN39PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50355210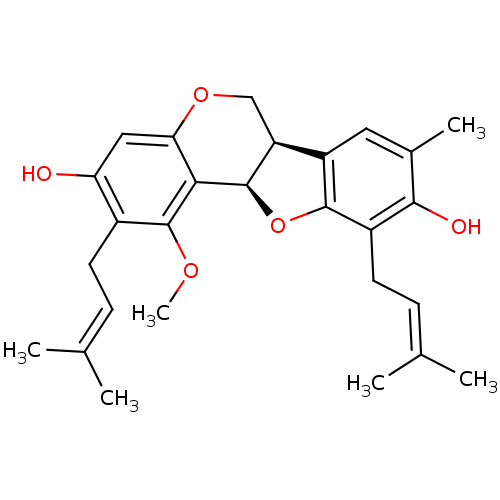 (CHEMBL1835715) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate by Linewe... | Bioorg Med Chem Lett 21: 6100-3 (2011) Article DOI: 10.1016/j.bmcl.2011.08.046 BindingDB Entry DOI: 10.7270/Q2C53M8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50355212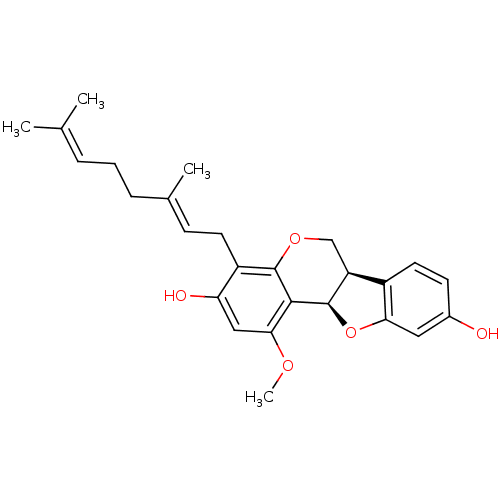 (CHEMBL1835717) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate by Linewe... | Bioorg Med Chem Lett 21: 6100-3 (2011) Article DOI: 10.1016/j.bmcl.2011.08.046 BindingDB Entry DOI: 10.7270/Q2C53M8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50278346 (CHEMBL470844 | Cudratricusxanthone) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Apparent binding affinity at Clostridium perfringens neuraminidase by fluorimetry | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082250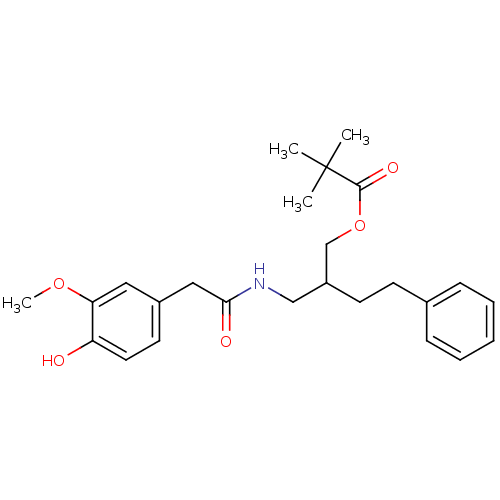 (2,2-Dimethyl-propionic acid 2-{[2-(4-hydroxy-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082239 (2,2-Dimethyl-propionic acid 2-benzyl-3-[2-(4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 404 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50175019 (2,3,8-trihydroxy-6-methoxy-1-(3-methylbut-2-enyl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 436 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Apparent binding affinity at Clostridium perfringens neuraminidase by fluorimetry | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50378020 (MACLURAXANTHONE) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 477 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Apparent binding affinity at Clostridium perfringens neuraminidase by fluorimetry | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082243 ((E)-4-Benzyl-5-[2-(4-hydroxy-3-methoxy-phenyl)-ace...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50175018 (4,7,8-trihydroxy-2,3,3-trimethyl-9-(3-methylbut-2-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Apparent binding affinity at Clostridium perfringens neuraminidase by fluorimetry | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50175013 (1,3,6,7-tetrahydroxy-5-(3-methylbut-2-enyl)-2-(2-m...