 Found 1073 hits with Last Name = 'connor' and Initial = 'm'
Found 1073 hits with Last Name = 'connor' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50205990

(CHEMBL395429 | OXYTOCIN)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22-,25-,26-,27-,28-,29-,30-,31-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in HEK293 cells assessed as increase in IP1 accumulation preincubated for 1 hr followed by addi... |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from rat 5HT2A receptor expressed in mouse NIH3T3 cells |
J Nat Prod 60: 651-3 (1997)
Article DOI: 10.1021/np960644d
BindingDB Entry DOI: 10.7270/Q2ZC83R7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(RABBIT) | BDBM50020192
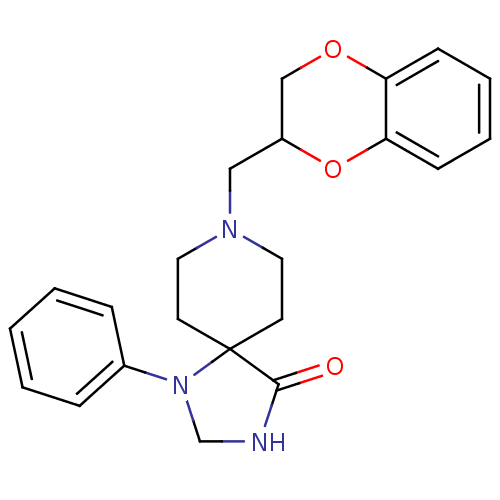
(8-(2,3-Dihydro-benzo[1,4]dioxin-2-ylmethyl)-1-phen...)Show SMILES O=C1NCN(c2ccccc2)C11CCN(CC2COc3ccccc3O2)CC1 Show InChI InChI=1S/C22H25N3O3/c26-21-22(25(16-23-21)17-6-2-1-3-7-17)10-12-24(13-11-22)14-18-15-27-19-8-4-5-9-20(19)28-18/h1-9,18H,10-16H2,(H,23,26) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by PDSP Ki Database
| |
Comp Biochem Physiol C, Pharmacol Toxicol Endocrinol 117: 19-24 (1997)
Article DOI: 10.1016/s0742-8413(97)00614-2
BindingDB Entry DOI: 10.7270/Q2PV6HWK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(RABBIT) | BDBM81444
(CAS_185453 | NSC_185453 | WB 4101 | WB-4101)Show InChI InChI=1S/C25H27NO5/c1-27-21-13-8-14-22(28-2)25(21)29-16-15-26-17-23-24(18-9-4-3-5-10-18)31-20-12-7-6-11-19(20)30-23/h3-14,23-24,26H,15-17H2,1-2H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by PDSP Ki Database
| |
Comp Biochem Physiol C, Pharmacol Toxicol Endocrinol 117: 19-24 (1997)
Article DOI: 10.1016/s0742-8413(97)00614-2
BindingDB Entry DOI: 10.7270/Q2PV6HWK |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM18771

((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside
Curated by ChEMBL
| Assay Description
Binding affinity against Thymidylate synthase was measured in vitro |
J Med Chem 33: 3060-7 (1990)
BindingDB Entry DOI: 10.7270/Q2ZW1JWX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50275934
((2-(2-(3,5-dimethylphenylamino)pyrimidin-4-yl)thia...)Show InChI InChI=1S/C16H16N4OS/c1-10-5-11(2)7-12(6-10)19-16-17-4-3-14(20-16)15-18-8-13(9-21)22-15/h3-8,21H,9H2,1-2H3,(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50562673
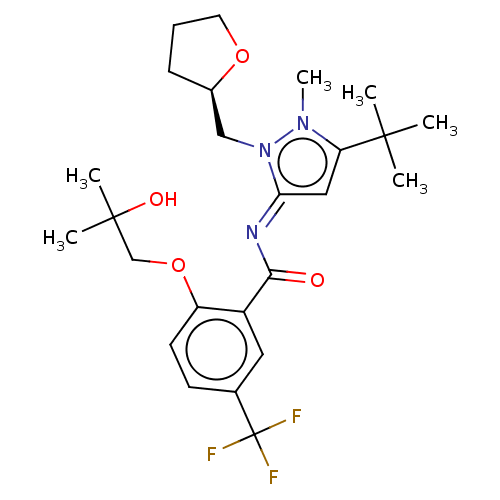
(CHEMBL4761606)Show SMILES Cn1c(c\c(=N/C(=O)c2cc(ccc2OCC(C)(C)O)C(F)(F)F)n1C[C@H]1CCCO1)C(C)(C)C |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-CP55940 from human CB2 expressed in CHO cell membrane incubated for 3 hrs by microbeta scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113087
BindingDB Entry DOI: 10.7270/Q26H4N58 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50276015
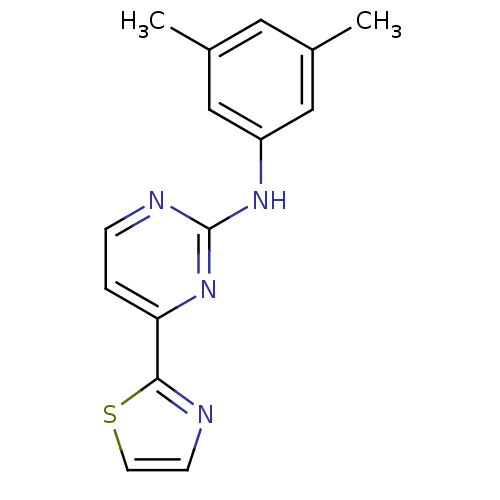
(CHEMBL509161 | N-(3,5-dimethylphenyl)-4-(thiazol-2...)Show InChI InChI=1S/C15H14N4S/c1-10-7-11(2)9-12(8-10)18-15-17-4-3-13(19-15)14-16-5-6-20-14/h3-9H,1-2H3,(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50275935
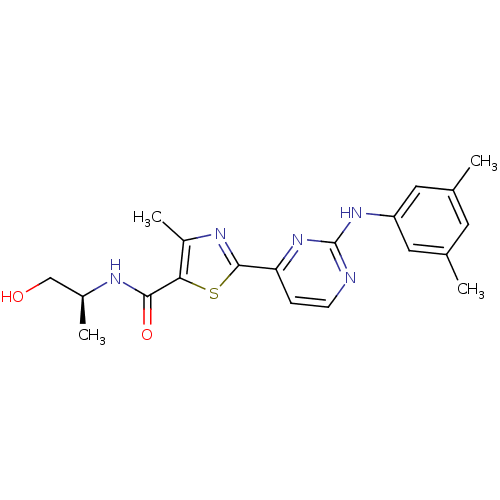
((S)-2-(2-(3,5-dimethylphenylamino)pyrimidin-4-yl)-...)Show SMILES C[C@@H](CO)NC(=O)c1sc(nc1C)-c1ccnc(Nc2cc(C)cc(C)c2)n1 |r| Show InChI InChI=1S/C20H23N5O2S/c1-11-7-12(2)9-15(8-11)24-20-21-6-5-16(25-20)19-23-14(4)17(28-19)18(27)22-13(3)10-26/h5-9,13,26H,10H2,1-4H3,(H,22,27)(H,21,24,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50275358
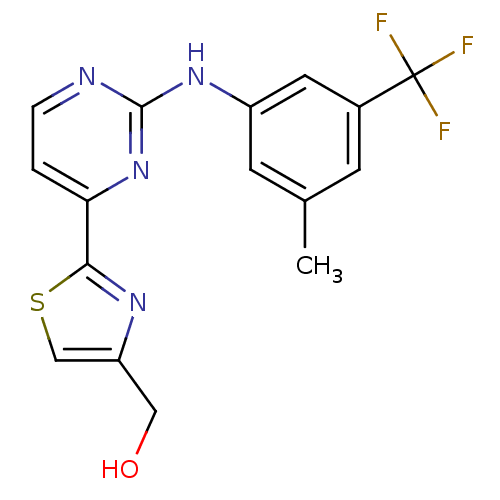
((2-(2-(3-methyl-5-(trifluoromethyl)phenylamino)pyr...)Show SMILES Cc1cc(Nc2nccc(n2)-c2nc(CO)cs2)cc(c1)C(F)(F)F Show InChI InChI=1S/C16H13F3N4OS/c1-9-4-10(16(17,18)19)6-11(5-9)22-15-20-3-2-13(23-15)14-21-12(7-24)8-25-14/h2-6,8,24H,7H2,1H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50006687

