 Found 26595 hits with Last Name = 'ott' and Initial = 'm'
Found 26595 hits with Last Name = 'ott' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50016326
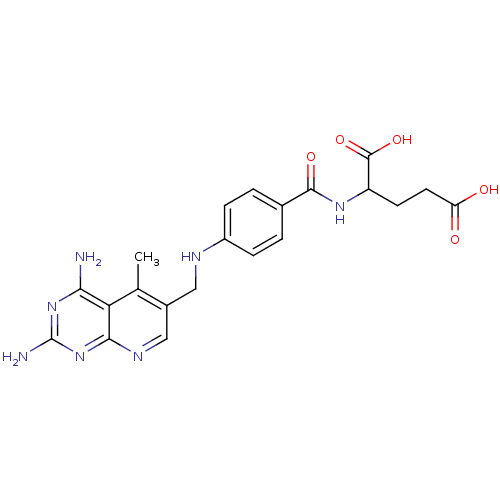
(2-{4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidin...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCC(O)=O)C(O)=O)cnc2nc(N)nc(N)c12 Show InChI InChI=1S/C21H23N7O5/c1-10-12(9-25-18-16(10)17(22)27-21(23)28-18)8-24-13-4-2-11(3-5-13)19(31)26-14(20(32)33)6-7-15(29)30/h2-5,9,14,24H,6-8H2,1H3,(H,26,31)(H,29,30)(H,32,33)(H4,22,23,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM66082

((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM14073
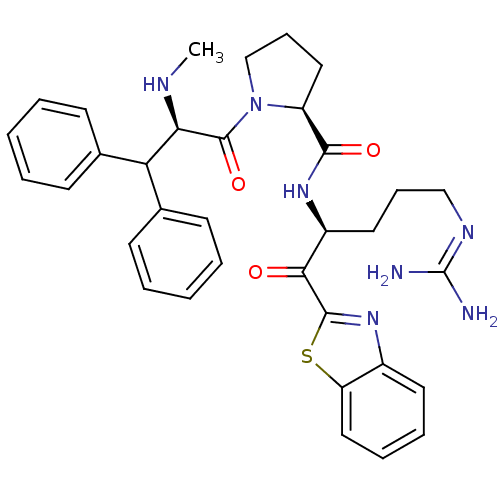
((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...)Show SMILES [#6]-[#7]-[#6@H](-[#6](-c1ccccc1)-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-c1nc2ccccc2s1 |r| Show InChI InChI=1S/C34H39N7O3S/c1-37-29(28(22-12-4-2-5-13-22)23-14-6-3-7-15-23)33(44)41-21-11-18-26(41)31(43)39-25(17-10-20-38-34(35)36)30(42)32-40-24-16-8-9-19-27(24)45-32/h2-9,12-16,19,25-26,28-29,37H,10-11,17-18,20-21H2,1H3,(H,39,43)(H4,35,36,38)/t25-,26-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.000650 | -72.4 | 4.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical
| Assay Description
Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... |
J Med Chem 48: 1984-2008 (2005)
Article DOI: 10.1021/jm0303857
BindingDB Entry DOI: 10.7270/Q2X0658X |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023681

(2-{4-[(2,4-Diamino-5,7-dimethyl-pyrido[2,3-d]pyrim...)Show SMILES Cc1nc2nc(N)nc(N)c2c(C)c1CNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C22H25N7O5/c1-10-14(11(2)26-19-17(10)18(23)28-22(24)29-19)9-25-13-5-3-12(4-6-13)20(32)27-15(21(33)34)7-8-16(30)31/h3-6,15,25H,7-9H2,1-2H3,(H,27,32)(H,30,31)(H,33,34)(H4,23,24,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093352
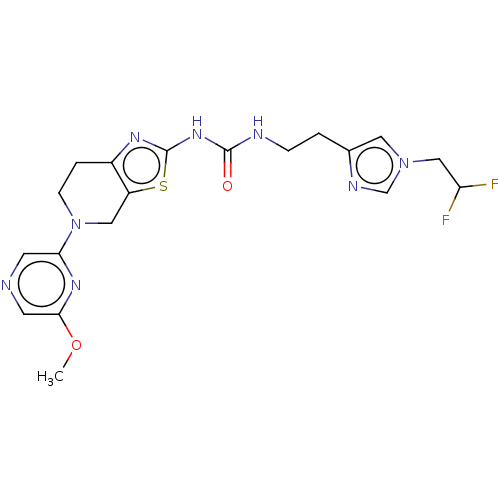
(CHEMBL3586678)Show SMILES COc1cncc(n1)N1CCc2nc(NC(=O)NCCc3cn(CC(F)F)cn3)sc2C1 Show InChI InChI=1S/C19H22F2N8O2S/c1-31-17-7-22-6-16(26-17)29-5-3-13-14(9-29)32-19(25-13)27-18(30)23-4-2-12-8-28(11-24-12)10-15(20)21/h6-8,11,15H,2-5,9-10H2,1H3,(H2,23,25,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093351
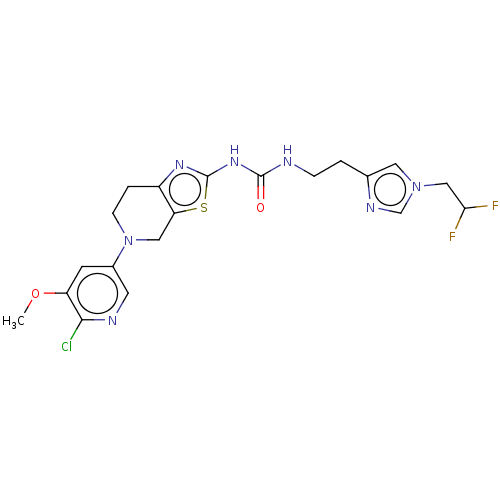
(CHEMBL3585362)Show SMILES COc1cc(cnc1Cl)N1CCc2nc(NC(=O)NCCc3cn(CC(F)F)cn3)sc2C1 Show InChI InChI=1S/C20H22ClF2N7O2S/c1-32-15-6-13(7-25-18(15)21)30-5-3-14-16(9-30)33-20(27-14)28-19(31)24-4-2-12-8-29(11-26-12)10-17(22)23/h6-8,11,17H,2-5,9-10H2,1H3,(H2,24,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093355
