

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547574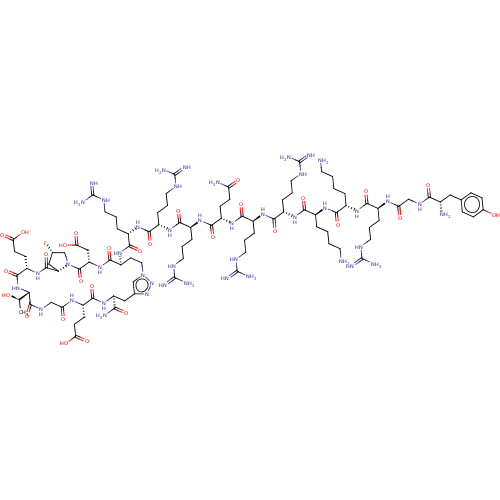 (CHEMBL4747964) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547568 (CHEMBL4791554) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547569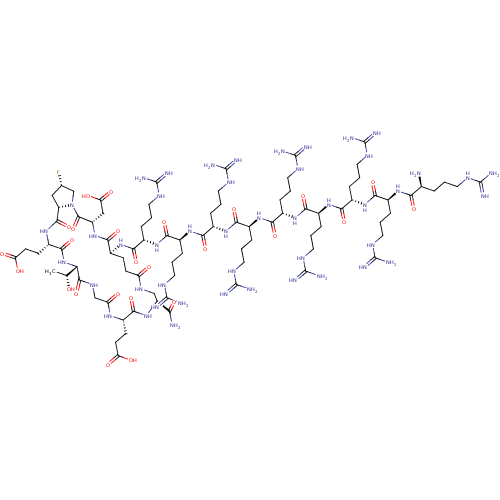 (CHEMBL4764193) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547572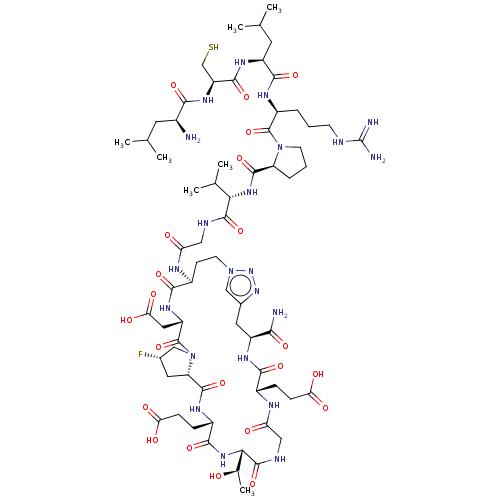 (CHEMBL4788971) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547573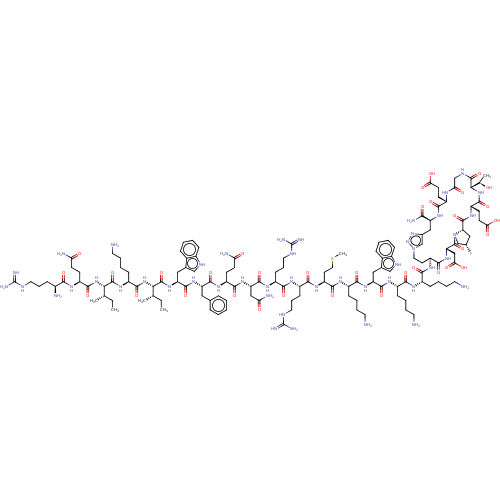 (CHEMBL4747827) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547571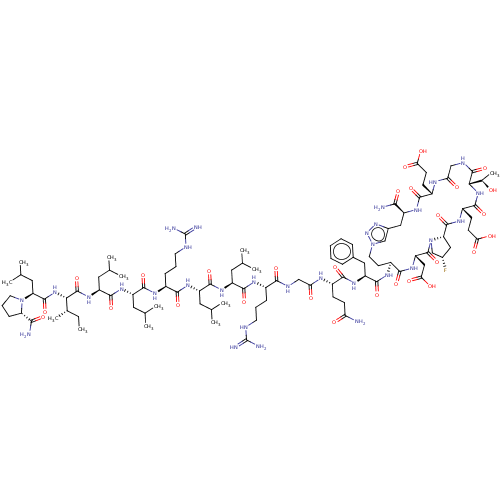 (CHEMBL4755277) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547566 (CHEMBL4790062) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547567 (CHEMBL4781886) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169942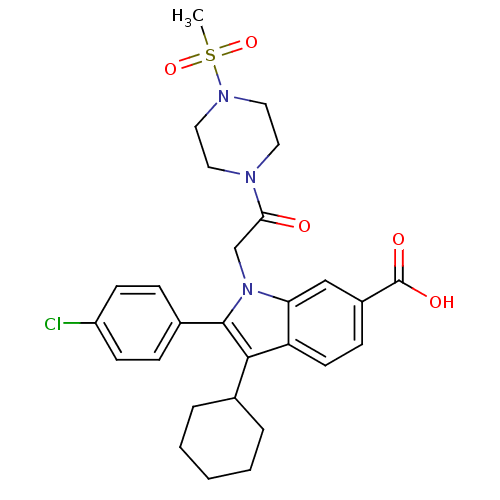 (2-(4-Chloro-phenyl)-3-cyclohexyl-1-[2-(4-methanesu...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547575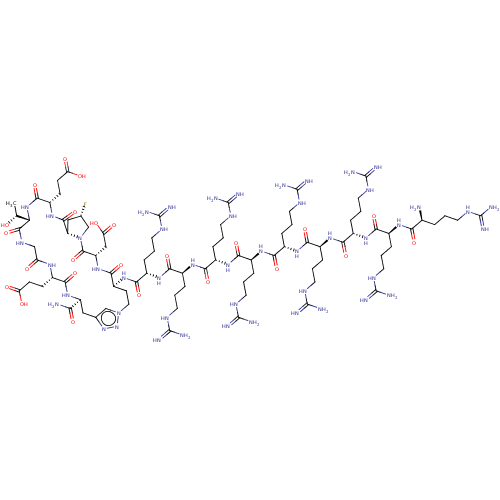 (CHEMBL4740349) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547565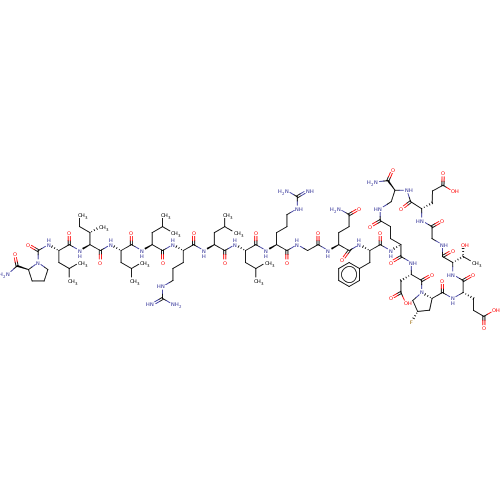 (CHEMBL4756743) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169930 (2-(4-Chloro-phenyl)-3-cyclohexyl-1-[(cyclopropylme...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169904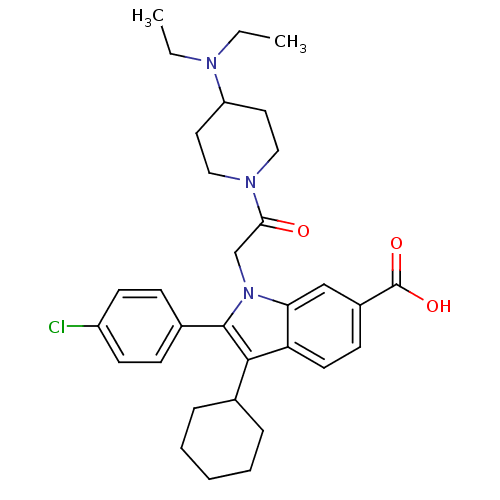 (2-(4-Chloro-phenyl)-3-cyclohexyl-1-[2-(4-diethylam...