((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H24N4O6/c1-3-12-29(14-16-4-9-20-19(13-16)24(33)27-15(2)26-20)18-7-5-17(6-8-18)23(32)28-21(25(34)35)10-11-22(30)31/h1,4-9,13,21H,10-12,14H2,2H3,(H,28,32)(H,30,31)(H,34,35)(H,26,27,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside
Curated by ChEMBL
| Assay Description
Binding affinity against Thymidylate synthase was measured in vitro |
J Med Chem 33: 3060-7 (1990)
BindingDB Entry DOI: 10.7270/Q2ZW1JWX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50276065
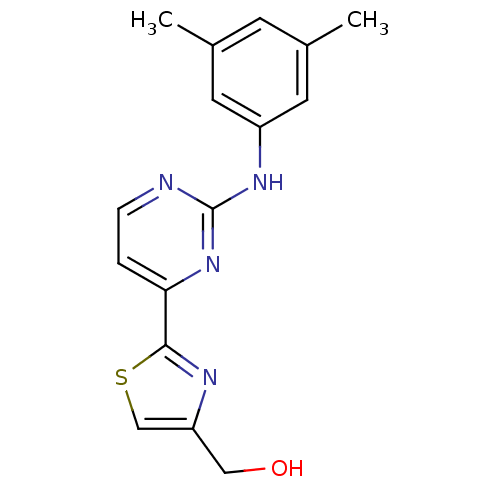
((2-(2-(3,5-dimethylphenylamino)pyrimidin-4-yl)thia...)Show InChI InChI=1S/C16H16N4OS/c1-10-5-11(2)7-12(6-10)19-16-17-4-3-14(20-16)15-18-13(8-21)9-22-15/h3-7,9,21H,8H2,1-2H3,(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50276018
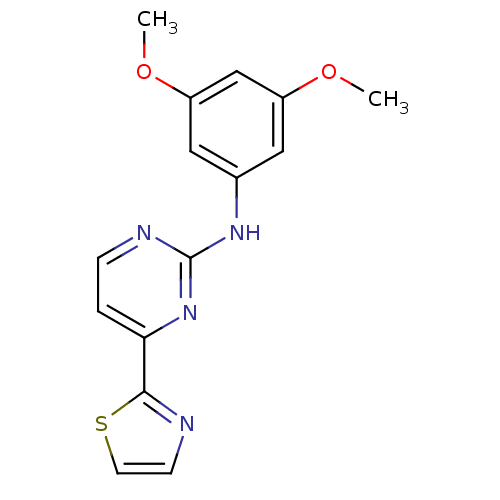
(1-(3-(cyclopenta-1,3-dienyl)benzyl)-3,5-diethylben...)Show InChI InChI=1S/C15H14N4O2S/c1-20-11-7-10(8-12(9-11)21-2)18-15-17-4-3-13(19-15)14-16-5-6-22-14/h3-9H,1-2H3,(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50562678
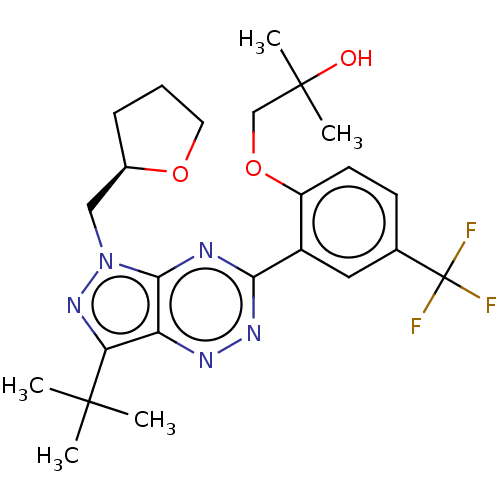
(CHEMBL4763456)Show SMILES CC(C)(O)COc1ccc(cc1-c1nnc2c(nn(C[C@H]3CCCO3)c2n1)C(C)(C)C)C(F)(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-CP55940 from human CB2 expressed in CHO cell membrane incubated for 3 hrs by microbeta scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113087
BindingDB Entry DOI: 10.7270/Q26H4N58 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249284
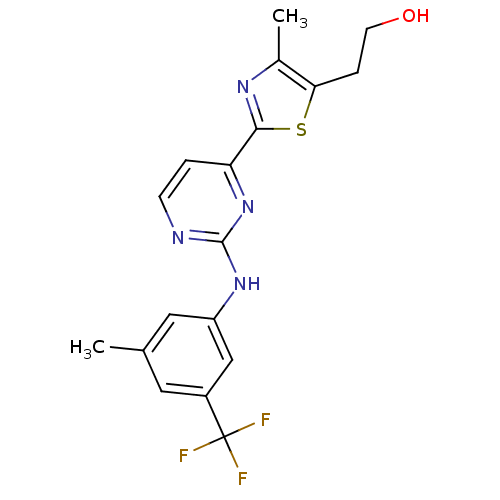
(2-(4-methyl-2-(2-(3-methyl-5-(trifluoromethyl)phen...)Show SMILES Cc1nc(sc1CCO)-c1ccnc(Nc2cc(C)cc(c2)C(F)(F)F)n1 Show InChI InChI=1S/C18H17F3N4OS/c1-10-7-12(18(19,20)21)9-13(8-10)24-17-22-5-3-14(25-17)16-23-11(2)15(27-16)4-6-26/h3,5,7-9,26H,4,6H2,1-2H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50276064
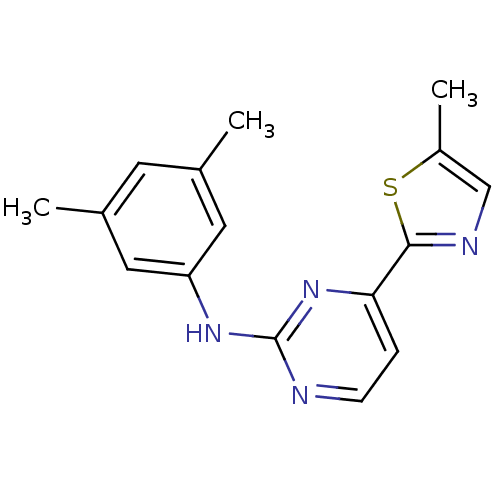
(CHEMBL470780 | N-(3,5-dimethylphenyl)-4-(5-methylt...)Show InChI InChI=1S/C16H16N4S/c1-10-6-11(2)8-13(7-10)19-16-17-5-4-14(20-16)15-18-9-12(3)21-15/h4-9H,1-3H3,(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50275357
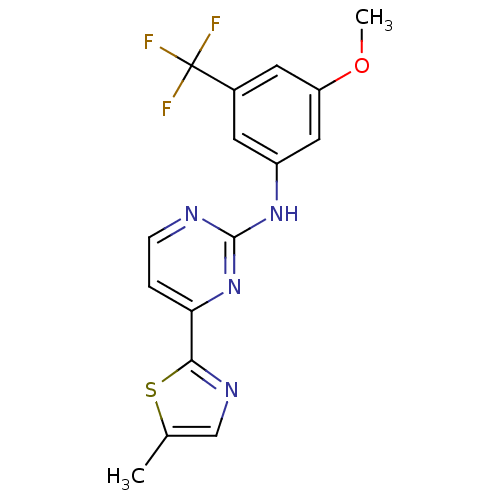
(CHEMBL451450 | N-(3-methoxy-5-(trifluoromethyl)phe...)Show SMILES COc1cc(Nc2nccc(n2)-c2ncc(C)s2)cc(c1)C(F)(F)F Show InChI InChI=1S/C16H13F3N4OS/c1-9-8-21-14(25-9)13-3-4-20-15(23-13)22-11-5-10(16(17,18)19)6-12(7-11)24-2/h3-8H,1-2H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249286
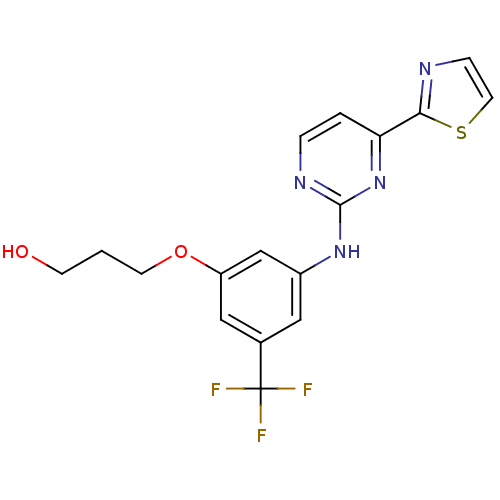
(3-(3-(4-(thiazol-2-yl)pyrimidin-2-ylamino)-5-(trif...)Show SMILES OCCCOc1cc(Nc2nccc(n2)-c2nccs2)cc(c1)C(F)(F)F Show InChI InChI=1S/C17H15F3N4O2S/c18-17(19,20)11-8-12(10-13(9-11)26-6-1-5-25)23-16-22-3-2-14(24-16)15-21-4-7-27-15/h2-4,7-10,25H,1,5-6H2,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50014480
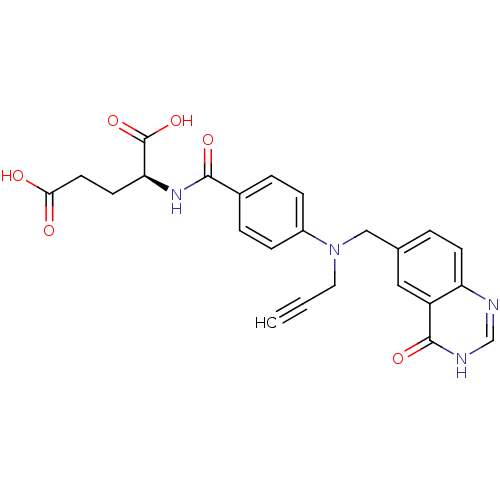
((S)-2-(4-(((4-oxo-3,4-dihydroquinazolin-6-yl)methy...)Show SMILES OC(=O)CC[C@H](NC(=O)c1ccc(cc1)N(CC#C)Cc1ccc2nc[nH]c(=O)c2c1)C(O)=O Show InChI InChI=1S/C24H22N4O6/c1-2-11-28(13-15-3-8-19-18(12-15)23(32)26-14-25-19)17-6-4-16(5-7-17)22(31)27-20(24(33)34)9-10-21(29)30/h1,3-8,12,14,20H,9-11,13H2,(H,27,31)(H,29,30)(H,33,34)(H,25,26,32)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside
Curated by ChEMBL
| Assay Description
Concentration required for in vitro inhibition of thymidylate synthase |
J Med Chem 33: 3060-7 (1990)
BindingDB Entry DOI: 10.7270/Q2ZW1JWX |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139178
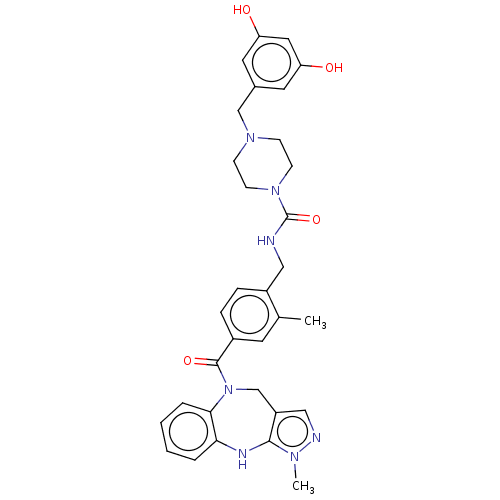
(CHEMBL3763342)Show SMILES Cc1cc(ccc1CNC(=O)N1CCN(Cc2cc(O)cc(O)c2)CC1)C(=O)N1Cc2cnn(C)c2Nc2ccccc12 Show InChI InChI=1S/C32H35N7O4/c1-21-13-23(31(42)39-20-25-18-34-36(2)30(25)35-28-5-3-4-6-29(28)39)7-8-24(21)17-33-32(43)38-11-9-37(10-12-38)19-22-14-26(40)16-27(41)15-22/h3-8,13-16,18,35,40-41H,9-12,17,19-20H2,1-2H3,(H,33,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay |
Eur J Med Chem 143: 1644-1656 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.059
BindingDB Entry DOI: 10.7270/Q2MS3W92 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139178

