 Found 285 hits with Last Name = 'pang' and Initial = 'l'
Found 285 hits with Last Name = 'pang' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aspartate--tRNA ligase
(Escherichia coli) | BDBM50339907
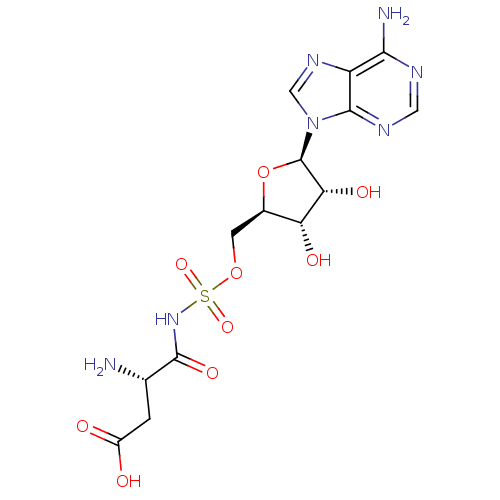
(((S)-2-amino-3-carboxypropanoyl)(((2R,3S,4R,5R)-5-...)Show SMILES N[C@@H](CC(O)=O)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H19N7O9S/c15-5(1-7(22)23)13(26)20-31(27,28)29-2-6-9(24)10(25)14(30-6)21-4-19-8-11(16)17-3-18-12(8)21/h3-6,9-10,14,24-25H,1-2,15H2,(H,20,26)(H,22,23)(H2,16,17,18)/t5-,6+,9+,10+,14+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli BL21(DE3) Aspartyl-tRNA synthetase assessed as reduction in tRNA aminoacylation preincubated for 10 mins with Escheric... |
Eur J Med Chem 148: 384-396 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.013
BindingDB Entry DOI: 10.7270/Q2057JJS |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50458119
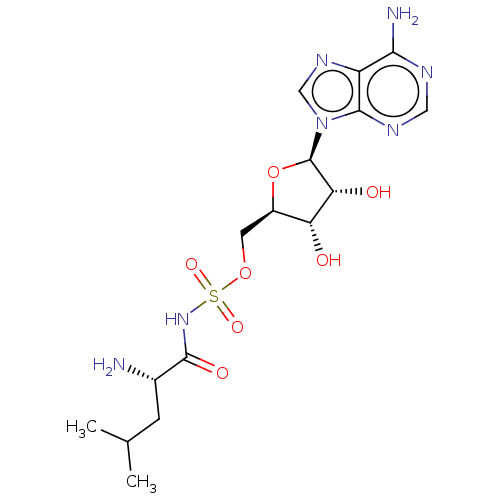
(Leusa)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C16H25N7O7S/c1-7(2)3-8(17)15(26)22-31(27,28)29-4-9-11(24)12(25)16(30-9)23-6-21-10-13(18)19-5-20-14(10)23/h5-9,11-12,16,24-25H,3-4,17H2,1-2H3,(H,22,26)(H2,18,19,20)/t8-,9+,11+,12+,16+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli BL21(DE3) Leucyl-tRNA synthetase assessed as reduction in tRNA aminoacylation preincubated for 10 mins with Escherichi... |
Eur J Med Chem 148: 384-396 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.013
BindingDB Entry DOI: 10.7270/Q2057JJS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50339906

(((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...)Show SMILES N[C@@H](CO)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C13H19N7O8S/c14-5(1-21)12(24)19-29(25,26)27-2-6-8(22)9(23)13(28-6)20-4-18-7-10(15)16-3-17-11(7)20/h3-6,8-9,13,21-23H,1-2,14H2,(H,19,24)(H2,15,16,17)/t5-,6+,8+,9+,13+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli BL21(DE3) Seryl-tRNA synthetase assessed as reduction in tRNA aminoacylation preincubated for 10 mins with Escherichia... |
Eur J Med Chem 148: 384-396 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.013
BindingDB Entry DOI: 10.7270/Q2057JJS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM85696
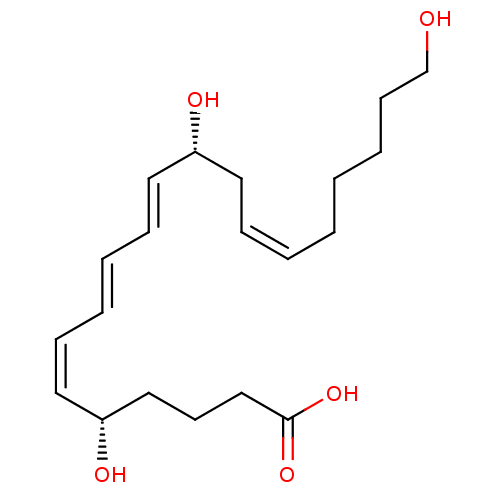
(LTB4-20-hydroxy)Show SMILES OCCCCC\C=C/C[C@@H](O)\C=C\C=C\C=C/[C@@H](O)CCCC(O)=O |r| Show InChI InChI=1S/C20H32O5/c21-17-10-6-2-1-3-7-12-18(22)13-8-4-5-9-14-19(23)15-11-16-20(24)25/h3-5,7-9,13-14,18-19,21-23H,1-2,6,10-12,15-17H2,(H,24,25)/b5-4+,7-3-,13-8+,14-9-/t18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
J Biol Chem 275: 40686-94 (2000)
Article DOI: 10.1074/jbc.M004512200
BindingDB Entry DOI: 10.7270/Q2XD1066 |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50458122
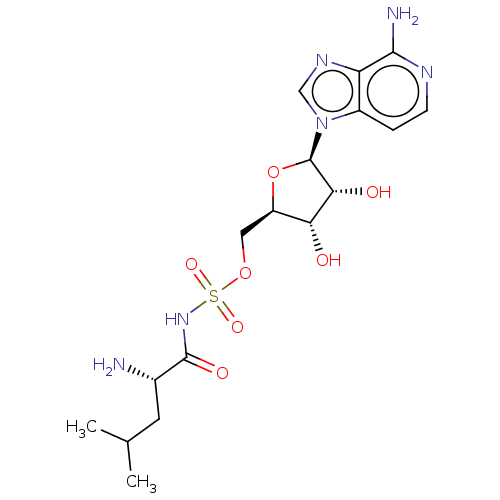
(CHEMBL4204314)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nccc12 |r| Show InChI InChI=1S/C17H26N6O7S/c1-8(2)5-9(18)16(26)22-31(27,28)29-6-11-13(24)14(25)17(30-11)23-7-21-12-10(23)3-4-20-15(12)19/h3-4,7-9,11,13-14,17,24-25H,5-6,18H2,1-2H3,(H2,19,20)(H,22,26)/t9-,11+,13+,14+,17+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.654 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli BL21(DE3) Leucyl-tRNA synthetase assessed as reduction in tRNA aminoacylation preincubated for 10 mins with Escherichi... |
Eur J Med Chem 148: 384-396 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.013
BindingDB Entry DOI: 10.7270/Q2057JJS |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50013889
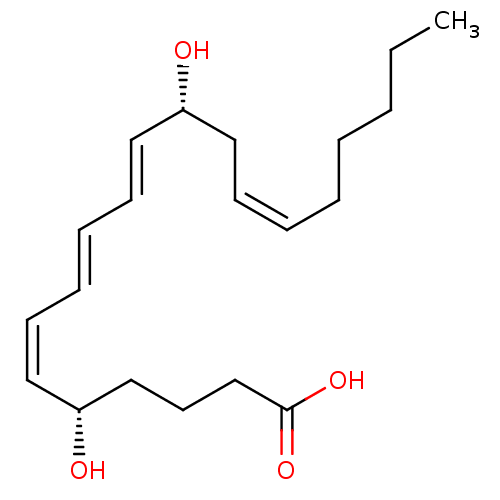
((5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,...)Show SMILES CCCCC\C=C/C[C@@H](O)\C=C\C=C\C=C/[C@@H](O)CCCC(O)=O |r| Show InChI InChI=1S/C20H32O4/c1-2-3-4-5-6-9-13-18(21)14-10-7-8-11-15-19(22)16-12-17-20(23)24/h6-11,14-15,18-19,21-22H,2-5,12-13,16-17H2,1H3,(H,23,24)/b8-7+,9-6-,14-10+,15-11-/t18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
J Biol Chem 275: 40686-94 (2000)
Article DOI: 10.1074/jbc.M004512200
BindingDB Entry DOI: 10.7270/Q2XD1066 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Isoleucine--tRNA ligase
(Escherichia coli) | BDBM50524451
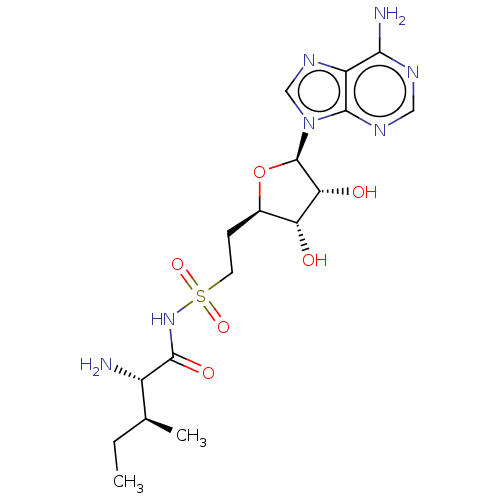
(CHEMBL4553017)Show SMILES CC[C@H](C)[C@H](N)C(=O)NS(=O)(=O)CC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C17H27N7O6S/c1-3-8(2)10(18)16(27)23-31(28,29)5-4-9-12(25)13(26)17(30-9)24-7-22-11-14(19)20-6-21-15(11)24/h6-10,12-13,17,25-26H,3-5,18H2,1-2H3,(H,23,27)(H2,19,20,21)/t8-,9+,10-,12+,13+,17+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Escherichia coli BL21(DE3) isoleucyl-tRNA synthetase expressed in Escherichia coli Rosetta 2 (DE3) assessed as reduction in... |
Eur J Med Chem 174: 252-264 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.045
BindingDB Entry DOI: 10.7270/Q26M3B73 |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Escherichia coli) | BDBM50222902