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS5B polymerase enzyme; Range=6-9 nM | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169922 (3-Cyclohexyl-1-[2-(4-diethylamino-piperidin-1-yl)-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS5B polymerase enzyme; Range=6-9 nM | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169906 (3-Cyclohexyl-1-[2-(2-diethylaminomethyl-morpholin-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS5B polymerase enzyme; Range=6-9 nM | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169899 (3-Cyclohexyl-1-[2-(4-dimethylamino-piperidin-1-yl)...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS5B polymerase enzyme; Range=6-9 nM | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169911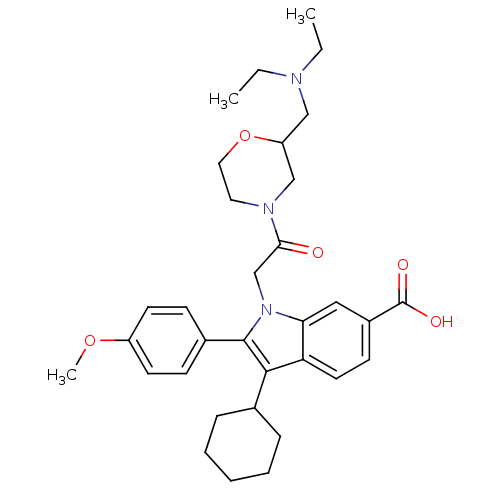 (3-Cyclohexyl-1-[2-(2-diethylaminomethyl-morpholin-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS5B polymerase enzyme; Range=6-9 nM | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 1b (isolate BK) (HCV)) | BDBM35554 (thiophene scaffold, 21) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 23 |