(CHEMBL3763342)Show SMILES Cc1cc(ccc1CNC(=O)N1CCN(Cc2cc(O)cc(O)c2)CC1)C(=O)N1Cc2cnn(C)c2Nc2ccccc12 Show InChI InChI=1S/C32H35N7O4/c1-21-13-23(31(42)39-20-25-18-34-36(2)30(25)35-28-5-3-4-6-29(28)39)7-8-24(21)17-33-32(43)38-11-9-37(10-12-38)19-22-14-26(40)16-27(41)15-22/h3-8,13-16,18,35,40-41H,9-12,17,19-20H2,1-2H3,(H,33,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in HEK293 cells assessed as increase in IP1 accumulation preincubated for 1 hr followed by addi... |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50276016
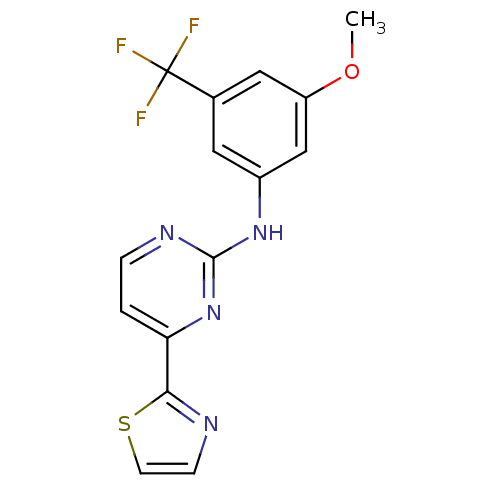
(CHEMBL512319 | N-(3-methoxy-5-(trifluoromethyl)phe...)Show InChI InChI=1S/C15H11F3N4OS/c1-23-11-7-9(15(16,17)18)6-10(8-11)21-14-20-3-2-12(22-14)13-19-4-5-24-13/h2-8H,1H3,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249318
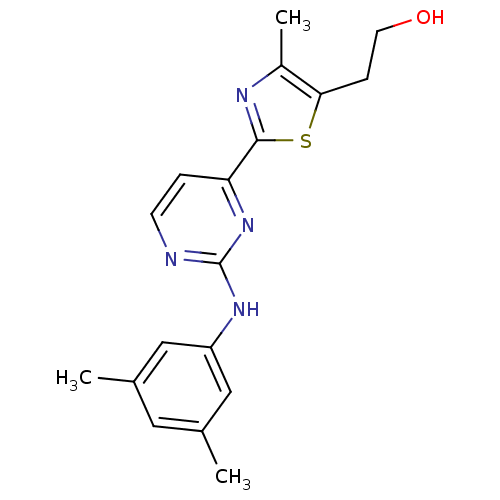
(2-(2-(2-(3,5-dimethylphenylamino)pyrimidin-4-yl)-4...)Show InChI InChI=1S/C18H20N4OS/c1-11-8-12(2)10-14(9-11)21-18-19-6-4-15(22-18)17-20-13(3)16(24-17)5-7-23/h4,6,8-10,23H,5,7H2,1-3H3,(H,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
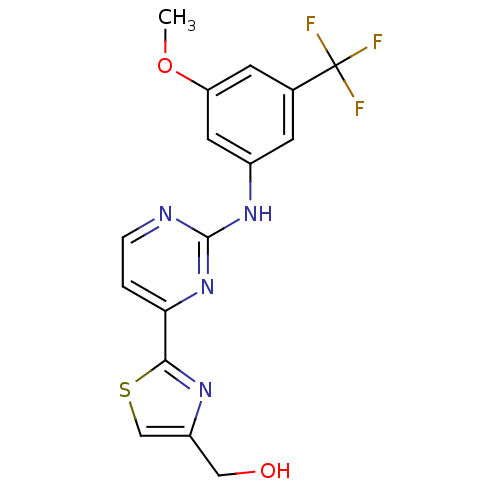
(Homo sapiens (Human)) | BDBM50275355
((2-(2-(3-methoxy-5-(trifluoromethyl)phenylamino)py...)Show SMILES COc1cc(Nc2nccc(n2)-c2nc(CO)cs2)cc(c1)C(F)(F)F Show InChI InChI=1S/C16H13F3N4O2S/c1-25-12-5-9(16(17,18)19)4-10(6-12)22-15-20-3-2-13(23-15)14-21-11(7-24)8-26-14/h2-6,8,24H,7H2,1H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
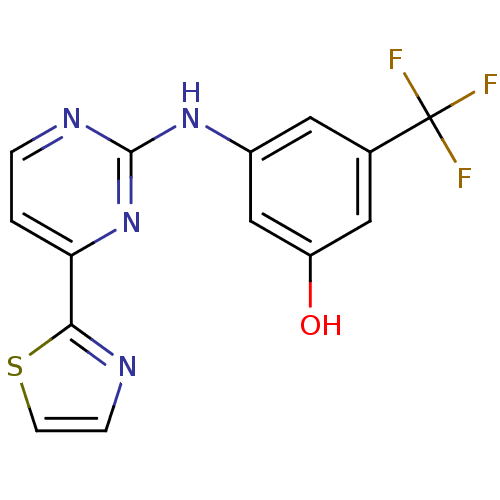
(Homo sapiens (Human)) | BDBM50276017
(3-(4-(thiazol-2-yl)pyrimidin-2-ylamino)-5-(trifluo...)Show InChI InChI=1S/C14H9F3N4OS/c15-14(16,17)8-5-9(7-10(22)6-8)20-13-19-2-1-11(21-13)12-18-3-4-23-12/h1-7,22H,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50275356
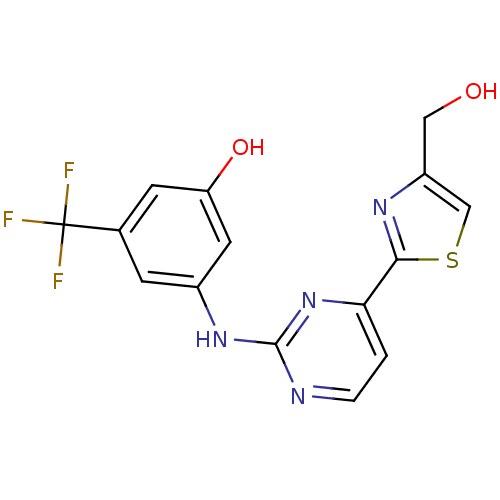
(3-(4-(4-(hydroxymethyl)thiazol-2-yl)pyrimidin-2-yl...)Show SMILES OCc1csc(n1)-c1ccnc(Nc2cc(O)cc(c2)C(F)(F)F)n1 Show InChI InChI=1S/C15H11F3N4O2S/c16-15(17,18)8-3-9(5-11(24)4-8)21-14-19-2-1-12(22-14)13-20-10(6-23)7-25-13/h1-5,7,23-24H,6H2,(H,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249356
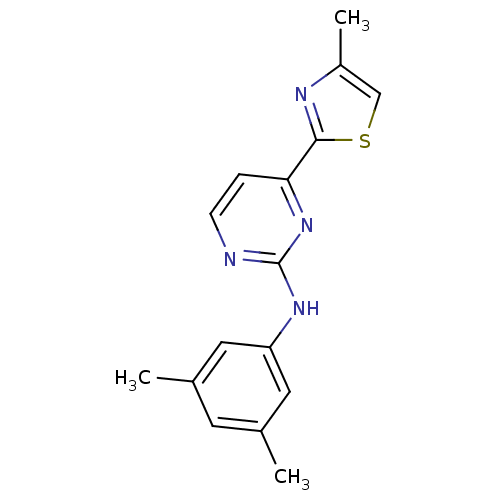
(CHEMBL453217 | N-(3,5-dimethylphenyl)-4-(4-methylt...)Show InChI InChI=1S/C16H16N4S/c1-10-6-11(2)8-13(7-10)19-16-17-5-4-14(20-16)15-18-12(3)9-21-15/h4-9H,1-3H3,(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50139178