(CHEMBL1163069)Show SMILES CC[C@H](C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C16H25N7O7S/c1-3-7(2)9(17)15(26)22-31(27,28)29-4-8-11(24)12(25)16(30-8)23-6-21-10-13(18)19-5-20-14(10)23/h5-9,11-12,16,24-25H,3-4,17H2,1-2H3,(H,22,26)(H2,18,19,20)/t7-,8+,9-,11+,12+,16+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli BL21(DE3) Isoleucyl-tRNA synthetase assessed as reduction in tRNA aminoacylation preincubated for 10 mins with Escheri... |
Eur J Med Chem 148: 384-396 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.013
BindingDB Entry DOI: 10.7270/Q2057JJS |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM50013889

((5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,...)Show SMILES CCCCC\C=C/C[C@@H](O)\C=C\C=C\C=C/[C@@H](O)CCCC(O)=O |r| Show InChI InChI=1S/C20H32O4/c1-2-3-4-5-6-9-13-18(21)14-10-7-8-11-15-19(22)16-12-17-20(23)24/h6-11,14-15,18-19,21-22H,2-5,12-13,16-17H2,1H3,(H,23,24)/b8-7+,9-6-,14-10+,15-11-/t18-,19-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
J Biol Chem 275: 40686-94 (2000)
Article DOI: 10.1074/jbc.M004512200
BindingDB Entry DOI: 10.7270/Q2XD1066 |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50569962
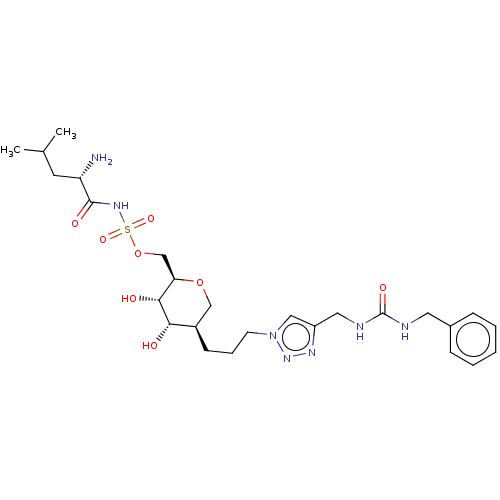
(CHEMBL4864835)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1OC[C@@H](CCCn2cc(CNC(=O)NCc3ccccc3)nn2)[C@H](O)[C@@H]1O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant Escherichia coli LeuRS assessed as inhibition of 14C-radiolabelled leucine transfer to tRNA leucine preincubated for 10 min... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113021
BindingDB Entry DOI: 10.7270/Q2MC93SZ |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50458121