IRBM-MRL Rome | Assay Description Primer-dependent assays were performed using the homopolymeric template/primer. Compounds, polymerase and template RNA were incubated at RT for 25 mi... | J Med Chem 52: 5217-27 (2009) Article DOI: 10.1021/jm900517t BindingDB Entry DOI: 10.7270/Q2X928P4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547516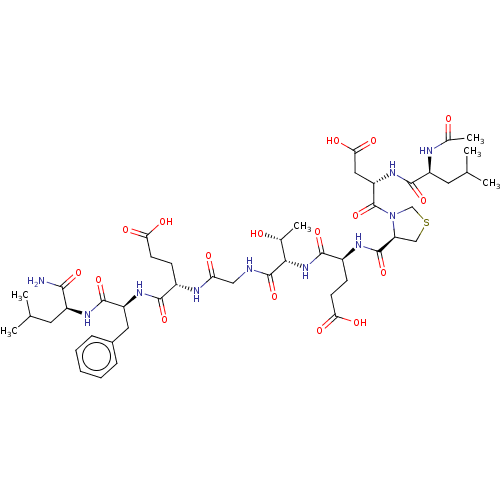 (CHEMBL4762897) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169903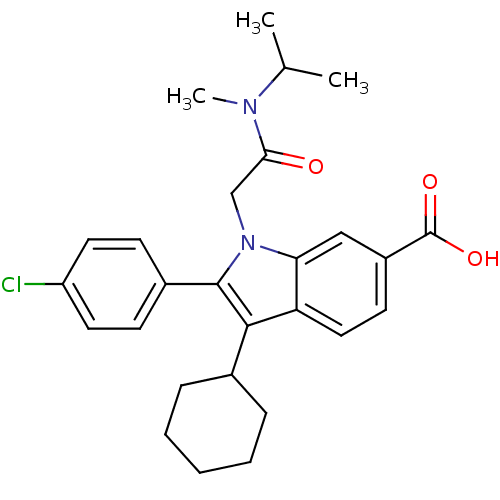 (2-(4-Chloro-phenyl)-3-cyclohexyl-1-[(isopropyl-met...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547550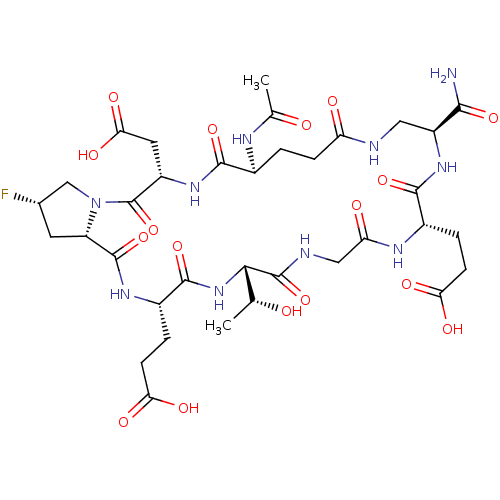 (CHEMBL4760303) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547560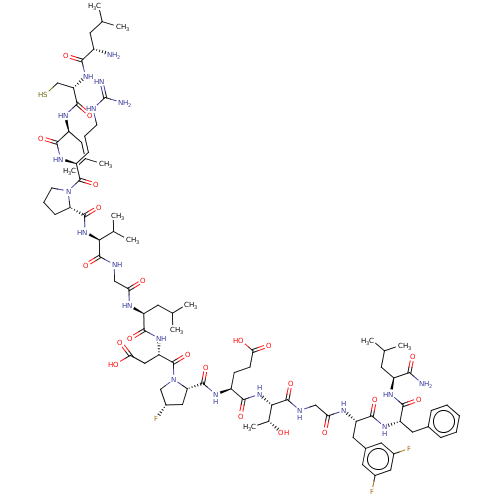 (CHEMBL4792032) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169915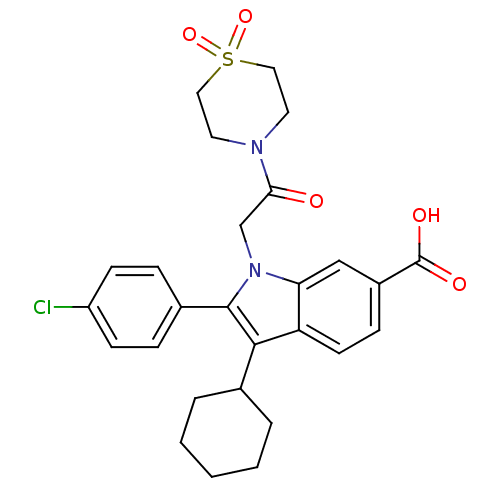 (2-(4-Chloro-phenyl)-3-cyclohexyl-1-[2-(1,1-dioxo-1...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169920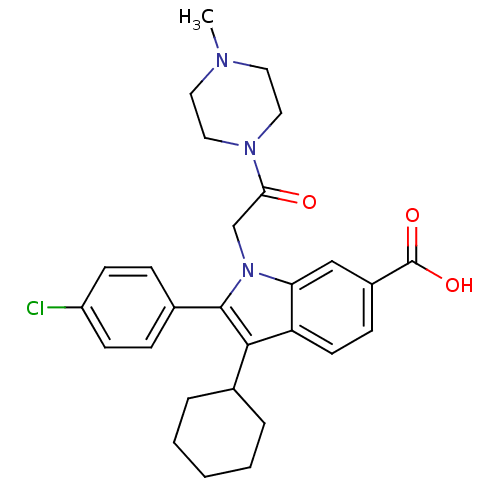 (2-(4-Chloro-phenyl)-3-cyclohexyl-1-[2-(4-methyl-pi...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169931 (2-(4-Chloro-phenyl)-3-cyclohexyl-1-{[methyl-(1-met...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547515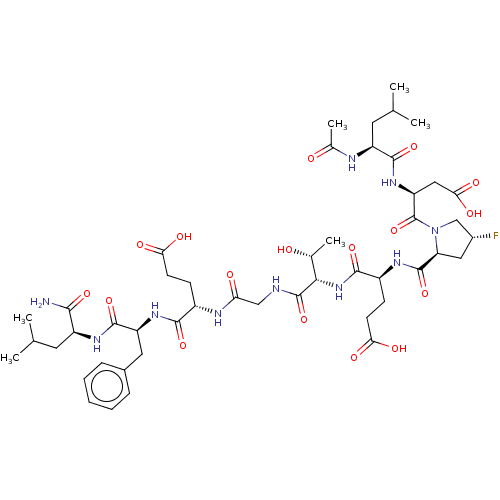 (CHEMBL4754176) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169919 (2-(4-Chloro-phenyl)-3-cyclohexyl-1-[2-(4-dimethyla...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 1b (isolate BK) (HCV)) | BDBM35570 (1H-benzo[de]isoquinoline-1,3(2H)-dione, 19) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <14 | n/a | 2.20E+3 | n/a | n/a | 7.5 | 23 |
