 Found 481 hits with Last Name = 'bower' and Initial = 'm'
Found 481 hits with Last Name = 'bower' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclin-A2/Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline
| Assay Description
In vitro CDK enzymatic assay using purified CDK mixed with cyclin A, was incubated with substrate, and test compounds in the presence of 1.4 uM ATP/ ... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline
| Assay Description
In vitro CDK enzymatic assay using purified CDK mixed with cyclin A, was incubated with substrate, and test compounds in the presence of 1.4 uM ATP/ ... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM17054
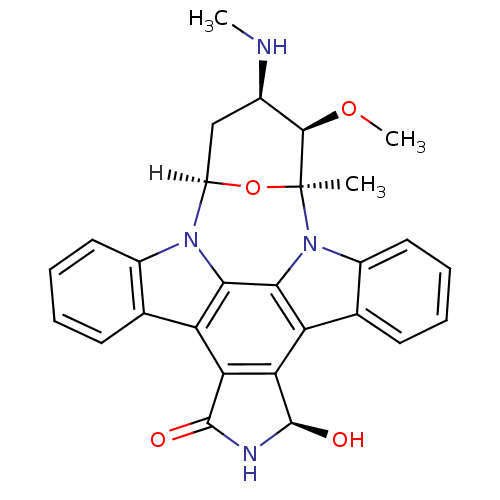
((2S,3R,4R,6R,18R)-18-hydroxy-3-methoxy-2-methyl-4-...)Show SMILES [H][C@]12C[C@@H](NC)[C@@H](OC)[C@](C)(O1)n1c3ccccc3c3c4[C@@H](O)NC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O4/c1-28-25(35-3)15(29-2)12-18(36-28)31-16-10-6-4-8-13(16)19-21-22(27(34)30-26(21)33)20-14-9-5-7-11-17(14)32(28)24(20)23(19)31/h4-11,15,18,25,27,29,34H,12H2,1-3H3,(H,30,33)/t15-,18-,25-,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.60 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
GlaxoSmithKline
| Assay Description
In vitro Chk1 enzymatic assay using purified enzyme, was incubated with substrate, and test compounds in the presence of 10 uM ATP/ [gamma-32P] ATP. ... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM17140
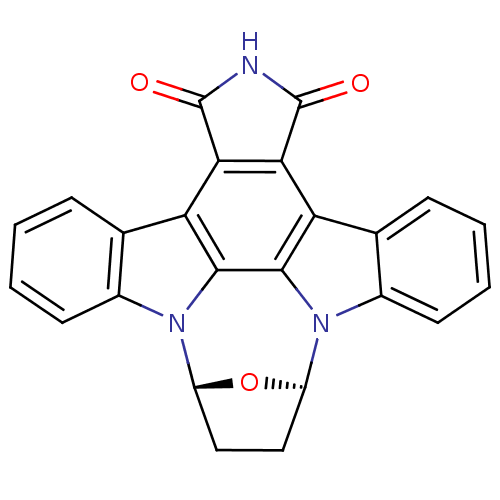
((15R,18R)-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3n3[C@H]4CC[C@@H](O4)n4c5ccccc5c2c4c13 |r| Show InChI InChI=1S/C24H15N3O3/c28-23-19-17-11-5-1-3-7-13(11)26-15-9-10-16(30-15)27-14-8-4-2-6-12(14)18(22(27)21(17)26)20(19)24(29)25-23/h1-8,15-16H,9-10H2,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 5.60 | -46.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline
| Assay Description
In vitro CDK enzymatic assay using purified CDK mixed with cyclin A, was incubated with substrate, and test compounds in the presence of 1.4 uM ATP/ ... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 7.80 | -48.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
GlaxoSmithKline
| Assay Description
In vitro Chk1 enzymatic assay using purified enzyme, was incubated with substrate, and test compounds in the presence of 10 uM ATP/ [gamma-32P] ATP. ... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM17140

((15R,18R)-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3n3[C@H]4CC[C@@H](O4)n4c5ccccc5c2c4c13 |r| Show InChI InChI=1S/C24H15N3O3/c28-23-19-17-11-5-1-3-7-13(11)26-15-9-10-16(30-15)27-14-8-4-2-6-12(14)18(22(27)21(17)26)20(19)24(29)25-23/h1-8,15-16H,9-10H2,(H,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 15 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
GlaxoSmithKline
| Assay Description
In vitro Chk1 enzymatic assay using purified enzyme, was incubated with substrate, and test compounds in the presence of 10 uM ATP/ [gamma-32P] ATP. ... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM17140

((15R,18R)-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3n3[C@H]4CC[C@@H](O4)n4c5ccccc5c2c4c13 |r| Show InChI InChI=1S/C24H15N3O3/c28-23-19-17-11-5-1-3-7-13(11)26-15-9-10-16(30-15)27-14-8-4-2-6-12(14)18(22(27)21(17)26)20(19)24(29)25-23/h1-8,15-16H,9-10H2,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 16 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
GlaxoSmithKline
| Assay Description
CDK4 and cyclin D1 were expressed in a baculovirus expression system and subsequently purified. The catalytic activity of the CDK4 protein was assaye... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM17140

((15R,18R)-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3n3[C@H]4CC[C@@H](O4)n4c5ccccc5c2c4c13 |r| Show InChI InChI=1S/C24H15N3O3/c28-23-19-17-11-5-1-3-7-13(11)26-15-9-10-16(30-15)27-14-8-4-2-6-12(14)18(22(27)21(17)26)20(19)24(29)25-23/h1-8,15-16H,9-10H2,(H,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 23 | -43.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline
| Assay Description
In vitro CDK enzymatic assay using purified CDK mixed with cyclin A, was incubated with substrate, and test compounds in the presence of 1.4 uM ATP/ ... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM17054

((2S,3R,4R,6R,18R)-18-hydroxy-3-methoxy-2-methyl-4-...)Show SMILES [H][C@]12C[C@@H](NC)[C@@H](OC)[C@](C)(O1)n1c3ccccc3c3c4[C@@H](O)NC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O4/c1-28-25(35-3)15(29-2)12-18(36-28)31-16-10-6-4-8-13(16)19-21-22(27(34)30-26(21)33)20-14-9-5-7-11-17(14)32(28)24(20)23(19)31/h4-11,15,18,25,27,29,34H,12H2,1-3H3,(H,30,33)/t15-,18-,25-,27-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 30 | -42.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline
| Assay Description
In vitro CDK enzymatic assay using purified CDK mixed with cyclin A, was incubated with substrate, and test compounds in the presence of 1.4 uM ATP/ ... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 41 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
GlaxoSmithKline
| Assay Description
CDK4 and cyclin D1 were expressed in a baculovirus expression system and subsequently purified. The catalytic activity of the CDK4 protein was assaye... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM17054