(CHEMBL1163085)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C19H23N7O8S/c20-11(5-9-1-3-10(27)4-2-9)18(30)25-35(31,32)33-6-12-14(28)15(29)19(34-12)26-8-24-13-16(21)22-7-23-17(13)26/h1-4,7-8,11-12,14-15,19,27-29H,5-6,20H2,(H,25,30)(H2,21,22,23)/t11-,12+,14+,15+,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli BL21(DE3) Tyrosyl-tRNA synthetase assessed as reduction in tRNA aminoacylation preincubated for 10 mins with Escherich... |
Eur J Med Chem 148: 384-396 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.013
BindingDB Entry DOI: 10.7270/Q2057JJS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM85702
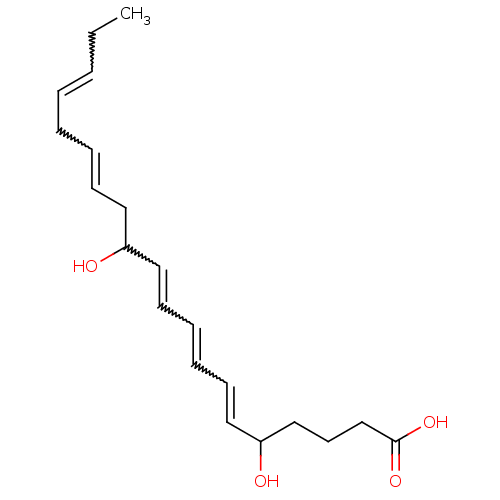
(CAS_5283125 | LTB5 | NSC_5283125)Show SMILES CCC=CCC=CCC(O)C=CC=CC=CC(O)CCCC(O)=O |w:2.1,5.4,10.9,12.11,14.13| Show InChI InChI=1S/C20H30O4/c1-2-3-4-5-6-9-13-18(21)14-10-7-8-11-15-19(22)16-12-17-20(23)24/h3-4,6-11,14-15,18-19,21-22H,2,5,12-13,16-17H2,1H3,(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
J Biol Chem 275: 40686-94 (2000)
Article DOI: 10.1074/jbc.M004512200
BindingDB Entry DOI: 10.7270/Q2XD1066 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM85692
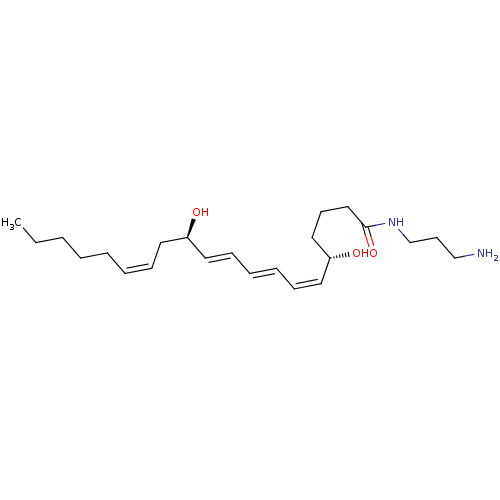
(LTB4-3-aminopropylamide)Show SMILES CCCCC\C=C/C[C@@H](O)\C=C\C=C\C=C/[C@@H](O)CCCC(=O)NCCCN |r| Show InChI InChI=1S/C23H40N2O3/c1-2-3-4-5-6-9-14-21(26)15-10-7-8-11-16-22(27)17-12-18-23(28)25-20-13-19-24/h6-11,15-16,21-22,26-27H,2-5,12-14,17-20,24H2,1H3,(H,25,28)/b8-7+,9-6-,15-10+,16-11-/t21-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
J Biol Chem 275: 40686-94 (2000)
Article DOI: 10.1074/jbc.M004512200
BindingDB Entry DOI: 10.7270/Q2XD1066 |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50569952
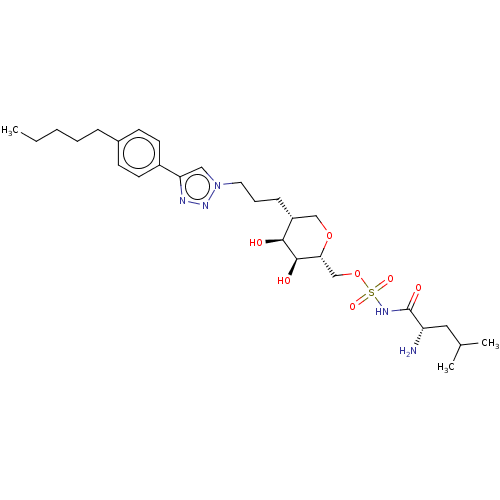
(CHEMBL4869028)Show SMILES CCCCCc1ccc(cc1)-c1cn(CCC[C@@H]2CO[C@H](COS(=O)(=O)NC(=O)[C@@H](N)CC(C)C)[C@@H](O)[C@H]2O)nn1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant Escherichia coli LeuRS assessed as inhibition of 14C-radiolabelled leucine transfer to tRNA leucine preincubated for 10 min... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113021
BindingDB Entry DOI: 10.7270/Q2MC93SZ |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM85702

