 Found 106 hits with Last Name = 'andrs' and Initial = 'm'
Found 106 hits with Last Name = 'andrs' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM412060
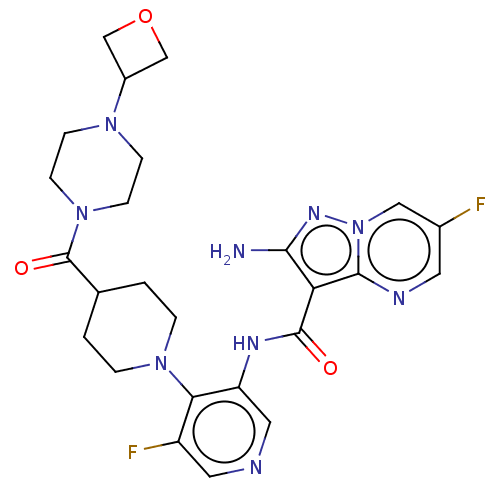
(2-amino-6-fluoro-N-[5-fluoro-4-[4-[4-(oxetan-3-yl)...)Show SMILES Nc1nn2cc(F)cnc2c1C(=O)Nc1cncc(F)c1N1CCC(CC1)C(=O)N1CCN(CC1)C1COC1 Show InChI InChI=1S/C25H29F2N9O3/c26-16-9-30-23-20(22(28)32-36(23)12-16)24(37)31-19-11-29-10-18(27)21(19)34-3-1-15(2-4-34)25(38)35-7-5-33(6-8-35)17-13-39-14-17/h9-12,15,17H,1-8,13-14H2,(H2,28,32)(H,31,37) | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
UniChem
| Article
PubMed
| <0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114580
BindingDB Entry DOI: 10.7270/Q261149W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM268079
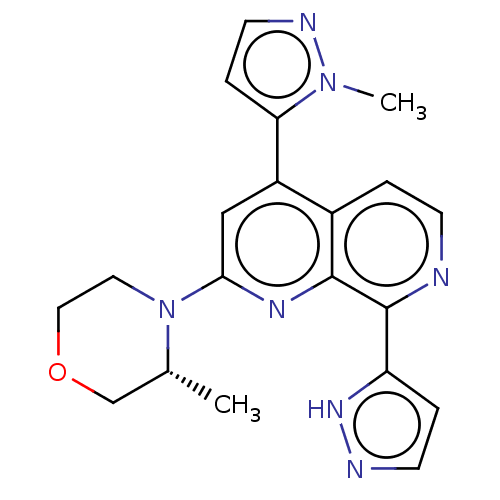
(2-[(3R)-3-methylmorpholin-4-yl]-4-(1-methyl-1H-pyr...)Show SMILES C[C@@H]1COCCN1c1cc(-c2ccnn2C)c2ccnc(-c3ccn[nH]3)c2n1 |r| | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114580
BindingDB Entry DOI: 10.7270/Q261149W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133473
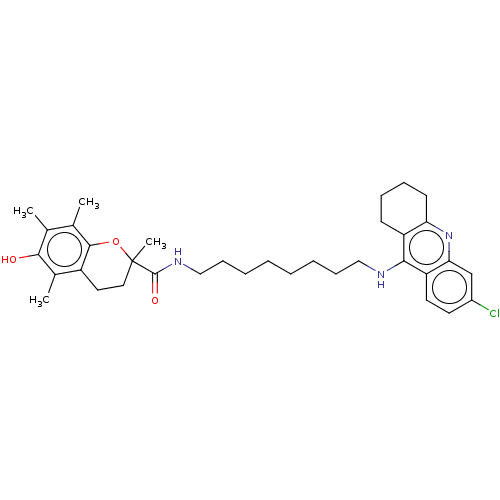
(CHEMBL3632994)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C35H46ClN3O3/c1-22-23(2)33-26(24(3)32(22)40)17-18-35(4,42-33)34(41)38-20-12-8-6-5-7-11-19-37-31-27-13-9-10-14-29(27)39-30-21-25(36)15-16-28(30)31/h15-16,21,40H,5-14,17-20H2,1-4H3,(H,37,39)(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50059637
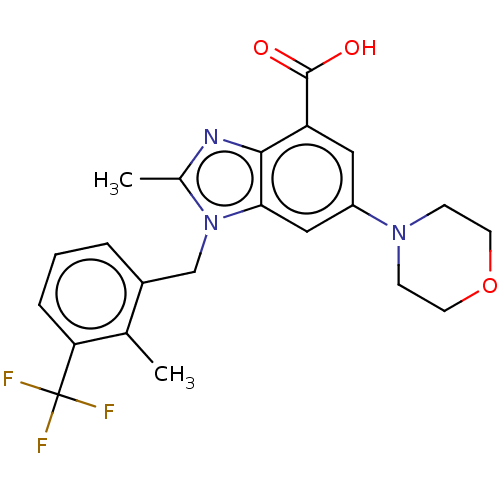
(GSK-2636771 | GSK2636771 | US10660898, Example 31)Show SMILES Cc1nc2c(cc(cc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1)C(O)=O Show InChI InChI=1S/C22H22F3N3O3/c1-13-15(4-3-5-18(13)22(23,24)25)12-28-14(2)26-20-17(21(29)30)10-16(11-19(20)28)27-6-8-31-9-7-27/h3-5,10-11H,6-9,12H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110beta (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308767
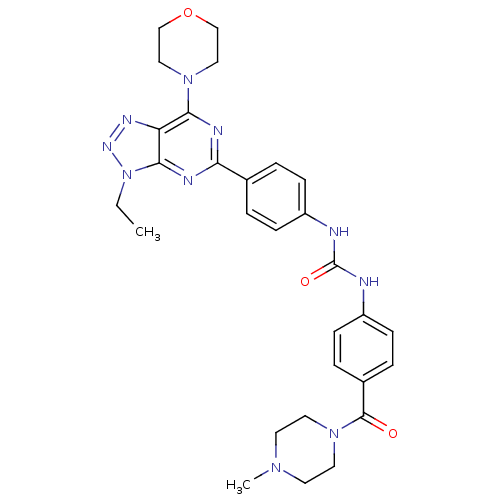
(1-[4-(3-Ethyl-7-morpholin-4-yl-3H-[1,2,3]triazolo-...)Show SMILES CCn1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N2CCN(C)CC2)cc1)N1CCOCC1 Show InChI InChI=1S/C29H34N10O3/c1-3-39-27-24(34-35-39)26(37-16-18-42-19-17-37)32-25(33-27)20-4-8-22(9-5-20)30-29(41)31-23-10-6-21(7-11-23)28(40)38-14-12-36(2)13-15-38/h4-11H,3,12-19H2,1-2H3,(H2,30,31,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50059642
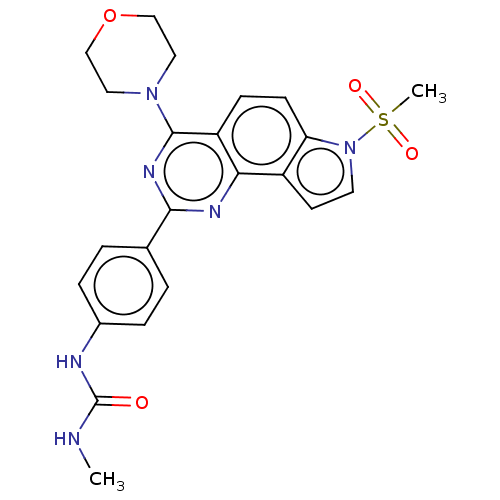
(CHEMBL3393593)Show SMILES CNC(=O)Nc1ccc(cc1)-c1nc(N2CCOCC2)c2ccc3n(ccc3c2n1)S(C)(=O)=O Show InChI InChI=1S/C23H24N6O4S/c1-24-23(30)25-16-5-3-15(4-6-16)21-26-20-17-9-10-29(34(2,31)32)19(17)8-7-18(20)22(27-21)28-11-13-33-14-12-28/h3-10H,11-14H2,1-2H3,(H2,24,25,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25018
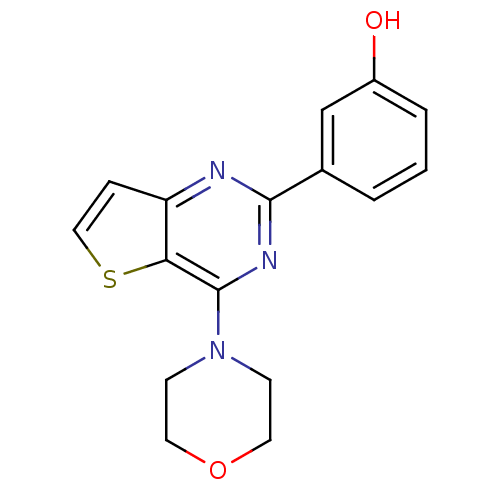
(3-(4-morpholin-4-ylthieno[3,2-d]pyrimidin-2-yl)phe...)Show InChI InChI=1S/C16H15N3O2S/c20-12-3-1-2-11(10-12)15-17-13-4-9-22-14(13)16(18-15)19-5-7-21-8-6-19/h1-4,9-10,20H,5-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308767