((2S,3R,4R,6R,18R)-18-hydroxy-3-methoxy-2-methyl-4-...)Show SMILES [H][C@]12C[C@@H](NC)[C@@H](OC)[C@](C)(O1)n1c3ccccc3c3c4[C@@H](O)NC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O4/c1-28-25(35-3)15(29-2)12-18(36-28)31-16-10-6-4-8-13(16)19-21-22(27(34)30-26(21)33)20-14-9-5-7-11-17(14)32(28)24(20)23(19)31/h4-11,15,18,25,27,29,34H,12H2,1-3H3,(H,30,33)/t15-,18-,25-,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 95 | -39.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline
| Assay Description
In vitro CDK enzymatic assay using purified CDK mixed with cyclin A, was incubated with substrate, and test compounds in the presence of 1.4 uM ATP/ ... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13465

((2S)-N-[(1S)-1-carbamoyl-2-{4-[(5S)-1,1,3-trioxo-1...)Show SMILES CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(cc1)[C@@H]1CC(=O)NS1(=O)=O)C(N)=O |r| Show InChI InChI=1S/C23H26N4O6S/c1-14(28)25-19(12-15-5-3-2-4-6-15)23(31)26-18(22(24)30)11-16-7-9-17(10-8-16)20-13-21(29)27-34(20,32)33/h2-10,18-20H,11-13H2,1H3,(H2,24,30)(H,25,28)(H,26,31)(H,27,29)/t18-,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant against protein-tyrosine phosphatase 1B by PNPP enzyme assay |
J Med Chem 48: 6544-8 (2005)
Article DOI: 10.1021/jm0504555
BindingDB Entry DOI: 10.7270/Q2805252 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50300312
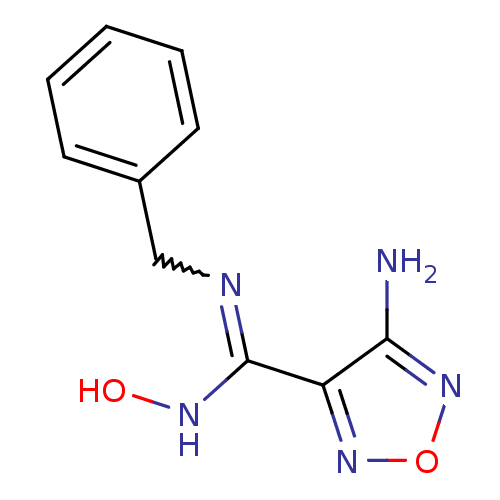
(4-amino-1,2,5-oxadiazole-3-carboximidamide | CHEMB...)Show InChI InChI=1S/C10H11N5O2/c11-9-8(14-17-15-9)10(13-16)12-6-7-4-2-1-3-5-7/h1-5,16H,6H2,(H2,11,15)(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal his-tagged human indoleamine 2,3-dioxygenase expressed in Escherichia coli assessed as N'-formylkynurenine formation by spec... |
J Med Chem 52: 7364-7 (2009)
Article DOI: 10.1021/jm900518f
BindingDB Entry DOI: 10.7270/Q29P32KW |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM17054

((2S,3R,4R,6R,18R)-18-hydroxy-3-methoxy-2-methyl-4-...)Show SMILES [H][C@]12C[C@@H](NC)[C@@H](OC)[C@](C)(O1)n1c3ccccc3c3c4[C@@H](O)NC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O4/c1-28-25(35-3)15(29-2)12-18(36-28)31-16-10-6-4-8-13(16)19-21-22(27(34)30-26(21)33)20-14-9-5-7-11-17(14)32(28)24(20)23(19)31/h4-11,15,18,25,27,29,34H,12H2,1-3H3,(H,30,33)/t15-,18-,25-,27-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.60E+3 | -31.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
GlaxoSmithKline
| Assay Description
CDK4 and cyclin D1 were expressed in a baculovirus expression system and subsequently purified. The catalytic activity of the CDK4 protein was assaye... |
J Biol Chem 277: 46609-15 (2002)
Article DOI: 10.1074/jbc.M201233200
BindingDB Entry DOI: 10.7270/Q2D798PF |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50300305
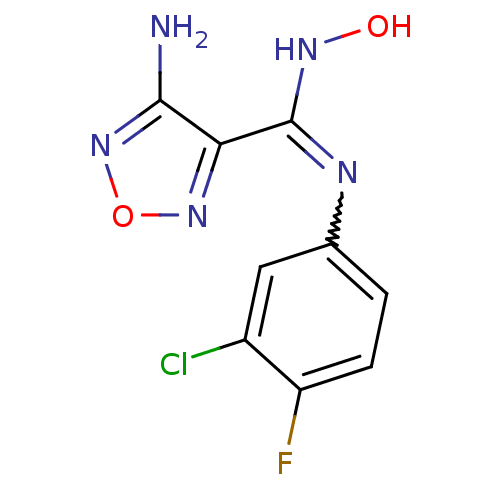
(4-Amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2...)Show InChI InChI=1S/C9H7ClFN5O2/c10-5-3-4(1-2-6(5)11)13-9(14-17)7-8(12)16-18-15-7/h1-3,17H,(H2,12,16)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Competitive inhibition of IDO1 (unknown origin) |
ACS Med Chem Lett 8: 486-491 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00391
BindingDB Entry DOI: 10.7270/Q2G73H0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM125902
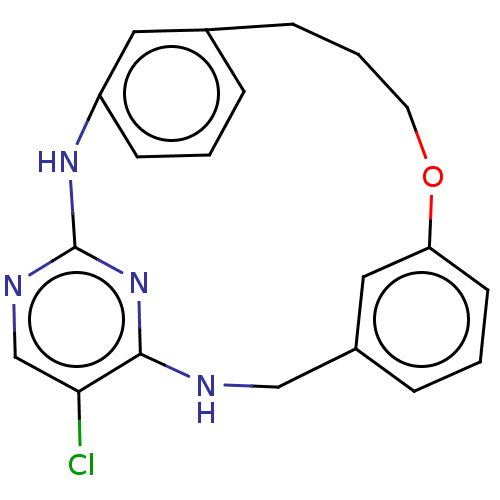
(US8765727, 5)Show InChI InChI=1S/C20H19ClN4O/c21-18-13-23-20-24-16-7-1-4-14(10-16)6-3-9-26-17-8-2-5-15(11-17)12-22-19(18)25-20/h1-2,4-5,7-8,10-11,13H,3,6,9,12H2,(H2,22,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Incyte Corporation
US Patent
| Assay Description
One or more compounds herein were tested for inhibitory activity of JAK targets according to the following in vitro assay described in Park et al., A... |
US Patent US8765727 (2014)
BindingDB Entry DOI: 10.7270/Q2FQ9V87 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM125902