(CAS_5283125 | LTB5 | NSC_5283125)Show SMILES CCC=CCC=CCC(O)C=CC=CC=CC(O)CCCC(O)=O |w:2.1,5.4,10.9,12.11,14.13| Show InChI InChI=1S/C20H30O4/c1-2-3-4-5-6-9-13-18(21)14-10-7-8-11-15-19(22)16-12-17-20(23)24/h3-4,6-11,14-15,18-19,21-22H,2,5,12-13,16-17H2,1H3,(H,23,24) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
J Biol Chem 275: 40686-94 (2000)
Article DOI: 10.1074/jbc.M004512200
BindingDB Entry DOI: 10.7270/Q2XD1066 |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50524359
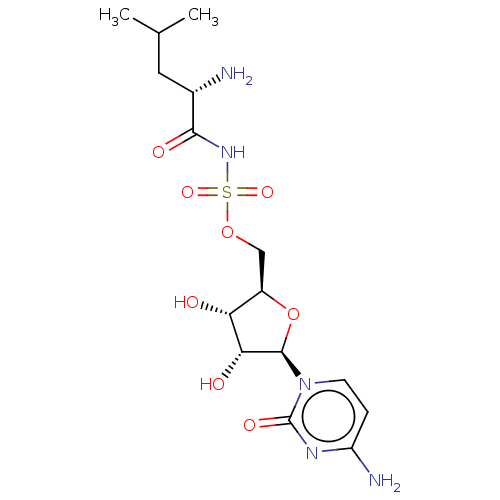
(CHEMBL4465674)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(N)nc1=O |r| Show InChI InChI=1S/C15H25N5O8S/c1-7(2)5-8(16)13(23)19-29(25,26)27-6-9-11(21)12(22)14(28-9)20-4-3-10(17)18-15(20)24/h3-4,7-9,11-12,14,21-22H,5-6,16H2,1-2H3,(H,19,23)(H2,17,18,24)/t8-,9+,11+,12+,14+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of purified Escherichia coli BL21 (DE3) leuRS incubated for 10 mins in presence of 14C-labeled leucine by radiolabel transfer assay |
Eur J Med Chem 173: 154-166 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.003
BindingDB Entry DOI: 10.7270/Q27M0CCG |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Escherichia coli) | BDBM50524360
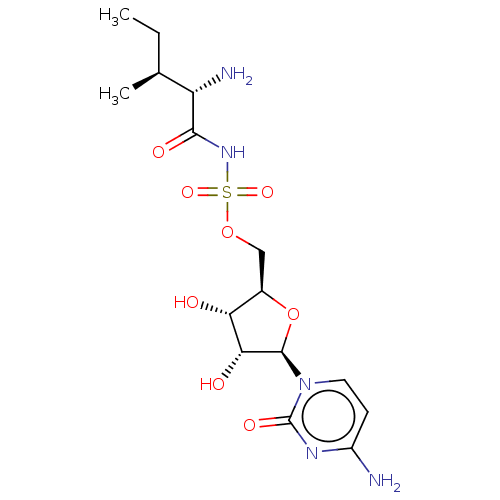
(CHEMBL3265242)Show SMILES CC[C@H](C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(N)nc1=O |r| Show InChI InChI=1S/C15H25N5O8S/c1-3-7(2)10(17)13(23)19-29(25,26)27-6-8-11(21)12(22)14(28-8)20-5-4-9(16)18-15(20)24/h4-5,7-8,10-12,14,21-22H,3,6,17H2,1-2H3,(H,19,23)(H2,16,18,24)/t7-,8+,10-,11+,12+,14+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of purified Escherichia coli BL21 (DE3) ileRS incubated for 10 mins in presence of 14C-labeled isoleucine by radiolabel transfer assay |
Eur J Med Chem 173: 154-166 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.003
BindingDB Entry DOI: 10.7270/Q27M0CCG |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50569957
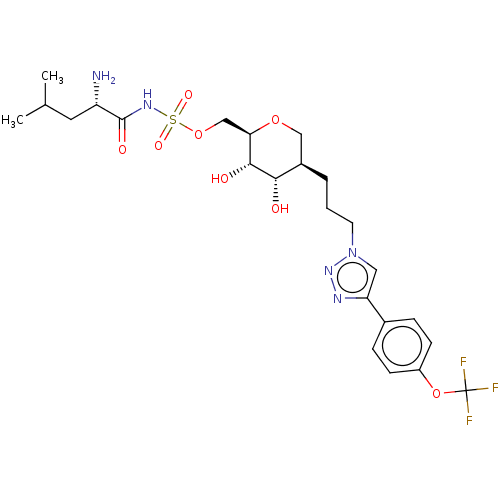
(CHEMBL4864965)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1OC[C@@H](CCCn2cc(nn2)-c2ccc(OC(F)(F)F)cc2)[C@H](O)[C@@H]1O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant Escherichia coli LeuRS assessed as inhibition of 14C-radiolabelled leucine transfer to tRNA leucine preincubated for 10 min... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113021
BindingDB Entry DOI: 10.7270/Q2MC93SZ |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM85697
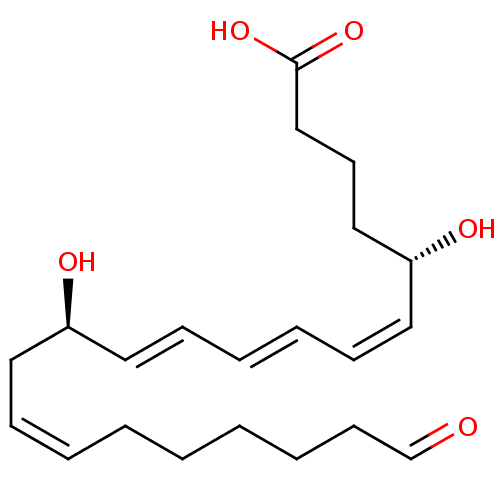
(LTB4-20-carboxy)Show SMILES O[C@@H](CCCC(O)=O)\C=C/C=C/C=C/[C@H](O)C\C=C/CCCCCC=O |r| Show InChI InChI=1S/C21H32O5/c22-18-11-7-3-1-2-4-8-13-19(23)14-9-5-6-10-15-20(24)16-12-17-21(25)26/h4-6,8-10,14-15,18-20,23-24H,1-3,7,11-13,16-17H2,(H,25,26)/b6-5+,8-4-,14-9+,15-10-/t19-,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
J Biol Chem 275: 40686-94 (2000)
Article DOI: 10.1074/jbc.M004512200
BindingDB Entry DOI: 10.7270/Q2XD1066 |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50524355
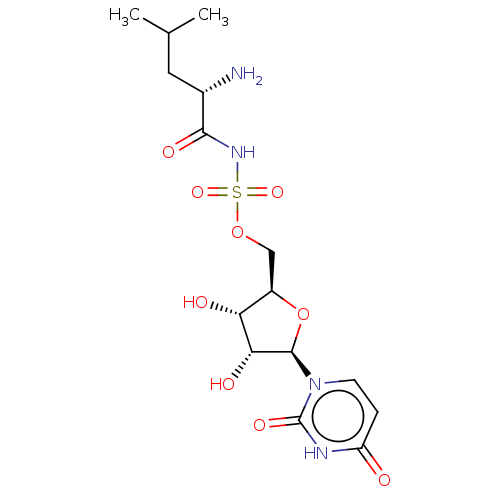
(CHEMBL4467328)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C15H24N4O9S/c1-7(2)5-8(16)13(23)18-29(25,26)27-6-9-11(21)12(22)14(28-9)19-4-3-10(20)17-15(19)24/h3-4,7-9,11-12,14,21-22H,5-6,16H2,1-2H3,(H,18,23)(H,17,20,24)/t8-,9+,11+,12+,14+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of purified Escherichia coli BL21 (DE3) leuRS incubated for 10 mins in presence of 14C-labeled leucine by radiolabel transfer assay |
Eur J Med Chem 173: 154-166 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.003
BindingDB Entry DOI: 10.7270/Q27M0CCG |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM79408
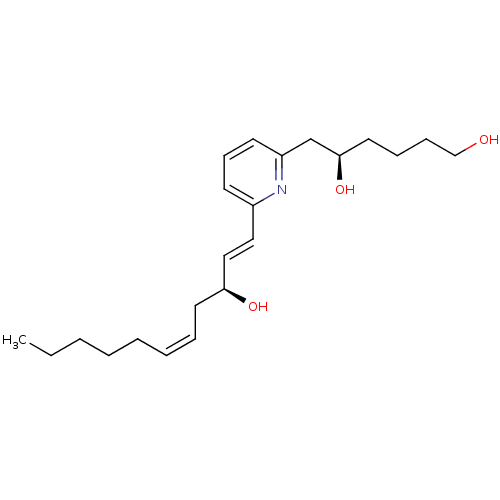
((5R)-6-[6-[(1E,3S,5Z)-3-hydroxyundeca-1,5-dienyl]-...)Show SMILES CCCCC\C=C/C[C@H](O)\C=C\c1cccc(C[C@H](O)CCCCO)n1 Show InChI InChI=1S/C22H35NO3/c1-2-3-4-5-6-7-13-21(25)16-15-19-11-10-12-20(23-19)18-22(26)14-8-9-17-24/h6-7,10-12,15-16,21-22,24-26H,2-5,8-9,13-14,17-18H2,1H3/b7-6-,16-15+/t21-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
J Biol Chem 275: 40686-94 (2000)
Article DOI: 10.1074/jbc.M004512200
BindingDB Entry DOI: 10.7270/Q2XD1066 |
More data for this
Ligand-Target Pair | |
Aspartate--tRNA ligase
(Escherichia coli) | BDBM50458117