IRBM-MRL Rome | Assay Description Primer-dependent assays were performed using the homopolymeric template/primer. Compounds, polymerase and template RNA were incubated at RT for 25 mi... | J Med Chem 52: 5217-27 (2009) Article DOI: 10.1021/jm900517t BindingDB Entry DOI: 10.7270/Q2X928P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547555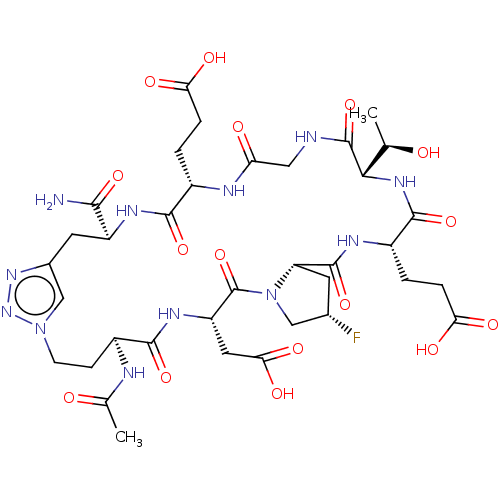 (CHEMBL4794133) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [2420-2989,M2833T,R2963Q] (Hepatitis C virus genotype 1b (isolate BK) (HCV)) | BDBM35554 (thiophene scaffold, 21) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.5 | 23 |
IRBM-MRL Rome | Assay Description Primer-dependent assays were performed using the homopolymeric template/primer. Compounds, polymerase and template RNA were incubated at RT for 25 mi... | J Med Chem 52: 5217-27 (2009) Article DOI: 10.1021/jm900517t BindingDB Entry DOI: 10.7270/Q2X928P4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547576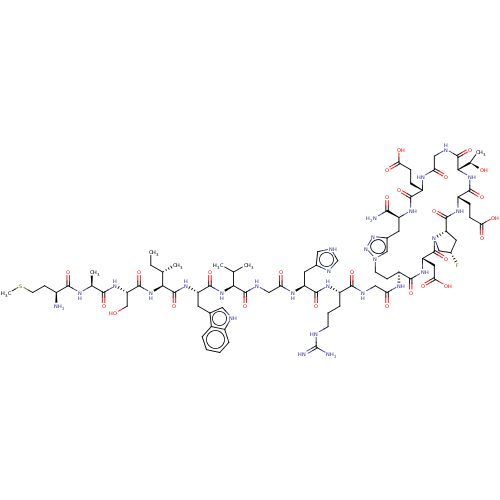 (CHEMBL4740544) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169932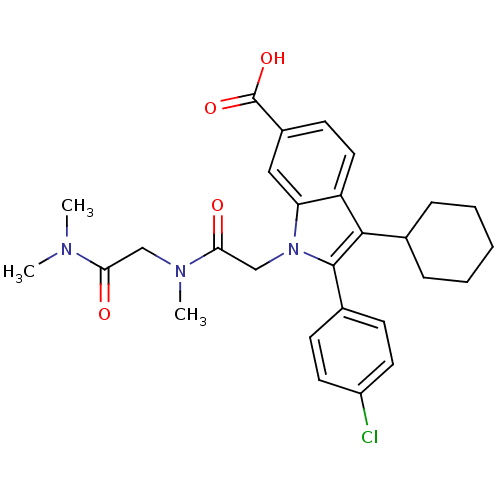 (2-(4-Chloro-phenyl)-3-cyclohexyl-1-[(dimethylcarba...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169921 (3-Cyclohexyl-2-[3-(3,3-difluoro-piperidin-1-ylmeth...