(US8765727, 5)Show InChI InChI=1S/C20H19ClN4O/c21-18-13-23-20-24-16-7-1-4-14(10-16)6-3-9-26-17-8-2-5-15(11-17)12-22-19(18)25-20/h1-2,4-5,7-8,10-11,13H,3,6,9,12H2,(H2,22,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Incyte Corporation
US Patent
| Assay Description
One or more compounds herein were tested for inhibitory activity of JAK targets according to the following in vitro assay described in Park et al., A... |
US Patent US8765727 (2014)
BindingDB Entry DOI: 10.7270/Q2FQ9V87 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM125902

(US8765727, 5)Show InChI InChI=1S/C20H19ClN4O/c21-18-13-23-20-24-16-7-1-4-14(10-16)6-3-9-26-17-8-2-5-15(11-17)12-22-19(18)25-20/h1-2,4-5,7-8,10-11,13H,3,6,9,12H2,(H2,22,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Incyte Corporation
US Patent
| Assay Description
One or more compounds herein were tested for inhibitory activity of JAK targets according to the following in vitro assay described in Park et al., A... |
US Patent US8765727 (2014)
BindingDB Entry DOI: 10.7270/Q2FQ9V87 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM125913
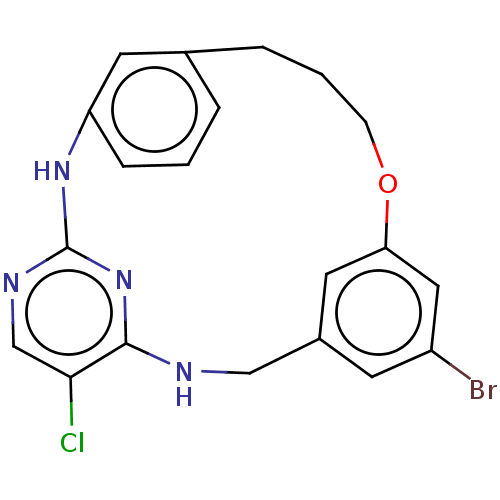
(US8765727, 16)Show SMILES Clc1cnc2Nc3cccc(CCCOc4cc(Br)cc(CNc1n2)c4)c3 Show InChI InChI=1S/C20H18BrClN4O/c21-15-7-14-9-17(10-15)27-6-2-4-13-3-1-5-16(8-13)25-20-24-12-18(22)19(26-20)23-11-14/h1,3,5,7-10,12H,2,4,6,11H2,(H2,23,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Incyte Corporation
US Patent
| Assay Description
One or more compounds herein were tested for inhibitory activity of JAK targets according to the following in vitro assay described in Park et al., A... |
US Patent US8765727 (2014)
BindingDB Entry DOI: 10.7270/Q2FQ9V87 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50203526
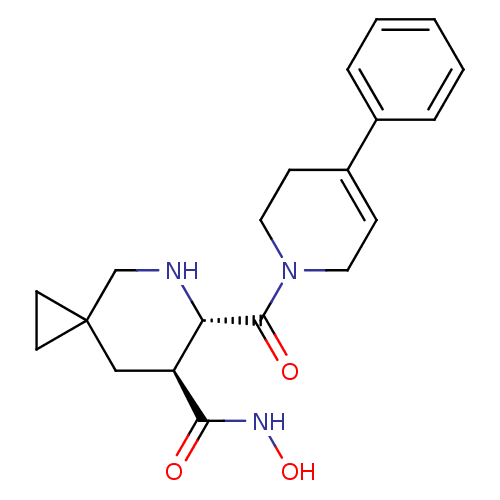
((6S,7S)-6-(4-phenyl-3,6-dihydro-2H-pyridine-1-carb...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CCC(=CC1)c1ccccc1 |c:19| Show InChI InChI=1S/C20H25N3O3/c24-18(22-26)16-12-20(8-9-20)13-21-17(16)19(25)23-10-6-15(7-11-23)14-4-2-1-3-5-14/h1-6,16-17,21,26H,7-13H2,(H,22,24)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50099348
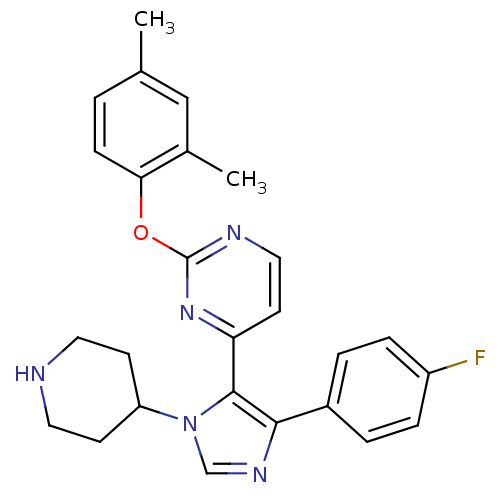
(2-(2,4-Dimethyl-phenoxy)-4-[5-(4-fluoro-phenyl)-3-...)Show SMILES Cc1ccc(Oc2nccc(n2)-c2c(ncn2C2CCNCC2)-c2ccc(F)cc2)c(C)c1 Show InChI InChI=1S/C26H26FN5O/c1-17-3-8-23(18(2)15-17)33-26-29-14-11-22(31-26)25-24(19-4-6-20(27)7-5-19)30-16-32(25)21-9-12-28-13-10-21/h3-8,11,14-16,21,28H,9-10,12-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 11: 1123-6 (2001)
BindingDB Entry DOI: 10.7270/Q2028QTS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM125904
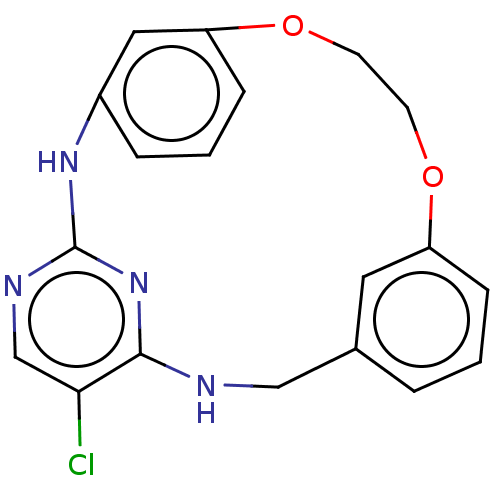
(US8765727, 7)Show InChI InChI=1S/C19H17ClN4O2/c20-17-12-22-19-23-14-4-2-6-16(10-14)26-8-7-25-15-5-1-3-13(9-15)11-21-18(17)24-19/h1-6,9-10,12H,7-8,11H2,(H2,21,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Incyte Corporation
US Patent
| Assay Description
One or more compounds herein were tested for inhibitory activity of JAK targets according to the following in vitro assay described in Park et al., A... |
US Patent US8765727 (2014)
BindingDB Entry DOI: 10.7270/Q2FQ9V87 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50099358
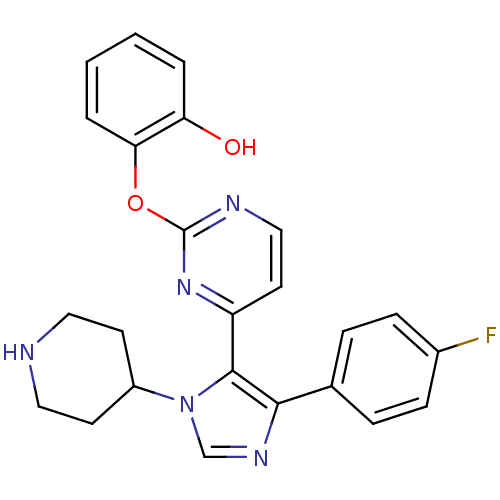
(2-{4-[5-(4-Fluoro-phenyl)-3-piperidin-4-yl-3H-imid...)Show SMILES Oc1ccccc1Oc1nccc(n1)-c1c(ncn1C1CCNCC1)-c1ccc(F)cc1 Show InChI InChI=1S/C24H22FN5O2/c25-17-7-5-16(6-8-17)22-23(30(15-28-22)18-9-12-26-13-10-18)19-11-14-27-24(29-19)32-21-4-2-1-3-20(21)31/h1-8,11,14-15,18,26,31H,9-10,12-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 11: 1123-6 (2001)
BindingDB Entry DOI: 10.7270/Q2028QTS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50099373
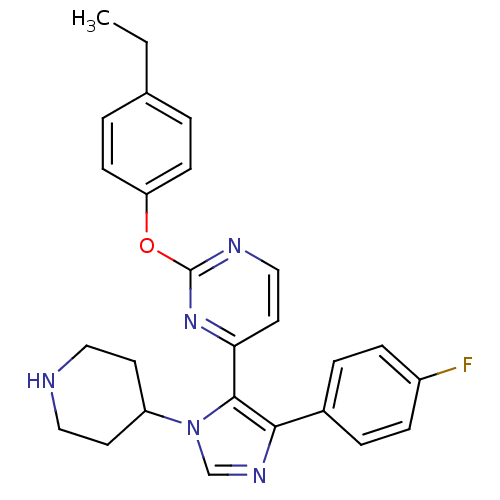
(2-(4-Ethyl-phenoxy)-4-[5-(4-fluoro-phenyl)-3-piper...)Show SMILES CCc1ccc(Oc2nccc(n2)-c2c(ncn2C2CCNCC2)-c2ccc(F)cc2)cc1 Show InChI InChI=1S/C26H26FN5O/c1-2-18-3-9-22(10-4-18)33-26-29-16-13-23(31-26)25-24(19-5-7-20(27)8-6-19)30-17-32(25)21-11-14-28-15-12-21/h3-10,13,16-17,21,28H,2,11-12,14-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 11: 1123-6 (2001)
BindingDB Entry DOI: 10.7270/Q2028QTS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrs |
ACS Med Chem Lett 8: 486-491 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00391
BindingDB Entry DOI: 10.7270/Q2G73H0Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM125904