(CHEMBL4203761)Show SMILES N[C@@H](CC(O)=O)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nccc12 |r| Show InChI InChI=1S/C15H20N6O9S/c16-6(3-9(22)23)14(26)20-31(27,28)29-4-8-11(24)12(25)15(30-8)21-5-19-10-7(21)1-2-18-13(10)17/h1-2,5-6,8,11-12,15,24-25H,3-4,16H2,(H2,17,18)(H,20,26)(H,22,23)/t6-,8+,11+,12+,15+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli BL21(DE3) Aspartyl-tRNA synthetase assessed as reduction in tRNA aminoacylation preincubated for 10 mins with Escheric... |
Eur J Med Chem 148: 384-396 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.013
BindingDB Entry DOI: 10.7270/Q2057JJS |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM85685
(LTB4-14,15-dehydro)Show SMILES CCCCCC(O)C(=O)C[C@@H](O)C=CC=C\C=C/[C@@H](O)CCCC(O)=O |r,w:15.15,13.13| Show InChI InChI=1S/C20H32O6/c1-2-3-6-13-18(23)19(24)15-17(22)11-8-5-4-7-10-16(21)12-9-14-20(25)26/h4-5,7-8,10-11,16-18,21-23H,2-3,6,9,12-15H2,1H3,(H,25,26)/b5-4?,10-7-,11-8?/t16-,17+,18?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
J Biol Chem 275: 40686-94 (2000)
Article DOI: 10.1074/jbc.M004512200
BindingDB Entry DOI: 10.7270/Q2XD1066 |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50458118
(CHEMBL4208126)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nccc12 |r| Show InChI InChI=1S/C20H24N6O8S/c21-12(7-10-1-3-11(27)4-2-10)19(30)25-35(31,32)33-8-14-16(28)17(29)20(34-14)26-9-24-15-13(26)5-6-23-18(15)22/h1-6,9,12,14,16-17,20,27-29H,7-8,21H2,(H2,22,23)(H,25,30)/t12-,14+,16+,17+,20+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli BL21(DE3) Tyrosyl-tRNA synthetase assessed as reduction in tRNA aminoacylation preincubated for 10 mins with Escherich... |
Eur J Med Chem 148: 384-396 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.013
BindingDB Entry DOI: 10.7270/Q2057JJS |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50524361
(CHEMBL4451258)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)n(C)c1=O |r| Show InChI InChI=1S/C16H26N4O9S/c1-8(2)6-9(17)14(24)18-30(26,27)28-7-10-12(22)13(23)15(29-10)20-5-4-11(21)19(3)16(20)25/h4-5,8-10,12-13,15,22-23H,6-7,17H2,1-3H3,(H,18,24)/t9-,10+,12+,13+,15+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of purified Escherichia coli BL21 (DE3) leuRS incubated for 10 mins in presence of 14C-labeled leucine by radiolabel transfer assay |
Eur J Med Chem 173: 154-166 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.003
BindingDB Entry DOI: 10.7270/Q27M0CCG |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50569960
(CHEMBL4852287)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1OC[C@@H](CCCn2cc(CNC(=O)c3ccccc3)nn2)[C@H](O)[C@@H]1O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant Escherichia coli LeuRS assessed as inhibition of 14C-radiolabelled leucine transfer to tRNA leucine preincubated for 10 min... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113021
BindingDB Entry DOI: 10.7270/Q2MC93SZ |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50569963
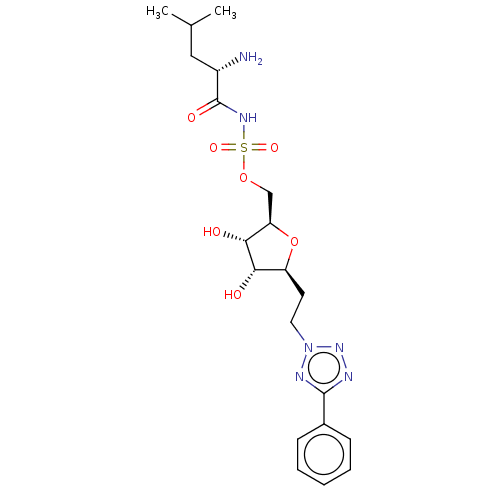
(CHEMBL4855635)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@@H](CCn2nnc(n2)-c2ccccc2)[C@H](O)[C@@H]1O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant Escherichia coli LeuRS assessed as inhibition of 14C-radiolabelled leucine transfer to tRNA leucine preincubated for 10 min... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113021
BindingDB Entry DOI: 10.7270/Q2MC93SZ |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50569958

(CHEMBL4864716)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1OC[C@@H](CCCn2cc(nn2)-c2cccnc2)[C@H](O)[C@@H]1O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant Escherichia coli LeuRS assessed as inhibition of 14C-radiolabelled leucine transfer to tRNA leucine preincubated for 10 min... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113021
BindingDB Entry DOI: 10.7270/Q2MC93SZ |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM85696

(LTB4-20-hydroxy)Show SMILES OCCCCC\C=C/C[C@@H](O)\C=C\C=C\C=C/[C@@H](O)CCCC(O)=O |r| Show InChI InChI=1S/C20H32O5/c21-17-10-6-2-1-3-7-12-18(22)13-8-4-5-9-14-19(23)15-11-16-20(24)25/h3-5,7-9,13-14,18-19,21-23H,1-2,6,10-12,15-17H2,(H,24,25)/b5-4+,7-3-,13-8+,14-9-/t18-,19-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
J Biol Chem 275: 40686-94 (2000)
Article DOI: 10.1074/jbc.M004512200
BindingDB Entry DOI: 10.7270/Q2XD1066 |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50524357
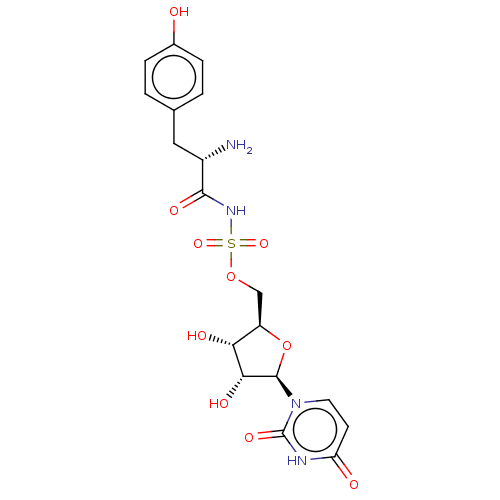
(CHEMBL4558119)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C18H22N4O10S/c19-11(7-9-1-3-10(23)4-2-9)16(27)21-33(29,30)31-8-12-14(25)15(26)17(32-12)22-6-5-13(24)20-18(22)28/h1-6,11-12,14-15,17,23,25-26H,7-8,19H2,(H,21,27)(H,20,24,28)/t11-,12+,14+,15+,17+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of purified Escherichia coli BL21 (DE3) tyrRS incubated for 10 mins in presence of 14C-labeled tyrosine by radiolabel transfer assay |
Eur J Med Chem 173: 154-166 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.003
BindingDB Entry DOI: 10.7270/Q27M0CCG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50569954
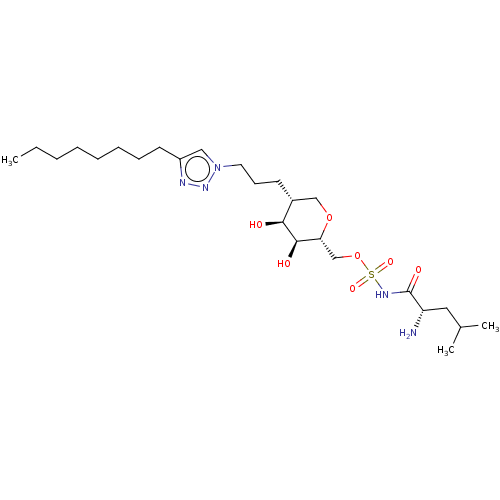
(CHEMBL4867227)Show SMILES CCCCCCCCc1cn(CCC[C@@H]2CO[C@H](COS(=O)(=O)NC(=O)[C@@H](N)CC(C)C)[C@@H](O)[C@H]2O)nn1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant Escherichia coli LeuRS assessed as inhibition of 14C-radiolabelled leucine transfer to tRNA leucine preincubated for 10 min... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113021
BindingDB Entry DOI: 10.7270/Q2MC93SZ |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Escherichia coli) | BDBM50524354

(CHEMBL3265243)Show SMILES CC[C@H](C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C15H24N4O9S/c1-3-7(2)10(16)13(23)18-29(25,26)27-6-8-11(21)12(22)14(28-8)19-5-4-9(20)17-15(19)24/h4-5,7-8,10-12,14,21-22H,3,6,16H2,1-2H3,(H,18,23)(H,17,20,24)/t7-,8+,10-,11+,12+,14+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of purified Escherichia coli BL21 (DE3) ileRS incubated for 10 mins in presence of 14C-labeled isoleucine by radiolabel transfer assay |
Eur J Med Chem 173: 154-166 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.003
BindingDB Entry DOI: 10.7270/Q27M0CCG |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Escherichia coli) | BDBM50544145