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169929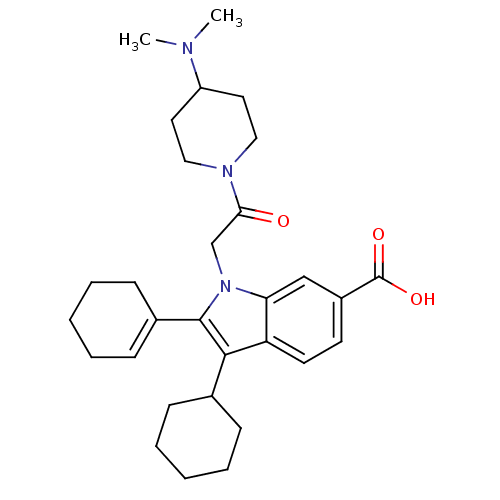 (2-Cyclohex-1-enyl-3-cyclohexyl-1-[2-(4-dimethylami...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169916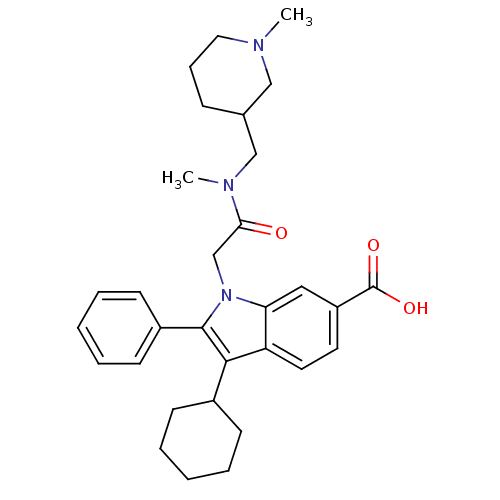 (3-Cyclohexyl-1-{[methyl-(1-methyl-piperidin-3-ylme...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547514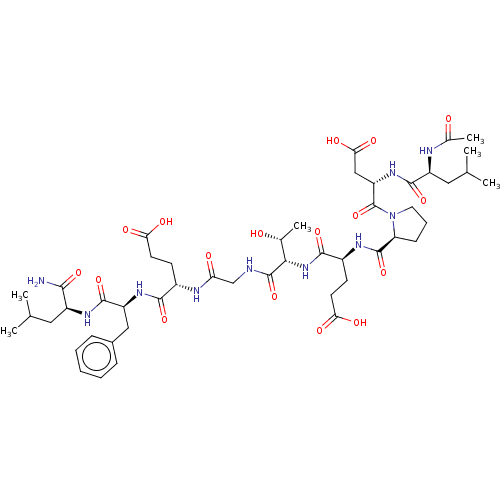 (CHEMBL4746924) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 1b (isolate BK) (HCV)) | BDBM35563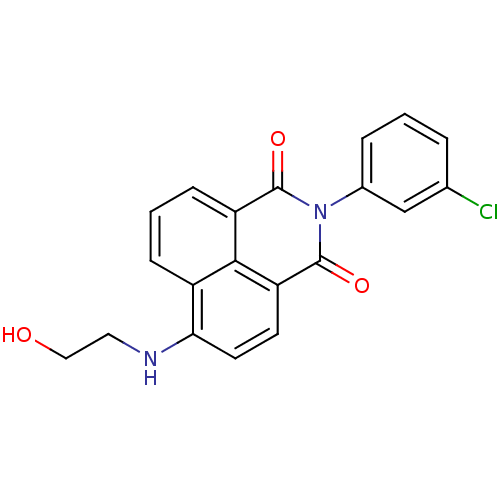 (1H-benzo[de]isoquinoline-1,3(2H)-dione, 12) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | 3.80E+3 | n/a | n/a | 7.5 | 23 |
IRBM-MRL Rome | Assay Description Primer-dependent assays were performed using the homopolymeric template/primer. Compounds, polymerase and template RNA were incubated at RT for 25 mi... | J Med Chem 52: 5217-27 (2009) Article DOI: 10.1021/jm900517t BindingDB Entry DOI: 10.7270/Q2X928P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169910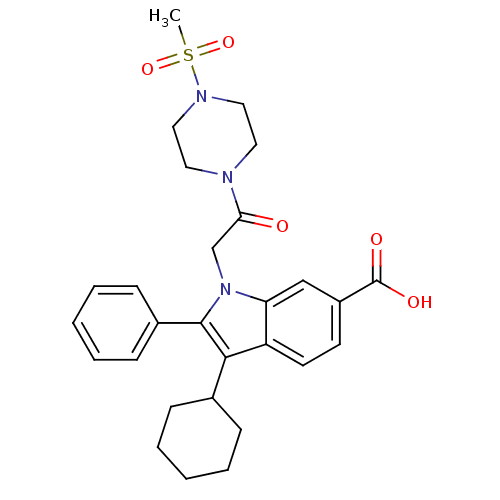 (3-Cyclohexyl-1-[2-(4-methanesulfonyl-piperazin-1-y...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 1b (isolate BK) (HCV)) | BDBM35553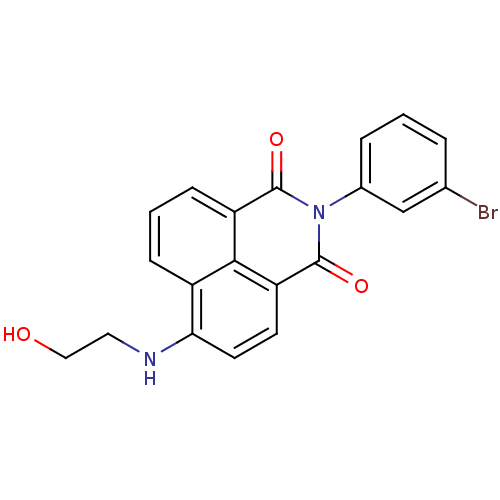 (1H-benzo[de]isoquinoline-1,3(2H)-dione, 1) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 20 | n/a | 7.10E+3 | n/a | n/a | 7.5 | 23 |