(US8765727, 7)Show InChI InChI=1S/C19H17ClN4O2/c20-17-12-22-19-23-14-4-2-6-16(10-14)26-8-7-25-15-5-1-3-13(9-15)11-21-18(17)24-19/h1-6,9-10,12H,7-8,11H2,(H2,21,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Incyte Corporation
US Patent
| Assay Description
One or more compounds herein were tested for inhibitory activity of JAK targets according to the following in vitro assay described in Park et al., A... |
US Patent US8765727 (2014)
BindingDB Entry DOI: 10.7270/Q2FQ9V87 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM125899
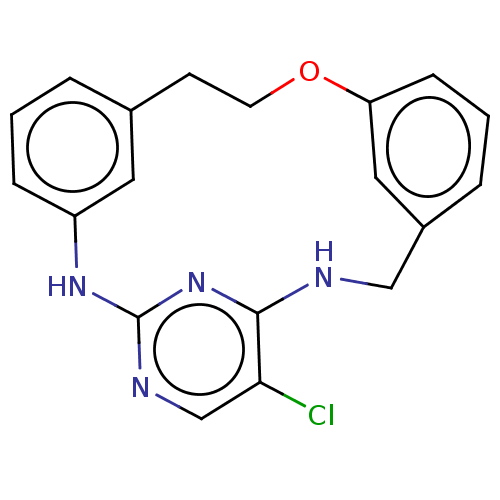
(US8765727, 2)Show InChI InChI=1S/C19H17ClN4O/c20-17-12-22-19-23-15-5-1-3-13(9-15)7-8-25-16-6-2-4-14(10-16)11-21-18(17)24-19/h1-6,9-10,12H,7-8,11H2,(H2,21,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Incyte Corporation
US Patent
| Assay Description
One or more compounds herein were tested for inhibitory activity of JAK targets according to the following in vitro assay described in Park et al., A... |
US Patent US8765727 (2014)
BindingDB Entry DOI: 10.7270/Q2FQ9V87 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50300306
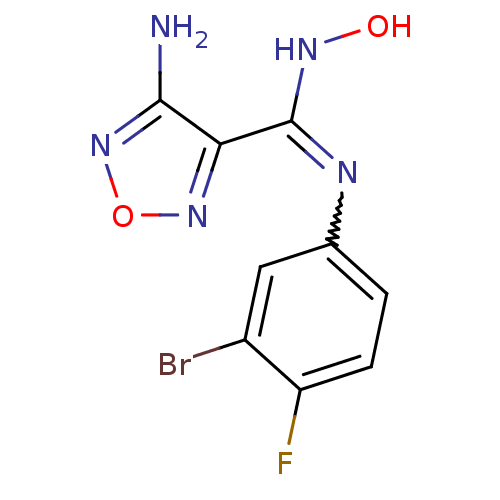
(4-Amino-N-(3-bromo-4-fluorophenyl)-N'-hydroxy-1,2,...)Show InChI InChI=1S/C9H7BrFN5O2/c10-5-3-4(1-2-6(5)11)13-9(14-17)7-8(12)16-18-15-7/h1-3,17H,(H2,12,16)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrs |
ACS Med Chem Lett 8: 486-491 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00391
BindingDB Entry DOI: 10.7270/Q2G73H0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50189670
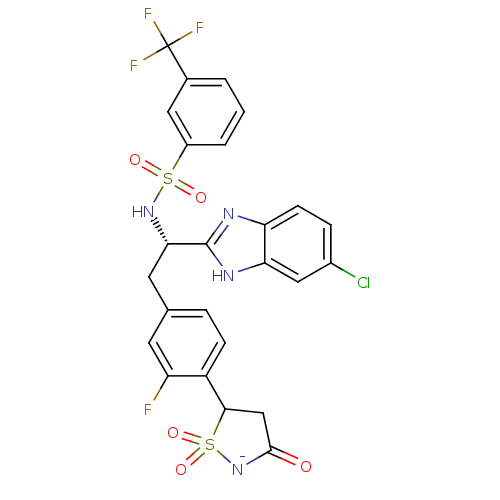
(5-{4-[(2S)-2-(5-chloro-1H-benzimidazol-2-yl)-2-({[...)Show SMILES Fc1cc(C[C@H](NS(=O)(=O)c2cccc(c2)C(F)(F)F)c2nc3ccc(Cl)cc3[nH]2)ccc1C1CC(=O)[N-]S1(=O)=O Show InChI InChI=1S/C25H19ClF4N4O5S2/c26-15-5-7-19-20(11-15)32-24(31-19)21(33-40(36,37)16-3-1-2-14(10-16)25(28,29)30)9-13-4-6-17(18(27)8-13)22-12-23(35)34-41(22,38)39/h1-8,10-11,21-22,33H,9,12H2,(H2,31,32,34,35)/p-1/t21-,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
J Med Chem 49: 3774-89 (2006)
Article DOI: 10.1021/jm0600904
BindingDB Entry DOI: 10.7270/Q2JQ10M8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM125899