(1-[4-(3-Ethyl-7-morpholin-4-yl-3H-[1,2,3]triazolo-...)Show SMILES CCn1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N2CCN(C)CC2)cc1)N1CCOCC1 Show InChI InChI=1S/C29H34N10O3/c1-3-39-27-24(34-35-39)26(37-16-18-42-19-17-37)32-25(33-27)20-4-8-22(9-5-20)30-29(41)31-23-10-6-21(7-11-23)28(40)38-14-12-36(2)13-15-38/h4-11H,3,12-19H2,1-2H3,(H2,30,31,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50392138
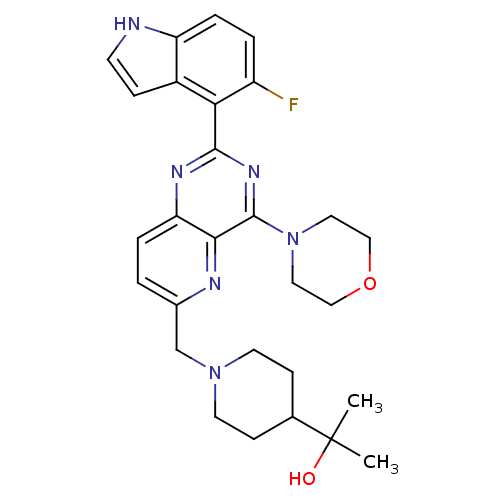
(CHEMBL2152768)Show SMILES CC(C)(O)C1CCN(Cc2ccc3nc(nc(N4CCOCC4)c3n2)-c2c(F)ccc3[nH]ccc23)CC1 Show InChI InChI=1S/C28H33FN6O2/c1-28(2,36)18-8-11-34(12-9-18)17-19-3-5-23-25(31-19)27(35-13-15-37-16-14-35)33-26(32-23)24-20-7-10-30-22(20)6-4-21(24)29/h3-7,10,18,30,36H,8-9,11-17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110delta (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50358208
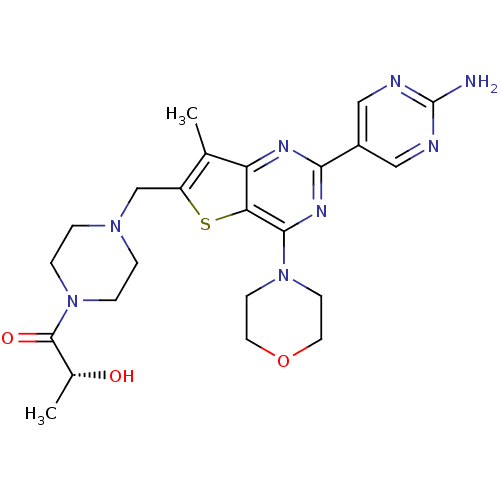
(CHEMBL1921986)Show SMILES C[C@@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50059639
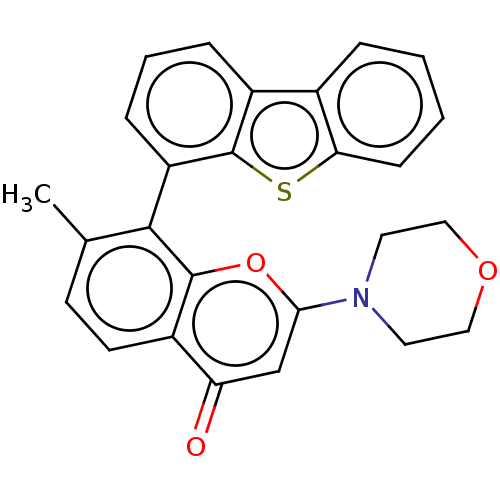
(CHEMBL3393500)Show SMILES Cc1ccc2c(oc(cc2=O)N2CCOCC2)c1-c1cccc2c3ccccc3sc12 |(-5,-3.04,;-3.93,-3.66,;-3.94,-5.2,;-2.6,-5.98,;-1.26,-5.22,;-1.25,-3.68,;.09,-2.92,;1.42,-3.7,;1.41,-5.24,;.07,-6,;.06,-7.23,;2.76,-2.93,;2.77,-1.39,;4.12,-.63,;5.45,-1.41,;5.43,-2.96,;4.09,-3.72,;-2.58,-2.9,;-2.56,-1.36,;-1.31,-.65,;-1.28,.82,;-2.51,1.58,;-3.82,.87,;-6.35,.92,;-7.61,1.68,;-8.93,.97,;-8.96,-.5,;-7.67,-1.26,;-6.37,-.55,;-5.13,-1.31,;-3.85,-.6,)| Show InChI InChI=1S/C26H21NO3S/c1-16-9-10-19-21(28)15-23(27-11-13-29-14-12-27)30-25(19)24(16)20-7-4-6-18-17-5-2-3-8-22(17)31-26(18)20/h2-10,15H,11-14H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of DNA-PK (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50315213
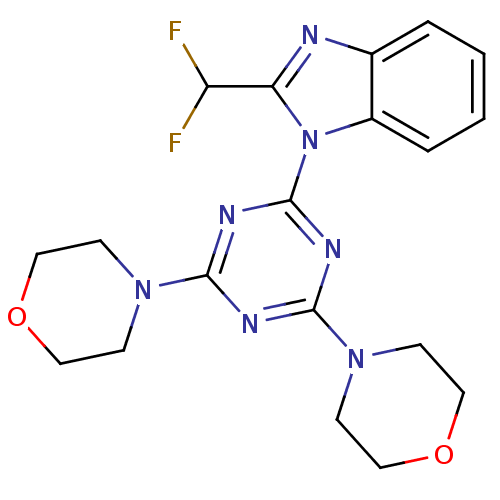
(2-(difluoromethyl)-1-(4,6-dimorpholin-4-yl-1,3,5-t...)Show SMILES FC(F)c1nc2ccccc2n1-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O2/c20-15(21)16-22-13-3-1-2-4-14(13)28(16)19-24-17(26-5-9-29-10-6-26)23-18(25-19)27-7-11-30-12-8-27/h1-4,15H,5-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110gamma (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50059639