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 568 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Apparent binding affinity at Clostridium perfringens neuraminidase by fluorimetry | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50442881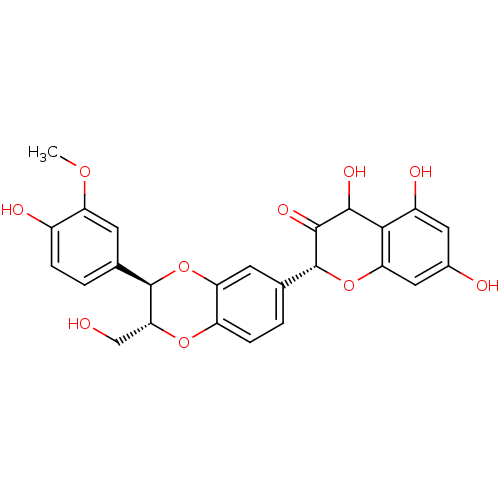 (SILYBIN A) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of monophenolase activity of mushroom tyrosinase assessed as reduction in dopachrome formation using L-Tyrosine substrate by UV... | Bioorg Med Chem 27: 2499-2507 (2019) Article DOI: 10.1016/j.bmc.2019.03.013 BindingDB Entry DOI: 10.7270/Q2PV6PTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50442881 (SILYBIN A) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of monophenolase activity of mushroom tyrosinase assessed as reduction in dopachrome formation using L-Tyrosine substrate by UV... | Bioorg Med Chem 27: 2499-2507 (2019) Article DOI: 10.1016/j.bmc.2019.03.013 BindingDB Entry DOI: 10.7270/Q2PV6PTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50442881 (SILYBIN A) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase assessed as inhibition constant for free enzyme-inhibitor complex by measuring reduction ... | Bioorg Med Chem 27: 2499-2507 (2019) Article DOI: 10.1016/j.bmc.2019.03.013 BindingDB Entry DOI: 10.7270/Q2PV6PTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50442881 (SILYBIN A) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase assessed as inhibition constant for free enzyme-inhibitor complex by measuring reduction ... | Bioorg Med Chem 27: 2499-2507 (2019) Article DOI: 10.1016/j.bmc.2019.03.013 BindingDB Entry DOI: 10.7270/Q2PV6PTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50278394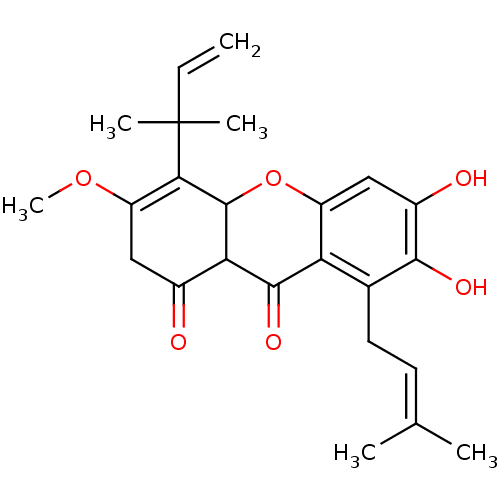 (CHEMBL469813 | Cudratricusxanthone F) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium perfringens neuraminidase by Lineweaver-Burke plot and Dixon plot | Bioorg Med Chem 17: 2744-50 (2009) Article DOI: 10.1016/j.bmc.2009.02.042 BindingDB Entry DOI: 10.7270/Q2V69KG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50526629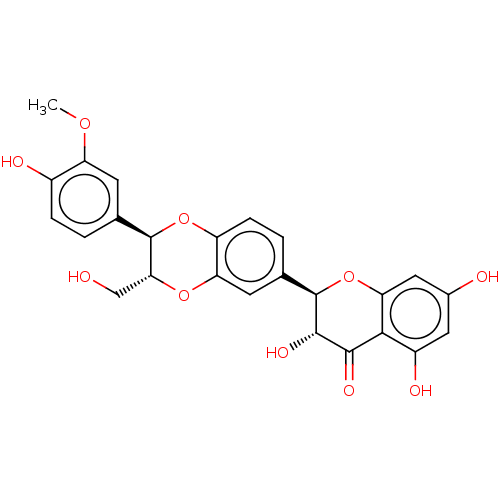 (ISOSILYBIN A) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase assessed as inhibition constant for free enzyme-inhibitor complex by measuring reduction ... | Bioorg Med Chem 27: 2499-2507 (2019) Article DOI: 10.1016/j.bmc.2019.03.013 BindingDB Entry DOI: 10.7270/Q2PV6PTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50526629 (ISOSILYBIN A) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of monophenolase activity of mushroom tyrosinase assessed as reduction in dopachrome formation using L-Tyrosine substrate by