(US8765727, 2)Show InChI InChI=1S/C19H17ClN4O/c20-17-12-22-19-23-15-5-1-3-13(9-15)7-8-25-16-6-2-4-14(10-16)11-21-18(17)24-19/h1-6,9-10,12H,7-8,11H2,(H2,21,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Incyte Corporation
US Patent
| Assay Description
One or more compounds herein were tested for inhibitory activity of JAK targets according to the following in vitro assay described in Park et al., A... |
US Patent US8765727 (2014)
BindingDB Entry DOI: 10.7270/Q2FQ9V87 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50099345
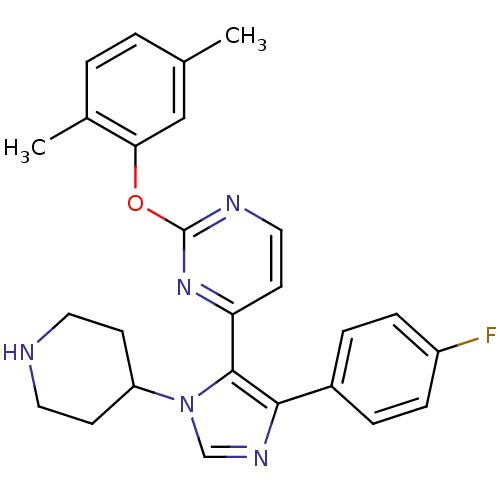
(2-(2,5-Dimethyl-phenoxy)-4-[5-(4-fluoro-phenyl)-3-...)Show SMILES Cc1ccc(C)c(Oc2nccc(n2)-c2c(ncn2C2CCNCC2)-c2ccc(F)cc2)c1 Show InChI InChI=1S/C26H26FN5O/c1-17-3-4-18(2)23(15-17)33-26-29-14-11-22(31-26)25-24(19-5-7-20(27)8-6-19)30-16-32(25)21-9-12-28-13-10-21/h3-8,11,14-16,21,28H,9-10,12-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 11: 1123-6 (2001)
BindingDB Entry DOI: 10.7270/Q2028QTS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50233431
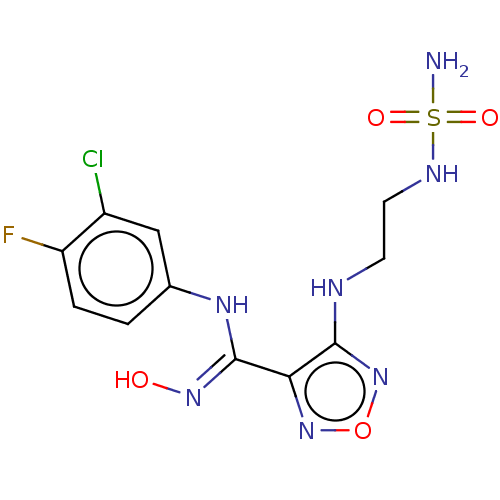
(CHEMBL3979473 | US9789094, 5)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Cl)c1)=N\O Show InChI InChI=1S/C11H13ClFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrs |
ACS Med Chem Lett 8: 486-491 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00391
BindingDB Entry DOI: 10.7270/Q2G73H0Q |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50300306

(4-Amino-N-(3-bromo-4-fluorophenyl)-N'-hydroxy-1,2,...)Show InChI InChI=1S/C9H7BrFN5O2/c10-5-3-4(1-2-6(5)11)13-9(14-17)7-8(12)16-18-15-7/h1-3,17H,(H2,12,16)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of indoleamine 2,3-dioxygenase in IFN-gamma-stimulated human HeLa cells assessed as kynurenine formation by spectrophotometry |
J Med Chem 52: 7364-7 (2009)
Article DOI: 10.1021/jm900518f
BindingDB Entry DOI: 10.7270/Q29P32KW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM125899