IRBM-MRL Rome | Assay Description Primer-dependent assays were performed using the homopolymeric template/primer. Compounds, polymerase and template RNA were incubated at RT for 25 mi... | J Med Chem 52: 5217-27 (2009) Article DOI: 10.1021/jm900517t BindingDB Entry DOI: 10.7270/Q2X928P4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein [2420-2989,P2914A,R2963Q] (Hepatitis C virus genotype 1b (isolate BK) (HCV)) | BDBM35553 (1H-benzo[de]isoquinoline-1,3(2H)-dione, 1) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | 23 |
IRBM-MRL Rome | Assay Description Primer-dependent assays were performed using the homopolymeric template/primer. Compounds, polymerase and template RNA were incubated at RT for 25 mi... | J Med Chem 52: 5217-27 (2009) Article DOI: 10.1021/jm900517t BindingDB Entry DOI: 10.7270/Q2X928P4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547562 (CHEMBL4751630) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 1b (isolate BK) (HCV)) | BDBM35560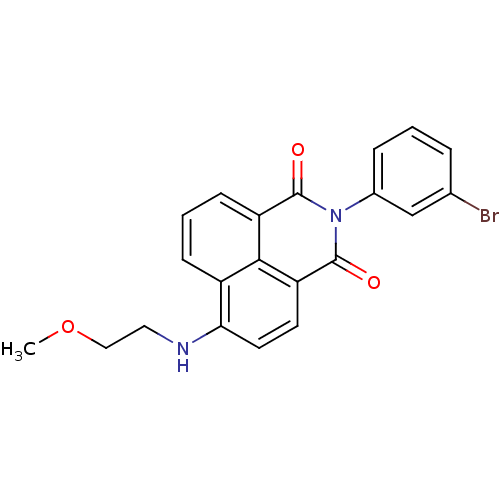 (1H-benzo[de]isoquinoline-1,3(2H)-dione, 9) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | 23 |
IRBM-MRL Rome | Assay Description Primer-dependent assays were performed using the homopolymeric template/primer. Compounds, polymerase and template RNA were incubated at RT for 25 mi... | J Med Chem 52: 5217-27 (2009) Article DOI: 