(CHEMBL3763342)Show SMILES Cc1cc(ccc1CNC(=O)N1CCN(Cc2cc(O)cc(O)c2)CC1)C(=O)N1Cc2cnn(C)c2Nc2ccccc12 Show InChI InChI=1S/C32H35N7O4/c1-21-13-23(31(42)39-20-25-18-34-36(2)30(25)35-28-5-3-4-6-29(28)39)7-8-24(21)17-33-32(43)38-11-9-37(10-12-38)19-22-14-26(40)16-27(41)15-22/h3-8,13-16,18,35,40-41H,9-12,17,19-20H2,1-2H3,(H,33,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from human vasopressin 1a receptor expressed in HEK293 cells after 90 mins by microbeta 2 microplate-reader method |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139179
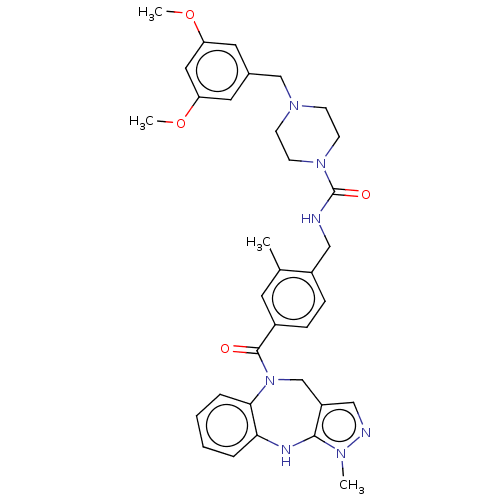
(CHEMBL3763951)Show SMILES COc1cc(CN2CCN(CC2)C(=O)NCc2ccc(cc2C)C(=O)N2Cc3cnn(C)c3Nc3ccccc23)cc(OC)c1 Show InChI InChI=1S/C34H39N7O4/c1-23-15-25(33(42)41-22-27-20-36-38(2)32(27)37-30-7-5-6-8-31(30)41)9-10-26(23)19-35-34(43)40-13-11-39(12-14-40)21-24-16-28(44-3)18-29(17-24)45-4/h5-10,15-18,20,37H,11-14,19,21-22H2,1-4H3,(H,35,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in HEK293 cells assessed as increase in IP1 accumulation preincubated for 1 hr followed by addi... |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139179

