

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50432760 (CHEMBL2348620) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to XIAP linker BIR2-BIR3 domain (unknown origin) after 3 hrs by competitive fluorescence polarization assay in presence of Smac-1F | J Med Chem 56: 3969-79 (2013) Article DOI: 10.1021/jm400216d BindingDB Entry DOI: 10.7270/Q2M61MNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237768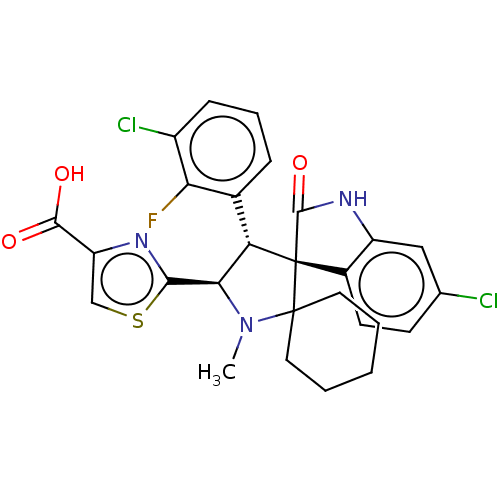 (CHEMBL4063804) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237747 (CHEMBL4083298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Evaluated for accumulation of cAMP in transfected HEK293 cells expressing human vasopressin V2 receptor | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237734 (CHEMBL4098834) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of [3H]-Ro- 15-1788 binding to recombinant human gamma-aminobutyric-acid A receptor alpha-3-beta-3-gamma-2 | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237759 (CHEMBL4064410) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V1a receptor | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237764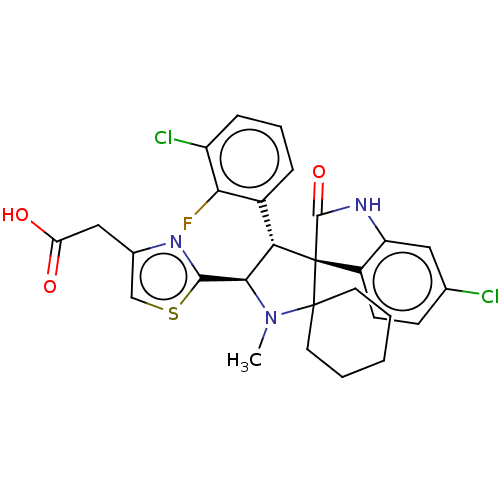 (CHEMBL4060006) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237744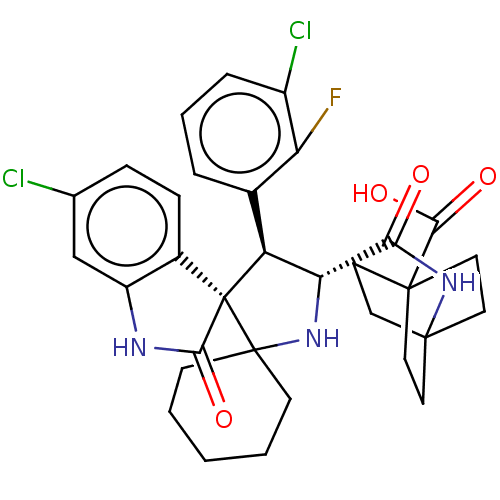 (CHEMBL4072857) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237766 (CHEMBL4085815) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of [3H]-Ro- 15-1788 binding to recombinant human gamma-aminobutyric-acid A receptor alpha-1-beta-3-gamma-2 | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237745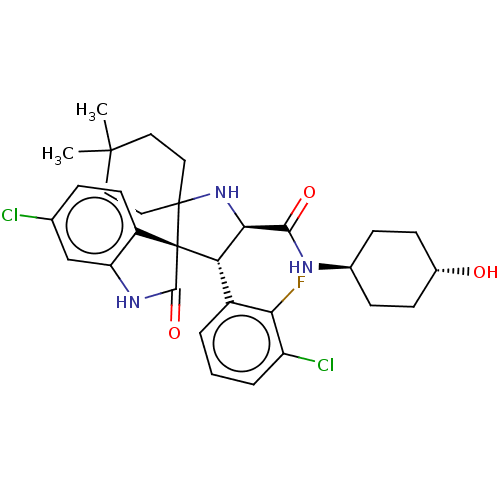 (CHEMBL4064790) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237763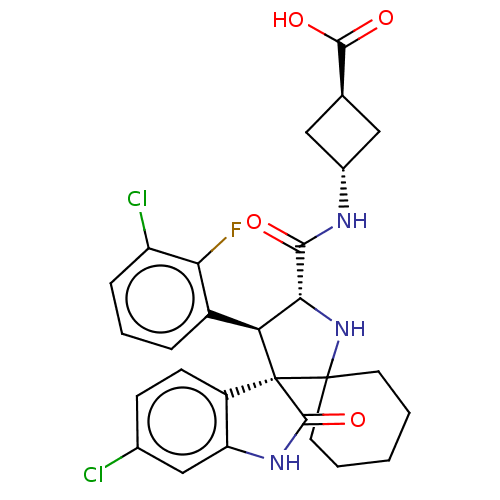 (CHEMBL4084366) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM112773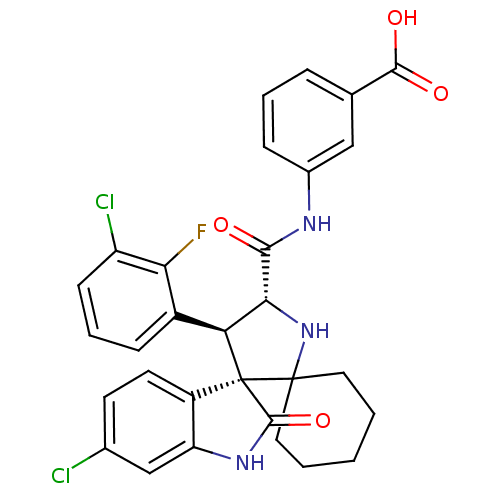 (US8629141, 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50236827 (CHEMBL4066694) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50393507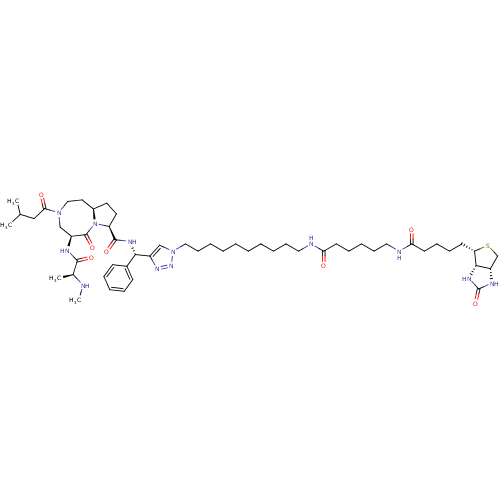 (CHEMBL2158053) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Competitive inhibition of human cIAP1 BIR3 domain expressed in Escherichia coli BL21(DE3) after 2 to 3 hrs by fluorescence polarization assay | J Med Chem 54: 2714-26 (2011) Article DOI: 10.1021/jm101505d BindingDB Entry DOI: 10.7270/Q2H70GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237739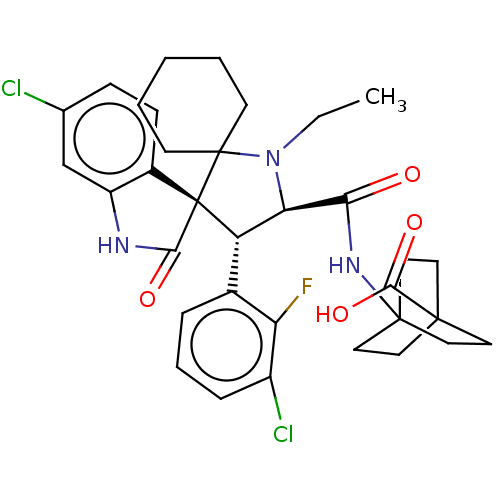 (CHEMBL4091801) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM112761 (US8629141, 31) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM112757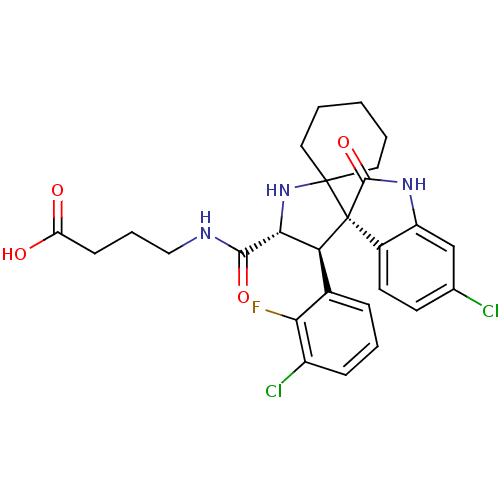 (US8629141, 27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM112759 (US8629141, 29) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237750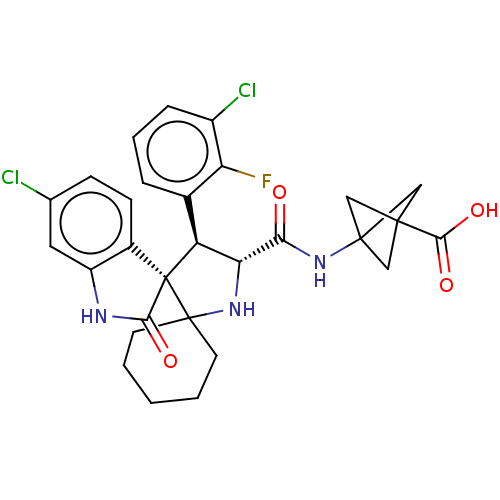 (CHEMBL4093562) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V1a receptor | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237738 (CHEMBL4064867) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V1a receptor | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM112766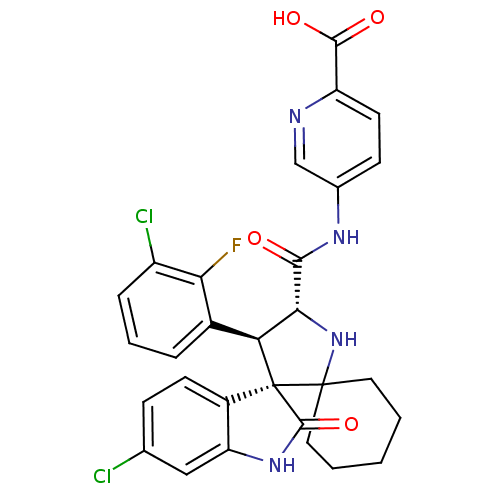 (US8629141, 36) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237765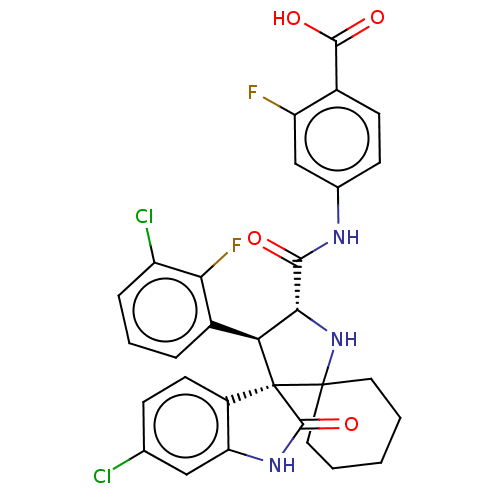 (CHEMBL4077687) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237752 (CHEMBL4095706) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V1a receptor | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24466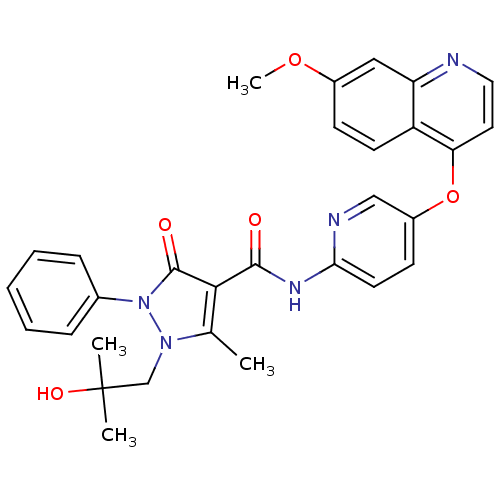 (1-(2-hydroxy-2-methylpropyl)-N-{5-[(7-methoxyquino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) | Eur J Med Chem 108: 495-504 (2016) Article DOI: 10.1016/j.ejmech.2015.12.016 BindingDB Entry DOI: 10.7270/Q2G73GMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237741 (CHEMBL4071840) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50432754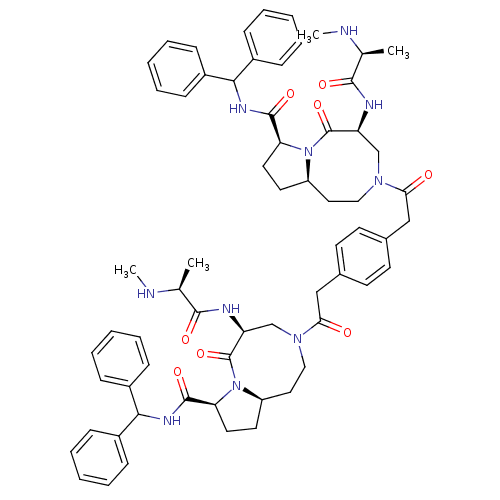 (CHEMBL2348614) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to XIAP linker BIR2-BIR3 domain (unknown origin) after 3 hrs by competitive fluorescence polarization assay in presence of Smac-1F | J Med Chem 56: 3969-79 (2013) Article DOI: 10.1021/jm400216d BindingDB Entry DOI: 10.7270/Q2M61MNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50432753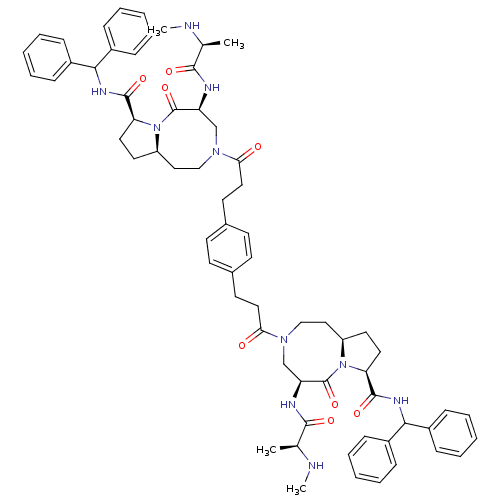 (CHEMBL2348613) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to XIAP linker BIR2-BIR3 domain (unknown origin) after 3 hrs by competitive fluorescence polarization assay in presence of Smac-1F | J Med Chem 56: 3969-79 (2013) Article DOI: 10.1021/jm400216d BindingDB Entry DOI: 10.7270/Q2M61MNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50432759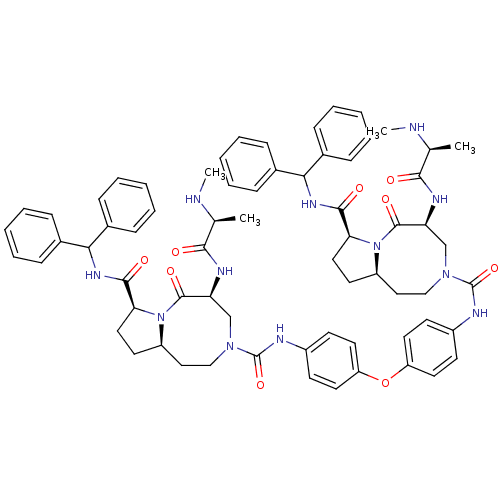 (CHEMBL2348619) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to cIAP1 BIR3 domain (unknown origin) after 3 hrs by competitive fluorescence polarization assay in presence of Smac-2F | J Med Chem 56: 3969-79 (2013) Article DOI: 10.1021/jm400216d BindingDB Entry DOI: 10.7270/Q2M61MNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50393507 (CHEMBL2158053) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Competitive inhibition of human cIAP2 BIR3 domain expressed in Escherichia coli BL21(DE3) after 2 to 3 hrs by fluorescence polarization assay | J Med Chem 54: 2714-26 (2011) Article DOI: 10.1021/jm101505d BindingDB Entry DOI: 10.7270/Q2H70GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50432762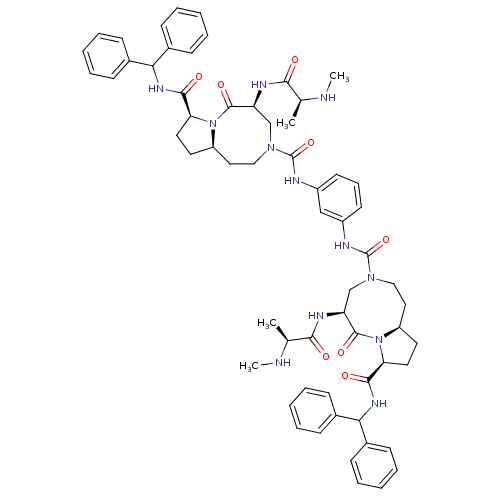 (CHEMBL2348622) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to XIAP linker BIR2-BIR3 domain (unknown origin) after 3 hrs by competitive fluorescence polarization assay in presence of Smac-1F | J Med Chem 56: 3969-79 (2013) Article DOI: 10.1021/jm400216d BindingDB Entry DOI: 10.7270/Q2M61MNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50432758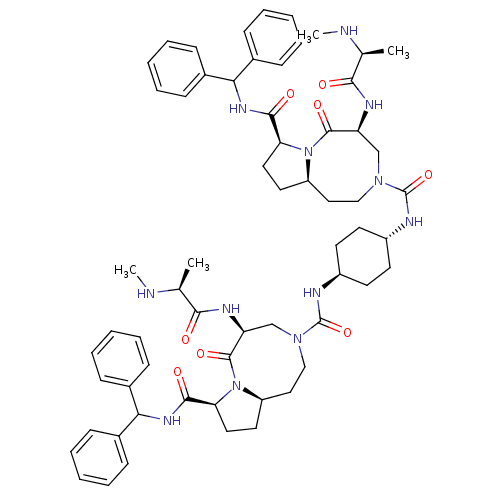 (CHEMBL2348618) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to XIAP linker BIR2-BIR3 domain (unknown origin) after 3 hrs by competitive fluorescence polarization assay in presence of Smac-1F | J Med Chem 56: 3969-79 (2013) Article DOI: 10.1021/jm400216d BindingDB Entry DOI: 10.7270/Q2M61MNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50393505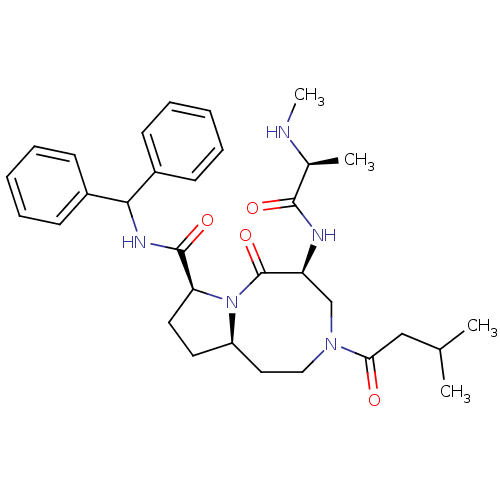 (CHEMBL2158051) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Competitive inhibition of human cIAP1 BIR3 domain expressed in Escherichia coli BL21(DE3) after 2 to 3 hrs by fluorescence polarization assay | J Med Chem 54: 2714-26 (2011) Article DOI: 10.1021/jm101505d BindingDB Entry DOI: 10.7270/Q2H70GXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237743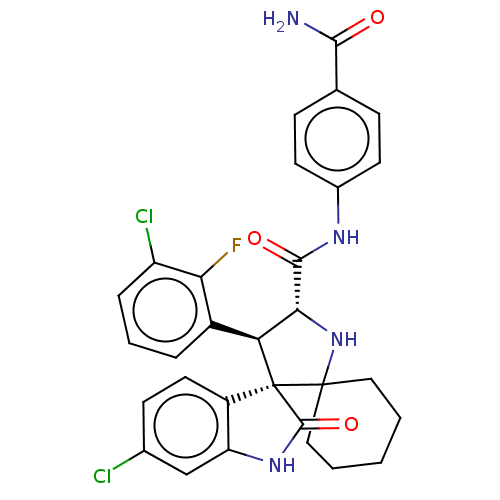 (CHEMBL4102205) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V2 receptor | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type IV secretion-like conjugative transfer relaxase protein TraI (Escherichia coli (strain SMS-3-5 / SECEC)) | BDBM50478336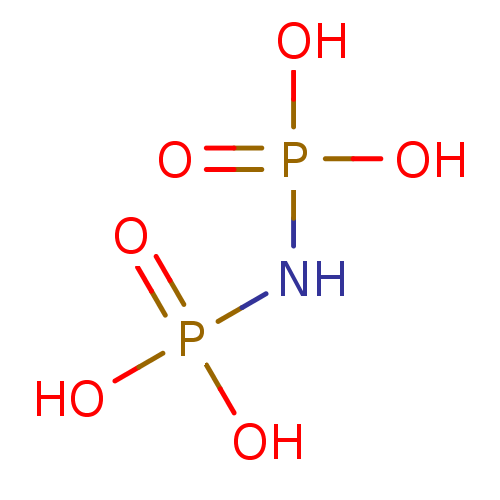 (Imidobisphosphate | Imidodiphosphate) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of Escherichia coli F plasmid TraI relaxase Y16F mutant assessed as oriT ssDNA cleavage by competitive inhibition assay | Proc Natl Acad Sci U S A 104: 12282-7 (2007) Article DOI: 10.1073/pnas.0702760104 BindingDB Entry DOI: 10.7270/Q2PK0JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50432759 (CHEMBL2348619) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to cIAP2 BIR3 domain (unknown origin) after 3 hrs by competitive fluorescence polarization assay in presence of Smac-2F | J Med Chem 56: 3969-79 (2013) Article DOI: 10.1021/jm400216d BindingDB Entry DOI: 10.7270/Q2M61MNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237757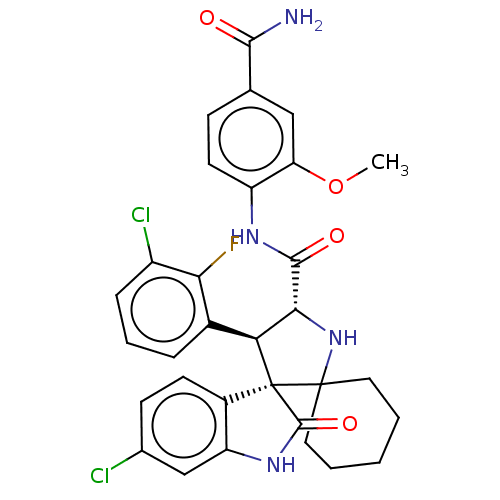 (CHEMBL4101295) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237762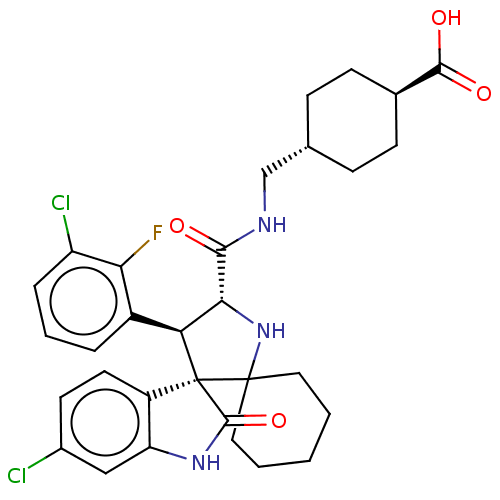 (CHEMBL4072993) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Evaluated for accumulation of cAMP in transfected HEK293 cells expressing human vasopressin V2 receptor | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237735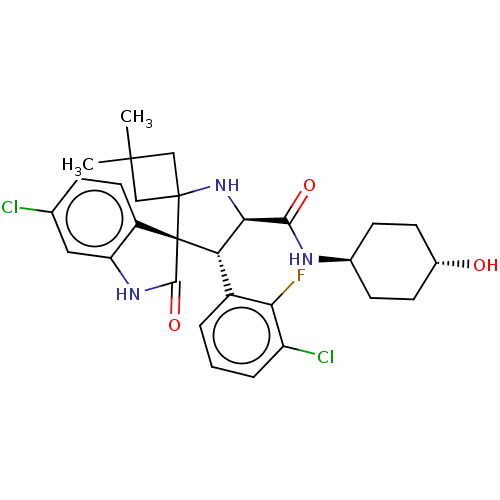 (CHEMBL4100233) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50236970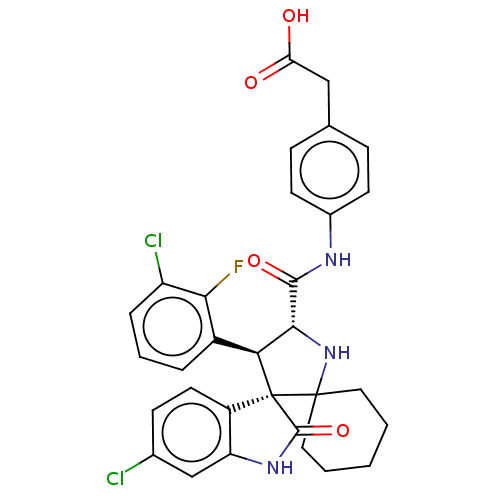 (CHEMBL4080118) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type IV secretion-like conjugative transfer relaxase protein TraI (Escherichia coli (strain SMS-3-5 / SECEC)) | BDBM50478336 (Imidobisphosphate | Imidodiphosphate) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of Escherichia coli F plasmid TraI relaxase Y16F mutant assessed as oriT ssDNA cleavage by uncompetitive inhibition assay | Proc Natl Acad Sci U S A 104: 12282-7 (2007) Article DOI: 10.1073/pnas.0702760104 BindingDB Entry DOI: 10.7270/Q2PK0JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237753 (CHEMBL4085388) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V2 receptor | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237736 (CHEMBL4103467) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type IV secretion-like conjugative transfer relaxase protein TraI (Escherichia coli (strain SMS-3-5 / SECEC)) | BDBM50115102 ((1-Hydroxy-1-phosphono-ethyl)-phosphonic acid | CH...