 Found 250 hits with Last Name = 'carballo' and Initial = 'lh'
Found 250 hits with Last Name = 'carballo' and Initial = 'lh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244020
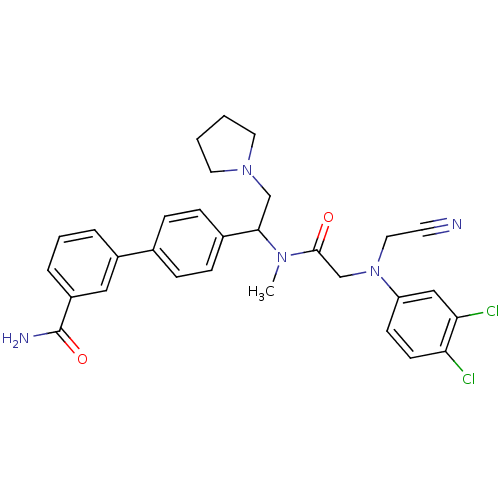
(4'-[1-({2-[Cyanomethyl-(3,4-dichloro-phenyl)-amino...)Show SMILES CN(C(CN1CCCC1)c1ccc(cc1)-c1cccc(c1)C(N)=O)C(=O)CN(CC#N)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C30H31Cl2N5O2/c1-35(29(38)20-37(16-13-33)25-11-12-26(31)27(32)18-25)28(19-36-14-2-3-15-36)22-9-7-21(8-10-22)23-5-4-6-24(17-23)30(34)39/h4-12,17-18,28H,2-3,14-16,19-20H2,1H3,(H2,34,39) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377220
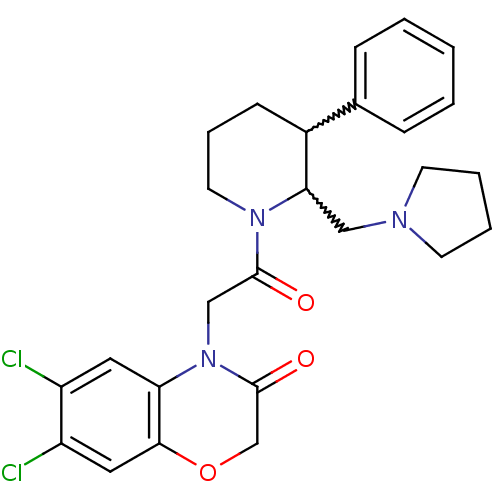
(CHEMBL255509)Show SMILES Clc1cc2OCC(=O)N(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2cc1Cl |w:17.18,16.25| Show InChI InChI=1S/C26H29Cl2N3O3/c27-20-13-22-24(14-21(20)28)34-17-26(33)31(22)16-25(32)30-12-6-9-19(18-7-2-1-3-8-18)23(30)15-29-10-4-5-11-29/h1-3,7-8,13-14,19,23H,4-6,9-12,15-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377218
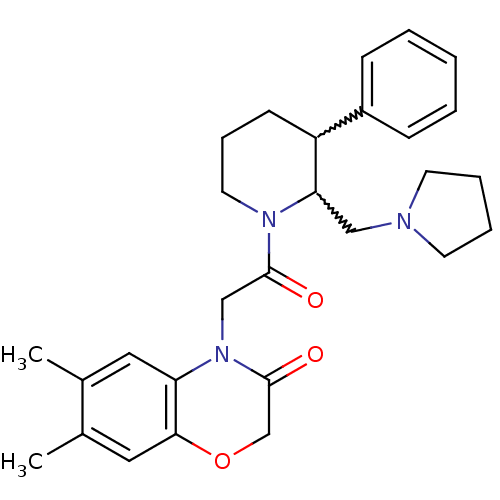
(CHEMBL257171)Show SMILES Cc1cc2OCC(=O)N(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2cc1C |w:17.18,16.25| Show InChI InChI=1S/C28H35N3O3/c1-20-15-24-26(16-21(20)2)34-19-28(33)31(24)18-27(32)30-14-8-11-23(22-9-4-3-5-10-22)25(30)17-29-12-6-7-13-29/h3-5,9-10,15-16,23,25H,6-8,11-14,17-19H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377217
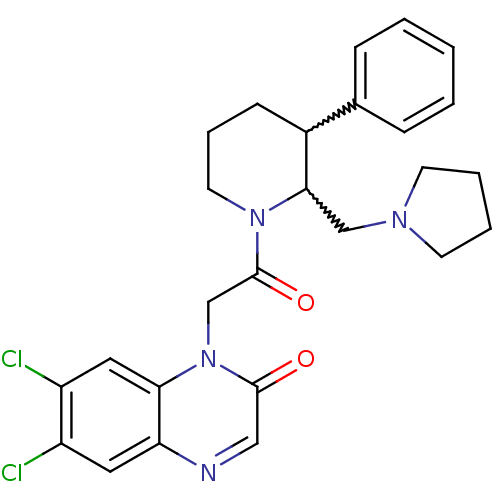
(CHEMBL256989)Show SMILES Clc1cc2ncc(=O)n(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2cc1Cl |w:17.18,16.25| Show InChI InChI=1S/C26H28Cl2N4O2/c27-20-13-22-23(14-21(20)28)32(25(33)15-29-22)17-26(34)31-12-6-9-19(18-7-2-1-3-8-18)24(31)16-30-10-4-5-11-30/h1-3,7-8,13-15,19,24H,4-6,9-12,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377215
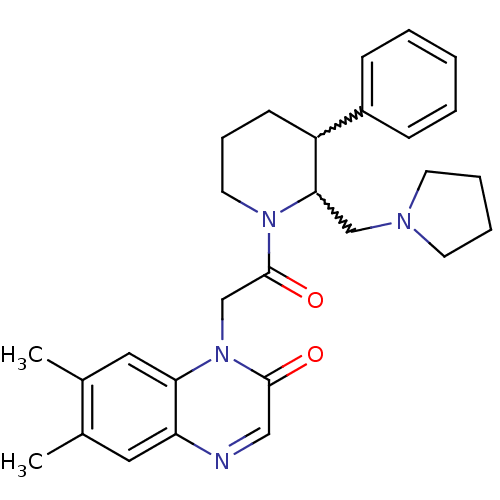
(CHEMBL257415)Show SMILES Cc1cc2ncc(=O)n(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2cc1C |w:17.18,16.25| Show InChI InChI=1S/C28H34N4O2/c1-20-15-24-25(16-21(20)2)32(27(33)17-29-24)19-28(34)31-14-8-11-23(22-9-4-3-5-10-22)26(31)18-30-12-6-7-13-30/h3-5,9-10,15-17,23,26H,6-8,11-14,18-19H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377227