(US8765727, 2)Show InChI InChI=1S/C19H17ClN4O/c20-17-12-22-19-23-15-5-1-3-13(9-15)7-8-25-16-6-2-4-14(10-16)11-21-18(17)24-19/h1-6,9-10,12H,7-8,11H2,(H2,21,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Incyte Corporation
US Patent
| Assay Description
One or more compounds herein were tested for inhibitory activity of JAK targets according to the following in vitro assay described in Park et al., A... |
US Patent US8765727 (2014)
BindingDB Entry DOI: 10.7270/Q2FQ9V87 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50099343
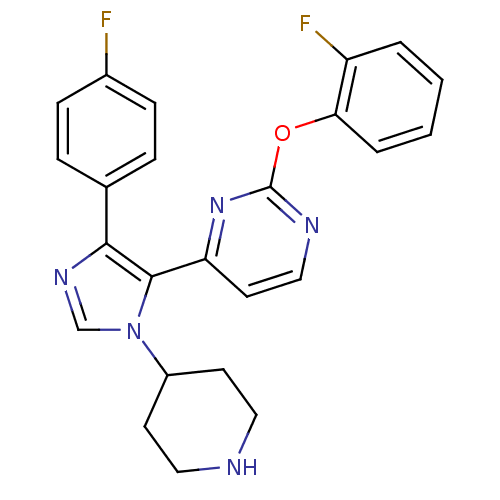
(2-(2-Fluoro-phenoxy)-4-[5-(4-fluoro-phenyl)-3-pipe...)Show SMILES Fc1ccc(cc1)-c1ncn(C2CCNCC2)c1-c1ccnc(Oc2ccccc2F)n1 Show InChI InChI=1S/C24H21F2N5O/c25-17-7-5-16(6-8-17)22-23(31(15-29-22)18-9-12-27-13-10-18)20-11-14-28-24(30-20)32-21-4-2-1-3-19(21)26/h1-8,11,14-15,18,27H,9-10,12-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 11: 1123-6 (2001)
BindingDB Entry DOI: 10.7270/Q2028QTS |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50203538
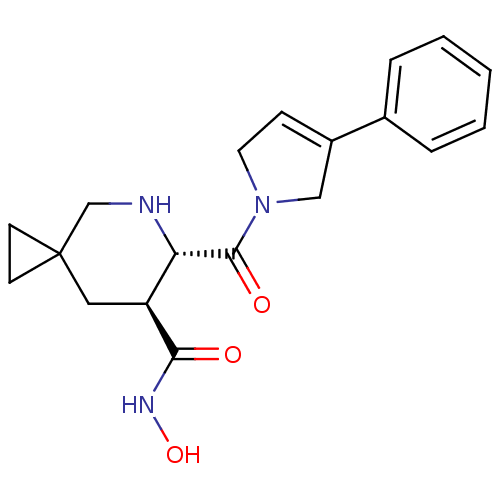
((6S,7S)-6-(3-phenyl-2,5-dihydro-pyrrole-1-carbonyl...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CC=C(C1)c1ccccc1 |c:18| Show InChI InChI=1S/C19H23N3O3/c23-17(21-25)15-10-19(7-8-19)12-20-16(15)18(24)22-9-6-14(11-22)13-4-2-1-3-5-13/h1-6,15-16,20,25H,7-12H2,(H,21,23)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50203538

((6S,7S)-6-(3-phenyl-2,5-dihydro-pyrrole-1-carbonyl...)Show SMILES ONC(=O)[C@H]1CC2(CC2)CN[C@@H]1C(=O)N1CC=C(C1)c1ccccc1 |c:18| Show InChI InChI=1S/C19H23N3O3/c23-17(21-25)15-10-19(7-8-19)12-20-16(15)18(24)22-9-6-14(11-22)13-4-2-1-3-5-13/h1-6,15-16,20,25H,7-12H2,(H,21,23)/t15-,16-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of Her2 sheddase activity in BT474 cell line |
J Med Chem 50: 603-6 (2007)
Article DOI: 10.1021/jm061344o
BindingDB Entry DOI: 10.7270/Q2JQ10P5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50099367
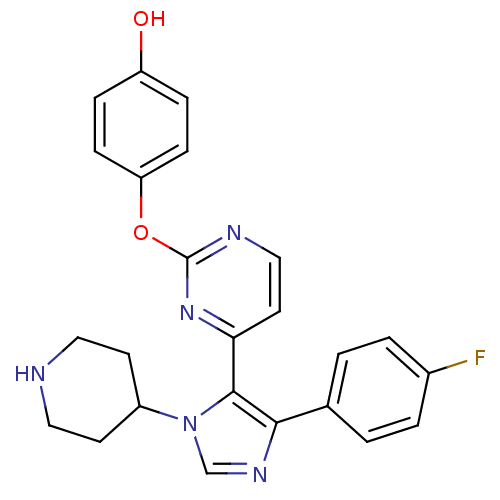
(4-{4-[5-(4-Fluoro-phenyl)-3-piperidin-4-yl-3H-imid...)Show SMILES Oc1ccc(Oc2nccc(n2)-c2c(ncn2C2CCNCC2)-c2ccc(F)cc2)cc1 Show InChI InChI=1S/C24H22FN5O2/c25-17-3-1-16(2-4-17)22-23(30(15-28-22)18-9-12-26-13-10-18)21-11-14-27-24(29-21)32-20-7-5-19(31)6-8-20/h1-8,11,14-15,18,26,31H,9-10,12-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 11: 1123-6 (2001)
BindingDB Entry DOI: 10.7270/Q2028QTS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50233424
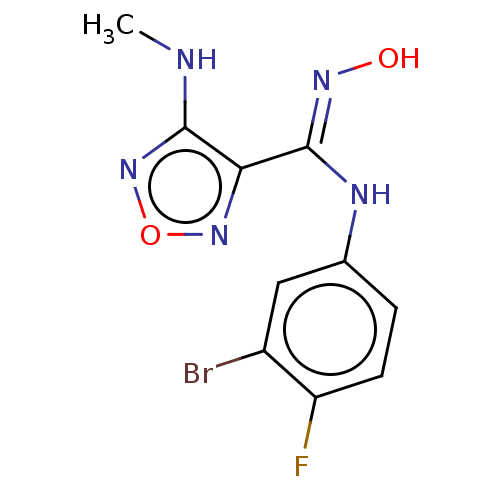
(CHEMBL4076756)Show InChI InChI=1S/C10H9BrFN5O2/c1-13-9-8(16-19-17-9)10(15-18)14-5-2-3-7(12)6(11)4-5/h2-4,18H,1H3,(H,13,17)(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrs |
ACS Med Chem Lett 8: 486-491 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00391
BindingDB Entry DOI: 10.7270/Q2G73H0Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50099349
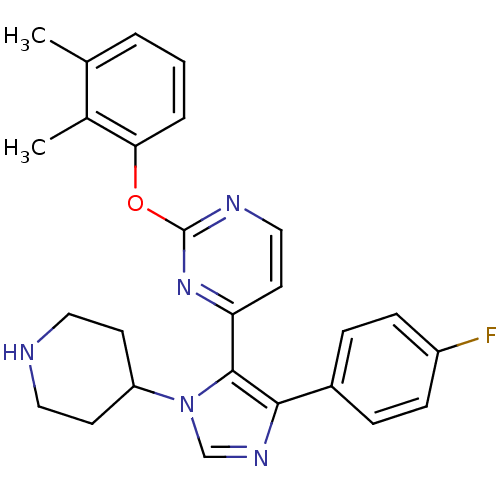
(2-(2,3-Dimethyl-phenoxy)-4-[5-(4-fluoro-phenyl)-3-...)Show SMILES Cc1cccc(Oc2nccc(n2)-c2c(ncn2C2CCNCC2)-c2ccc(F)cc2)c1C Show InChI InChI=1S/C26H26FN5O/c1-17-4-3-5-23(18(17)2)33-26-29-15-12-22(31-26)25-24(19-6-8-20(27)9-7-19)30-16-32(25)21-10-13-28-14-11-21/h3-9,12,15-16,21,28H,10-11,13-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 11: 1123-6 (2001)
BindingDB Entry DOI: 10.7270/Q2028QTS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50189686
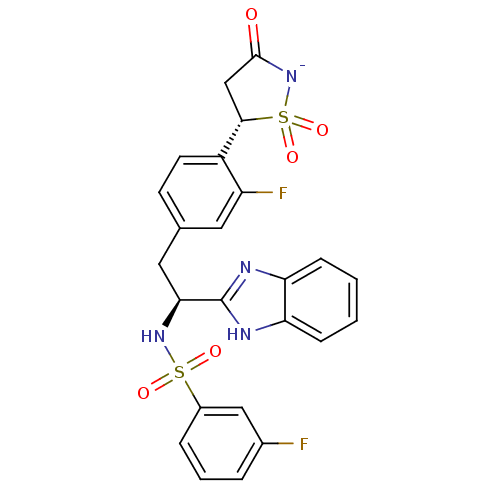
(CHEMBL381584 | N-(1S)-1-(1H-benzimidazol-2-yl)-2-{...)Show SMILES Fc1cccc(c1)S(=O)(=O)N[C@@H](Cc1ccc([C@@H]2CC(=O)[N-]S2(=O)=O)c(F)c1)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C24H20F2N4O5S2/c25-15-4-3-5-16(12-15)36(32,33)29-21(24-27-19-6-1-2-7-20(19)28-24)11-14-8-9-17(18(26)10-14)22-13-23(31)30-37(22,34)35/h1-10,12,21-22,29H,11,13H2,(H2,27,28,30,31)/p-1/t21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
J Med Chem 49: 3774-89 (2006)
Article DOI: 10.1021/jm0600904
BindingDB Entry DOI: 10.7270/Q2JQ10M8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50099342