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of Escherichia coli F plasmid TraI relaxase Y16F mutant assessed as oriT ssDNA cleavage by competitive inhibition assay | Proc Natl Acad Sci U S A 104: 12282-7 (2007) Article DOI: 10.1073/pnas.0702760104 BindingDB Entry DOI: 10.7270/Q2PK0JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type IV secretion-like conjugative transfer relaxase protein TraI (Escherichia coli (strain SMS-3-5 / SECEC)) | BDBM50162816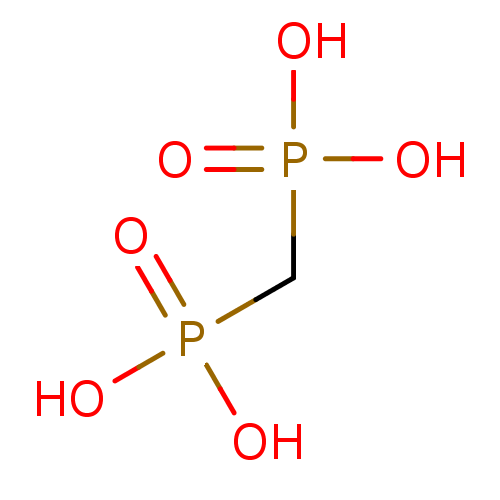 (CHEMBL180570 | Medronic acid | Methylene diphsphon...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of Escherichia coli F plasmid TraI relaxase Y16F mutant assessed as oriT ssDNA cleavage by competitive inhibition assay | Proc Natl Acad Sci U S A 104: 12282-7 (2007) Article DOI: 10.1073/pnas.0702760104 BindingDB Entry DOI: 10.7270/Q2PK0JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type IV secretion-like conjugative transfer relaxase protein TraI (Escherichia coli (strain SMS-3-5 / SECEC)) | BDBM50216172 ((Dichloro-phosphono-methyl)-phosphonic acid | CHEM...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of Escherichia coli F plasmid TraI relaxase Y16F mutant assessed as oriT ssDNA cleavage by competitive inhibition assay | Proc Natl Acad Sci U S A 104: 12282-7 (2007) Article DOI: 10.1073/pnas.0702760104 BindingDB Entry DOI: 10.7270/Q2PK0JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50237751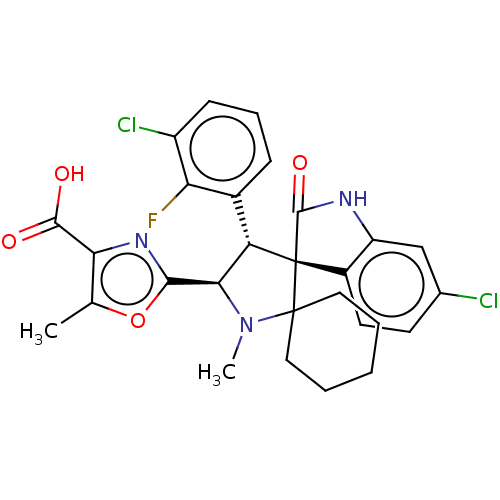 (CHEMBL4066543) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of [3H]-Ro- 15-1788 binding to recombinant human gamma-aminobutyric-acid A receptor alpha-1-beta-3-gamma-2 | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM112767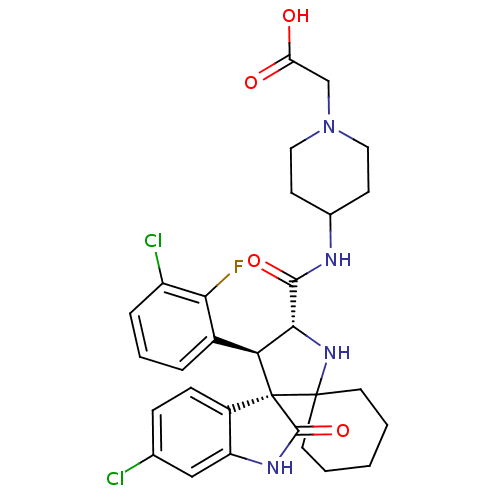 (US8629141, 37) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM112768 (US8629141, 38) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Comprehensive Cancer Center Curated by ChEMBL | Assay Description Inhibition of FAM-tagged p53-based PMDM6-F peptide binding to human recombinant His-tagged MDM2 (1 to 118 residues) after 30 mins by fluorescence pol... | J Med Chem 60: 2819-2839 (2017) Article DOI: 10.1021/acs.jmedchem.6b01665 BindingDB Entry DOI: 10.7270/Q25Q4ZCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50432763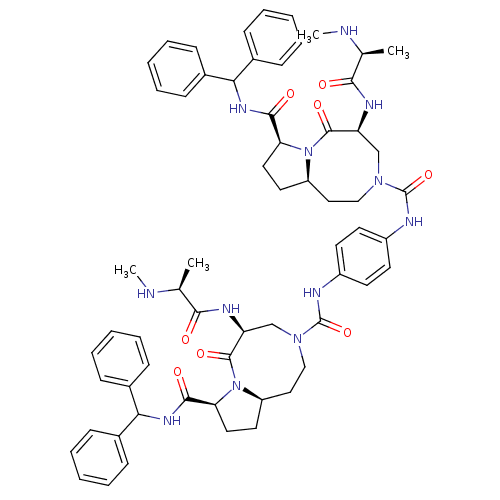 (CHEMBL2348623) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to cIAP1 BIR3 domain (unknown origin) after 3 hrs by competitive fluorescence polarization assay in presence of Smac-2F | J Med Chem 56: 3969-79 (2013) Article DOI: 10.1021/jm400216d BindingDB Entry DOI: 10.7270/Q2M61MNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50432756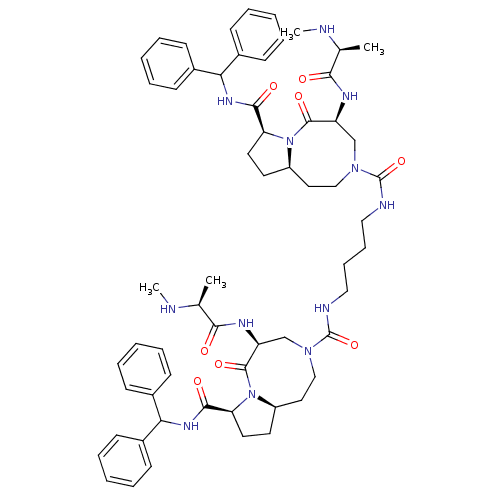 (CHEMBL2348616) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Binding affinity to cIAP1 BIR3 domain (unknown origin) after 3 hrs by competitive fluorescence polarization assay in presence of Smac-2F | J Med Chem 56: 3969-79 (2013) Article DOI: 10.1021/jm400216d BindingDB Entry DOI: 10.7270/Q2M61MNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50306682 ((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) | Eur J Med Chem 108: 495-504 (2016) Article DOI: 10.1016/j.ejmech.2015.12.016 BindingDB Entry DOI: 10.7270/Q2G73GMC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 1017 total ) | Next | Last >> |