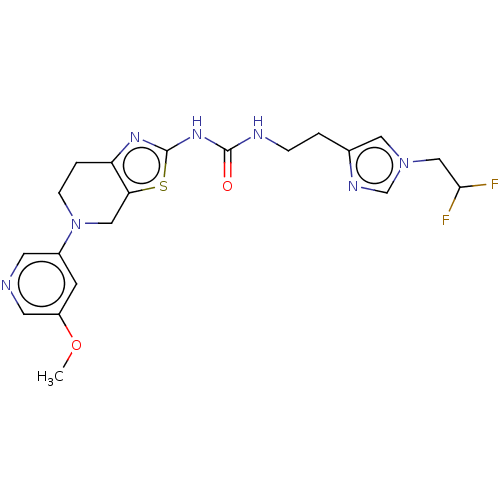
(CHEMBL3586677)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CC(F)F)cn3)sc2C1 Show InChI InChI=1S/C20H23F2N7O2S/c1-31-15-6-14(7-23-8-15)29-5-3-16-17(10-29)32-20(26-16)27-19(30)24-4-2-13-9-28(12-25-13)11-18(21)22/h6-9,12,18H,2-5,10-11H2,1H3,(H2,24,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043393
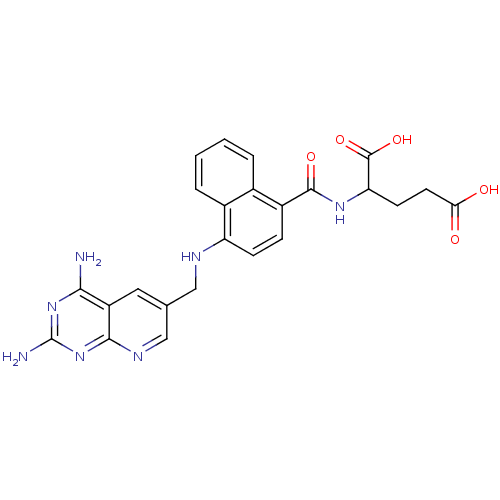
(2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(C(=O)NC(CCC(O)=O)C(O)=O)c4ccccc34)cnc2n1 Show InChI InChI=1S/C24H23N7O5/c25-20-16-9-12(11-28-21(16)31-24(26)30-20)10-27-17-6-5-15(13-3-1-2-4-14(13)17)22(34)29-18(23(35)36)7-8-19(32)33/h1-6,9,11,18,27H,7-8,10H2,(H,29,34)(H,32,33)(H,35,36)(H4,25,26,28,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00365 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023680

(2-{4-[(2,4-Diamino-7-phenyl-pyrido[2,3-d]pyrimidin...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)c(nc2n1)-c1ccccc1 Show InChI InChI=1S/C26H25N7O5/c27-22-18-12-16(21(14-4-2-1-3-5-14)31-23(18)33-26(28)32-22)13-29-17-8-6-15(7-9-17)24(36)30-19(25(37)38)10-11-20(34)35/h1-9,12,19,29H,10-11,13H2,(H,30,36)(H,34,35)(H,37,38)(H4,27,28,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023684
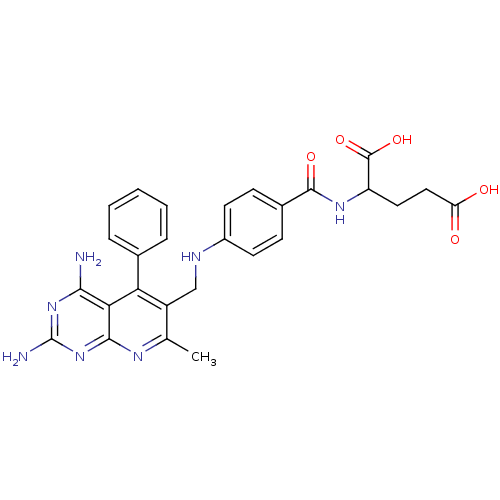
(2-{4-[(2,4-Diamino-7-methyl-5-phenyl-pyrido[2,3-d]...)Show SMILES Cc1nc2nc(N)nc(N)c2c(-c2ccccc2)c1CNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C27H27N7O5/c1-14-18(21(15-5-3-2-4-6-15)22-23(28)33-27(29)34-24(22)31-14)13-30-17-9-7-16(8-10-17)25(37)32-19(26(38)39)11-12-20(35)36/h2-10,19,30H,11-13H2,1H3,(H,32,37)(H,35,36)(H,38,39)(H4,28,29,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023683
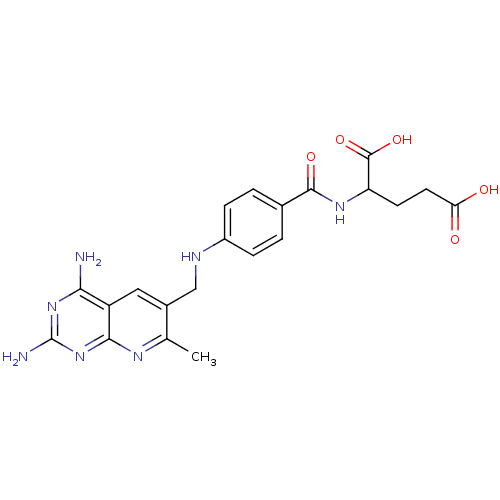
(2-{4-[(2,4-Diamino-7-methyl-pyrido[2,3-d]pyrimidin...)Show SMILES Cc1nc2nc(N)nc(N)c2cc1CNc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C21H23N7O5/c1-10-12(8-14-17(22)27-21(23)28-18(14)25-10)9-24-13-4-2-11(3-5-13)19(31)26-15(20(32)33)6-7-16(29)30/h2-5,8,15,24H,6-7,9H2,1H3,(H,26,31)(H,29,30)(H,32,33)(H4,22,23,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50023682

(2-{4-[(2,4-Diamino-5-methyl-7-phenyl-pyrido[2,3-d]...)Show SMILES Cc1c(CNc2ccc(cc2)C(=O)NC(CCC(O)=O)C(O)=O)c(nc2nc(N)nc(N)c12)-c1ccccc1 Show InChI InChI=1S/C27H27N7O5/c1-14-18(22(15-5-3-2-4-6-15)32-24-21(14)23(28)33-27(29)34-24)13-30-17-9-7-16(8-10-17)25(37)31-19(26(38)39)11-12-20(35)36/h2-10,19,30H,11-13H2,1H3,(H,31,37)(H,35,36)(H,38,39)(H4,28,29,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by ChEMBL