10.1021/jm900517t BindingDB Entry DOI: 10.7270/Q2X928P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547563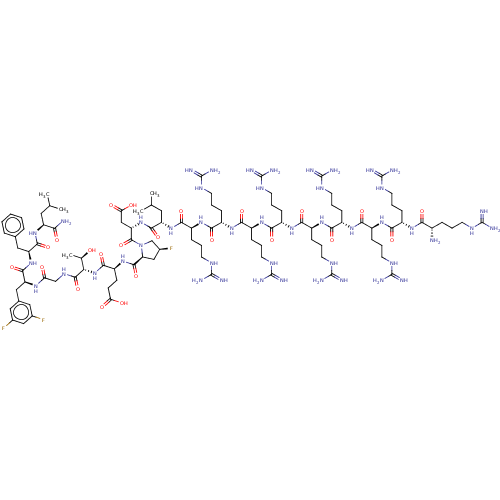 (CHEMBL4788462) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547570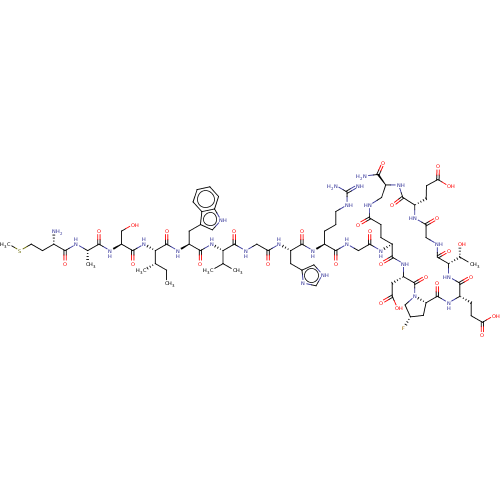 (CHEMBL4785277) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 1b (isolate BK) (HCV)) | BDBM35558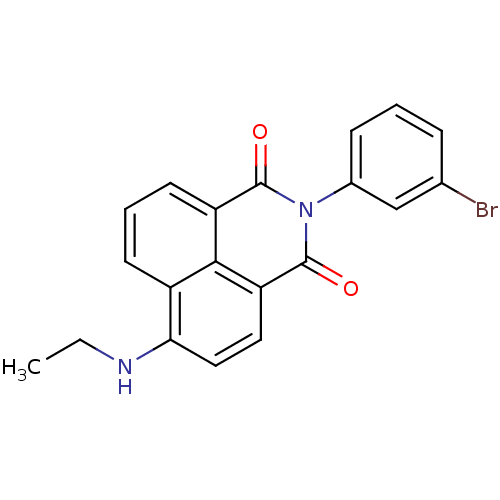 (1H-benzo[de]isoquinoline-1,3(2H)-dione, 7) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | 7.5 | 23 |
IRBM-MRL Rome | Assay Description Primer-dependent assays were performed using the homopolymeric template/primer. Compounds, polymerase and template RNA were incubated at RT for 25 mi... | J Med Chem 52: 5217-27 (2009) Article DOI: 10.1021/jm900517t BindingDB Entry DOI: 10.7270/Q2X928P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 1b (isolate BK) (HCV)) | BDBM35569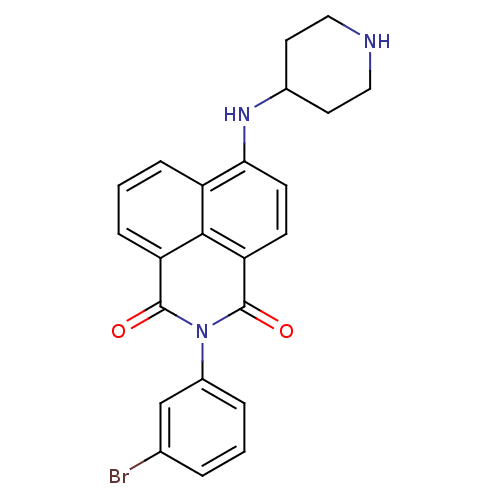 (1H-benzo[de]isoquinoline-1,3(2H)-dione, 18) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | 1.90E+3 | n/a | n/a | 7.5 | 23 |
IRBM-MRL Rome | Assay Description Primer-dependent assays were performed using the homopolymeric template/primer. Compounds, polymerase and template RNA were incubated at RT for 25 mi... | J Med Chem 52: 5217-27 (2009) Article DOI: 10.1021/jm900517t BindingDB Entry DOI: 