(CHEMBL255462)Show SMILES Clc1ccc2sc(=O)n(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2c1 |w:16.25,17.18| Show InChI InChI=1S/C25H28ClN3O2S/c26-19-10-11-23-21(15-19)29(25(31)32-23)17-24(30)28-14-6-9-20(18-7-2-1-3-8-18)22(28)16-27-12-4-5-13-27/h1-3,7-8,10-11,15,20,22H,4-6,9,12-14,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50417599
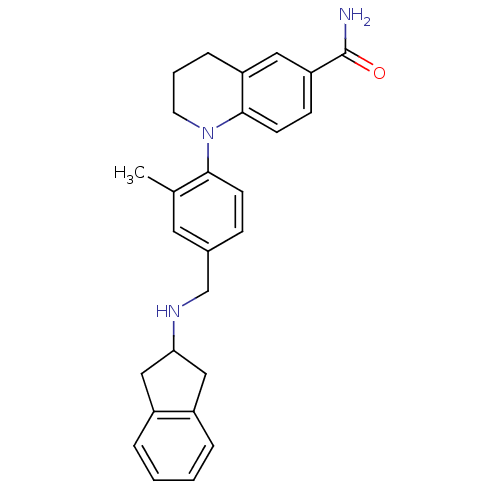
(CHEMBL1642758)Show SMILES Cc1cc(CNC2Cc3ccccc3C2)ccc1N1CCCc2cc(ccc12)C(N)=O Show InChI InChI=1S/C27H29N3O/c1-18-13-19(17-29-24-15-20-5-2-3-6-21(20)16-24)8-10-25(18)30-12-4-7-22-14-23(27(28)31)9-11-26(22)30/h2-3,5-6,8-11,13-14,24,29H,4,7,12,15-17H2,1H3,(H2,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000787

((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10,17-dihydr...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377224
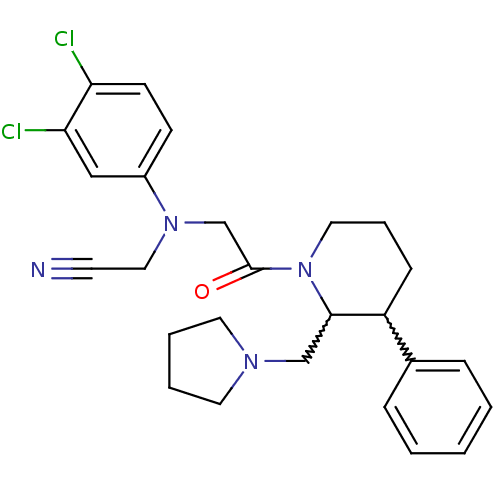
(CHEMBL257767)Show SMILES Clc1ccc(cc1Cl)N(CC#N)CC(=O)N1CCCC(C1CN1CCCC1)c1ccccc1 |w:19.29,20.22| Show InChI InChI=1S/C26H30Cl2N4O/c27-23-11-10-21(17-24(23)28)31(16-12-29)19-26(33)32-15-6-9-22(20-7-2-1-3-8-20)25(32)18-30-13-4-5-14-30/h1-3,7-8,10-11,17,22,25H,4-6,9,13-16,18-19H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377219
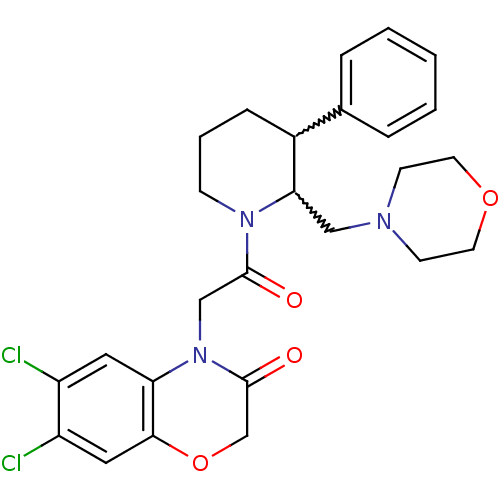
(CHEMBL402520)Show SMILES Clc1cc2OCC(=O)N(CC(=O)N3CCCC(C3CN3CCOCC3)c3ccccc3)c2cc1Cl |w:16.26,17.18| Show InChI InChI=1S/C26H29Cl2N3O4/c27-20-13-22-24(14-21(20)28)35-17-26(33)31(22)16-25(32)30-8-4-7-19(18-5-2-1-3-6-18)23(30)15-29-9-11-34-12-10-29/h1-3,5-6,13-14,19,23H,4,7-12,15-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244019
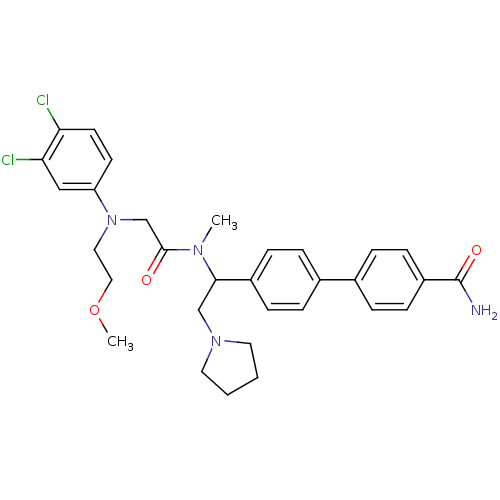
(4'-[1-({2-[(3,4-Dichloro-phenyl)-(2-methoxy-ethyl)...)Show SMILES COCCN(CC(=O)N(C)C(CN1CCCC1)c1ccc(cc1)-c1ccc(cc1)C(N)=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C31H36Cl2N4O3/c1-35(30(38)21-37(17-18-40-2)26-13-14-27(32)28(33)19-26)29(20-36-15-3-4-16-36)24-9-5-22(6-10-24)23-7-11-25(12-8-23)31(34)39/h5-14,19,29H,3-4,15-18,20-21H2,1-2H3,(H2,34,39) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244022
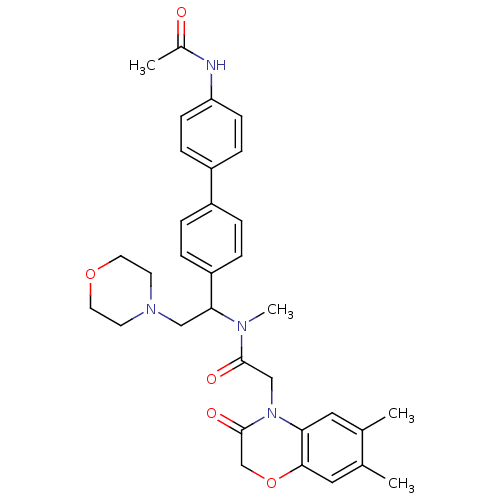
(CHEMBL449192 | N-[1-(4'-Acetylamino-biphenyl-4-yl)...)Show SMILES CN(C(CN1CCOCC1)c1ccc(cc1)-c1ccc(NC(C)=O)cc1)C(=O)CN1C(=O)COc2cc(C)c(C)cc12 Show InChI InChI=1S/C33H38N4O5/c1-22-17-29-31(18-23(22)2)42-21-33(40)37(29)20-32(39)35(4)30(19-36-13-15-41-16-14-36)27-7-5-25(6-8-27)26-9-11-28(12-10-26)34-24(3)38/h5-12,17-18,30H,13-16,19-21H2,1-4H3,(H,34,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377216
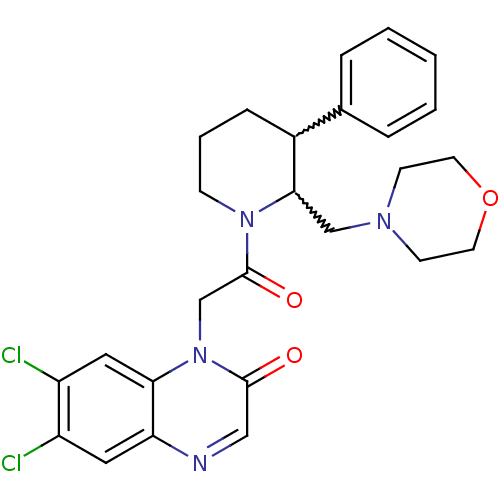