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) of L-1210 cells |
J Med Chem 31: 1209-15 (1988)
BindingDB Entry DOI: 10.7270/Q2930S6N |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043396
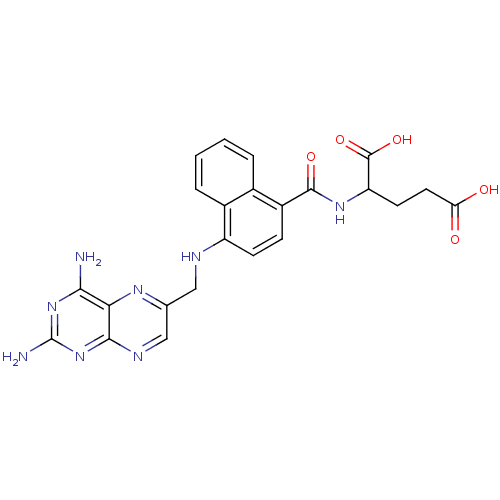
(2-({4-[(2,4-Diamino-pteridin-6-ylmethyl)-amino]-na...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(C(=O)NC(CCC(O)=O)C(O)=O)c4ccccc34)cnc2n1 Show InChI InChI=1S/C23H22N8O5/c24-19-18-20(31-23(25)30-19)27-10-11(28-18)9-26-15-6-5-14(12-3-1-2-4-13(12)15)21(34)29-16(22(35)36)7-8-17(32)33/h1-6,10,16,26H,7-9H2,(H,29,34)(H,32,33)(H,35,36)(H4,24,25,27,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00455 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043399
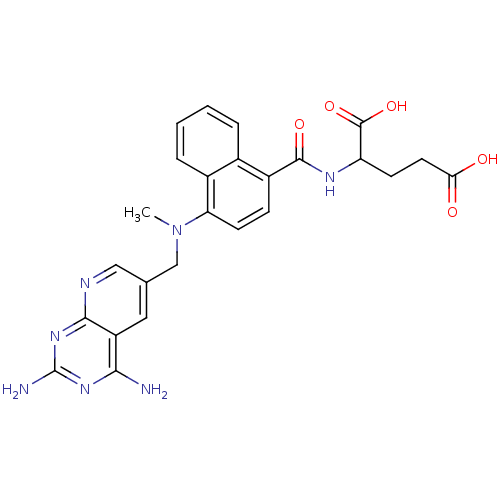
(2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2c1)c1ccc(C(=O)NC(CCC(O)=O)C(O)=O)c2ccccc12 Show InChI InChI=1S/C25H25N7O5/c1-32(12-13-10-17-21(26)30-25(27)31-22(17)28-11-13)19-8-6-16(14-4-2-3-5-15(14)19)23(35)29-18(24(36)37)7-9-20(33)34/h2-6,8,10-11,18H,7,9,12H2,1H3,(H,29,35)(H,33,34)(H,36,37)(H4,26,27,28,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00465 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for the inhibition of dihydrofolate reductase (DHFR) in L1210 cells |
J Med Chem 35: 3002-6 (1992)
BindingDB Entry DOI: 10.7270/Q2MP527Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.00482 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043395
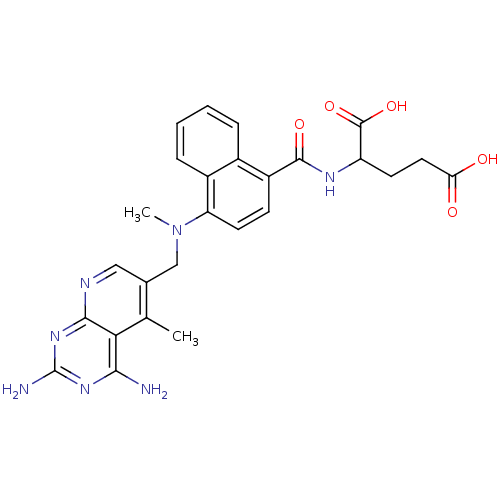
(2-({4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidi...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2c1C)c1ccc(C(=O)NC(CCC(O)=O)C(O)=O)c2ccccc12 Show InChI InChI=1S/C26H27N7O5/c1-13-14(11-29-23-21(13)22(27)31-26(28)32-23)12-33(2)19-9-7-17(15-5-3-4-6-16(15)19)24(36)30-18(25(37)38)8-10-20(34)35/h3-7,9,11,18H,8,10,12H2,1-2H3,(H,30,36)(H,34,35)(H,37,38)(H4,27,28,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00484 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043400
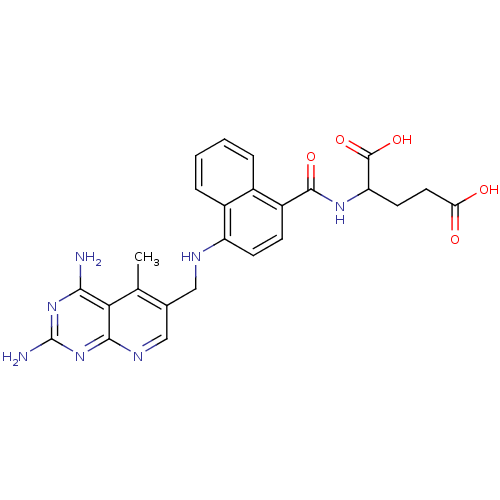
(2-({4-[(2,4-Diamino-5-methyl-pyrido[2,3-d]pyrimidi...)Show SMILES Cc1c(CNc2ccc(C(=O)NC(CCC(O)=O)C(O)=O)c3ccccc23)cnc2nc(N)nc(N)c12 Show InChI InChI=1S/C25H25N7O5/c1-12-13(11-29-22-20(12)21(26)31-25(27)32-22)10-28-17-7-6-16(14-4-2-3-5-15(14)17)23(35)30-18(24(36)37)8-9-19(33)34/h2-7,11,18,28H,8-10H2,1H3,(H,30,35)(H,33,34)(H,36,37)(H4,26,27,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00508 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043398