(4-[5-(4-Fluoro-phenyl)-3-piperidin-4-yl-3H-imidazo...)Show SMILES Cc1ccccc1Oc1nccc(n1)-c1c(ncn1C1CCNCC1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H24FN5O/c1-17-4-2-3-5-22(17)32-25-28-15-12-21(30-25)24-23(18-6-8-19(26)9-7-18)29-16-31(24)20-10-13-27-14-11-20/h2-9,12,15-16,20,27H,10-11,13-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 11: 1123-6 (2001)
BindingDB Entry DOI: 10.7270/Q2028QTS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM125913

(US8765727, 16)Show SMILES Clc1cnc2Nc3cccc(CCCOc4cc(Br)cc(CNc1n2)c4)c3 Show InChI InChI=1S/C20H18BrClN4O/c21-15-7-14-9-17(10-15)27-6-2-4-13-3-1-5-16(8-13)25-20-24-12-18(22)19(26-20)23-11-14/h1,3,5,7-10,12H,2,4,6,11H2,(H2,23,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Incyte Corporation
US Patent
| Assay Description
One or more compounds herein were tested for inhibitory activity of JAK targets according to the following in vitro assay described in Park et al., A... |
US Patent US8765727 (2014)
BindingDB Entry DOI: 10.7270/Q2FQ9V87 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM125913