(CHEMBL3393500)Show SMILES Cc1ccc2c(oc(cc2=O)N2CCOCC2)c1-c1cccc2c3ccccc3sc12 |(-5,-3.04,;-3.93,-3.66,;-3.94,-5.2,;-2.6,-5.98,;-1.26,-5.22,;-1.25,-3.68,;.09,-2.92,;1.42,-3.7,;1.41,-5.24,;.07,-6,;.06,-7.23,;2.76,-2.93,;2.77,-1.39,;4.12,-.63,;5.45,-1.41,;5.43,-2.96,;4.09,-3.72,;-2.58,-2.9,;-2.56,-1.36,;-1.31,-.65,;-1.28,.82,;-2.51,1.58,;-3.82,.87,;-6.35,.92,;-7.61,1.68,;-8.93,.97,;-8.96,-.5,;-7.67,-1.26,;-6.37,-.55,;-5.13,-1.31,;-3.85,-.6,)| Show InChI InChI=1S/C26H21NO3S/c1-16-9-10-19-21(28)15-23(27-11-13-29-14-12-27)30-25(19)24(16)20-7-4-6-18-17-5-2-3-8-22(17)31-26(18)20/h2-10,15H,11-14H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of DNA-PK (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50059640
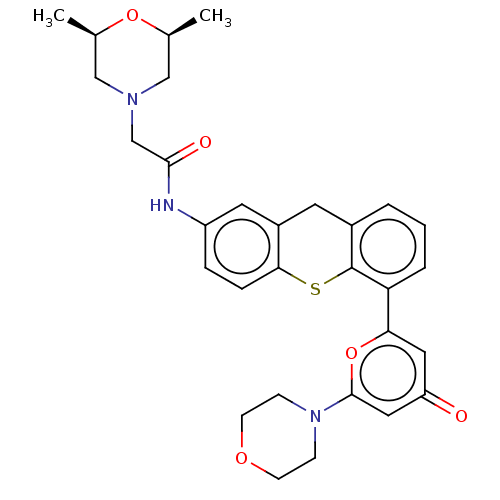
(CHEMBL2140173)Show SMILES C[C@H]1CN(CC(=O)Nc2ccc3Sc4c(Cc3c2)cccc4-c2cc(=O)cc(o2)N2CCOCC2)C[C@@H](C)O1 Show InChI InChI=1S/C30H33N3O5S/c1-19-16-32(17-20(2)37-19)18-28(35)31-23-6-7-27-22(13-23)12-21-4-3-5-25(30(21)39-27)26-14-24(34)15-29(38-26)33-8-10-36-11-9-33/h3-7,13-15,19-20H,8-12,16-18H2,1-2H3,(H,31,35)/t19-,20+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of ATM kinase in human glioma cells |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50358208