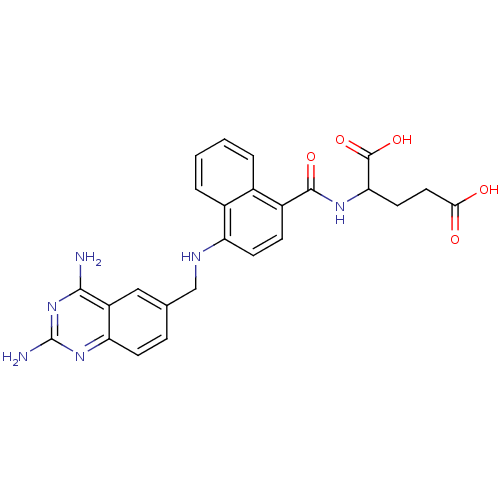
(2-({4-[(2,4-Diamino-quinazolin-6-ylmethyl)-amino]-...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(C(=O)NC(CCC(O)=O)C(O)=O)c4ccccc34)ccc2n1 Show InChI InChI=1S/C25H24N6O5/c26-22-17-11-13(5-7-19(17)30-25(27)31-22)12-28-18-8-6-16(14-3-1-2-4-15(14)18)23(34)29-20(24(35)36)9-10-21(32)33/h1-8,11,20,28H,9-10,12H2,(H,29,34)(H,32,33)(H,35,36)(H4,26,27,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043394
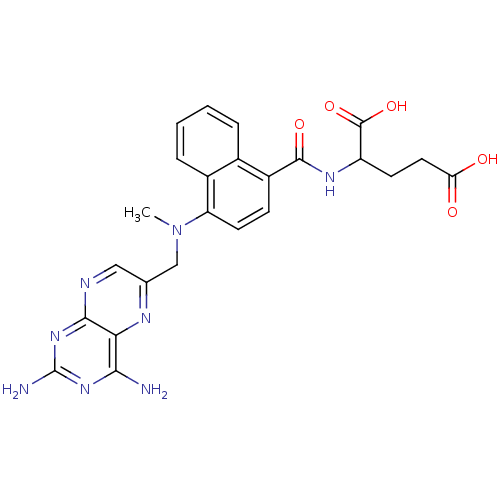
(2-({4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-am...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(C(=O)NC(CCC(O)=O)C(O)=O)c2ccccc12 Show InChI InChI=1S/C24H24N8O5/c1-32(11-12-10-27-21-19(28-12)20(25)30-24(26)31-21)17-8-6-15(13-4-2-3-5-14(13)17)22(35)29-16(23(36)37)7-9-18(33)34/h2-6,8,10,16H,7,9,11H2,1H3,(H,29,35)(H,33,34)(H,36,37)(H4,25,26,27,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00522 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM14065
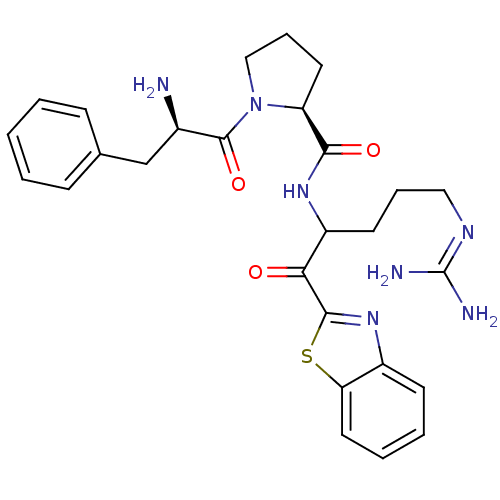
((2S)-1-[(2R)-2-amino-3-phenylpropanoyl]-N-[1-(1,3-...)Show SMILES [#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-c1nc2ccccc2s1 |r| Show InChI InChI=1S/C27H33N7O3S/c28-18(16-17-8-2-1-3-9-17)26(37)34-15-7-12-21(34)24(36)32-20(11-6-14-31-27(29)30)23(35)25-33-19-10-4-5-13-22(19)38-25/h1-5,8-10,13,18,20-21H,6-7,11-12,14-16,28H2,(H,32,36)(H4,29,30,31)/t18-,20?,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00550 | -66.9 | 21 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical
| Assay Description
Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... |
J Med Chem 48: 1984-2008 (2005)
Article DOI: 10.1021/jm0303857
BindingDB Entry DOI: 10.7270/Q2X0658X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093356
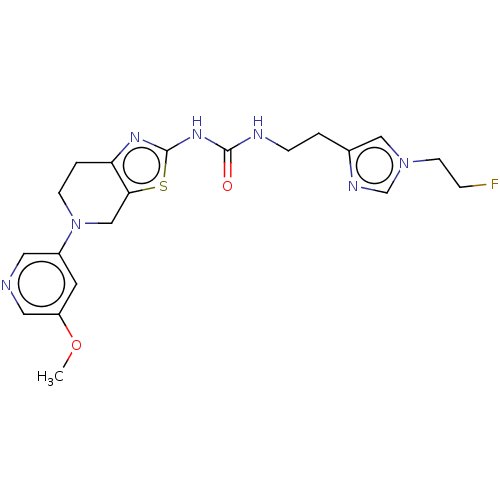
(CHEMBL3586676)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CCF)cn3)sc2C1 Show InChI InChI=1S/C20H24FN7O2S/c1-30-16-8-15(9-22-10-16)28-6-3-17-18(12-28)31-20(25-17)26-19(29)23-5-2-14-11-27(7-4-21)13-24-14/h8-11,13H,2-7,12H2,1H3,(H2,23,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM14127
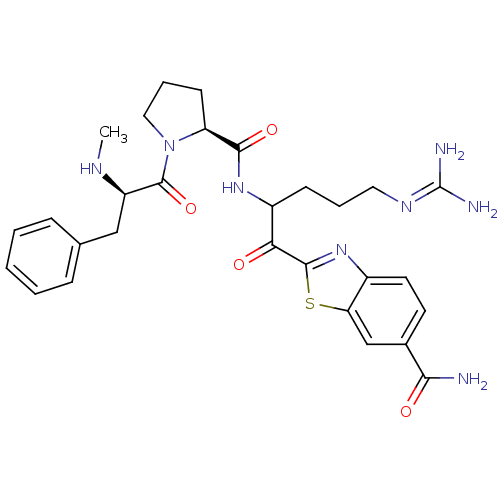
(2-(5-carbamimidamido-2-{[(2S)-1-[(2R)-2-(methylami...)Show SMILES [#6]-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-c1nc2ccc(cc2s1)-[#6](-[#7])=O |r| Show InChI InChI=1S/C29H36N8O4S/c1-33-21(15-17-7-3-2-4-8-17)28(41)37-14-6-10-22(37)26(40)35-20(9-5-13-34-29(31)32)24(38)27-36-19-12-11-18(25(30)39)16-23(19)42-27/h2-4,7-8,11-12,16,20-22,33H,5-6,9-10,13-15H2,1H3,(H2,30,39)(H,35,40)(H4,31,32,34)/t20?,21-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00700 | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical
| Assay Description
Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... |
J Med Chem 48: 1984-2008 (2005)
Article DOI: 10.1021/jm0303857
BindingDB Entry DOI: 10.7270/Q2X0658X |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische Chemie der Philipps-Universit£t Marburg
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards (alpha-4)2(beta-2)3 neuronal nicotinic acetylcholine receptor in P2 membrane fractions of rat forebrain |
J Med Chem 46: 2031-48 (2003)
Article DOI: 10.1021/jm020859m
BindingDB Entry DOI: 10.7270/Q2N87DHM |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093354

(CHEMBL3586679)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CC(F)(F)F)cn3)sc2C1 Show InChI InChI=1S/C20H22F3N7O2S/c1-32-15-6-14(7-24-8-15)30-5-3-16-17(10-30)33-19(27-16)28-18(31)25-4-2-13-9-29(12-26-13)11-20(21,22)23/h6-9,12H,2-5,10-11H2,1H3,(H2,25,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische Chemie der Philipps-Universit£t Marburg
Curated by ChEMBL
| Assay Description
Binding affinity to Nicotinic acetylcholine receptor alpha4-beta2 using +/-[3H]epibatidine as radioligand in rat brain |
J Med Chem 45: 1064-72 (2002)
BindingDB Entry DOI: 10.7270/Q2J96736 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50161085
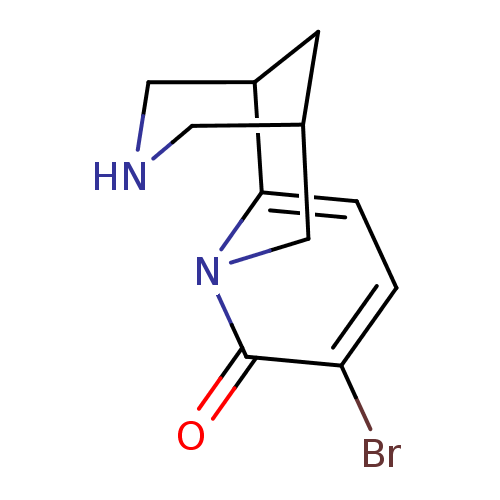
(9-Bromo-1,2,3,4,5,6-hexahydro-1,5-methano-pyrido[1...)Show SMILES Brc1ccc2C3CNCC(C3)Cn2c1=O |TLB:13:12:10:7.6.8,THB:3:4:10:7.6.8| Show InChI InChI=1S/C11H13BrN2O/c12-9-1-2-10-8-3-7(4-13-5-8)6-14(10)11(9)15/h1-2,7-8,13H,3-6H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische Chemie der Philipps-Universit£t Marburg
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards (alpha-4)2(beta-2)3 neuronal nicotinic acetylcholine receptor in P2 membrane fractions of rat forebrain |
J Med Chem 46: 2031-48 (2003)
Article DOI: 10.1021/jm020859m
BindingDB Entry DOI: 10.7270/Q2N87DHM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093395
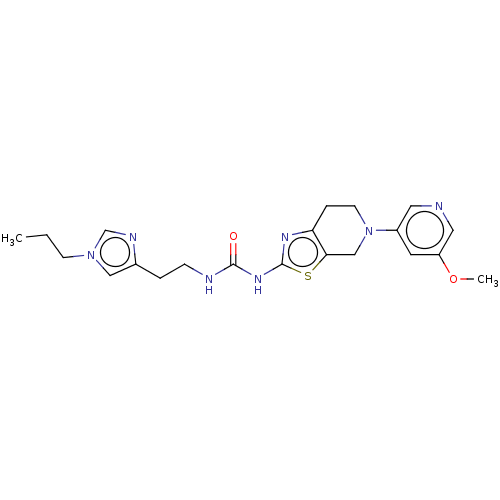
(CHEMBL3586674)Show SMILES CCCn1cnc(CCNC(=O)Nc2nc3CCN(Cc3s2)c2cncc(OC)c2)c1 Show InChI InChI=1S/C21H27N7O2S/c1-3-7-27-12-15(24-14-27)4-6-23-20(29)26-21-25-18-5-8-28(13-19(18)31-21)16-9-17(30-2)11-22-10-16/h9-12,14H,3-8,13H2,1-2H3,(H2,23,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50157823
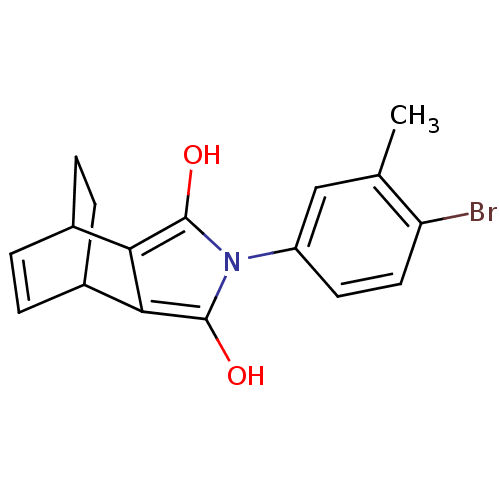
