

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM254887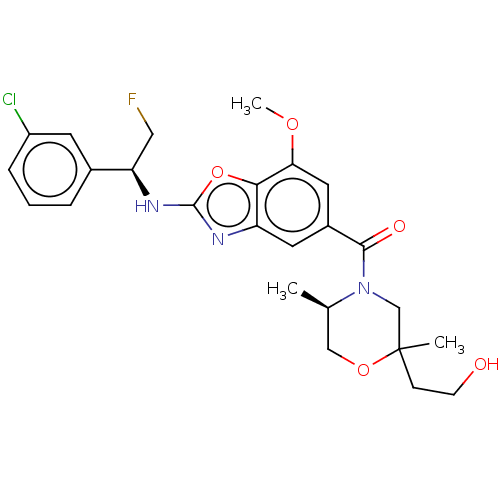 (US9493472, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of purified human plasma thrombin using Boc-Asp(OBzl)-Pro-Arg-AMC substrate incubated for 30 mins by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01035 BindingDB Entry DOI: 10.7270/Q2Z60SND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50547821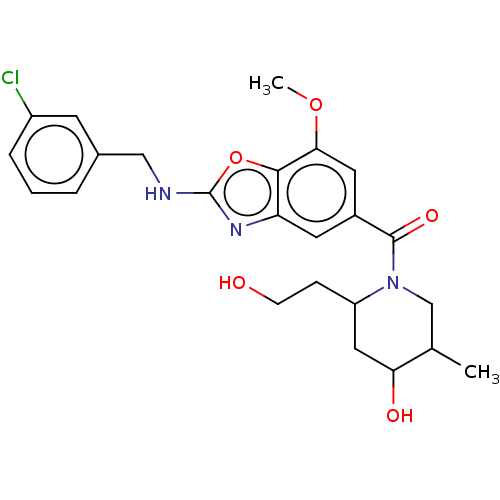 (CHEMBL4761137) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of purified human plasma thrombin using Boc-Asp(OBzl)-Pro-Arg-AMC substrate incubated for 30 mins by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01035 BindingDB Entry DOI: 10.7270/Q2Z60SND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM254899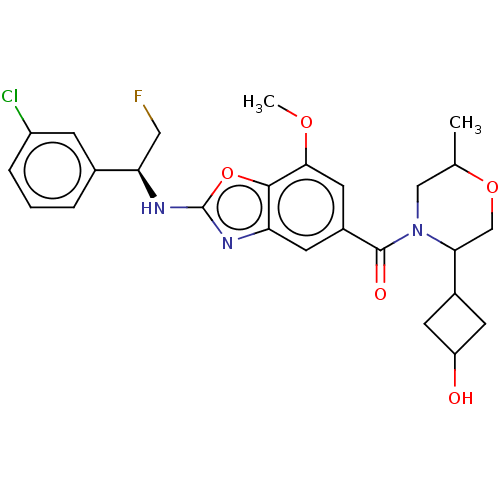 (US9493472, 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of purified human plasma thrombin using Boc-Asp(OBzl)-Pro-Arg-AMC substrate incubated for 30 mins by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01035 BindingDB Entry DOI: 10.7270/Q2Z60SND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50547825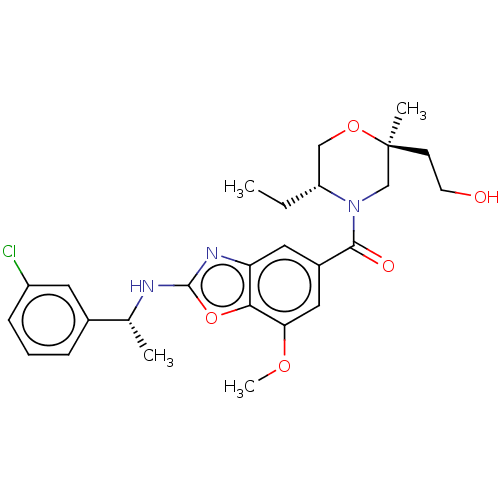 (CHEMBL4749829) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of purified human plasma thrombin using Boc-Asp(OBzl)-Pro-Arg-AMC substrate incubated for 30 mins by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01035 BindingDB Entry DOI: 10.7270/Q2Z60SND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50547824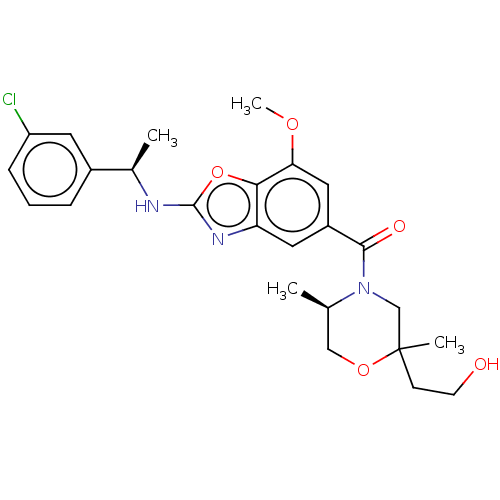 (CHEMBL4754786) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of purified human plasma thrombin using Boc-Asp(OBzl)-Pro-Arg-AMC substrate incubated for 30 mins by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01035 BindingDB Entry DOI: 10.7270/Q2Z60SND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50547836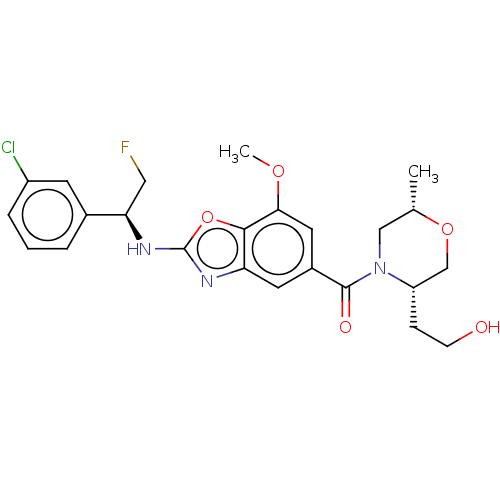 (CHEMBL4763593) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of purified human plasma thrombin using Boc-Asp(OBzl)-Pro-Arg-AMC substrate incubated for 30 mins by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01035 BindingDB Entry DOI: 10.7270/Q2Z60SND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM333401 (2-{[3-(Furo[2,3-c]pyridin-2-yl)imidazo[1,2-b]pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description A recombinant fusion protein of Glutathione-S-Transferase (GST, N-terminally) and human full-length MKNK1 (amino acids 1-424 and T344D of accession n... | US Patent US9730929 (2017) BindingDB Entry DOI: 10.7270/Q28S4S1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM259295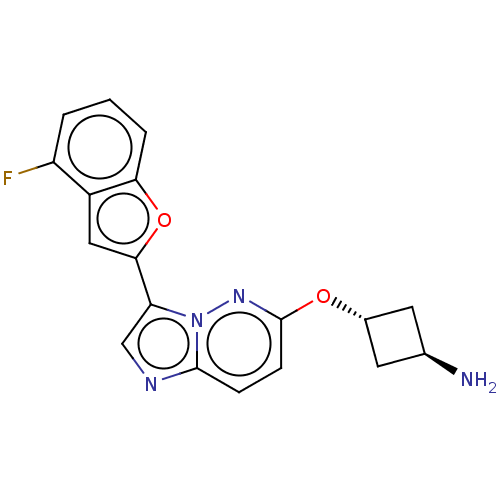 (US9499547, 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description A recombinant fusion protein of Glutathione-S-Transferase (GST, N-terminally) and human full-length MKNK1 (amino acids 1-424 and T344D of accession n... | US Patent US9499547 (2016) BindingDB Entry DOI: 10.7270/Q2736PVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM333402 (2-{[3-(Furo[3,2-c]pyridin-2-yl)imidazo[1,2-b]pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description A recombinant fusion protein of Glutathione-S-Transferase (GST, N-terminally) and human full-length MKNK1 (amino acids 1-424 and T344D of accession n... | US Patent US9730929 (2017) BindingDB Entry DOI: 10.7270/Q28S4S1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50547829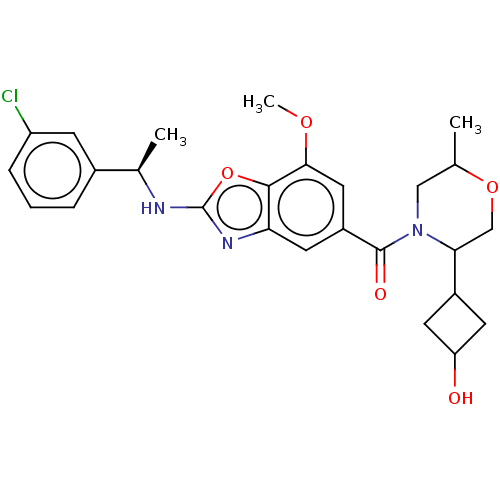 (CHEMBL4776051) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of purified human plasma thrombin using Boc-Asp(OBzl)-Pro-Arg-AMC substrate incubated for 30 mins by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01035 BindingDB Entry DOI: 