(CHEMBL256988)Show SMILES Clc1cc2ncc(=O)n(CC(=O)N3CCCC(C3CN3CCOCC3)c3ccccc3)c2cc1Cl |w:17.18,16.26| Show InChI InChI=1S/C26H28Cl2N4O3/c27-20-13-22-23(14-21(20)28)32(25(33)15-29-22)17-26(34)31-8-4-7-19(18-5-2-1-3-6-18)24(31)16-30-9-11-35-12-10-30/h1-3,5-6,13-15,19,24H,4,7-12,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244021
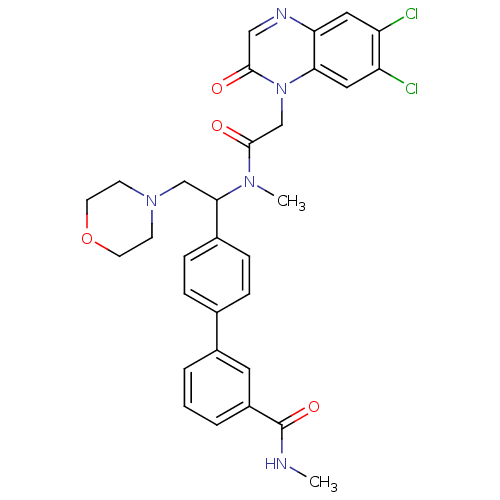
(4'-(1-{[2-(6,7-Dichloro-2-oxo-2H-quinoxalin-1-yl)-...)Show SMILES CNC(=O)c1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)Cn1c2cc(Cl)c(Cl)cc2ncc1=O Show InChI InChI=1S/C31H31Cl2N5O4/c1-34-31(41)23-5-3-4-22(14-23)20-6-8-21(9-7-20)28(18-37-10-12-42-13-11-37)36(2)30(40)19-38-27-16-25(33)24(32)15-26(27)35-17-29(38)39/h3-9,14-17,28H,10-13,18-19H2,1-2H3,(H,34,41) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377229
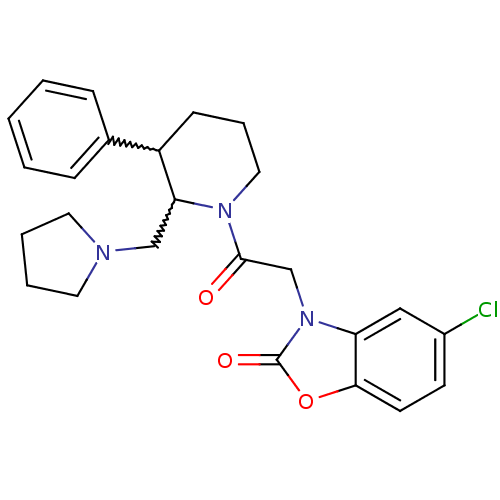
(CHEMBL257150)Show SMILES Clc1ccc2oc(=O)n(CC(=O)N3CCCC(C3CN3CCCC3)c3ccccc3)c2c1 |w:16.25,17.18| Show InChI InChI=1S/C25H28ClN3O3/c26-19-10-11-23-21(15-19)29(25(31)32-23)17-24(30)28-14-6-9-20(18-7-2-1-3-8-18)22(28)16-27-12-4-5-13-27/h1-3,7-8,10-11,15,20,22H,4-6,9,12-14,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50417589

(CHEMBL1642747)Show SMILES NC(=O)c1ccc2N(CCCc2c1)c1ccc(CNC2Cc3ccccc3C2)cc1 Show InChI InChI=1S/C26H27N3O/c27-26(30)22-9-12-25-21(14-22)6-3-13-29(25)24-10-7-18(8-11-24)17-28-23-15-19-4-1-2-5-20(19)16-23/h1-2,4-5,7-12,14,23,28H,3,6,13,15-17H2,(H2,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244065
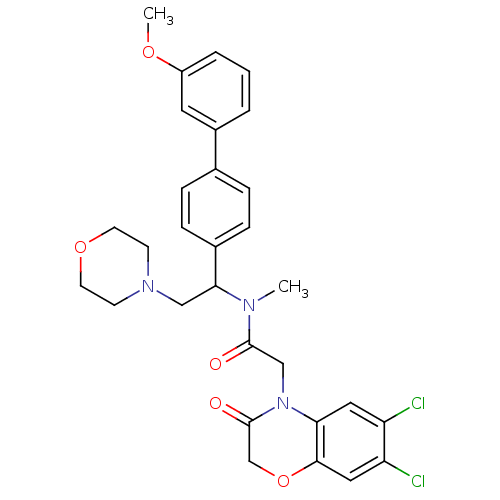
(2-(6,7-Dichloro-3-oxo-2,3-dihydro-benzo[1,4]oxazin...)Show SMILES COc1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 Show InChI InChI=1S/C30H31Cl2N3O5/c1-33(29(36)18-35-26-15-24(31)25(32)16-28(26)40-19-30(35)37)27(17-34-10-12-39-13-11-34)21-8-6-20(7-9-21)22-4-3-5-23(14-22)38-2/h3-9,14-16,27H,10-13,17-19H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377214
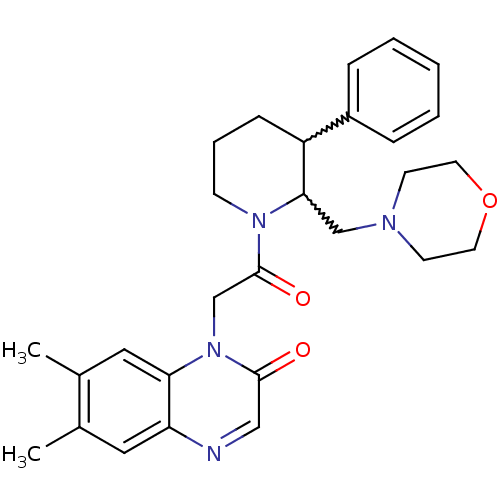
(CHEMBL256721)Show SMILES Cc1cc2ncc(=O)n(CC(=O)N3CCCC(C3CN3CCOCC3)c3ccccc3)c2cc1C |w:17.18,16.26| Show InChI InChI=1S/C28H34N4O3/c1-20-15-24-25(16-21(20)2)32(27(33)17-29-24)19-28(34)31-10-6-9-23(22-7-4-3-5-8-22)26(31)18-30-11-13-35-14-12-30/h3-5,7-8,15-17,23,26H,6,9-14,18-19H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50239135
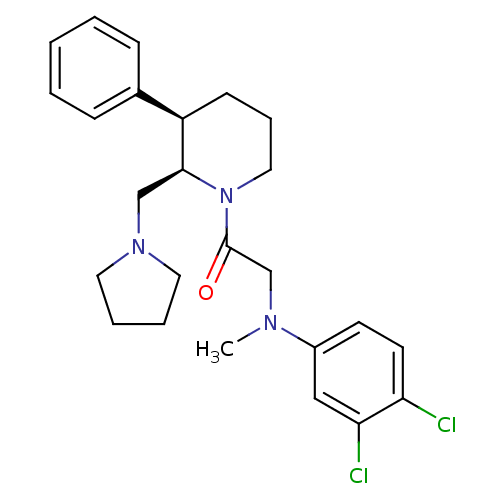