(CHEMBL4636583)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1OC[C@@H](CCCn2cc(nn2)-c2ccccc2)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C23H35N5O7S/c1-3-15(2)20(24)23(31)26-36(32,33)35-14-19-22(30)21(29)17(13-34-19)10-7-11-28-12-18(25-27-28)16-8-5-4-6-9-16/h4-6,8-9,12,15,17,19-22,29-30H,3,7,10-11,13-14,24H2,1-2H3,(H,26,31)/t15?,17-,19-,20+,21+,22-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli BL21(DE3) ileRS overexpressed in Escherichia coli Rosetta2(DE3) assessed as reduction in tRNA aminoacylation preincuba... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115580
BindingDB Entry DOI: 10.7270/Q2XK8K4Q |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50569955
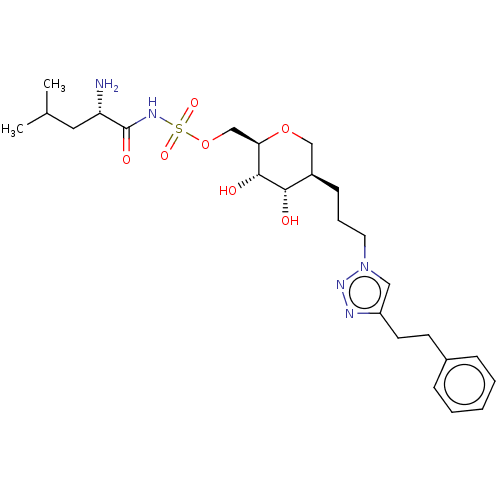
(CHEMBL4876966)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1OC[C@@H](CCCn2cc(CCc3ccccc3)nn2)[C@H](O)[C@@H]1O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant Escherichia coli LeuRS assessed as inhibition of 14C-radiolabelled leucine transfer to tRNA leucine preincubated for 10 min... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113021
BindingDB Entry DOI: 10.7270/Q2MC93SZ |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Escherichia coli) | BDBM50544146

(CHEMBL4648043)Show SMILES CCC(C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1OC[C@@H](OCCn2cc(nn2)-c2ccccc2)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C22H33N5O8S/c1-3-14(2)19(23)22(30)25-36(31,32)35-13-18-21(29)20(28)17(12-34-18)33-10-9-27-11-16(24-26-27)15-7-5-4-6-8-15/h4-8,11,14,17-21,28-29H,3,9-10,12-13,23H2,1-2H3,(H,25,30)/t14?,17-,18-,19+,20+,21-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli BL21(DE3) ileRS overexpressed in Escherichia coli Rosetta2(DE3) assessed as reduction in tRNA aminoacylation preincuba... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115580
BindingDB Entry DOI: 10.7270/Q2XK8K4Q |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50524353