(CHEMBL1921986)Show SMILES C[C@@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110gamma (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50308767

(1-[4-(3-Ethyl-7-morpholin-4-yl-3H-[1,2,3]triazolo-...)Show SMILES CCn1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N2CCN(C)CC2)cc1)N1CCOCC1 Show InChI InChI=1S/C29H34N10O3/c1-3-39-27-24(34-35-39)26(37-16-18-42-19-17-37)32-25(33-27)20-4-8-22(9-5-20)30-29(41)31-23-10-6-21(7-11-23)28(40)38-14-12-36(2)13-15-38/h4-11H,3,12-19H2,1-2H3,(H2,30,31,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110beta (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50059638
(CHEMBL3393499)Show SMILES O=c1cc(nc2c(cccn12)-c1cccc2c3ccccc3sc12)N1CCOCC1 Show InChI InChI=1S/C24H19N3O2S/c28-22-15-21(26-11-13-29-14-12-26)25-24-19(8-4-10-27(22)24)18-7-3-6-17-16-5-1-2-9-20(16)30-23(17)18/h1-10,15H,11-14H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of DNA-PK (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50358208

(CHEMBL1921986)Show SMILES C[C@@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110delta (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM25045
(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of DNA-PK (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50156495
(8-(dibenzo[b,d]thiophen-4-yl)-2-morpholino-4H-chro...)Show SMILES O=c1cc(oc2c(cccc12)-c1cccc2c3ccccc3sc12)N1CCOCC1 Show InChI InChI=1S/C25H19NO3S/c27-21-15-23(26-11-13-28-14-12-26)29-24-17(6-3-9-20(21)24)19-8-4-7-18-16-5-1-2-10-22(16)30-25(18)19/h1-10,15H,11-14H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of DNA-PK (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50308767

(1-[4-(3-Ethyl-7-morpholin-4-yl-3H-[1,2,3]triazolo-...)Show SMILES CCn1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N2CCN(C)CC2)cc1)N1CCOCC1 Show InChI InChI=1S/C29H34N10O3/c1-3-39-27-24(34-35-39)26(37-16-18-42-19-17-37)32-25(33-27)20-4-8-22(9-5-20)30-29(41)31-23-10-6-21(7-11-23)28(40)38-14-12-36(2)13-15-38/h4-11H,3,12-19H2,1-2H3,(H2,30,31,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110delta (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50308767

(1-[4-(3-Ethyl-7-morpholin-4-yl-3H-[1,2,3]triazolo-...)Show SMILES CCn1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N2CCN(C)CC2)cc1)N1CCOCC1 Show InChI InChI=1S/C29H34N10O3/c1-3-39-27-24(34-35-39)26(37-16-18-42-19-17-37)32-25(33-27)20-4-8-22(9-5-20)30-29(41)31-23-10-6-21(7-11-23)28(40)38-14-12-36(2)13-15-38/h4-11H,3,12-19H2,1-2H3,(H2,30,31,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110gamma (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50315213

(2-(difluoromethyl)-1-(4,6-dimorpholin-4-yl-1,3,5-t...)Show SMILES FC(F)c1nc2ccccc2n1-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O2/c20-15(21)16-22-13-3-1-2-4-14(13)28(16)19-24-17(26-5-9-29-10-6-26)23-18(25-19)27-7-11-30-12-8-27/h1-4,15H,5-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50059635
(CHEMBL3393066)Show SMILES CC(C)n1c(C)nc2c(nc(nc12)N1CCOCC1)-c1cnc(N)nc1 Show InChI InChI=1S/C17H22N8O/c1-10(2)25-11(3)21-14-13(12-8-19-16(18)20-9-12)22-17(23-15(14)25)24-4-6-26-7-5-24/h8-10H,4-7H2,1-3H3,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110alpha (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50358208