(US8765727, 16)Show SMILES Clc1cnc2Nc3cccc(CCCOc4cc(Br)cc(CNc1n2)c4)c3 Show InChI InChI=1S/C20H18BrClN4O/c21-15-7-14-9-17(10-15)27-6-2-4-13-3-1-5-16(8-13)25-20-24-12-18(22)19(26-20)23-11-14/h1,3,5,7-10,12H,2,4,6,11H2,(H2,23,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Incyte Corporation
US Patent
| Assay Description
One or more compounds herein were tested for inhibitory activity of JAK targets according to the following in vitro assay described in Park et al., A... |
US Patent US8765727 (2014)
BindingDB Entry DOI: 10.7270/Q2FQ9V87 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50233430
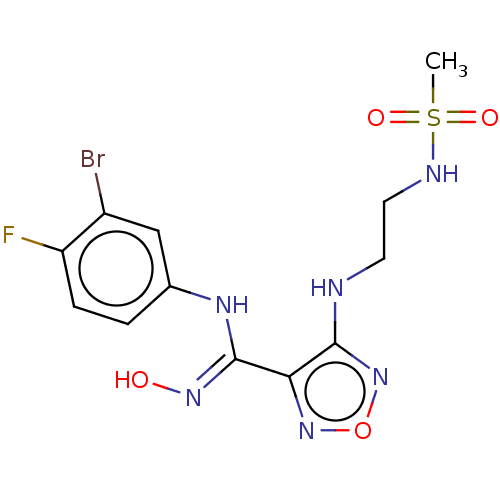
(CHEMBL3955791 | US11207302, Example 2)Show SMILES CS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C12H14BrFN6O4S/c1-25(22,23)16-5-4-15-11-10(19-24-20-11)12(18-21)17-7-2-3-9(14)8(13)6-7/h2-3,6,16,21H,4-5H2,1H3,(H,15,20)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as decrease in kynurenine levels after 48 hrs |
ACS Med Chem Lett 8: 486-491 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00391
BindingDB Entry DOI: 10.7270/Q2G73H0Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50099355
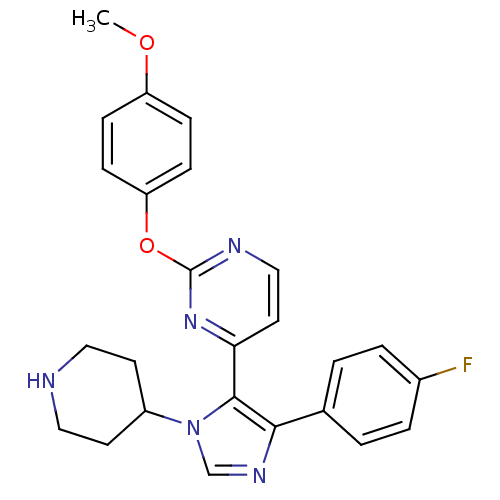
(4-[5-(4-Fluoro-phenyl)-3-piperidin-4-yl-3H-imidazo...)Show SMILES COc1ccc(Oc2nccc(n2)-c2c(ncn2C2CCNCC2)-c2ccc(F)cc2)cc1 Show InChI InChI=1S/C25H24FN5O2/c1-32-20-6-8-21(9-7-20)33-25-28-15-12-22(30-25)24-23(17-2-4-18(26)5-3-17)29-16-31(24)19-10-13-27-14-11-19/h2-9,12,15-16,19,27H,10-11,13-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 11: 1123-6 (2001)
BindingDB Entry DOI: 10.7270/Q2028QTS |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50125604
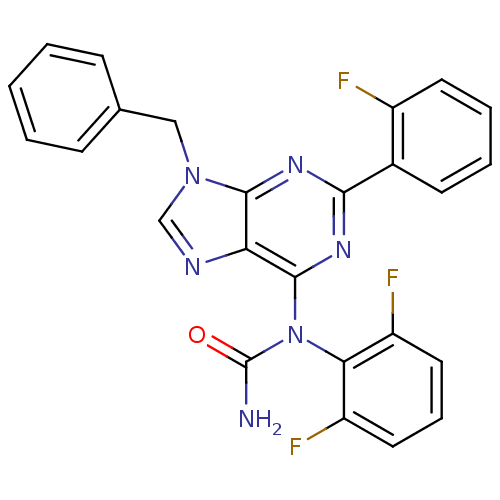
(1-[9-Benzyl-2-(2-fluoro-phenyl)-9H-purin-6-yl]-1-(...)Show SMILES NC(=O)N(c1nc(nc2n(Cc3ccccc3)cnc12)-c1ccccc1F)c1c(F)cccc1F Show InChI InChI=1S/C25H17F3N6O/c26-17-10-5-4-9-16(17)22-31-23-20(30-14-33(23)13-15-7-2-1-3-8-15)24(32-22)34(25(29)35)21-18(27)11-6-12-19(21)28/h1-12,14H,13H2,(H2,29,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human MAP p38-alpha kinase in vitro. |
Bioorg Med Chem Lett 13: 1191-4 (2003)
BindingDB Entry DOI: 10.7270/Q2MG7NW6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50189659
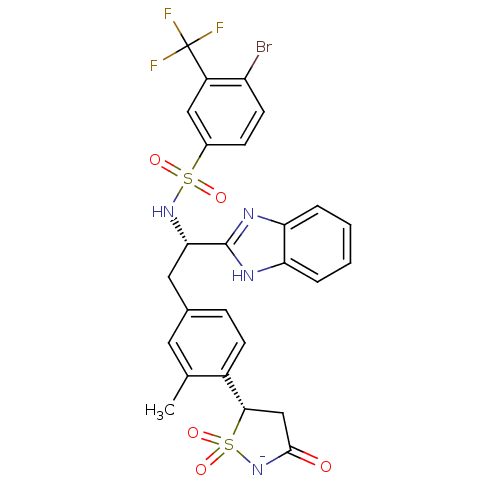
(CHEMBL378614 | N-((1S)-1-(1H-benzimidazol-2-yl)-2-...)Show SMILES Cc1cc(C[C@H](NS(=O)(=O)c2ccc(Br)c(c2)C(F)(F)F)c2nc3ccccc3[nH]2)ccc1[C@@H]1CC(=O)[N-]S1(=O)=O Show InChI InChI=1S/C26H22BrF3N4O5S2/c1-14-10-15(6-8-17(14)23-13-24(35)34-41(23,38)39)11-22(25-31-20-4-2-3-5-21(20)32-25)33-40(36,37)16-7-9-19(27)18(12-16)26(28,29)30/h2-10,12,22-23,33H,11,13H2,1H3,(H2,31,32,34,35)/p-1/t22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B by pNPP assay |
J Med Chem 49: 3774-89 (2006)
Article DOI: 10.1021/jm0600904
BindingDB Entry DOI: 10.7270/Q2JQ10M8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM125904

(US8765727, 7)Show InChI InChI=1S/C19H17ClN4O2/c20-17-12-22-19-23-14-4-2-6-16(10-14)26-8-7-25-15-5-1-3-13(9-15)11-21-18(17)24-19/h1-6,9-10,12H,7-8,11H2,(H2,21,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Incyte Corporation
US Patent
| Assay Description
One or more compounds herein were tested for inhibitory activity of JAK targets according to the following in vitro assay described in Park et al., A... |
US Patent US8765727 (2014)
BindingDB Entry DOI: 10.7270/Q2FQ9V87 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM125898
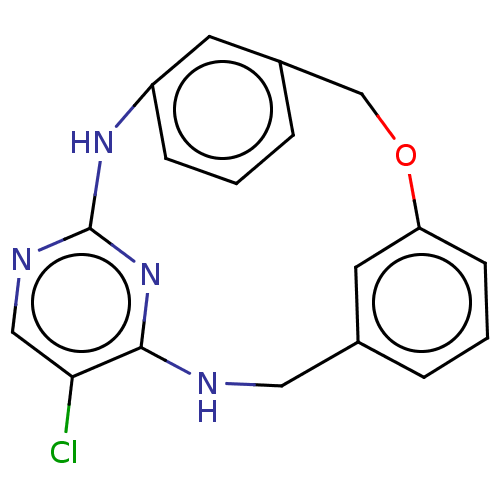
(US8765727, 1)Show InChI InChI=1S/C18H15ClN4O/c19-16-10-21-18-22-14-5-1-4-13(7-14)11-24-15-6-2-3-12(8-15)9-20-17(16)23-18/h1-8,10H,9,11H2,(H2,20,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Incyte Corporation
US Patent
| Assay Description
One or more compounds herein were tested for inhibitory activity of JAK targets according to the following in vitro assay described in Park et al., A... |
US Patent US8765727 (2014)
BindingDB Entry DOI: 10.7270/Q2FQ9V87 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data