(2-((3,4-dichlorophenyl)(methyl)amino)-1-((2R,3R)-3...)Show SMILES CN(CC(=O)N1CCC[C@@H]([C@@H]1CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C25H31Cl2N3O/c1-28(20-11-12-22(26)23(27)16-20)18-25(31)30-15-7-10-21(19-8-3-2-4-9-19)24(30)17-29-13-5-6-14-29/h2-4,8-9,11-12,16,21,24H,5-7,10,13-15,17-18H2,1H3/t21-,24+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377226

(CHEMBL255460)Show SMILES Clc1ccc2sc(=O)n(CC(=O)N3CCCC(C3CN3CCOCC3)c3ccccc3)c2c1 |w:16.26,17.18| Show InChI InChI=1S/C25H28ClN3O3S/c26-19-8-9-23-21(15-19)29(25(31)33-23)17-24(30)28-10-4-7-20(18-5-2-1-3-6-18)22(28)16-27-11-13-32-14-12-27/h1-3,5-6,8-9,15,20,22H,4,7,10-14,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377223
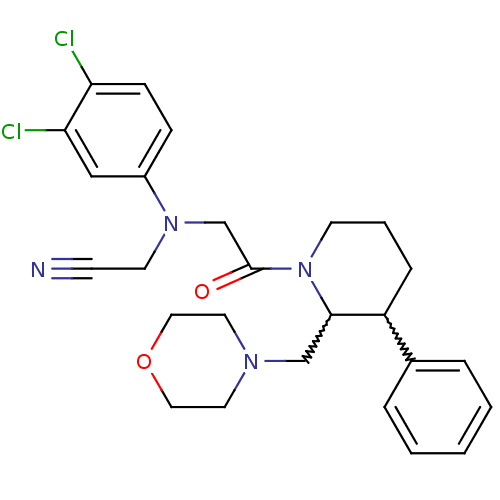
(CHEMBL258251)Show SMILES Clc1ccc(cc1Cl)N(CC#N)CC(=O)N1CCCC(C1CN1CCOCC1)c1ccccc1 |w:19.30,20.22| Show InChI InChI=1S/C26H30Cl2N4O2/c27-23-9-8-21(17-24(23)28)31(12-10-29)19-26(33)32-11-4-7-22(20-5-2-1-3-6-20)25(32)18-30-13-15-34-16-14-30/h1-3,5-6,8-9,17,22,25H,4,7,11-16,18-19H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244018
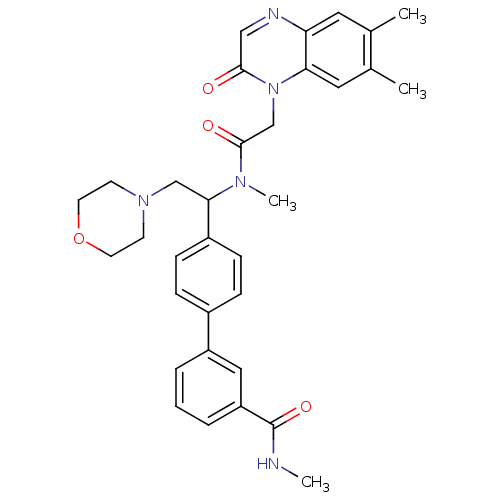
(4'-(1-{[2-(6,7-Dimethyl-2-oxo-2H-quinoxalin-1-yl)-...)Show SMILES CNC(=O)c1cccc(c1)-c1ccc(cc1)C(CN1CCOCC1)N(C)C(=O)Cn1c2cc(C)c(C)cc2ncc1=O Show InChI InChI=1S/C33H37N5O4/c1-22-16-28-29(17-23(22)2)38(31(39)19-35-28)21-32(40)36(4)30(20-37-12-14-42-15-13-37)25-10-8-24(9-11-25)26-6-5-7-27(18-26)33(41)34-3/h5-11,16-19,30H,12-15,20-21H2,1-4H3,(H,34,41) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50417594
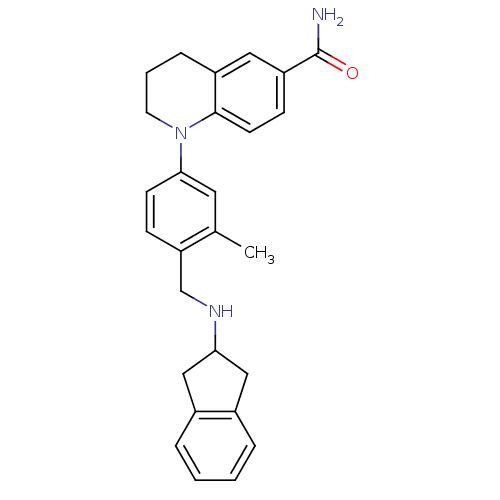
(CHEMBL1642753)Show SMILES Cc1cc(ccc1CNC1Cc2ccccc2C1)N1CCCc2cc(ccc12)C(N)=O Show InChI InChI=1S/C27H29N3O/c1-18-13-25(30-12-4-7-21-14-22(27(28)31)9-11-26(21)30)10-8-23(18)17-29-24-15-19-5-2-3-6-20(19)16-24/h2-3,5-6,8-11,13-14,24,29H,4,7,12,15-17H2,1H3,(H2,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50417595
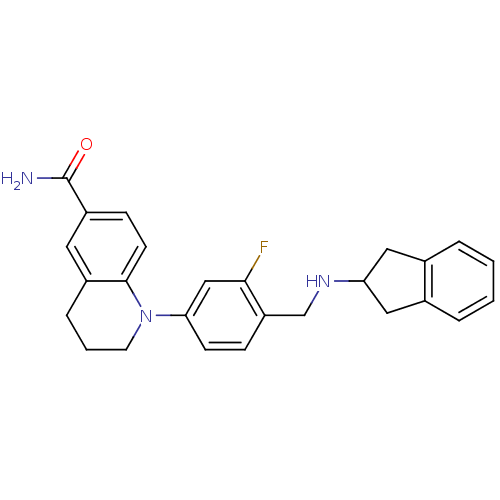
(CHEMBL1642754)Show SMILES NC(=O)c1ccc2N(CCCc2c1)c1ccc(CNC2Cc3ccccc3C2)c(F)c1 Show InChI InChI=1S/C26H26FN3O/c27-24-15-23(30-11-3-6-19-12-20(26(28)31)8-10-25(19)30)9-7-21(24)16-29-22-13-17-4-1-2-5-18(17)14-22/h1-2,4-5,7-10,12,15,22,29H,3,6,11,13-14,16H2,(H2,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377222
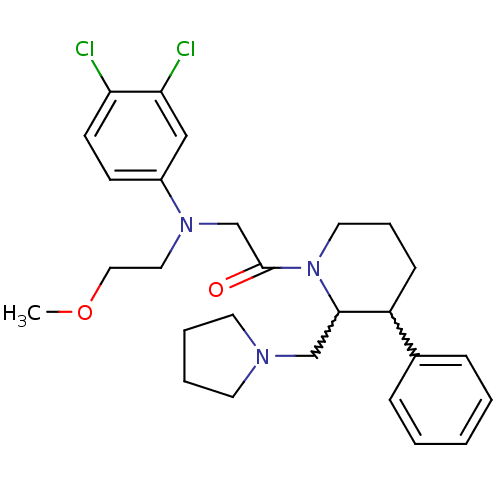