(CHEMBL1921986)Show SMILES C[C@@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8987
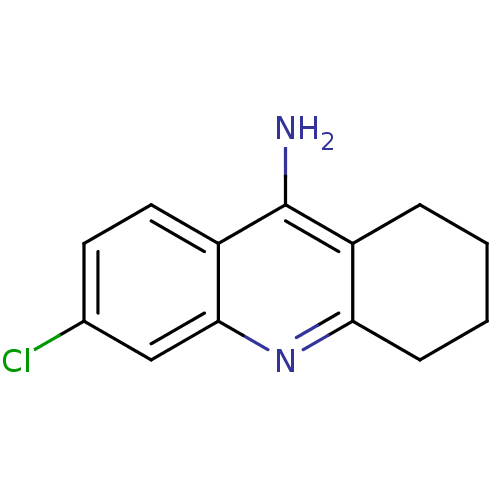
(6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...)Show InChI InChI=1S/C13H13ClN2/c14-8-5-6-10-12(7-8)16-11-4-2-1-3-9(11)13(10)15/h5-7H,1-4H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
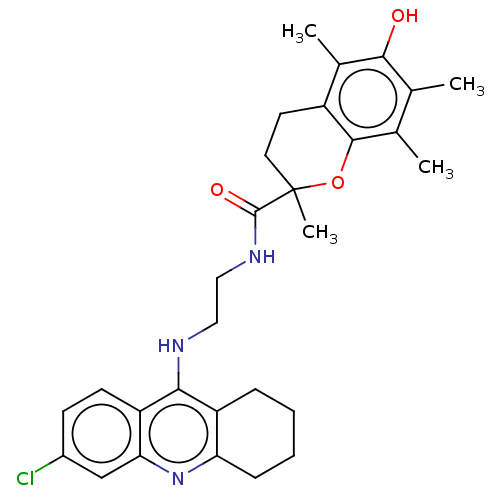
Cholinesterase
(Homo sapiens (Human)) | BDBM50133449
(CHEMBL3632988)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C29H34ClN3O3/c1-16-17(2)27-20(18(3)26(16)34)11-12-29(4,36-27)28(35)32-14-13-31-25-21-7-5-6-8-23(21)33-24-15-19(30)9-10-22(24)25/h9-10,15,34H,5-8,11-14H2,1-4H3,(H,31,33)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
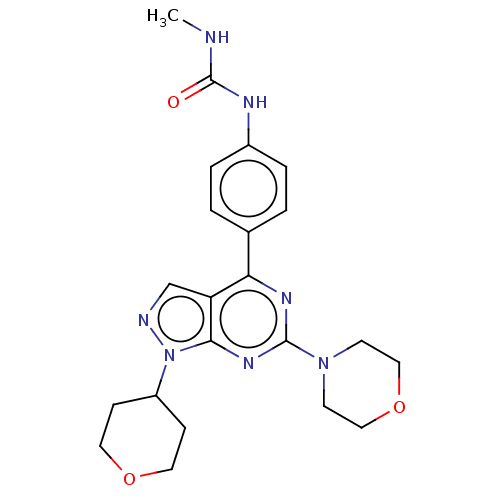
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50059648
(CHEMBL3393594)Show SMILES CNC(=O)Nc1ccc(cc1)-c1nc(nc2n(ncc12)C1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C22H27N7O3/c1-23-22(30)25-16-4-2-15(3-5-16)19-18-14-24-29(17-6-10-31-11-7-17)20(18)27-21(26-19)28-8-12-32-13-9-28/h2-5,14,17H,6-13H2,1H3,(H2,23,25,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50059635

(CHEMBL3393066)Show SMILES CC(C)n1c(C)nc2c(nc(nc12)N1CCOCC1)-c1cnc(N)nc1 Show InChI InChI=1S/C17H22N8O/c1-10(2)25-11(3)21-14-13(12-8-19-16(18)20-9-12)22-17(23-15(14)25)24-4-6-26-7-5-24/h8-10H,4-7H2,1-3H3,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110gamma (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50358208

(CHEMBL1921986)Show SMILES C[C@@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110beta (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
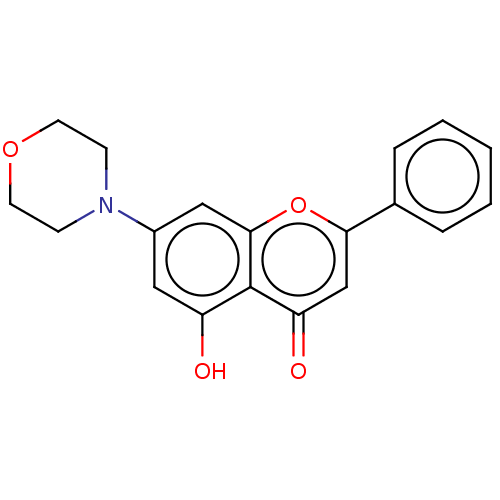
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50017675
(CHEMBL3288951)Show InChI InChI=1S/C19H17NO4/c21-15-10-14(20-6-8-23-9-7-20)11-18-19(15)16(22)12-17(24-18)13-4-2-1-3-5-13/h1-5,10-12,21H,6-9H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of DNA-PK purified from HeLa nuclear extracts assessed as EPPLSQEAFADLWKKR peptide substrate phosphorylation |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50059641
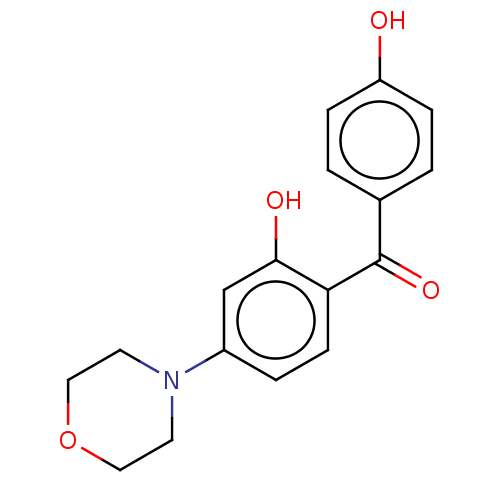
(CHEMBL3393501)Show InChI InChI=1S/C17H17NO4/c19-14-4-1-12(2-5-14)17(21)15-6-3-13(11-16(15)20)18-7-9-22-10-8-18/h1-6,11,19-20H,7-10H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of DNA-PK purified from HeLa nuclear extracts assessed as EPPLSQEAFADLWKKR peptide substrate phosphorylation |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50059635