(CHEMBL3763951)Show SMILES COc1cc(CN2CCN(CC2)C(=O)NCc2ccc(cc2C)C(=O)N2Cc3cnn(C)c3Nc3ccccc23)cc(OC)c1 Show InChI InChI=1S/C34H39N7O4/c1-23-15-25(33(42)41-22-27-20-36-38(2)32(27)37-30-7-5-6-8-31(30)41)9-10-26(23)19-35-34(43)40-13-11-39(12-14-40)21-24-16-28(44-3)18-29(17-24)45-4/h5-10,15-18,20,37H,11-14,19,21-22H2,1-4H3,(H,35,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay |
Eur J Med Chem 143: 1644-1656 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.059
BindingDB Entry DOI: 10.7270/Q2MS3W92 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139373
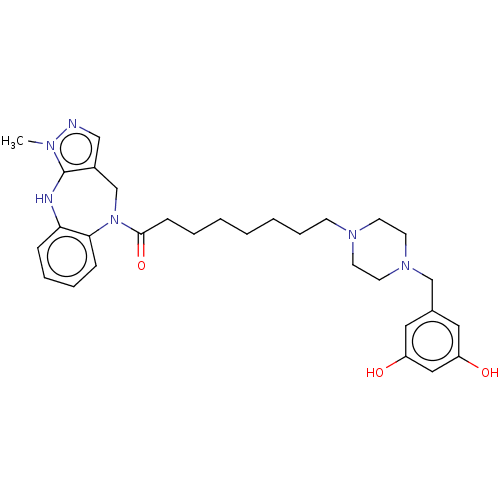
(CHEMBL3763823)Show SMILES Cn1ncc2CN(C(=O)CCCCCCCN3CCN(Cc4cc(O)cc(O)c4)CC3)c3ccccc3Nc12 Show InChI InChI=1S/C30H40N6O3/c1-33-30-24(20-31-33)22-36(28-10-7-6-9-27(28)32-30)29(39)11-5-3-2-4-8-12-34-13-15-35(16-14-34)21-23-17-25(37)19-26(38)18-23/h6-7,9-10,17-20,32,37-38H,2-5,8,11-16,21-22H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Antagonist activity at human vasopressin 1a receptor expressed in HEK293 cells assessed as inhibition of vasopressin induced IP1 accumulation pretrea... |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139373