(CHEMBL256937)Show SMILES COCCN(CC(=O)N1CCCC(C1CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 |w:12.21,13.14| Show InChI InChI=1S/C27H35Cl2N3O2/c1-34-17-16-31(22-11-12-24(28)25(29)18-22)20-27(33)32-15-7-10-23(21-8-3-2-4-9-21)26(32)19-30-13-5-6-14-30/h2-4,8-9,11-12,18,23,26H,5-7,10,13-17,19-20H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50417588
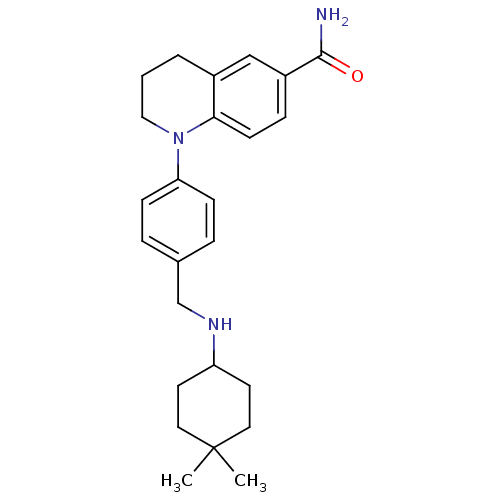
(CHEMBL1642746)Show SMILES CC1(C)CCC(CC1)NCc1ccc(cc1)N1CCCc2cc(ccc12)C(N)=O Show InChI InChI=1S/C25H33N3O/c1-25(2)13-11-21(12-14-25)27-17-18-5-8-22(9-6-18)28-15-3-4-19-16-20(24(26)29)7-10-23(19)28/h5-10,16,21,27H,3-4,11-15,17H2,1-2H3,(H2,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000787

((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10,17-dihydr...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human kappa opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243970
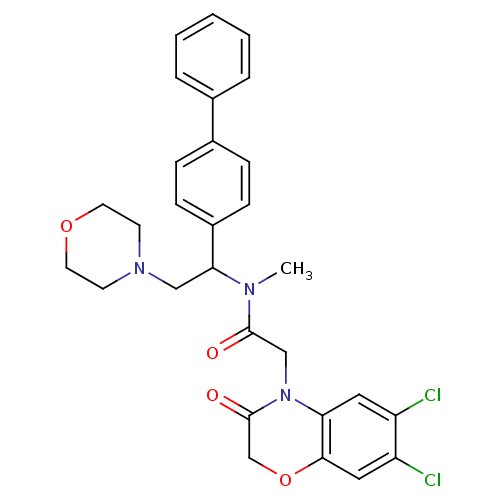
((+/-)N-(1-Biphenyl-4-yl-2-morpholin-4-yl-ethyl)-2-...)Show SMILES CN(C(CN1CCOCC1)c1ccc(cc1)-c1ccccc1)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 Show InChI InChI=1S/C29H29Cl2N3O4/c1-32(28(35)18-34-25-15-23(30)24(31)16-27(25)38-19-29(34)36)26(17-33-11-13-37-14-12-33)22-9-7-21(8-10-22)20-5-3-2-4-6-20/h2-10,15-16,26H,11-14,17-19H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50417600
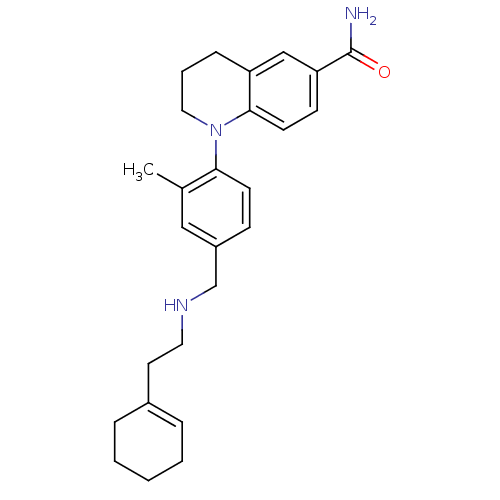
(CHEMBL1642759)Show SMILES Cc1cc(CNCCC2=CCCCC2)ccc1N1CCCc2cc(ccc12)C(N)=O |t:8| Show InChI InChI=1S/C26H33N3O/c1-19-16-21(18-28-14-13-20-6-3-2-4-7-20)9-11-24(19)29-15-5-8-22-17-23(26(27)30)10-12-25(22)29/h6,9-12,16-17,28H,2-5,7-8,13-15,18H2,1H3,(H2,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243919
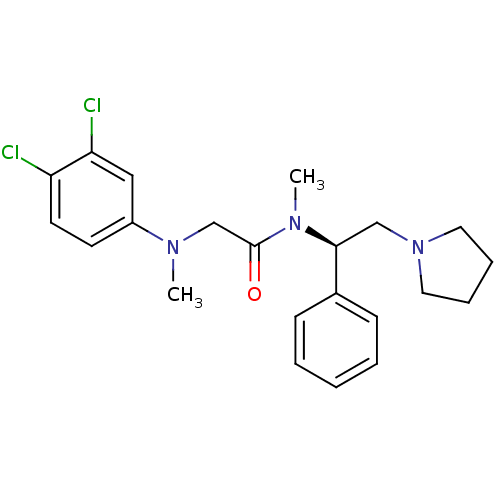
((R)-2-((3,4-dichlorophenyl)(methyl)amino)-N-methyl...)Show SMILES CN(CC(=O)N(C)[C@@H](CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C22H27Cl2N3O/c1-25(18-10-11-19(23)20(24)14-18)16-22(28)26(2)21(15-27-12-6-7-13-27)17-8-4-3-5-9-17/h3-5,8-11,14,21H,6-7,12-13,15-16H2,1-2H3/t21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50417599

(CHEMBL1642758)Show SMILES Cc1cc(CNC2Cc3ccccc3C2)ccc1N1CCCc2cc(ccc12)C(N)=O Show InChI InChI=1S/C27H29N3O/c1-18-13-19(17-29-24-15-20-5-2-3-6-21(20)16-24)8-10-25(18)30-12-4-7-22-14-23(27(28)31)9-11-26(22)30/h2-3,5-6,8-11,13-14,24,29H,4,7,12,15-17H2,1H3,(H2,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human delta opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50417590
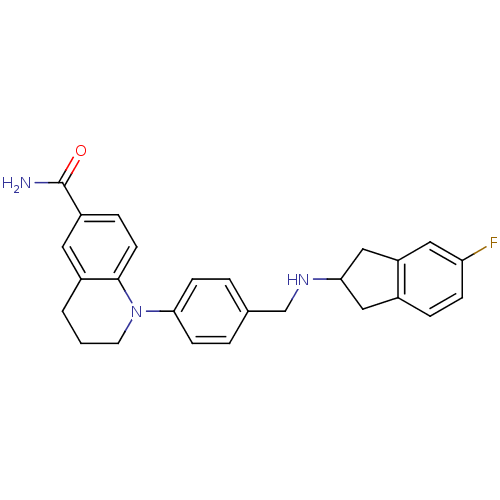