((2R,6S)-4-(4-Bromo-3-methyl-phenyl)-4-aza-tricyclo...)Show SMILES Cc1cc(ccc1Br)-n1c(O)c2C3CCC(C=C3)c2c1O |c:17,THB:9:11:16.17:13.14| Show InChI InChI=1S/C17H16BrNO2/c1-9-8-12(6-7-13(9)18)19-16(20)14-10-2-3-11(5-4-10)15(14)17(19)21/h2-3,6-8,10-11,20-21H,4-5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity for mutant T877A Androgen receptor in human LNCaP cells |
Bioorg Med Chem Lett 15: 271-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.085
BindingDB Entry DOI: 10.7270/Q2N58KVH |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50066789
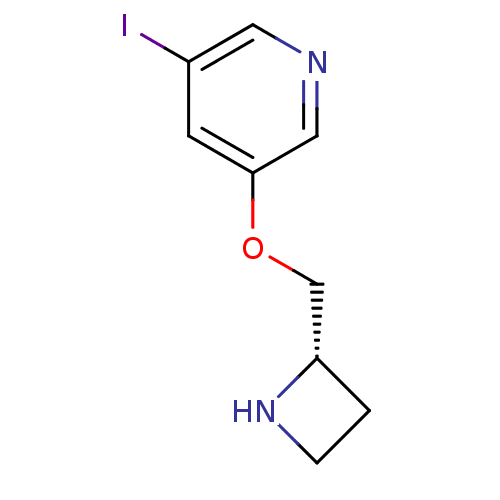
(3-((S)-1-Azetidin-2-ylmethoxy)-5-iodo-pyridine | A...)Show InChI InChI=1S/C9H11IN2O/c10-7-3-9(5-11-4-7)13-6-8-1-2-12-8/h3-5,8,12H,1-2,6H2/t8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50049750

((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...)Show InChI InChI=1S/C9H12N2O/c1-2-9(6-10-4-1)12-7-8-3-5-11-8/h1-2,4,6,8,11H,3,5,7H2/t8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50066789

(3-((S)-1-Azetidin-2-ylmethoxy)-5-iodo-pyridine | A...)Show InChI InChI=1S/C9H11IN2O/c10-7-3-9(5-11-4-7)13-6-8-1-2-12-8/h3-5,8,12H,1-2,6H2/t8-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50049750

((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...)Show InChI InChI=1S/C9H12N2O/c1-2-9(6-10-4-1)12-7-8-3-5-11-8/h1-2,4,6,8,11H,3,5,7H2/t8-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093417
(CHEMBL3586672)Show InChI InChI=1S/C18H25N5O3S/c1-3-7-26-8-5-20-17(24)22-18-21-15-4-6-23(12-16(15)27-18)13-9-14(25-2)11-19-10-13/h9-11H,3-8,12H2,1-2H3,(H2,20,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093437
(CHEMBL3586668)Show InChI InChI=1S/C20H32O2/c1-19-7-5-14(21)10-13(19)3-4-15-16(19)6-8-20(2)17(15)9-12-11-22-18(12)20/h12-18,21H,3-11H2,1-2H3/t12-,13+,14-,15-,16+,17+,18+,19+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50043397
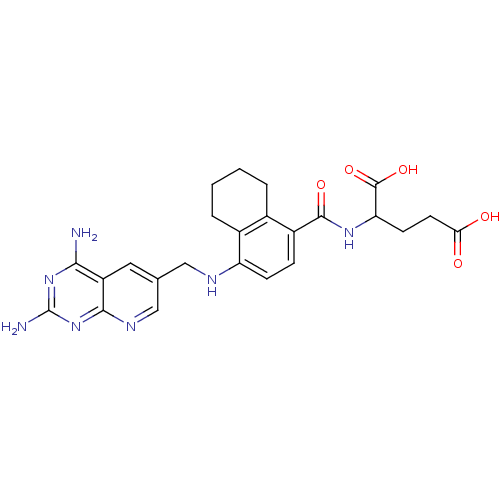
(2-({4-[(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylmet...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(C(=O)NC(CCC(O)=O)C(O)=O)c4CCCCc34)cnc2n1 Show InChI InChI=1S/C24H27N7O5/c25-20-16-9-12(11-28-21(16)31-24(26)30-20)10-27-17-6-5-15(13-3-1-2-4-14(13)17)22(34)29-18(23(35)36)7-8-19(32)33/h5-6,9,11,18,27H,1-4,7-8,10H2,(H,29,34)(H,32,33)(H,35,36)(H4,25,26,28,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of Dihydrofolate reductase in L1210 cells |
J Med Chem 36: 4161-71 (1994)
BindingDB Entry DOI: 10.7270/Q2BC3XNB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093399