(CHEMBL4518549)Show SMILES Cn1c(=O)ccn([C@@H]2O[C@H](COS(=O)(=O)NC(=O)[C@@H](N)Cc3ccc(O)cc3)[C@@H](O)[C@H]2O)c1=O |r| Show InChI InChI=1S/C19H24N4O10S/c1-22-14(25)6-7-23(19(22)29)18-16(27)15(26)13(33-18)9-32-34(30,31)21-17(28)12(20)8-10-2-4-11(24)5-3-10/h2-7,12-13,15-16,18,24,26-27H,8-9,20H2,1H3,(H,21,28)/t12-,13+,15+,16+,18+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of purified Escherichia coli BL21 (DE3) tyrRS incubated for 10 mins in presence of 14C-labeled tyrosine by radiolabel transfer assay |
Eur J Med Chem 173: 154-166 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.003
BindingDB Entry DOI: 10.7270/Q27M0CCG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM85703
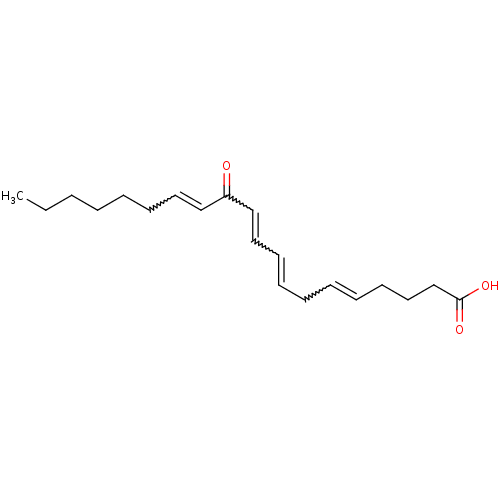
(CAS_5283162 | ETE-12-Oxo | NSC_5283162)Show SMILES CCCCCCC=CC(=O)C=CC=CCC=CCCCC(O)=O |w:6.5,10.9,12.11,15.14| Show InChI InChI=1S/C20H30O3/c1-2-3-4-5-10-13-16-19(21)17-14-11-8-6-7-9-12-15-18-20(22)23/h7-9,11,13-14,16-17H,2-6,10,12,15,18H2,1H3,(H,22,23) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
J Biol Chem 275: 40686-94 (2000)
Article DOI: 10.1074/jbc.M004512200
BindingDB Entry DOI: 10.7270/Q2XD1066 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM50025845
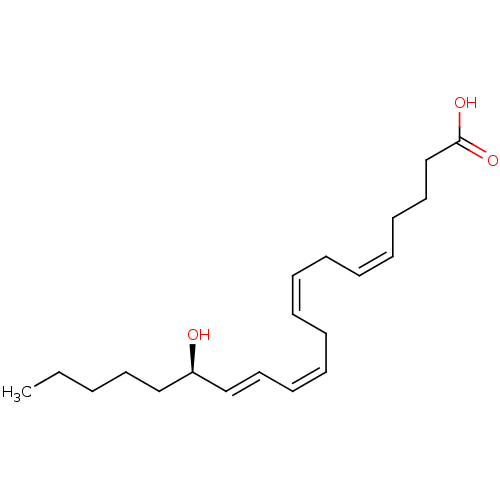
(115-hydroxy-(5Z,8Z,11Z,13E,15R)-5,8,11,13-icosatet...)Show SMILES CCCCC[C@@H](O)\C=C\C=C/C\C=C/C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O3/c1-2-3-13-16-19(21)17-14-11-9-7-5-4-6-8-10-12-15-18-20(22)23/h4-5,8-11,14,17,19,21H,2-3,6-7,12-13,15-16,18H2,1H3,(H,22,23)/b5-4-,10-8-,11-9-,17-14+/t19-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 173 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
J Biol Chem 275: 40686-94 (2000)
Article DOI: 10.1074/jbc.M004512200
BindingDB Entry DOI: 10.7270/Q2XD1066 |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50569961
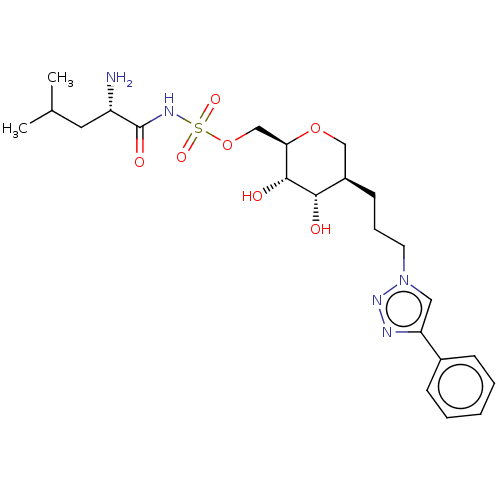
(CHEMBL4861722)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1OC[C@@H](CCCn2cc(nn2)-c2ccccc2)[C@H](O)[C@@H]1O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant Escherichia coli LeuRS assessed as inhibition of 14C-radiolabelled leucine transfer to tRNA leucine preincubated for 10 min... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113021
BindingDB Entry DOI: 10.7270/Q2MC93SZ |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase
(Escherichia coli) | BDBM50458120
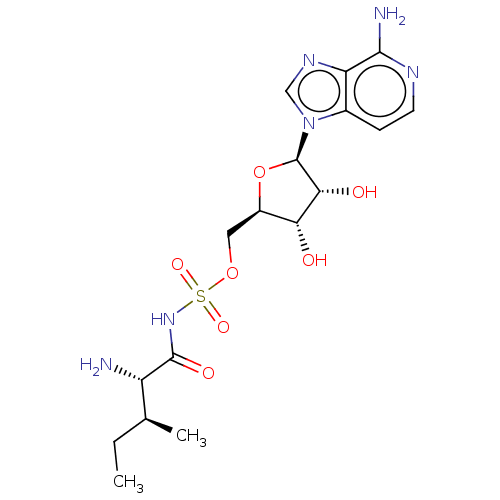
(CHEMBL4215986)Show SMILES CC[C@H](C)[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nccc12 |r| Show InChI InChI=1S/C17H26N6O7S/c1-3-8(2)11(18)16(26)22-31(27,28)29-6-10-13(24)14(25)17(30-10)23-7-21-12-9(23)4-5-20-15(12)19/h4-5,7-8,10-11,13-14,17,24-25H,3,6,18H2,1-2H3,(H2,19,20)(H,22,26)/t8-,10+,11-,13+,14+,17+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 213 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli BL21(DE3) Isoleucyl-tRNA synthetase assessed as reduction in tRNA aminoacylation preincubated for 10 mins with Escheri... |
Eur J Med Chem 148: 384-396 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.013
BindingDB Entry DOI: 10.7270/Q2057JJS |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM85689
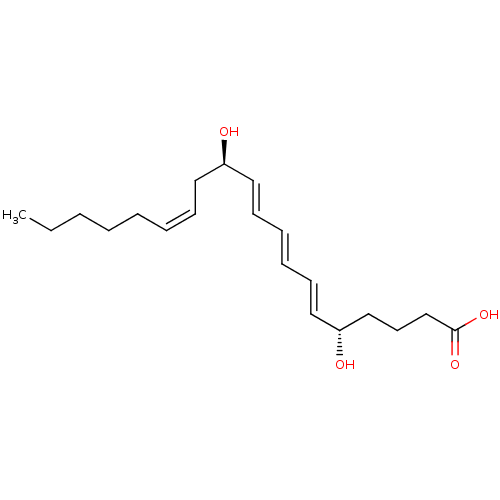
(LTB4-6-trans)Show SMILES CCCCC\C=C/C[C@@H](O)\C=C\C=C\C=C\[C@@H](O)CCCC(O)=O |r| Show InChI InChI=1S/C20H32O4/c1-2-3-4-5-6-9-13-18(21)14-10-7-8-11-15-19(22)16-12-17-20(23)24/h6-11,14-15,18-19,21-22H,2-5,12-13,16-17H2,1H3,(H,23,24)/b8-7+,9-6-,14-10+,15-11+/t18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 336 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
J Biol Chem 275: 40686-94 (2000)
Article DOI: 10.1074/jbc.M004512200
BindingDB Entry DOI: 10.7270/Q2XD1066 |
More data for this
Ligand-Target Pair | |
Leucine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50569956
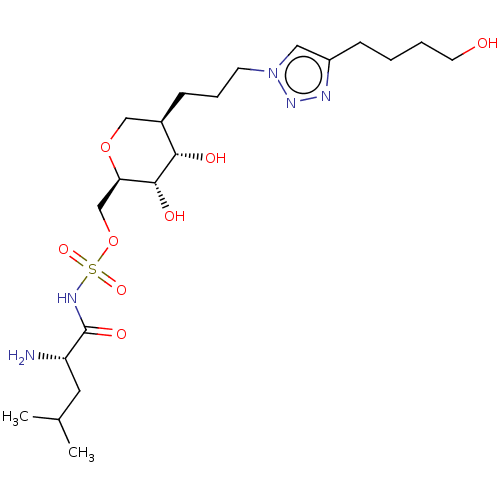
(CHEMBL4850408)Show SMILES CC(C)C[C@H](N)C(=O)NS(=O)(=O)OC[C@H]1OC[C@@H](CCCn2cc(CCCCO)nn2)[C@H](O)[C@@H]1O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 388 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant Escherichia coli LeuRS assessed as inhibition of 14C-radiolabelled leucine transfer to tRNA leucine preincubated for 10 min... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113021
BindingDB Entry DOI: 10.7270/Q2MC93SZ |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM85685

(LTB4-14,15-dehydro)Show SMILES CCCCCC(O)C(=O)C[C@@H](O)C=CC=C\C=C/[C@@H](O)CCCC(O)=O |r,w:15.15,13.13| Show InChI InChI=1S/C20H32O6/c1-2-3-6-13-18(23)19(24)15-17(22)11-8-5-4-7-10-16(21)12-9-14-20(25)26/h4-5,7-8,10-11,16-18,21-23H,2-3,6,9,12-15H2,1H3,(H,25,26)/b5-4?,10-7-,11-8?/t16-,17+,18?/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 473 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
J Biol Chem 275: 40686-94 (2000)
Article DOI: 10.1074/jbc.M004512200
BindingDB Entry DOI: 10.7270/Q2XD1066 |
More data for this
Ligand-Target Pair | |
Serine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50458116
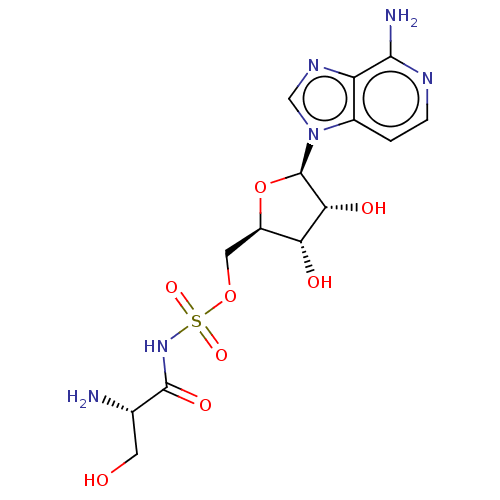
(CHEMBL4208640)Show SMILES N[C@@H](CO)C(=O)NS(=O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nccc12 |r| Show InChI InChI=1S/C14H20N6O8S/c15-6(3-21)13(24)19-29(25,26)27-4-8-10(22)11(23)14(28-8)20-5-18-9-7(20)1-2-17-12(9)16/h1-2,5-6,8,10-11,14,21-23H,3-4,15H2,(H2,16,17)(H,19,24)/t6-,8+,10+,11+,14+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 523 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli BL21(DE3) Seryl-tRNA synthetase assessed as reduction in tRNA aminoacylation preincubated for 10 mins with Escherichia... |
Eur J Med Chem 148: 384-396 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.013
BindingDB Entry DOI: 10.7270/Q2057JJS |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM85689