10.7270/Q2Z60SND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) 344D] (Homo sapiens (Human)) | BDBM223030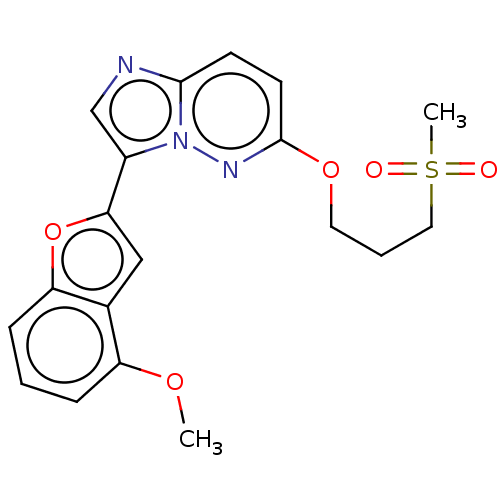 (US9320737, III.3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 mL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9320737 (2016) BindingDB Entry DOI: 10.7270/Q22V2F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM333390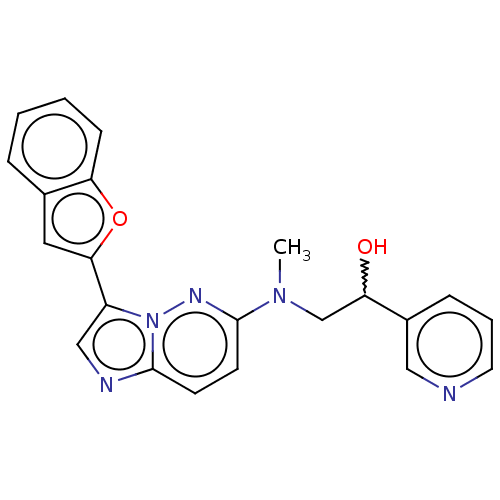 (2-{[3-(1-Benzofuran-2-yl)imidazo[1,2-b]pyridazin-6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description A recombinant fusion protein of Glutathione-S-Transferase (GST, N-terminally) and human full-length MKNK1 (amino acids 1-424 and T344D of accession n... | US Patent US9730929 (2017) BindingDB Entry DOI: 10.7270/Q28S4S1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) 344D] (Homo sapiens (Human)) | BDBM223024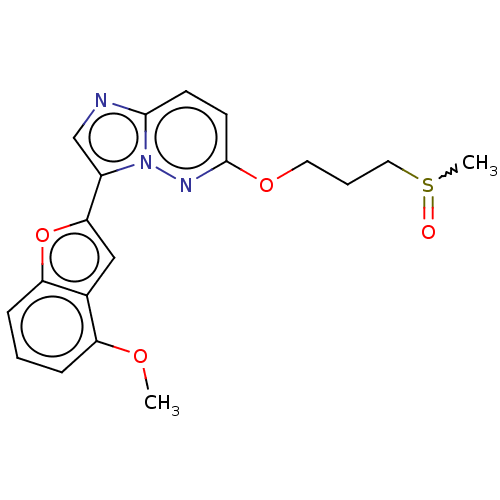 (US9320737, II.1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 mL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9320737 (2016) BindingDB Entry DOI: 10.7270/Q22V2F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM170016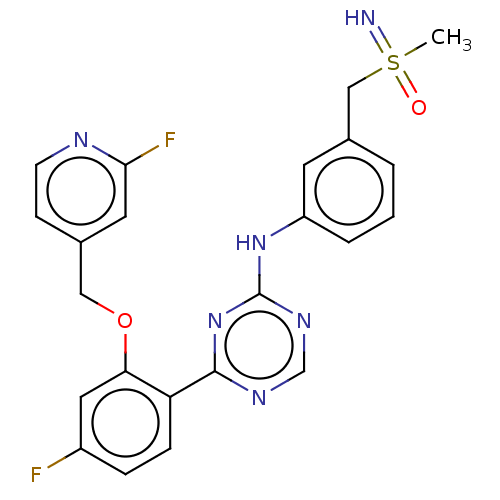 (US9669034, 82 (rac)-4-{4-Fluoro-2-[(2-fluoropyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 8.0 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9669034 (2017) BindingDB Entry DOI: 10.7270/Q22Z13PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM333382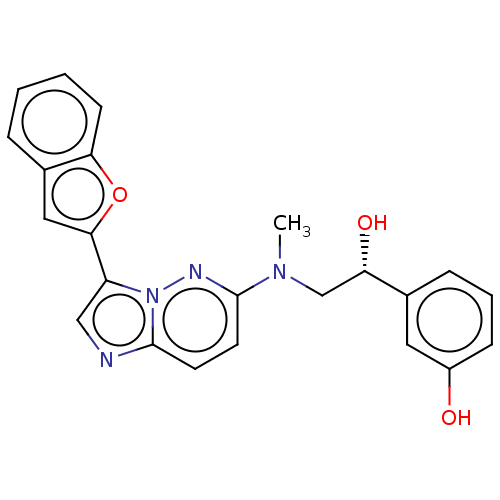 (3-[(1R)-1-{[3-(1- Benzofuran-2- yl)imidazo[1,2-b]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description A recombinant fusion protein of Glutathione-S-Transferase (GST, N-terminally) and human full-length MKNK1 (amino acids 1-424 and T344D of accession n... | US Patent US9730929 (2017) BindingDB Entry DOI: 10.7270/Q28S4S1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM175041 (US9669034, 99 (rac)-4-{4-Fluoro-2-[(2-methylprop-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 8.0 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9669034 (2017) BindingDB Entry DOI: 10.7270/Q22Z13PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) 344D] (Homo sapiens (Human)) | BDBM223043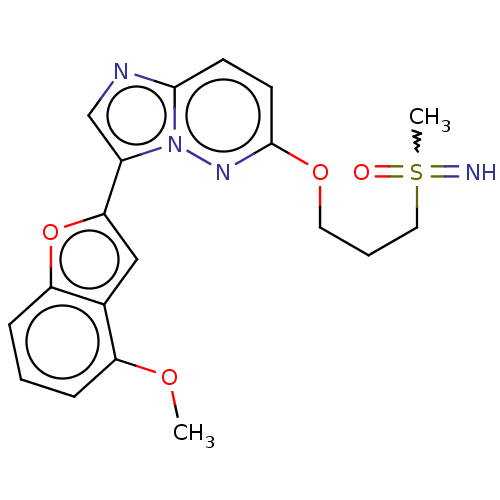 (US9320737, IV.2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 mL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9320737 (2016) BindingDB Entry DOI: 10.7270/Q22V2F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM176781 (US9669034, 104 (rac)-4-{4-Fluoro-2-[(3,4,5-trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 8.0 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9669034 (2017) BindingDB Entry DOI: 10.7270/Q22Z13PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM179848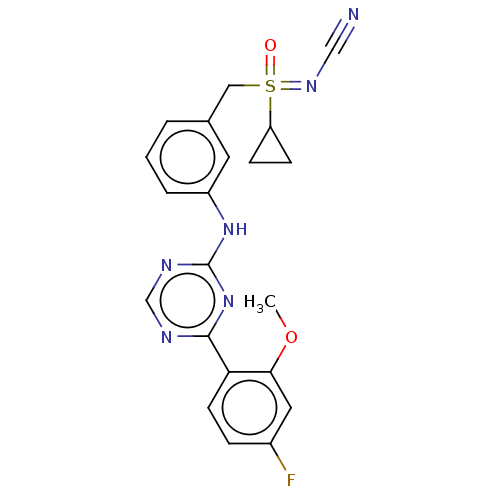 (US9669034, 113 (rac)-[Cyclopropyl(3-{[4-(4-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 8.0 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9669034 (2017) BindingDB Entry DOI: 10.7270/Q22Z13PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM259295 (US9499547, 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description MKNK1-inhibitory activity at high ATP of compounds of the present invention after their preincubation with MKNK1 was quantified employing the TR-FRET... | US Patent US9499547 (2016) BindingDB Entry DOI: 10.7270/Q2736PVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM170515 (US9669034, 90 (rac)-4-{4-Fluoro-2-[(3-fluorobenzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 8.0 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9669034 (2017) BindingDB Entry DOI: 10.7270/Q22Z13PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM378995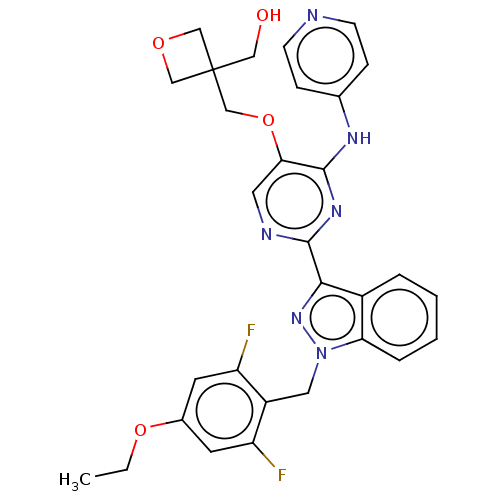 (Preparation of {3-[({2-[1-(4-ethoxy-2,6-difluorobe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50547830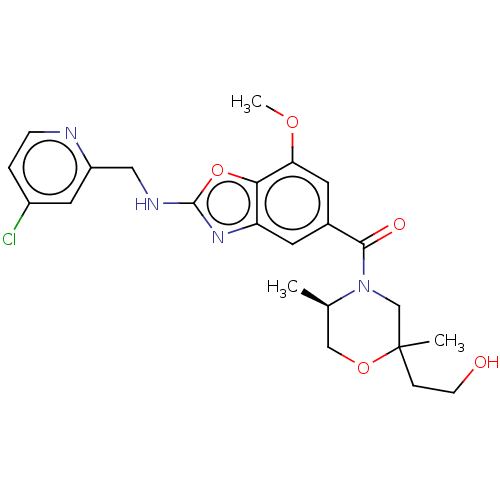 (CHEMBL4796689) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of purified human plasma thrombin using Boc-Asp(OBzl)-Pro-Arg-AMC substrate incubated for 30 mins by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01035 BindingDB Entry DOI: 10.7270/Q2Z60SND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM378915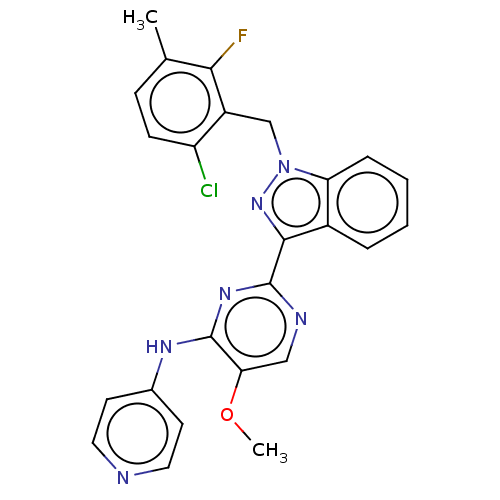 (2-[1-(6-chloro-2-fluoro-3-methylbenzyl)-1H-indazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM378932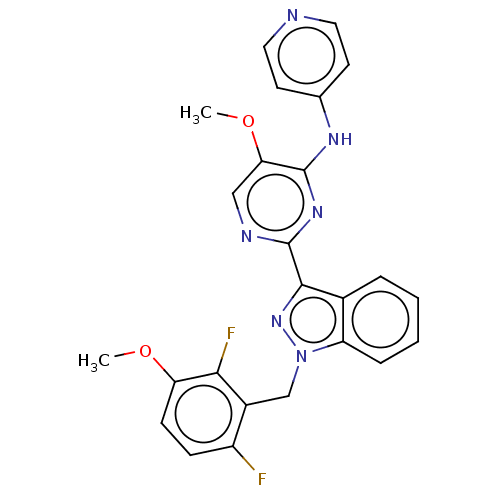 (2-[1-(6-chloro-2- fluoro-3- methoxybenzyl)- 1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM259260 (US9499547, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description A recombinant fusion protein of Glutathione-S-Transferase (GST, N-terminally) and human full-length MKNK1 (amino acids 1-424 and T344D of accession n... | US Patent US9499547 (2016) BindingDB Entry DOI: 10.7270/Q2736PVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM97722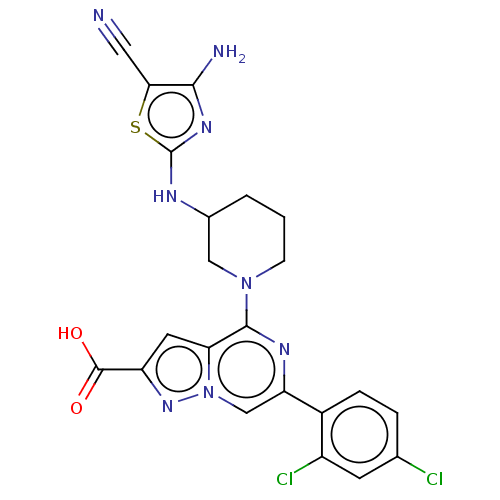 (US8476267, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 30 |
Bayer Intellectual Property GmbH US Patent | Assay Description The inhibitory activity of active substance is determined in a biochemical assay. | US Patent US8476267 (2013) BindingDB Entry DOI: 10.7270/Q20863X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM259298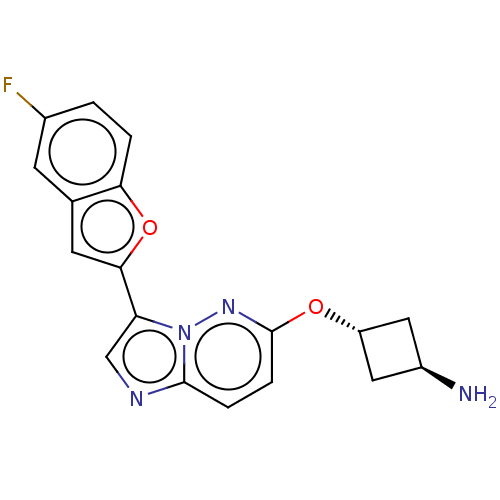 (US9499547, 39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description A recombinant fusion protein of Glutathione-S-Transferase (GST, N-terminally) and human full-length MKNK1 (amino acids 1-424 and T344D of accession n... | US Patent US9499547 (2016) BindingDB Entry DOI: 10.7270/Q2736PVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) [T344D] (Homo sapiens (Human)) | BDBM259297 (US9499547, 38) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description A recombinant fusion protein of Glutathione-S-Transferase (GST, N-terminally) and human full-length MKNK1 (amino acids 1-424 and T344D of accession n... | US Patent US9499547 (2016) BindingDB Entry DOI: 10.7270/Q2736PVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM174971 (US9669034, 98 (rac)-3-({5-Fluoro-2-[4-({3-[(S-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9669034 (2017) BindingDB Entry DOI: 10.7270/Q22Z13PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM379026 (Preparation of 1-[3,5-difluoro-4-({3-[5-methoxy-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM169242 (US9669034, 73 (rac)-4-[2-(But-2-yn-1-yloxy)-4-fluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9669034 (2017) BindingDB Entry DOI: 10.7270/Q22Z13PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM333403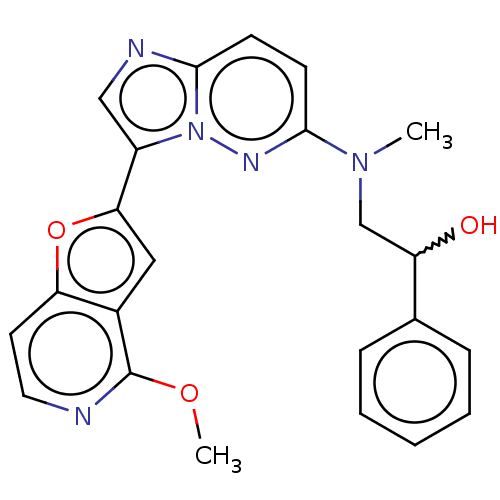 (2-{[3-(4-Methoxyfuro[3,2-c]pyridin-2-yl)imidazo[1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description A recombinant fusion protein of Glutathione-S-Transferase (GST, N-terminally) and human full-length MKNK1 (amino acids 1-424 and T344D of accession n... | US Patent US9730929 (2017) BindingDB Entry DOI: 10.7270/Q28S4S1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM179392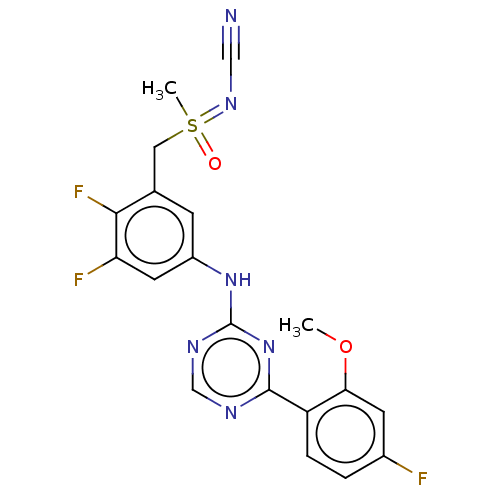 (US9669034, 105 (rac)-[(2,3-Difluoro-5-{[4-(4-fluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9669034 (2017) BindingDB Entry DOI: 10.7270/Q22Z13PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM179686 (US9669034, 111 (rac)-[(3-{[4-(4-Fluoro-2-methoxyph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9669034 (2017) BindingDB Entry DOI: 10.7270/Q22Z13PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM168387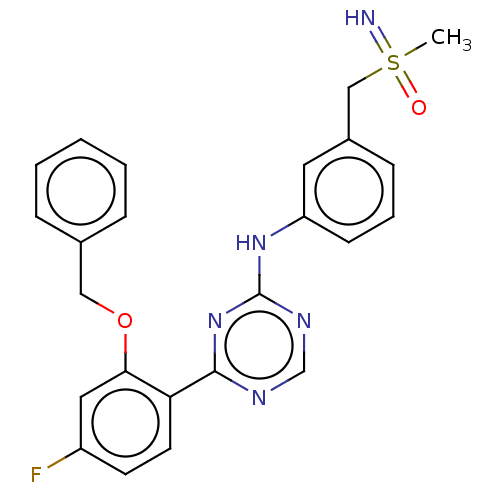 (US9669034, 6 (rac)-4-[2-(Benzyloxy)-4-fluorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9669034 (2017) BindingDB Entry DOI: 10.7270/Q22Z13PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM170016 (US9669034, 82 (rac)-4-{4-Fluoro-2-[(2-fluoropyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9669034 (2017) BindingDB Entry DOI: 10.7270/Q22Z13PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM170507 (US9669034, 87 (rac)-4-{2-[(4-Chlorobenzyl)oxy]-4-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9669034 (2017) BindingDB Entry DOI: 10.7270/Q22Z13PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM170520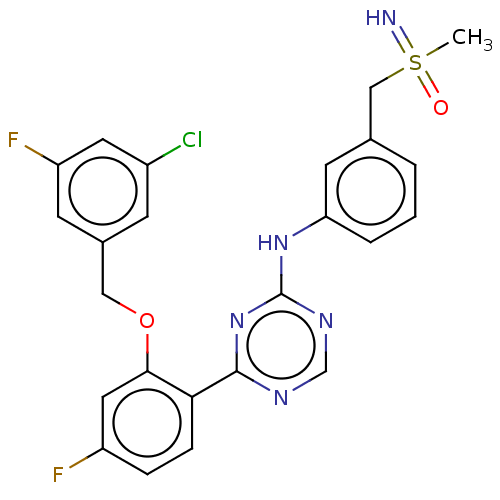 (US9669034, 94 (rac)-4-{2-[(3-Chloro-5-fluorobenzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9669034 (2017) BindingDB Entry DOI: 10.7270/Q22Z13PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM174598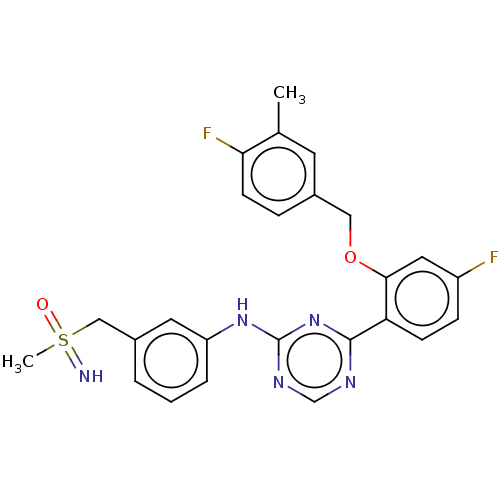 (US9669034, 96 (rac)-4-{4-Fluoro-2-[(4-fluoro-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9669034 (2017) BindingDB Entry DOI: 10.7270/Q22Z13PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM174850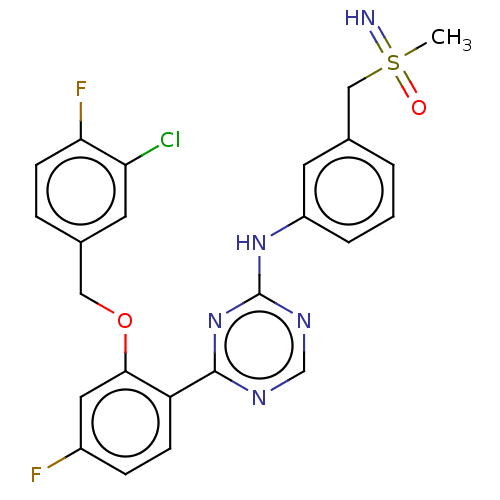 (US9669034, 97 (rac)-4-{2-[(3-Chloro-4-fluorobenzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9669034 (2017) BindingDB Entry DOI: 10.7270/Q22Z13PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM379006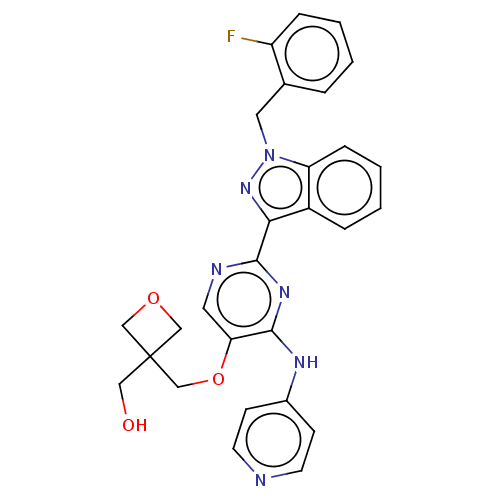 (US10266548, Example 4-2-1 | {3[({2-[1-(2- fluorobe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM379042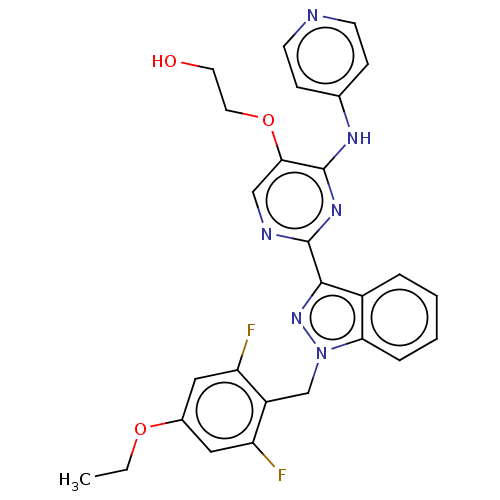 (Preparation of 2-({2-[1-(4-ethoxy-2,6-difluorobenz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM379108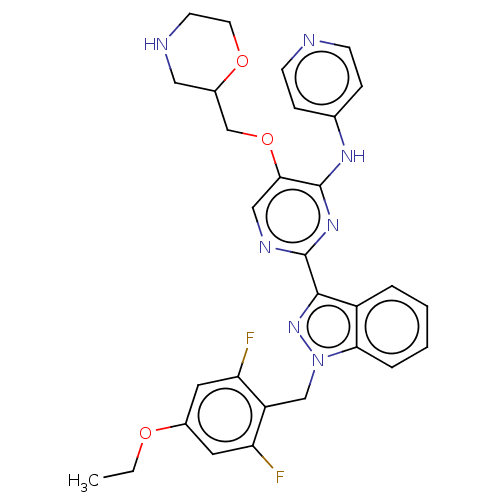 (2-[1-(4-ethoxy-2,6- difluorobenzyl)-1H- indazol-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM379107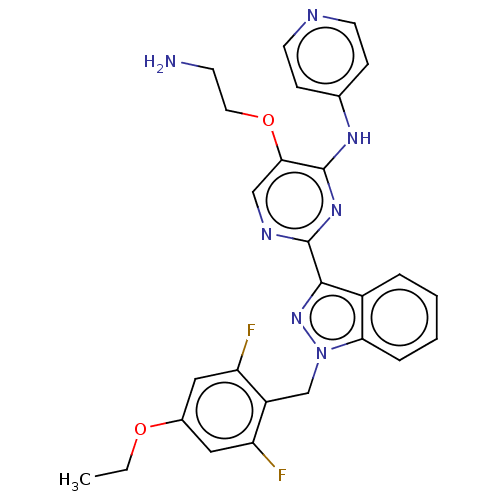 (Preparation of 5-(2-aminoethoxy)-2-[1-(4-ethoxy-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitotic checkpoint serine/threonine-protein kinase BUB1 (Homo sapiens (Human)) | BDBM379024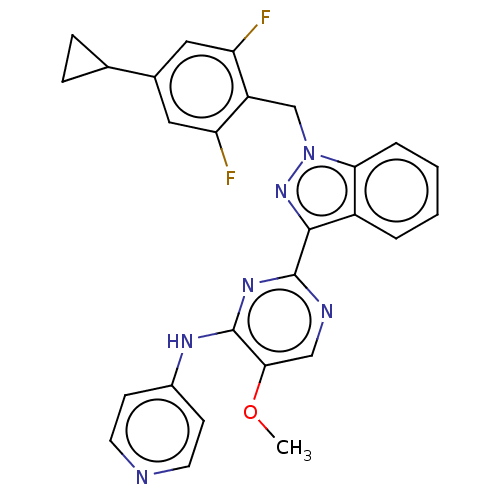 (Preparation of 2-[1-(4-cyclopropyl-2,6-difluoroben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRE... | J Med Chem 51: 4150-69 (2008) BindingDB Entry DOI: 10.7270/Q2RV0R1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM333388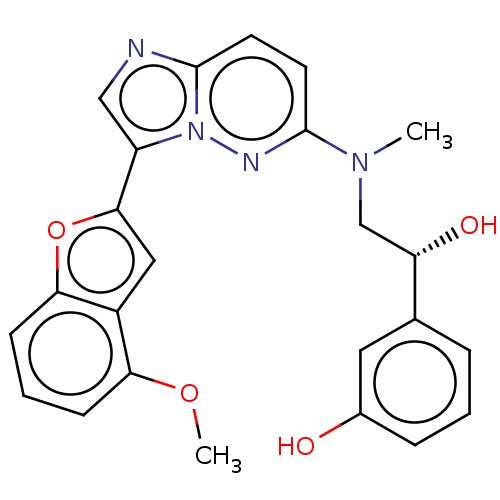 (3-[(1R)-1-Hydroxy-2- {[3-(4-methoxy-1- benzofuran-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description A recombinant fusion protein of Glutathione-S-Transferase (GST, N-terminally) and human full-length MKNK1 (amino acids 1-424 and T344D of accession n... | US Patent US9730929 (2017) BindingDB Entry DOI: 10.7270/Q28S4S1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM168386 (US9669034, 5 (rac)-Ethyl{[3-({4-[2-(benzyloxy)-4-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.0 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9669034 (2017) BindingDB Entry DOI: 10.7270/Q22Z13PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform 2 of MAP kinase-interacting serine/threonine-protein kinase 1 (MNK1a) 344D] (Homo sapiens (Human)) | BDBM223045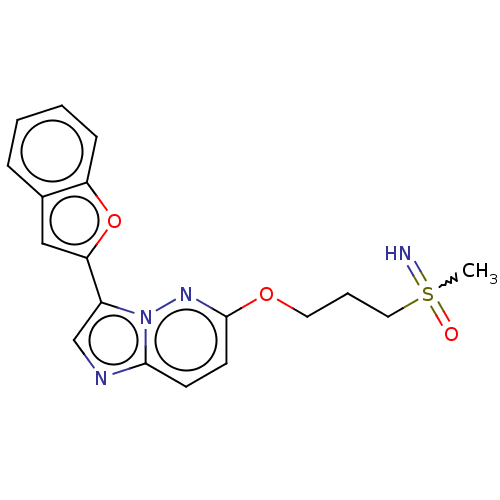 (US9320737, IV.4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 mL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9320737 (2016) BindingDB Entry DOI: 10.7270/Q22V2F00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM170518 (US9669034, 92 (rac)-4-{2-[(3-Chlorobenzyl)oxy]-4-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.0 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (... | US Patent US9669034 (2017) BindingDB Entry DOI: 10.7270/Q22Z13PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 742 total ) | Next | Last >> |