(CHEMBL3393066)Show SMILES CC(C)n1c(C)nc2c(nc(nc12)N1CCOCC1)-c1cnc(N)nc1 Show InChI InChI=1S/C17H22N8O/c1-10(2)25-11(3)21-14-13(12-8-19-16(18)20-9-12)22-17(23-15(14)25)24-4-6-26-7-5-24/h8-10H,4-7H2,1-3H3,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110delta (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
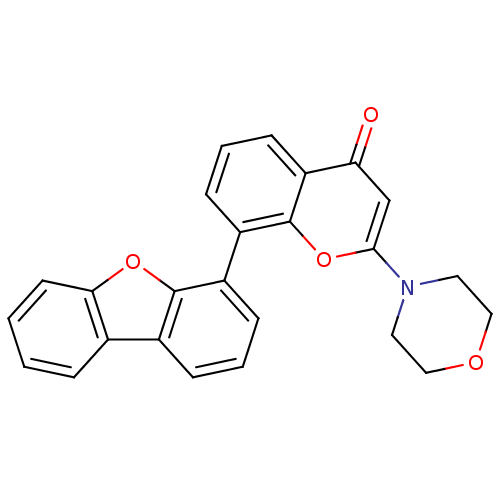
(Homo sapiens (Human)) | BDBM50156503
(8-Dibenzofuran-4-yl-2-morpholin-4-yl-chromen-4-one...)Show SMILES O=c1cc(oc2c(cccc12)-c1cccc2c1oc1ccccc21)N1CCOCC1 Show InChI InChI=1S/C25H19NO4/c27-21-15-23(26-11-13-28-14-12-26)30-25-19(8-4-9-20(21)25)18-7-3-6-17-16-5-1-2-10-22(16)29-24(17)18/h1-10,15H,11-14H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of DNA-PK (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50315213

(2-(difluoromethyl)-1-(4,6-dimorpholin-4-yl-1,3,5-t...)Show SMILES FC(F)c1nc2ccccc2n1-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O2/c20-15(21)16-22-13-3-1-2-4-14(13)28(16)19-24-17(26-5-9-29-10-6-26)23-18(25-19)27-7-11-30-12-8-27/h1-4,15H,5-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110beta (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50315213

(2-(difluoromethyl)-1-(4,6-dimorpholin-4-yl-1,3,5-t...)Show SMILES FC(F)c1nc2ccccc2n1-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O2/c20-15(21)16-22-13-3-1-2-4-14(13)28(16)19-24-17(26-5-9-29-10-6-26)23-18(25-19)27-7-11-30-12-8-27/h1-4,15H,5-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110delta (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50073117
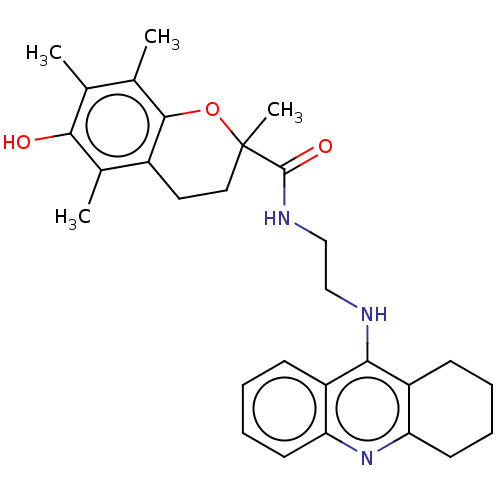
(CHEMBL3410951)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H35N3O3/c1-17-18(2)27-20(19(3)26(17)33)13-14-29(4,35-27)28(34)31-16-15-30-25-21-9-5-7-11-23(21)32-24-12-8-6-10-22(24)25/h5,7,9,11,33H,6,8,10,12-16H2,1-4H3,(H,30,32)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50059635