(CHEMBL1642748)Show SMILES NC(=O)c1ccc2N(CCCc2c1)c1ccc(CNC2Cc3ccc(F)cc3C2)cc1 Show InChI InChI=1S/C26H26FN3O/c27-22-7-5-18-14-23(15-21(18)13-22)29-16-17-3-8-24(9-4-17)30-11-1-2-19-12-20(26(28)31)6-10-25(19)30/h3-10,12-13,23,29H,1-2,11,14-16H2,(H2,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243868
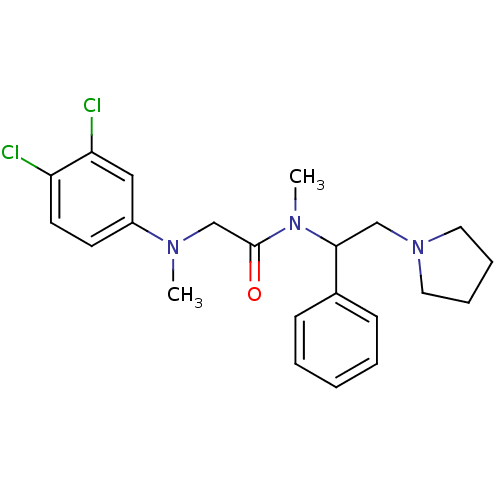
((+/-)-2-((3,4-dichlorophenyl)(methyl)amino)-N-meth...)Show SMILES CN(CC(=O)N(C)C(CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H27Cl2N3O/c1-25(18-10-11-19(23)20(24)14-18)16-22(28)26(2)21(15-27-12-6-7-13-27)17-8-4-3-5-9-17/h3-5,8-11,14,21H,6-7,12-13,15-16H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50240153
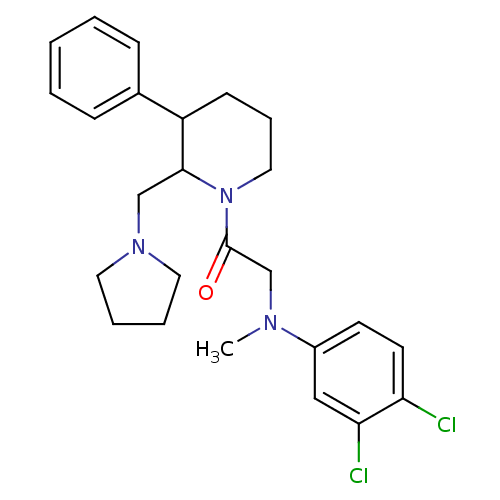
((+/-)-2-((3,4-dichlorophenyl)(methyl)amino)-1-(3-p...)Show SMILES CN(CC(=O)N1CCCC(C1CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C25H31Cl2N3O/c1-28(20-11-12-22(26)23(27)16-20)18-25(31)30-15-7-10-21(19-8-3-2-4-9-19)24(30)17-29-13-5-6-14-29/h2-4,8-9,11-12,16,21,24H,5-7,10,13-15,17-18H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243971
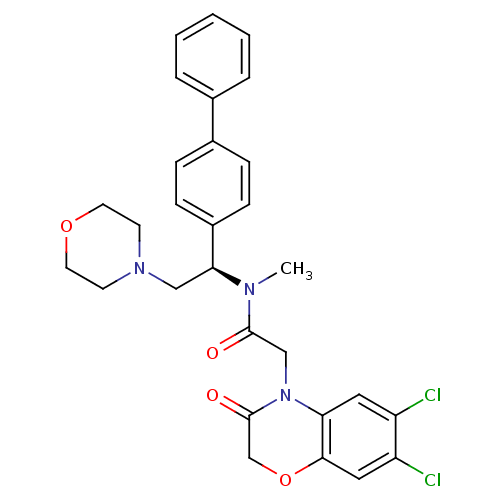
(CHEMBL453075 | N-((R)-1-Biphenyl-4-yl-2-morpholin-...)Show SMILES CN([C@@H](CN1CCOCC1)c1ccc(cc1)-c1ccccc1)C(=O)CN1C(=O)COc2cc(Cl)c(Cl)cc12 |r| Show InChI InChI=1S/C29H29Cl2N3O4/c1-32(28(35)18-34-25-15-23(30)24(31)16-27(25)38-19-29(34)36)26(17-33-11-13-37-14-12-33)22-9-7-21(8-10-22)20-5-3-2-4-6-20/h2-10,15-16,26H,11-14,17-19H2,1H3/t26-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50240153

((+/-)-2-((3,4-dichlorophenyl)(methyl)amino)-1-(3-p...)Show SMILES CN(CC(=O)N1CCCC(C1CN1CCCC1)c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C25H31Cl2N3O/c1-28(20-11-12-22(26)23(27)16-20)18-25(31)30-15-7-10-21(19-8-3-2-4-9-19)24(30)17-29-13-5-6-14-29/h2-4,8-9,11-12,16,21,24H,5-7,10,13-15,17-18H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50417598
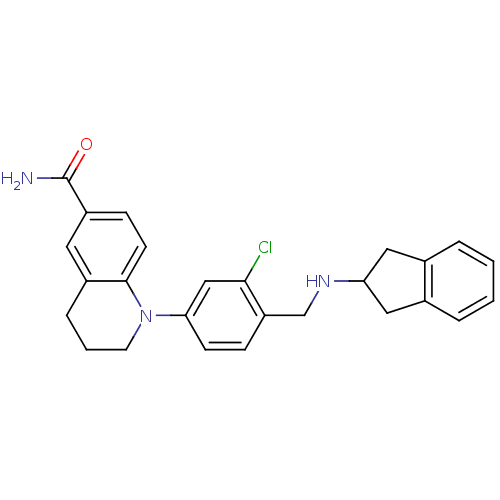
(CHEMBL1642757)Show SMILES NC(=O)c1ccc2N(CCCc2c1)c1ccc(CNC2Cc3ccccc3C2)c(Cl)c1 Show InChI InChI=1S/C26H26ClN3O/c27-24-15-23(30-11-3-6-19-12-20(26(28)31)8-10-25(19)30)9-7-21(24)16-29-22-13-17-4-1-2-5-18(17)14-22/h1-2,4-5,7-10,12,15,22,29H,3,6,11,13-14,16H2,(H2,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50243921
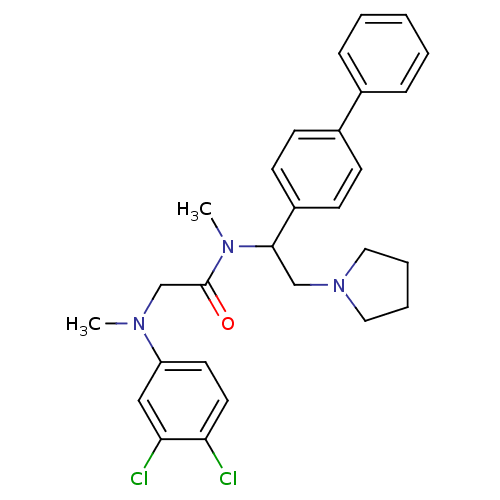