10.7270/Q2X928P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547561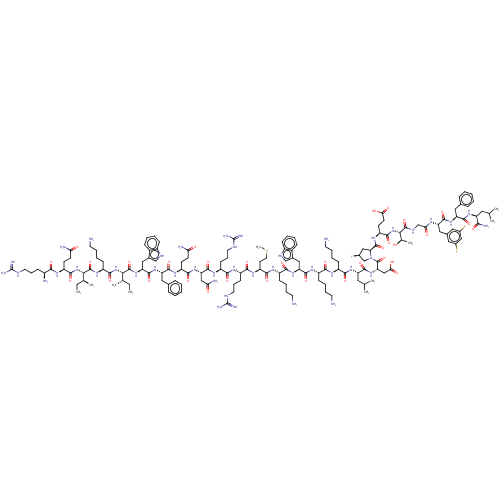 (CHEMBL4750320) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50169927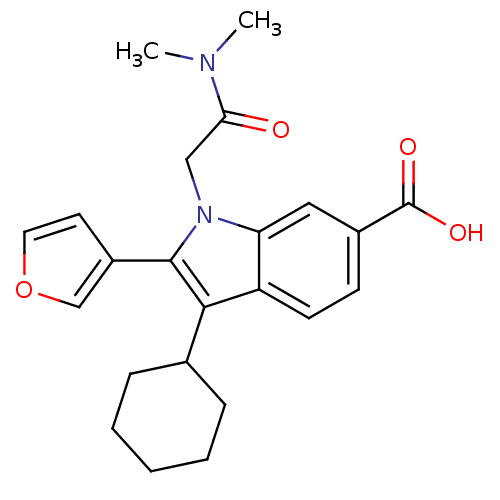 (3-Cyclohexyl-1-dimethylcarbamoylmethyl-2-furan-3-y...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against by hepatitis C virus NS5B polymerase | J Med Chem 48: 4547-57 (2005) Article DOI: 10.1021/jm050056+ BindingDB Entry DOI: 10.7270/Q24B30VN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kelch-like ECH-associated protein 1 (Homo sapiens (Human)) | BDBM50547552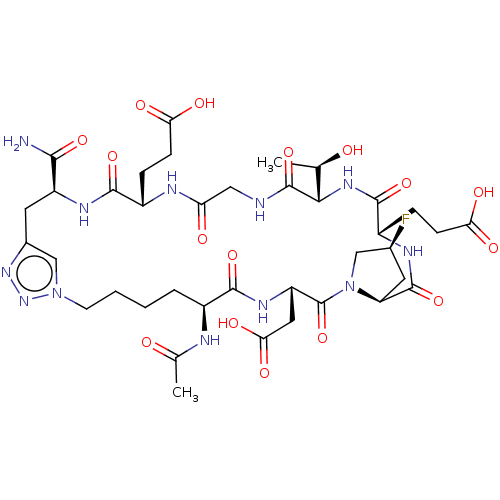 (CHEMBL4754751) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human KEAP1 Kelch domain (322 to 609 residues) incubated for 60 mins by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115738 BindingDB Entry DOI: 10.7270/Q2QV3R4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50158845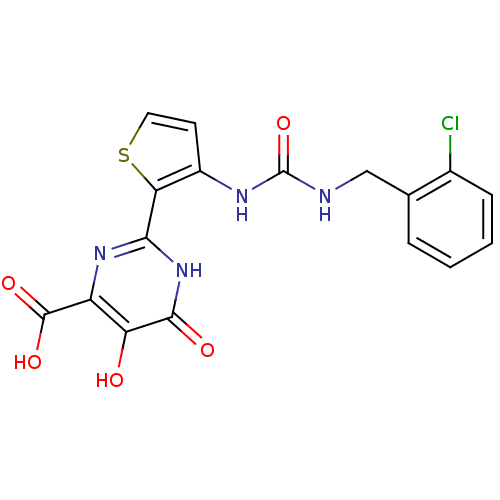 (2-(3-(3-(2-chlorobenzyl)ureido)thiophen-2-yl)-5,6-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
P. Angeletti S.p.A. (Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HCV 1b BK NS5B deltaC55 RNA polymerase involving manganese chelation | J Med Chem 49: 1693-705 (2006) Article DOI: 10.1021/jm051064t BindingDB Entry DOI: 10.7270/Q2QJ7GWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 271 total ) | Next | Last >> |