(CHEMBL3393066)Show SMILES CC(C)n1c(C)nc2c(nc(nc12)N1CCOCC1)-c1cnc(N)nc1 Show InChI InChI=1S/C17H22N8O/c1-10(2)25-11(3)21-14-13(12-8-19-16(18)20-9-12)22-17(23-15(14)25)24-4-6-26-7-5-24/h8-10H,4-7H2,1-3H3,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110beta (unknown origin) |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50073116
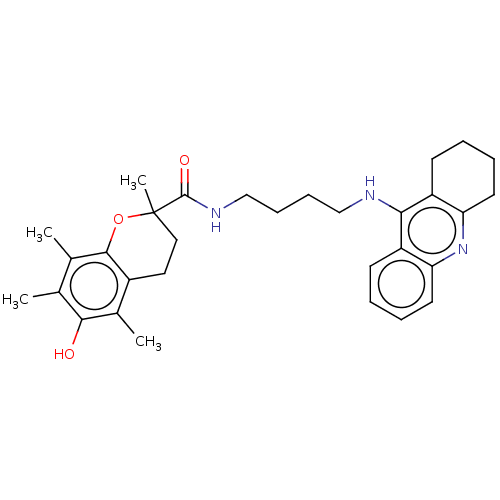
(CHEMBL3410952)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C31H39N3O3/c1-19-20(2)29-22(21(3)28(19)35)15-16-31(4,37-29)30(36)33-18-10-9-17-32-27-23-11-5-7-13-25(23)34-26-14-8-6-12-24(26)27/h5,7,11,13,35H,6,8-10,12,14-18H2,1-4H3,(H,32,34)(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133450
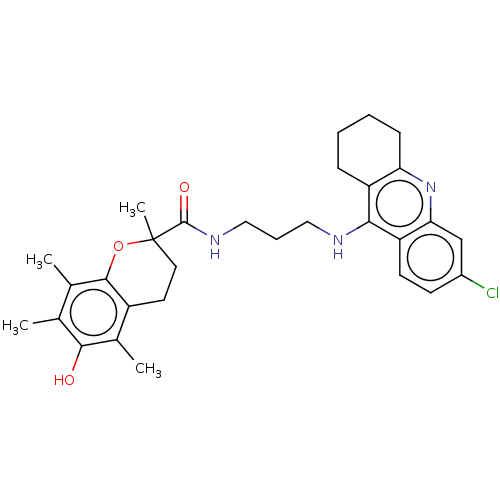
(CHEMBL3632989)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C30H36ClN3O3/c1-17-18(2)28-21(19(3)27(17)35)12-13-30(4,37-28)29(36)33-15-7-14-32-26-22-8-5-6-9-24(22)34-25-16-20(31)10-11-23(25)26/h10-11,16,35H,5-9,12-15H2,1-4H3,(H,32,34)(H,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133472
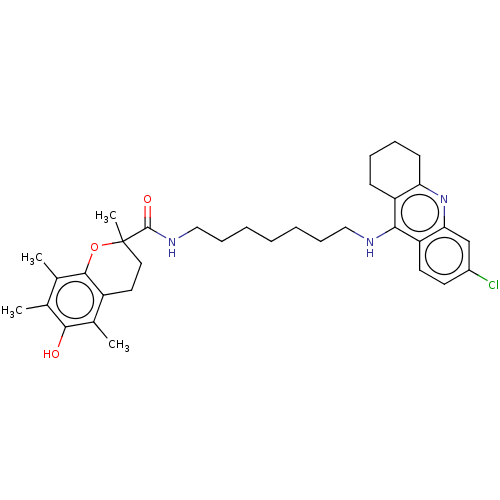
(CHEMBL3632993)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C34H44ClN3O3/c1-21-22(2)32-25(23(3)31(21)39)16-17-34(4,41-32)33(40)37-19-11-7-5-6-10-18-36-30-26-12-8-9-13-28(26)38-29-20-24(35)14-15-27(29)30/h14-15,20,39H,5-13,16-19H2,1-4H3,(H,36,38)(H,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133473