(CHEMBL488642 | N-(1-Biphenyl-4-yl-2-pyrrolidin-1-y...)Show SMILES CN(CC(=O)N(C)C(CN1CCCC1)c1ccc(cc1)-c1ccccc1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C28H31Cl2N3O/c1-31(24-14-15-25(29)26(30)18-24)20-28(34)32(2)27(19-33-16-6-7-17-33)23-12-10-22(11-13-23)21-8-4-3-5-9-21/h3-5,8-15,18,27H,6-7,16-17,19-20H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50417599

(CHEMBL1642758)Show SMILES Cc1cc(CNC2Cc3ccccc3C2)ccc1N1CCCc2cc(ccc12)C(N)=O Show InChI InChI=1S/C27H29N3O/c1-18-13-19(17-29-24-15-20-5-2-3-6-21(20)16-24)8-10-25(18)30-12-4-7-22-14-23(27(28)31)9-11-26(22)30/h2-3,5-6,8-11,13-14,24,29H,4,7,12,15-17H2,1H3,(H2,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human kappa opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50417596

(CHEMBL1642755)Show SMILES NC(=O)c1ccc2N(CCCc2c1)c1cc(F)c(CNC2Cc3ccccc3C2)c(F)c1 Show InChI InChI=1S/C26H25F2N3O/c27-23-13-21(31-9-3-6-18-10-19(26(29)32)7-8-25(18)31)14-24(28)22(23)15-30-20-11-16-4-1-2-5-17(16)12-20/h1-2,4-5,7-8,10,13-14,20,30H,3,6,9,11-12,15H2,(H2,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50417584
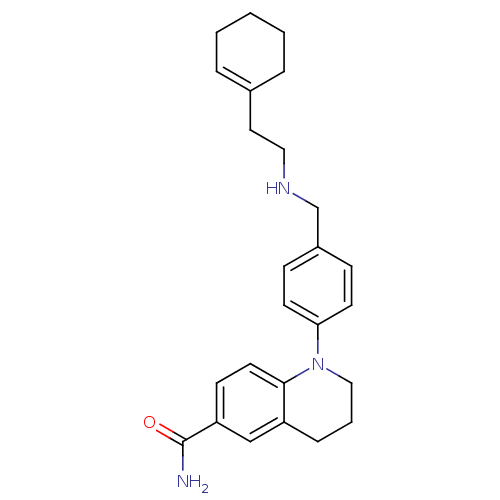
(CHEMBL1642742)Show SMILES NC(=O)c1ccc2N(CCCc2c1)c1ccc(CNCCC2=CCCCC2)cc1 |t:23| Show InChI InChI=1S/C25H31N3O/c26-25(29)22-10-13-24-21(17-22)7-4-16-28(24)23-11-8-20(9-12-23)18-27-15-14-19-5-2-1-3-6-19/h5,8-13,17,27H,1-4,6-7,14-16,18H2,(H2,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50417585

(CHEMBL1642743)Show SMILES NC(=O)c1ccc2N(CCCc2c1)c1ccc(CNCCc2cccs2)cc1 Show InChI InChI=1S/C23H25N3OS/c24-23(27)19-7-10-22-18(15-19)3-1-13-26(22)20-8-5-17(6-9-20)16-25-12-11-21-4-2-14-28-21/h2,4-10,14-15,25H,1,3,11-13,16H2,(H2,24,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50000787

((1S,5R,13R,17S)-4-(cyclopropylmethyl)-10,17-dihydr...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2/t15-,18+,19+,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human delta opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50417589

(CHEMBL1642747)Show SMILES NC(=O)c1ccc2N(CCCc2c1)c1ccc(CNC2Cc3ccccc3C2)cc1 Show InChI InChI=1S/C26H27N3O/c27-26(30)22-9-12-25-21(14-22)6-3-13-29(25)24-10-7-18(8-11-24)17-28-23-15-19-4-1-2-5-20(19)16-23/h1-2,4-5,7-12,14,23,28H,3,6,13,15-17H2,(H2,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human kappa opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50417587
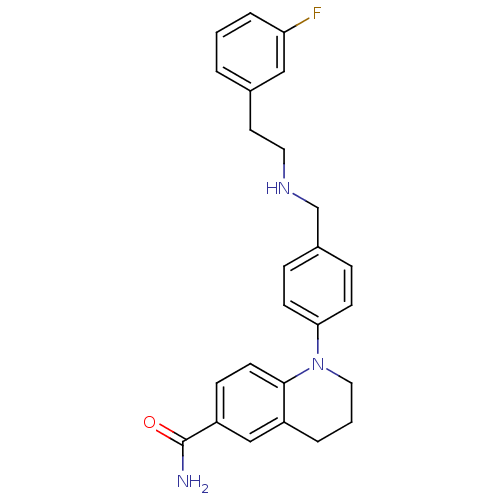