(CHEMBL3763823)Show SMILES Cn1ncc2CN(C(=O)CCCCCCCN3CCN(Cc4cc(O)cc(O)c4)CC3)c3ccccc3Nc12 Show InChI InChI=1S/C30H40N6O3/c1-33-30-24(20-31-33)22-36(28-10-7-6-9-27(28)32-30)29(39)11-5-3-2-4-8-12-34-13-15-35(16-14-34)21-23-17-25(37)19-26(38)18-23/h6-7,9-10,17-20,32,37-38H,2-5,8,11-16,21-22H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay |
Eur J Med Chem 143: 1644-1656 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.059
BindingDB Entry DOI: 10.7270/Q2MS3W92 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18771

((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside
Curated by ChEMBL
| Assay Description
Inhibitory activity against Dihydrofolate reductase in rat liver |
J Med Chem 33: 3060-7 (1990)
BindingDB Entry DOI: 10.7270/Q2ZW1JWX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(RABBIT) | BDBM25761

(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by PDSP Ki Database
| |
Comp Biochem Physiol C, Pharmacol Toxicol Endocrinol 117: 19-24 (1997)
Article DOI: 10.1016/s0742-8413(97)00614-2
BindingDB Entry DOI: 10.7270/Q2PV6HWK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50276063
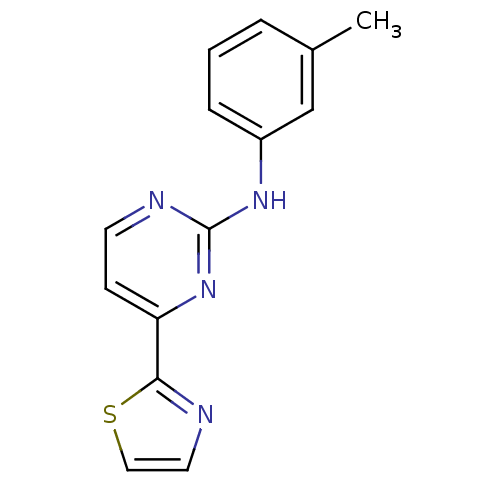
(4-(thiazol-2-yl)-N-m-tolylpyrimidin-2-amine | CHEM...)Show InChI InChI=1S/C14H12N4S/c1-10-3-2-4-11(9-10)17-14-16-6-5-12(18-14)13-15-7-8-19-13/h2-9H,1H3,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50249356