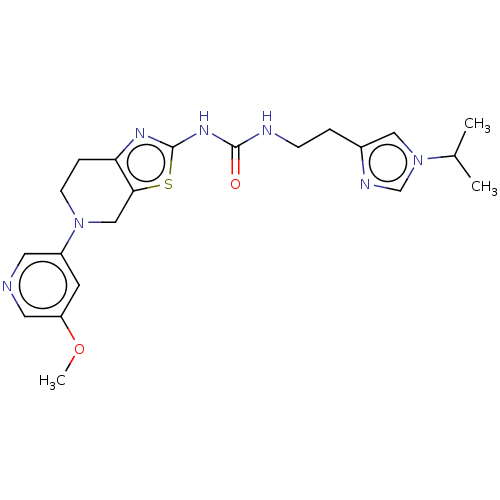
(CHEMBL3586673)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(cn3)C(C)C)sc2C1 Show InChI InChI=1S/C21H27N7O2S/c1-14(2)28-11-15(24-13-28)4-6-23-20(29)26-21-25-18-5-7-27(12-19(18)31-21)16-8-17(30-3)10-22-9-16/h8-11,13-14H,4-7,12H2,1-3H3,(H2,23,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
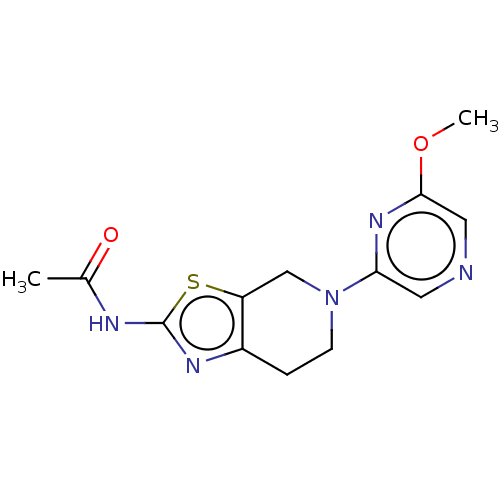
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093434
(CHEMBL3586670)Show InChI InChI=1S/C12H11NO8S2/c1-6(14)13-10-4-8(22(16,17)18)2-7-3-9(23(19,20)21)5-11(15)12(7)10/h2-5,15H,1H3,(H,13,14)(H,16,17,18)(H,19,20,21)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50161086
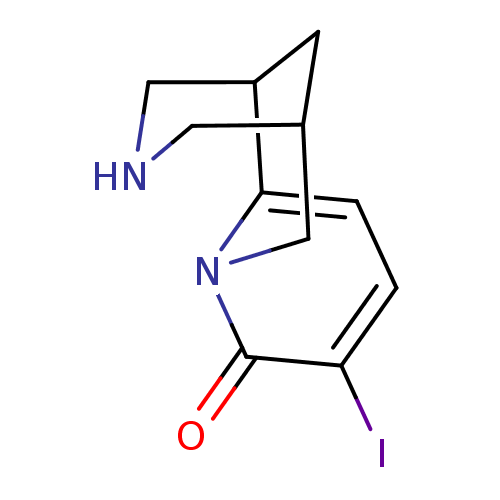
(9-Iodo-1,2,3,4,5,6-hexahydro-1,5-methano-pyrido[1,...)Show SMILES Ic1ccc2C3CNCC(C3)Cn2c1=O |THB:3:4:10:7.6.8| Show InChI InChI=1S/C11H13IN2O/c12-9-1-2-10-8-3-7(4-13-5-8)6-14(10)11(9)15/h1-2,7-8,13H,3-6H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische Chemie der Philipps-Universit£t Marburg
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards (alpha-4)2(beta-2)3 neuronal nicotinic acetylcholine receptor in P2 membrane fractions of rat forebrain |
J Med Chem 46: 2031-48 (2003)
Article DOI: 10.1021/jm020859m
BindingDB Entry DOI: 10.7270/Q2N87DHM |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM14076
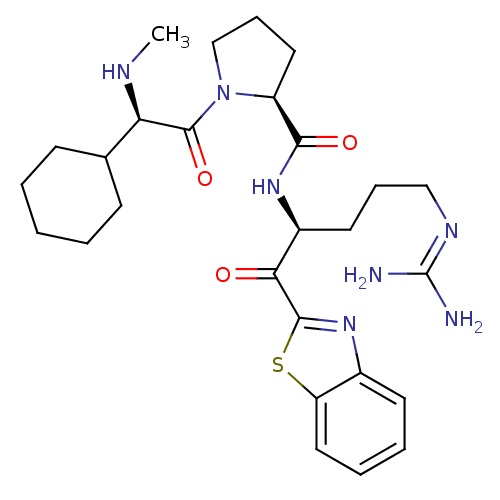
((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...)Show SMILES [#6]-[#7]-[#6@H](-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-c1nc2ccccc2s1 |r| Show InChI InChI=1S/C27H39N7O3S/c1-30-22(17-9-3-2-4-10-17)26(37)34-16-8-13-20(34)24(36)32-19(12-7-15-31-27(28)29)23(35)25-33-18-11-5-6-14-21(18)38-25/h5-6,11,14,17,19-20,22,30H,2-4,7-10,12-13,15-16H2,1H3,(H,32,36)(H4,28,29,31)/t19-,20-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0180 | -63.8 | 5.30 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical
| Assay Description
Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... |
J Med Chem 48: 1984-2008 (2005)
Article DOI: 10.1021/jm0303857
BindingDB Entry DOI: 10.7270/Q2X0658X |
More data for this
Ligand-Target Pair | |
WD repeat-containing protein 5
(Homo sapiens (Human)) | BDBM50520131
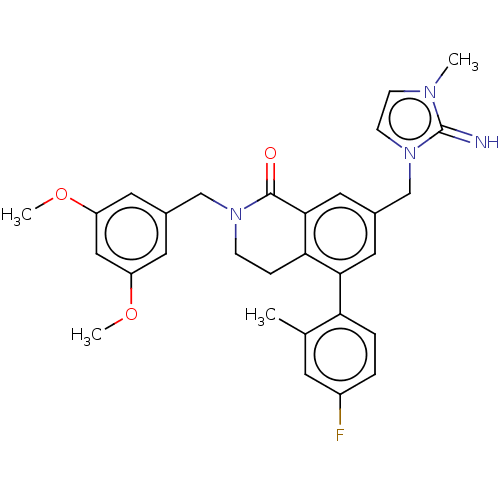
(CHEMBL4588661)Show SMILES COc1cc(CN2CCc3c(cc(Cn4ccn(C)c4=N)cc3-c3ccc(F)cc3C)C2=O)cc(OC)c1 Show InChI InChI=1S/C30H31FN4O3/c1-19-11-22(31)5-6-25(19)27-14-21(18-35-10-9-33(2)30(35)32)15-28-26(27)7-8-34(29(28)36)17-20-12-23(37-3)16-24(13-20)38-4/h5-6,9-16,32H,7-8,17-18H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00195