(CHEMBL1642745)Show SMILES NC(=O)c1ccc2N(CCCc2c1)c1ccc(CNCCc2cccc(F)c2)cc1 Show InChI InChI=1S/C25H26FN3O/c26-22-5-1-3-18(15-22)12-13-28-17-19-6-9-23(10-7-19)29-14-2-4-20-16-21(25(27)30)8-11-24(20)29/h1,3,5-11,15-16,28H,2,4,12-14,17H2,(H2,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50417581
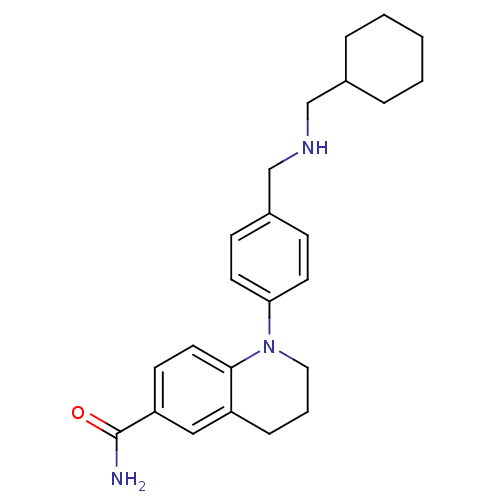
(CHEMBL1642739)Show SMILES NC(=O)c1ccc2N(CCCc2c1)c1ccc(CNCC2CCCCC2)cc1 Show InChI InChI=1S/C24H31N3O/c25-24(28)21-10-13-23-20(15-21)7-4-14-27(23)22-11-8-19(9-12-22)17-26-16-18-5-2-1-3-6-18/h8-13,15,18,26H,1-7,14,16-17H2,(H2,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50377228
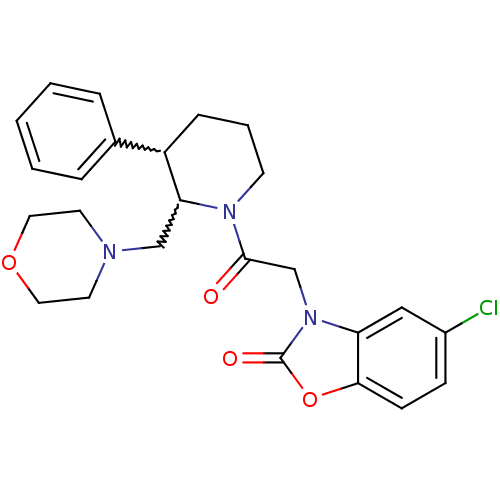
(CHEMBL404289)Show SMILES Clc1ccc2oc(=O)n(CC(=O)N3CCCC(C3CN3CCOCC3)c3ccccc3)c2c1 |w:16.26,17.18| Show InChI InChI=1S/C25H28ClN3O4/c26-19-8-9-23-21(15-19)29(25(31)33-23)17-24(30)28-10-4-7-20(18-5-2-1-3-6-18)22(28)16-27-11-13-32-14-12-27/h1-3,5-6,8-9,15,20,22H,4,7,10-14,16-17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3500-3 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.027
BindingDB Entry DOI: 10.7270/Q29024Q7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50417589

(CHEMBL1642747)Show SMILES NC(=O)c1ccc2N(CCCc2c1)c1ccc(CNC2Cc3ccccc3C2)cc1 Show InChI InChI=1S/C26H27N3O/c27-26(30)22-9-12-25-21(14-22)6-3-13-29(25)24-10-7-18(8-11-24)17-28-23-15-19-4-1-2-5-20(19)16-23/h1-2,4-5,7-12,14,23,28H,3,6,13,15-17H2,(H2,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human delta opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50417597
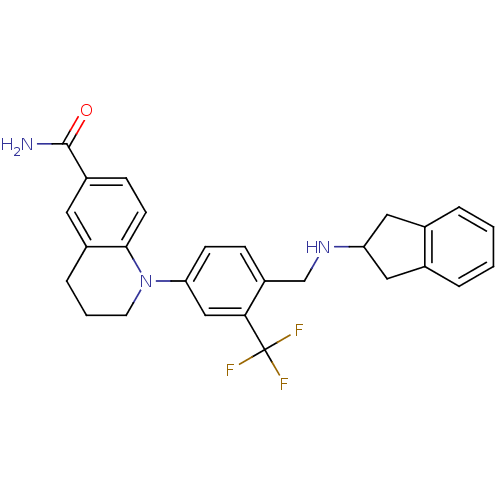
(CHEMBL1642756)Show SMILES NC(=O)c1ccc2N(CCCc2c1)c1ccc(CNC2Cc3ccccc3C2)c(c1)C(F)(F)F Show InChI InChI=1S/C27H26F3N3O/c28-27(29,30)24-15-23(33-11-3-6-19-12-20(26(31)34)8-10-25(19)33)9-7-21(24)16-32-22-13-17-4-1-2-5-18(17)14-22/h1-2,4-5,7-10,12,15,22,32H,3,6,11,13-14,16H2,(H2,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
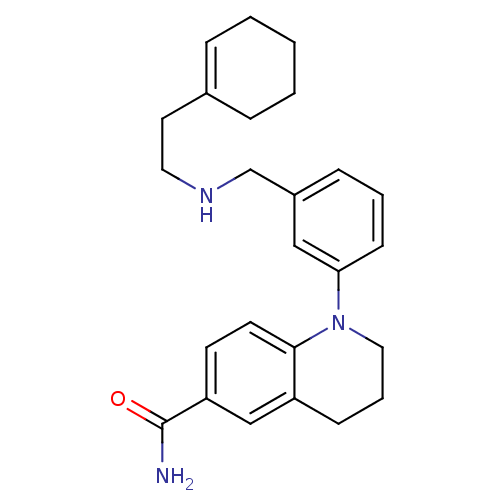
(Homo sapiens (Human)) | BDBM50417593
(CHEMBL1642751)Show SMILES NC(=O)c1ccc2N(CCCc2c1)c1cccc(CNCCC2=CCCCC2)c1 |t:24| Show InChI InChI=1S/C25H31N3O/c26-25(29)22-11-12-24-21(17-22)9-5-15-28(24)23-10-4-8-20(16-23)18-27-14-13-19-6-2-1-3-7-19/h4,6,8,10-12,16-17,27H,1-3,5,7,9,13-15,18H2,(H2,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by SPA |
Bioorg Med Chem Lett 21: 670-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.010
BindingDB Entry DOI: 10.7270/Q2KD2051 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data