(LTB4-6-trans)Show SMILES CCCCC\C=C/C[C@@H](O)\C=C\C=C\C=C\[C@@H](O)CCCC(O)=O |r| Show InChI InChI=1S/C20H32O4/c1-2-3-4-5-6-9-13-18(21)14-10-7-8-11-15-19(22)16-12-17-20(23)24/h6-11,14-15,18-19,21-22H,2-5,12-13,16-17H2,1H3,(H,23,24)/b8-7+,9-6-,14-10+,15-11+/t18-,19-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
J Biol Chem 275: 40686-94 (2000)
Article DOI: 10.1074/jbc.M004512200
BindingDB Entry DOI: 10.7270/Q2XD1066 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM85699
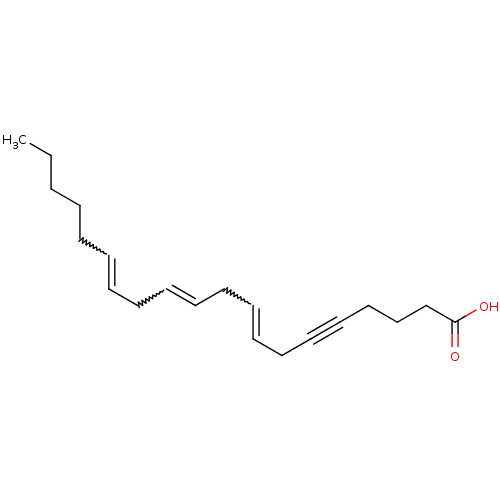
(5, 6-Dehydroarachidonic acid | CAS_1777 | NSC_1777)Show SMILES CCCCCC=CCC=CCC=CCC#CCCCC(O)=O |w:5.4,8.7,11.10| Show InChI InChI=1S/C20H30O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22/h6-7,9-10,12-13H,2-5,8,11,14,17-19H2,1H3,(H,21,22) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
J Biol Chem 275: 40686-94 (2000)
Article DOI: 10.1074/jbc.M004512200
BindingDB Entry DOI: 10.7270/Q2XD1066 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM50024447
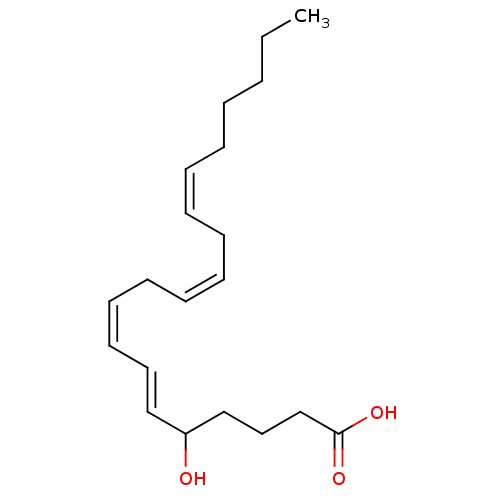
((6E,8Z,11Z,14Z)-5-Hydroxy-icosa-6,8,11,14-tetraeno...)Show InChI InChI=1S/C20H32O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-16-19(21)17-15-18-20(22)23/h6-7,9-10,12-14,16,19,21H,2-5,8,11,15,17-18H2,1H3,(H,22,23)/b7-6-,10-9-,13-12-,16-14+ | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
J Biol Chem 275: 40686-94 (2000)
Article DOI: 10.1074/jbc.M004512200
BindingDB Entry DOI: 10.7270/Q2XD1066 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM85695
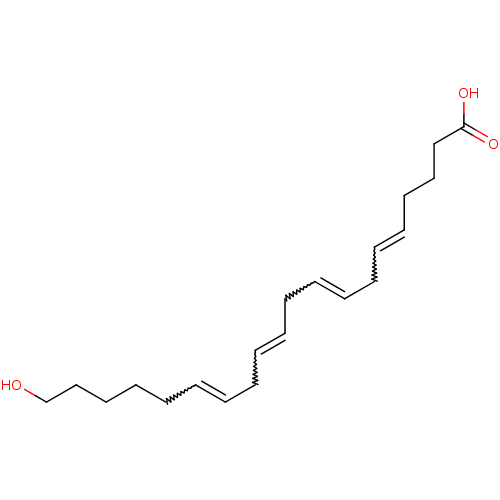
(CAS_5283157 | HETE-20 | NSC_5283157)Show SMILES OCCCCCC=CCC=CCC=CCC=CCCCC(O)=O |w:6.5,9.8,12.11,15.14| Show InChI InChI=1S/C20H32O3/c21-19-17-15-13-11-9-7-5-3-1-2-4-6-8-10-12-14-16-18-20(22)23/h1,3-4,6-7,9-10,12,21H,2,5,8,11,13-19H2,(H,22,23) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
J Biol Chem 275: 40686-94 (2000)
Article DOI: 10.1074/jbc.M004512200
BindingDB Entry DOI: 10.7270/Q2XD1066 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM85684
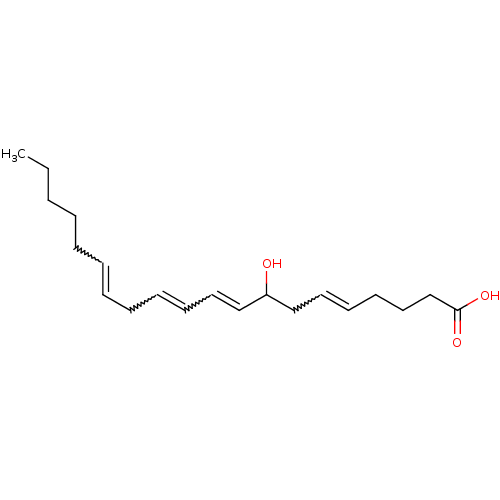
(CAS_5283163 | HETE-8(R ) | NSC_5283163)Show SMILES CCCCCC=CCC=CC=CC(O)CC=CCCCC(O)=O |w:5.4,8.7,10.9,15.14| Show InChI InChI=1S/C20H32O3/c1-2-3-4-5-6-7-8-9-10-13-16-19(21)17-14-11-12-15-18-20(22)23/h6-7,9-11,13-14,16,19,21H,2-5,8,12,15,17-18H2,1H3,(H,22,23) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
J Biol Chem 275: 40686-94 (2000)
Article DOI: 10.1074/jbc.M004512200
BindingDB Entry DOI: 10.7270/Q2XD1066 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 2
(Homo sapiens (Human)) | BDBM85697

(LTB4-20-carboxy)Show SMILES O[C@@H](CCCC(O)=O)\C=C/C=C/C=C/[C@H](O)C\C=C/CCCCCC=O |r| Show InChI InChI=1S/C21H32O5/c22-18-11-7-3-1-2-4-8-13-19(23)14-9-5-6-10-15-20(24)16-12-17-21(25)26/h4-6,8-10,14-15,18-20,23-24H,1-3,7,11-13,16-17H2,(H,25,26)/b6-5+,8-4-,14-9+,15-10-/t19-,20-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
J Biol Chem 275: 40686-94 (2000)
Article DOI: 10.1074/jbc.M004512200
BindingDB Entry DOI: 10.7270/Q2XD1066 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data