(CHEMBL453217 | N-(3,5-dimethylphenyl)-4-(4-methylt...)Show InChI InChI=1S/C16H16N4S/c1-10-6-11(2)8-13(7-10)19-16-17-5-4-14(20-16)15-18-12(3)9-21-15/h4-9H,1-3H3,(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249416
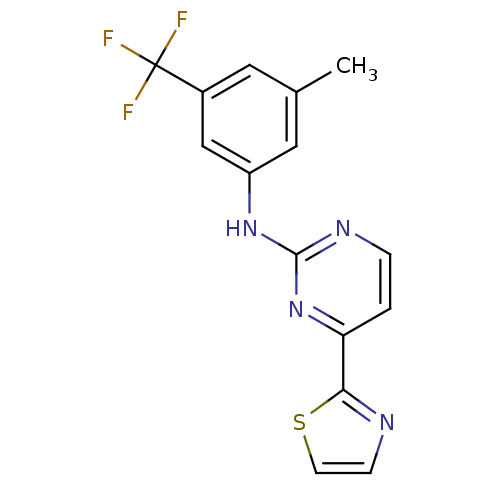
(CHEMBL471776 | N-(3-methyl-5-(trifluoromethyl)phen...)Show InChI InChI=1S/C15H11F3N4S/c1-9-6-10(15(16,17)18)8-11(7-9)21-14-20-3-2-12(22-14)13-19-4-5-23-13/h2-8H,1H3,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139178

(CHEMBL3763342)Show SMILES Cc1cc(ccc1CNC(=O)N1CCN(Cc2cc(O)cc(O)c2)CC1)C(=O)N1Cc2cnn(C)c2Nc2ccccc12 Show InChI InChI=1S/C32H35N7O4/c1-21-13-23(31(42)39-20-25-18-34-36(2)30(25)35-28-5-3-4-6-29(28)39)7-8-24(21)17-33-32(43)38-11-9-37(10-12-38)19-22-14-26(40)16-27(41)15-22/h3-8,13-16,18,35,40-41H,9-12,17,19-20H2,1-2H3,(H,33,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from human vasopressin 1a receptor expressed in HEK293 cells after 90 mins by microbeta 2 microplate-reader method |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139183
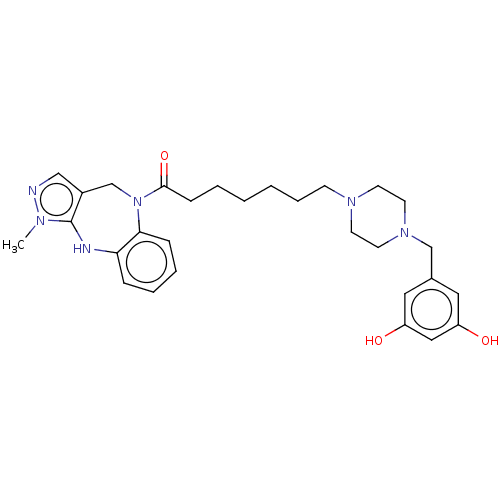
(CHEMBL3763217)Show SMILES Cn1ncc2CN(C(=O)CCCCCCN3CCN(Cc4cc(O)cc(O)c4)CC3)c3ccccc3Nc12 Show InChI InChI=1S/C29H38N6O3/c1-32-29-23(19-30-32)21-35(27-9-6-5-8-26(27)31-29)28(38)10-4-2-3-7-11-33-12-14-34(15-13-33)20-22-16-24(36)18-25(37)17-22/h5-6,8-9,16-19,31,36-37H,2-4,7,10-15,20-21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Antagonist activity at human vasopressin 1a receptor expressed in HEK293 cells assessed as inhibition of vasopressin induced IP1 accumulation pretrea... |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(RABBIT) | BDBM50007406
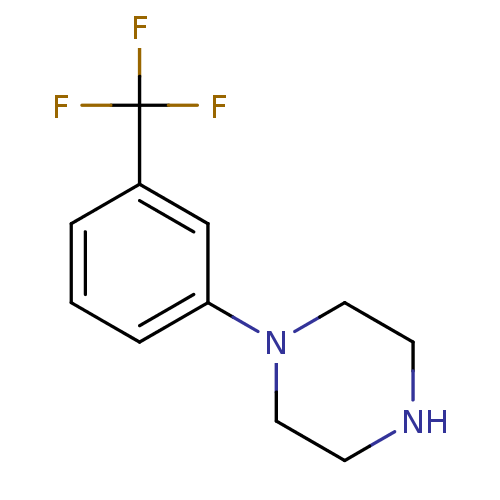
(1-(3-(trifluoromethyl)phenyl)piperazine | 1-(3-Tri...)Show InChI InChI=1S/C11H13F3N2/c12-11(13,14)9-2-1-3-10(8-9)16-6-4-15-5-7-16/h1-3,8,15H,4-7H2 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by PDSP Ki Database
| |
Comp Biochem Physiol C, Pharmacol Toxicol Endocrinol 117: 19-24 (1997)
Article DOI: 10.1016/s0742-8413(97)00614-2
BindingDB Entry DOI: 10.7270/Q2PV6HWK |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50249258
(CHEMBL475570 | N-(2,5-dimethylphenyl)-4-(thiazol-2...)Show InChI InChI=1S/C15H14N4S/c1-10-3-4-11(2)13(9-10)19-15-17-6-5-12(18-15)14-16-7-8-20-14/h3-9H,1-2H3,(H,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SRC (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139176
(CHEMBL3763174)Show SMILES COc1cc(CN2CCN(CCCCCCC(=O)N3Cc4cnn(C)c4Nc4ccccc34)CC2)cc(OC)c1 Show InChI InChI=1S/C31H42N6O3/c1-34-31-25(21-32-34)23-37(29-11-8-7-10-28(29)33-31)30(38)12-6-4-5-9-13-35-14-16-36(17-15-35)22-24-18-26(39-2)20-27(19-24)40-3/h7-8,10-11,18-21,33H,4-6,9,12-17,22-23H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Antagonist activity at human vasopressin 1a receptor expressed in HEK293 cells assessed as inhibition of vasopressin induced IP1 accumulation pretrea... |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139176