BindingDB Entry DOI: 10.7270/Q2JQ153M |
More data for this
Ligand-Target Pair | |
WD repeat-containing protein 5
(Homo sapiens (Human)) | BDBM50605350
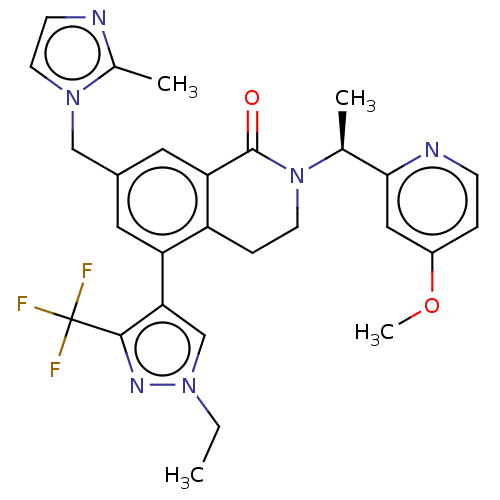
(CHEMBL5177656)Show SMILES CCn1cc(c(n1)C(F)(F)F)-c1cc(Cn2ccnc2C)cc2C(=O)N(CCc12)[C@@H](C)c1cc(OC)ccn1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00195
BindingDB Entry DOI: 10.7270/Q2JQ153M |
More data for this
Ligand-Target Pair | |
WD repeat-containing protein 5
(Homo sapiens (Human)) | BDBM50605353
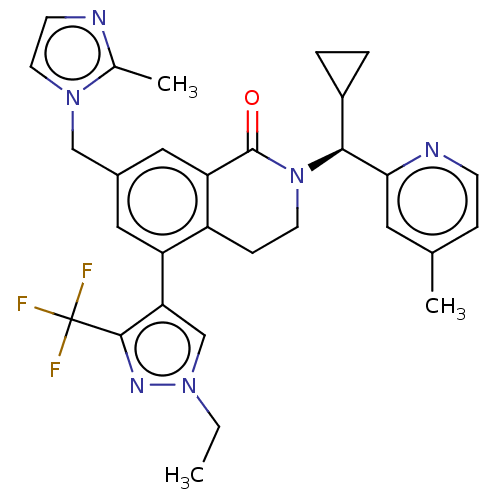
(CHEMBL5190717)Show SMILES CCn1cc(c(n1)C(F)(F)F)-c1cc(Cn2ccnc2C)cc2C(=O)N(CCc12)[C@@H](C1CC1)c1cc(C)ccn1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00195
BindingDB Entry DOI: 10.7270/Q2JQ153M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
WD repeat-containing protein 5
(Homo sapiens (Human)) | BDBM50605352

(CHEMBL5198319)Show SMILES CCn1cc(c(n1)C(F)(F)F)-c1cc(Cn2ccnc2C)cc2C(=O)N(CCc12)[C@H](C1CC1)c1cc(OC)ccn1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00195
BindingDB Entry DOI: 10.7270/Q2JQ153M |
More data for this
Ligand-Target Pair | |
WD repeat-containing protein 5
(Homo sapiens (Human)) | BDBM50605323
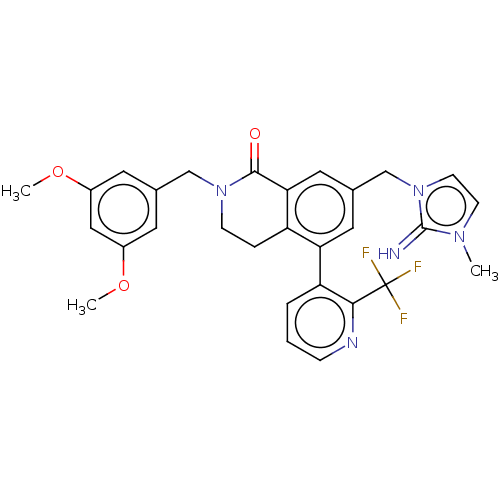
(CHEMBL5174357)Show SMILES COc1cc(CN2CCc3c(cc(Cn4ccn(C)c4=N)cc3-c3cccnc3C(F)(F)F)C2=O)cc(OC)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00195
BindingDB Entry DOI: 10.7270/Q2JQ153M |
More data for this
Ligand-Target Pair | |
WD repeat-containing protein 5
(Homo sapiens (Human)) | BDBM50605350

(CHEMBL5177656)Show SMILES CCn1cc(c(n1)C(F)(F)F)-c1cc(Cn2ccnc2C)cc2C(=O)N(CCc12)[C@@H](C)c1cc(OC)ccn1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00195
BindingDB Entry DOI: 10.7270/Q2JQ153M |
More data for this
Ligand-Target Pair | |
WD repeat-containing protein 5
(Homo sapiens (Human)) | BDBM50605349
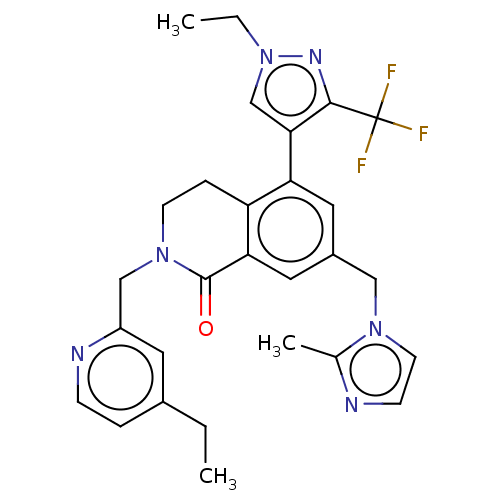
(CHEMBL5191181)Show SMILES CCc1ccnc(CN2CCc3c(cc(Cn4ccnc4C)cc3C2=O)-c2cn(CC)nc2C(F)(F)F)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00195
BindingDB Entry DOI: 10.7270/Q2JQ153M |
More data for this
Ligand-Target Pair | |
WD repeat-containing protein 5
(Homo sapiens (Human)) | BDBM50605348
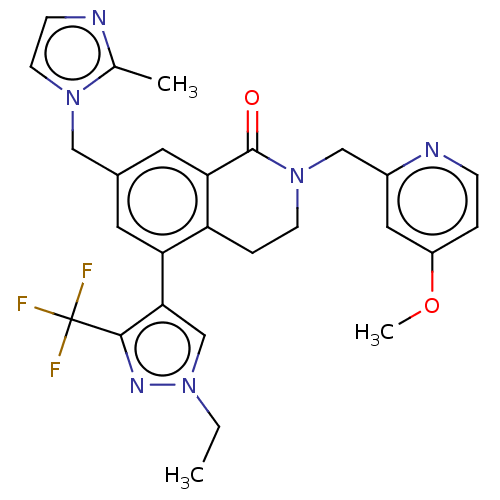
(CHEMBL5183319)Show SMILES CCn1cc(c(n1)C(F)(F)F)-c1cc(Cn2ccnc2C)cc2C(=O)N(Cc3cc(OC)ccn3)CCc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00195
BindingDB Entry DOI: 10.7270/Q2JQ153M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data