UV... | Bioorg Med Chem 27: 2499-2507 (2019) Article DOI: 10.1016/j.bmc.2019.03.013 BindingDB Entry DOI: 10.7270/Q2PV6PTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50526629 (ISOSILYBIN A) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of monophenolase activity of mushroom tyrosinase assessed as reduction in dopachrome formation using L-Tyrosine substrate by UV... | Bioorg Med Chem 27: 2499-2507 (2019) Article DOI: 10.1016/j.bmc.2019.03.013 BindingDB Entry DOI: 10.7270/Q2PV6PTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50526629 (ISOSILYBIN A) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of monophenolase activity of mushroom tyrosinase assessed as inhibition constant for free enzyme-inhibitor complex by measuring reduction ... | Bioorg Med Chem 27: 2499-2507 (2019) Article DOI: 10.1016/j.bmc.2019.03.013 BindingDB Entry DOI: 10.7270/Q2PV6PTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50355211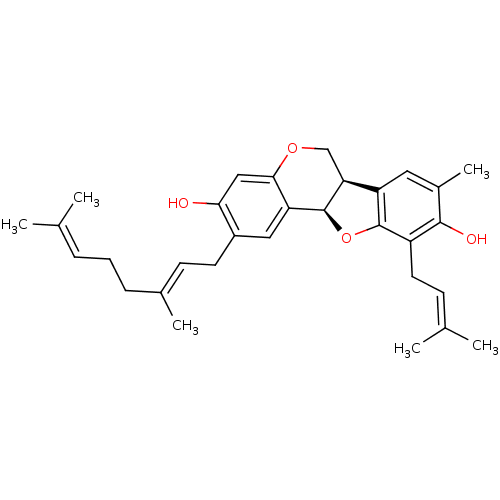 (CHEMBL1835716) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate by Linewe... | Bioorg Med Chem Lett 21: 6100-3 (2011) Article DOI: 10.1016/j.bmcl.2011.08.046 BindingDB Entry DOI: 10.7270/Q2C53M8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082248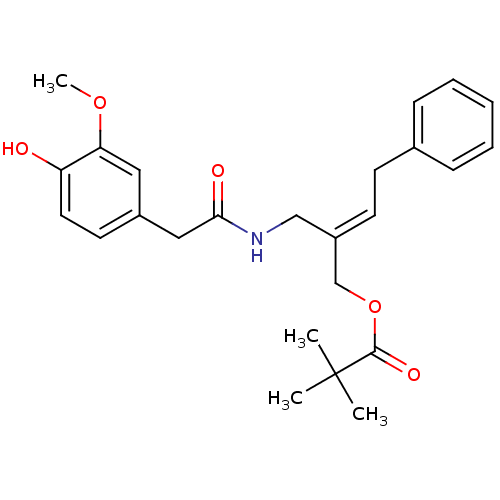 (2,2-Dimethyl-propionic acid (E)-2-{[2-(4-hydroxy-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50355213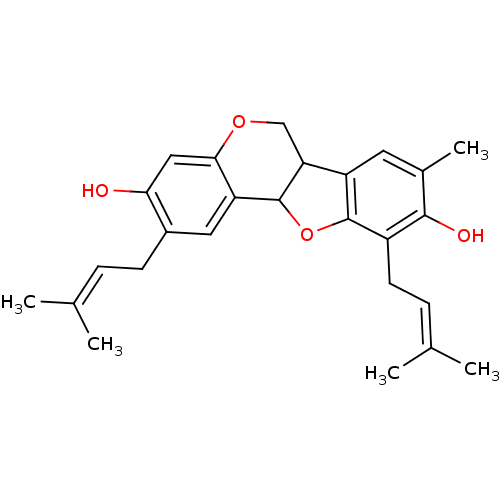 (CHEMBL1835792) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Non-competitive inhibition of Clostridium perfringens neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid as substrate by Linewe... | Bioorg Med Chem Lett 21: 6100-3 (2011) Article DOI: 10.1016/j.bmcl.2011.08.046 BindingDB Entry DOI: 10.7270/Q2C53M8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50082242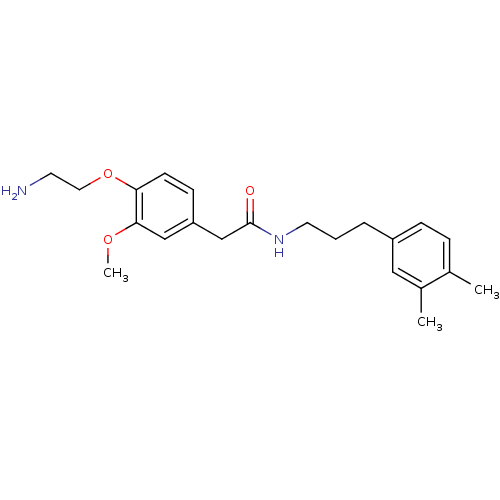 (2-[4-(2-Amino-ethoxy)-3-methoxy-phenyl]-N-[3-(3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50526625 (CHEMBL4592595) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University Curated by ChEMBL | Assay Description Mixed type inhibition of monophenolase activity of mushroom tyrosinase assessed as reduction in dopachrome formation using L-Tyrosine substrate by UV... | Bioorg Med Chem 27: 2499-2507 (2019) Article DOI: 10.1016/j.bmc.2019.03.013 BindingDB Entry DOI: 10.7270/Q2PV6PTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1924 total ) | Next | Last >> |