(CHEMBL3763174)Show SMILES COc1cc(CN2CCN(CCCCCCC(=O)N3Cc4cnn(C)c4Nc4ccccc34)CC2)cc(OC)c1 Show InChI InChI=1S/C31H42N6O3/c1-34-31-25(21-32-34)23-37(29-11-8-7-10-28(29)33-31)30(38)12-6-4-5-9-13-35-14-16-36(17-15-35)22-24-18-26(39-2)20-27(19-24)40-3/h7-8,10-11,18-21,33H,4-6,9,12-17,22-23H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay |
Eur J Med Chem 143: 1644-1656 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.059
BindingDB Entry DOI: 10.7270/Q2MS3W92 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249540
((R)-2-(2-(3,5-dimethylphenylamino)pyrimidin-4-yl)-...)Show SMILES C[C@H](CO)NC(=O)c1sc(nc1C)-c1ccnc(Nc2cc(C)cc(C)c2)n1 |r| Show InChI InChI=1S/C20H23N5O2S/c1-11-7-12(2)9-15(8-11)24-20-21-6-5-16(25-20)19-23-14(4)17(28-19)18(27)22-13(3)10-26/h5-9,13,26H,10H2,1-4H3,(H,22,27)(H,21,24,25)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50139178

(CHEMBL3763342)Show SMILES Cc1cc(ccc1CNC(=O)N1CCN(Cc2cc(O)cc(O)c2)CC1)C(=O)N1Cc2cnn(C)c2Nc2ccccc12 Show InChI InChI=1S/C32H35N7O4/c1-21-13-23(31(42)39-20-25-18-34-36(2)30(25)35-28-5-3-4-6-29(28)39)7-8-24(21)17-33-32(43)38-11-9-37(10-12-38)19-22-14-26(40)16-27(41)15-22/h3-8,13-16,18,35,40-41H,9-12,17,19-20H2,1-2H3,(H,33,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in HEK293 cells assessed as increase in IP1 accumulation preincubated for 1 hr followed by addi... |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50139178

(CHEMBL3763342)Show SMILES Cc1cc(ccc1CNC(=O)N1CCN(Cc2cc(O)cc(O)c2)CC1)C(=O)N1Cc2cnn(C)c2Nc2ccccc12 Show InChI InChI=1S/C32H35N7O4/c1-21-13-23(31(42)39-20-25-18-34-36(2)30(25)35-28-5-3-4-6-29(28)39)7-8-24(21)17-33-32(43)38-11-9-37(10-12-38)19-22-14-26(40)16-27(41)15-22/h3-8,13-16,18,35,40-41H,9-12,17,19-20H2,1-2H3,(H,33,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-oxytocin from human OTR expressed in HEK293 cell membranes after 90 mins by radioligand binding assay |
Eur J Med Chem 143: 1644-1656 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.059
BindingDB Entry DOI: 10.7270/Q2MS3W92 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50275935

((S)-2-(2-(3,5-dimethylphenylamino)pyrimidin-4-yl)-...)Show SMILES C[C@@H](CO)NC(=O)c1sc(nc1C)-c1ccnc(Nc2cc(C)cc(C)c2)n1 |r| Show InChI InChI=1S/C20H23N5O2S/c1-11-7-12(2)9-15(8-11)24-20-21-6-5-16(25-20)19-23-14(4)17(28-19)18(27)22-13(3)10-26/h5-9,13,26H,10H2,1-4H3,(H,22,27)(H,21,24,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ZAP70 (unknown origin) |
Bioorg Med Chem Lett 18: 6231-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.106
BindingDB Entry DOI: 10.7270/Q25Q4VZT |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139177
(CHEMBL3765689)Show SMILES COc1cc(CN2CCN(CCCCCCCC(=O)N3Cc4cnn(C)c4Nc4ccccc34)CC2)cc(OC)c1 Show InChI InChI=1S/C32H44N6O3/c1-35-32-26(22-33-35)24-38(30-12-9-8-11-29(30)34-32)31(39)13-7-5-4-6-10-14-36-15-17-37(18-16-36)23-25-19-27(40-2)21-28(20-25)41-3/h8-9,11-12,19-22,34H,4-7,10,13-18,23-24H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Agonist activity at human oxytocin receptor expressed in HEK293 cells assessed as increase in IP1 accumulation preincubated for 1 hr followed by addi... |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50283980
(CHEMBL4166008)Show SMILES Cn1ncc2CN(C(=O)CCc3ccccc3N3CCN(Cc4cc(O)cc(O)c4)CC3)c3ccccc3Nc12 Show InChI InChI=1S/C31H34N6O3/c1-34-31-24(19-32-34)21-37(29-9-5-3-7-27(29)33-31)30(40)11-10-23-6-2-4-8-28(23)36-14-12-35(13-15-36)20-22-16-25(38)18-26(39)17-22/h2-9,16-19,33,38-39H,10-15,20-21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from human vasopressin V1a receptor expressed in HEK293 cell membranes after 90 mins by radioligand binding assay |
Eur J Med Chem 143: 1644-1656 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.059
BindingDB Entry DOI: 10.7270/Q2MS3W92 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50139146
(CHEMBL3765356)Show SMILES COc1cc(CN2CCN(CCCCCC(=O)N3Cc4cnn(C)c4Nc4ccccc34)CC2)cc(OC)c1 Show InChI InChI=1S/C30H40N6O3/c1-33-30-24(20-31-33)22-36(28-10-7-6-9-27(28)32-30)29(37)11-5-4-8-12-34-13-15-35(16-14-34)21-23-17-25(38-2)19-26(18-23)39-3/h6-7,9-10,17-20,32H,4-5,8,11-16,21-22H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 266 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Antagonist activity at human vasopressin 1a receptor expressed in HEK293 cells assessed as inhibition of vasopressin induced IP1 accumulation pretrea... |
Eur J Med Chem 108: 730-40 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.050
BindingDB Entry DOI: 10.7270/Q2XW4MNF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data