(CHEMBL3632994)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C35H46ClN3O3/c1-22-23(2)33-26(24(3)32(22)40)17-18-35(4,42-33)34(41)38-20-12-8-6-5-7-11-19-37-31-27-13-9-10-14-29(27)39-30-21-25(36)15-16-28(30)31/h15-16,21,40H,5-14,17-20H2,1-4H3,(H,37,39)(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50133421
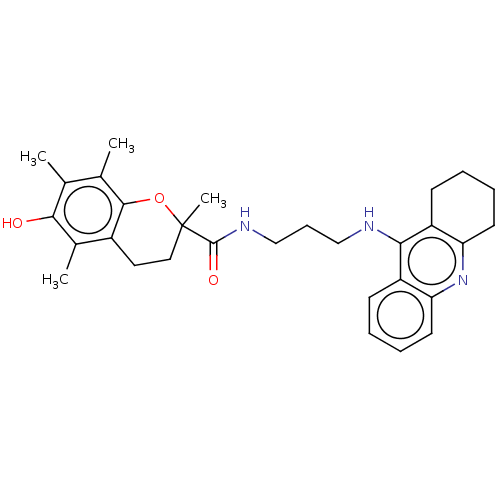
(CHEMBL3632987)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H37N3O3/c1-18-19(2)28-21(20(3)27(18)34)14-15-30(4,36-28)29(35)32-17-9-16-31-26-22-10-5-7-12-24(22)33-25-13-8-6-11-23(25)26/h5,7,10,12,34H,6,8-9,11,13-17H2,1-4H3,(H,31,33)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50133469
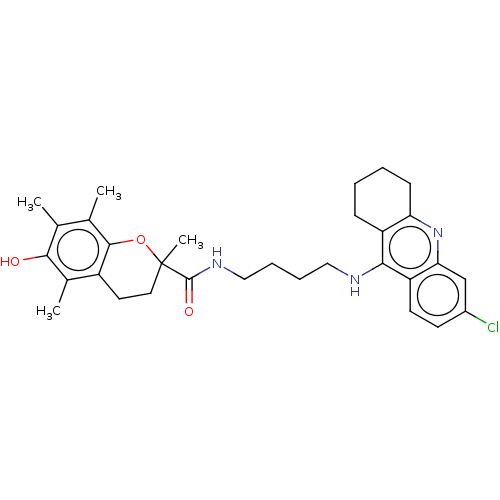
(CHEMBL3632990)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C31H38ClN3O3/c1-18-19(2)29-22(20(3)28(18)36)13-14-31(4,38-29)30(37)34-16-8-7-15-33-27-23-9-5-6-10-25(23)35-26-17-21(32)11-12-24(26)27/h11-12,17,36H,5-10,13-16H2,1-4H3,(H,33,35)(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133471
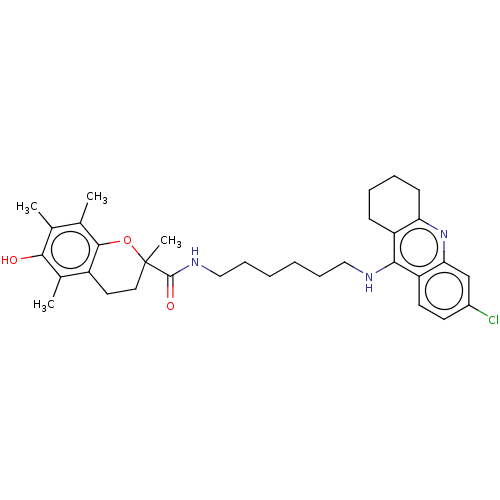
(CHEMBL3632992)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C33H42ClN3O3/c1-20-21(2)31-24(22(3)30(20)38)15-16-33(4,40-31)32(39)36-18-10-6-5-9-17-35-29-25-11-7-8-12-27(25)37-28-19-23(34)13-14-26(28)29/h13-14,19,38H,5-12,15-18H2,1-4H3,(H,35,37)(H,36,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133470
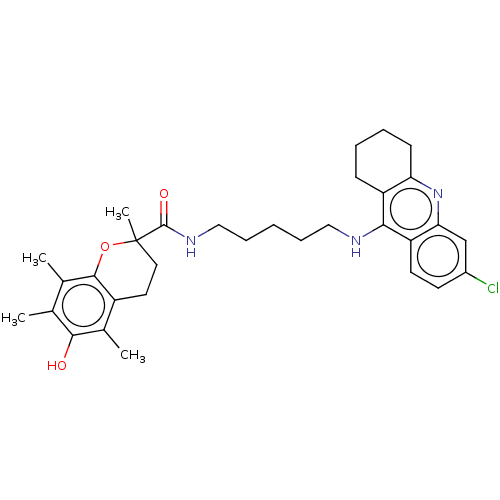
(CHEMBL3632991)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C32H40ClN3O3/c1-19-20(2)30-23(21(3)29(19)37)14-15-32(4,39-30)31(38)35-17-9-5-8-16-34-28-24-10-6-7-11-26(24)36-27-18-22(33)12-13-25(27)28/h12-13,18,37H,5-11,14-17H2,1-4H3,(H,34,36)(H,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50398036
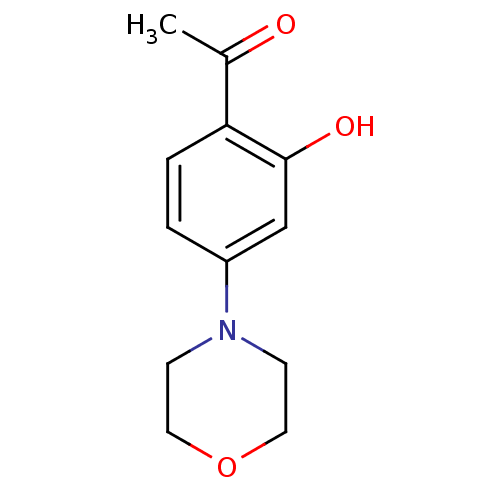
(CHEMBL1317546)Show InChI InChI=1S/C12H15NO3/c1-9(14)11-3-2-10(8-12(11)15)13-4-6-16-7-5-13/h2-3,8,15H,4-7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of DNA-PK purified from HeLa nuclear extracts assessed as EPPLSQEAFADLWKKR peptide substrate phosphorylation |
J Med Chem 58: 41-71 (2015)
Article DOI: 10.1021/jm501026z
BindingDB Entry DOI: 10.7270/Q2P84DJ7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50133470

(CHEMBL3632991)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C32H40ClN3O3/c1-19-20(2)30-23(21(3)29(19)37)14-15-32(4,39-30)31(38)35-17-9-5-8-16-34-28-24-10-6-7-11-26(24)36-27-18-22(33)12-13-25(27)28/h12-13,18,37H,5-11,14-17H2,1-4H3,(H,34,36)(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50073113
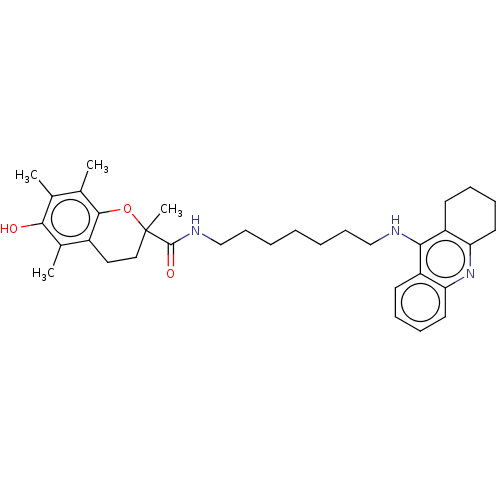
(CHEMBL3410955)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H45N3O3/c1-22-23(2)32-25(24(3)31(22)38)18-19-34(4,40-32)33(39)36-21-13-7-5-6-12-20-35-30-26-14-8-10-16-28(26)37-29-17-11-9-15-27(29)30/h8,10,14,16,38H,5-7,9,11-13,15,17-21H2,1-4H3,(H,35,37)(H,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data