

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4560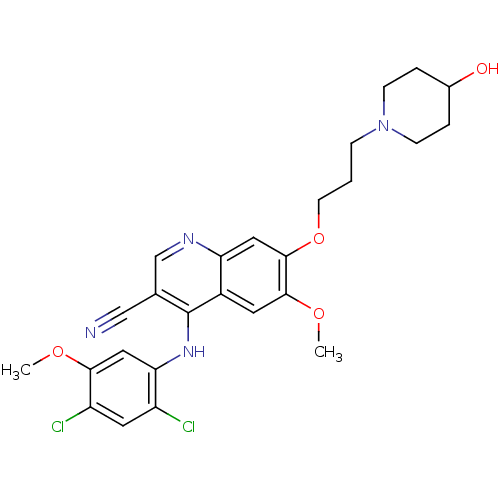 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31i |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4557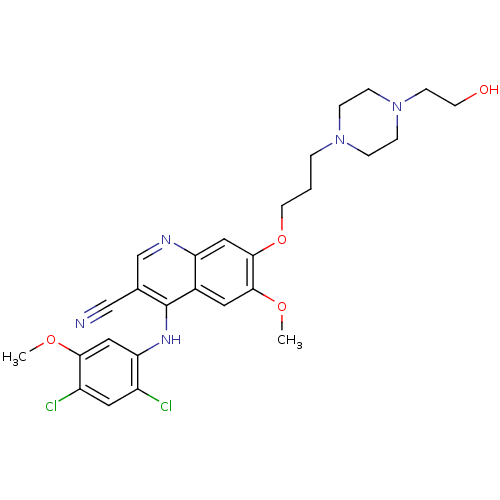 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31f |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4553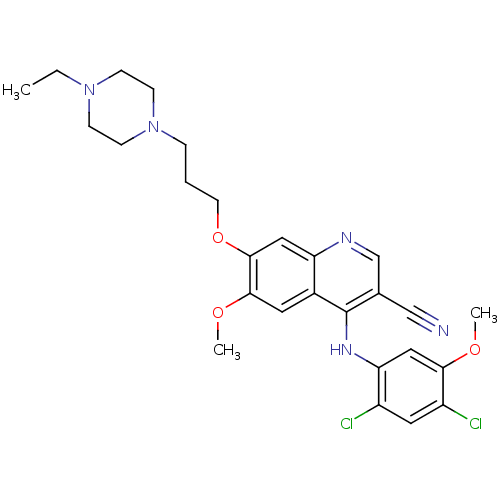 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31b |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4543 (4-Phenylamino-3-quinolinecarbonitrile deriv. 2c | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4544 (4-Phenylamino-3-quinolinecarbonitrile deriv. 2d | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4547 (4-Phenylamino-3-quinolinecarbonitrile deriv. 2g | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4556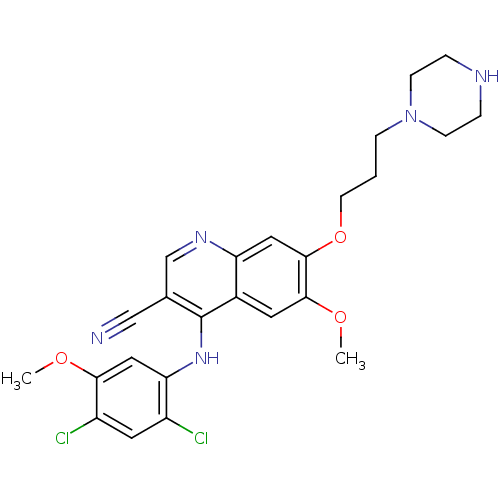 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31e |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4555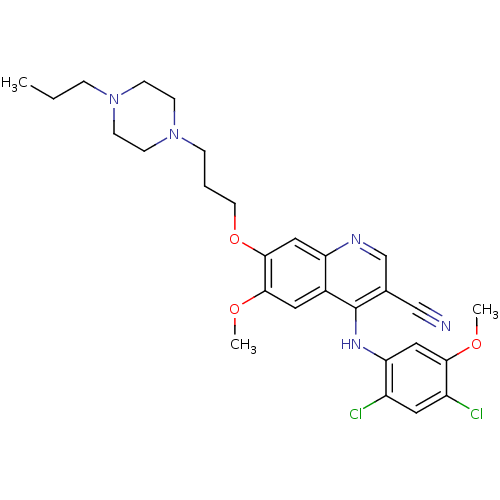 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31d |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4552 (4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4559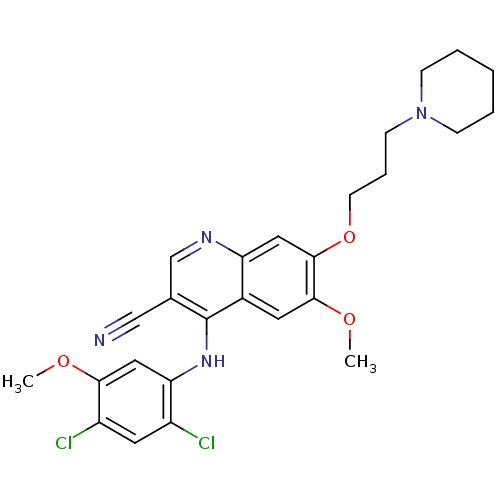 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31h |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4545 (4-Phenylamino-3-quinolinecarbonitrile deriv. 2e | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4550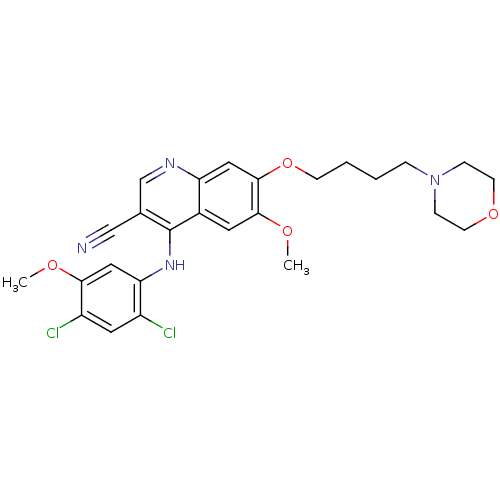 (4-Phenylamino 3-quinolinecarbonitrile deriv. 27 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4554 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31c |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4558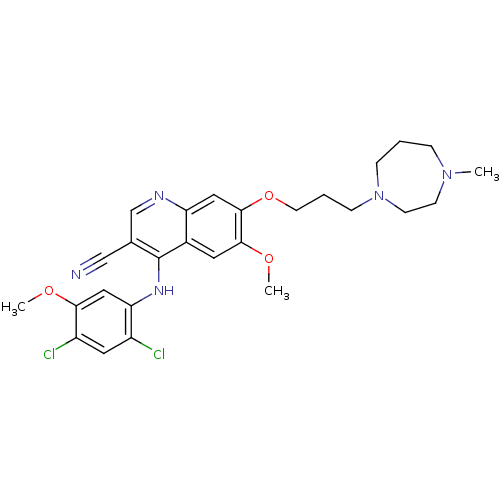 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31g |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4542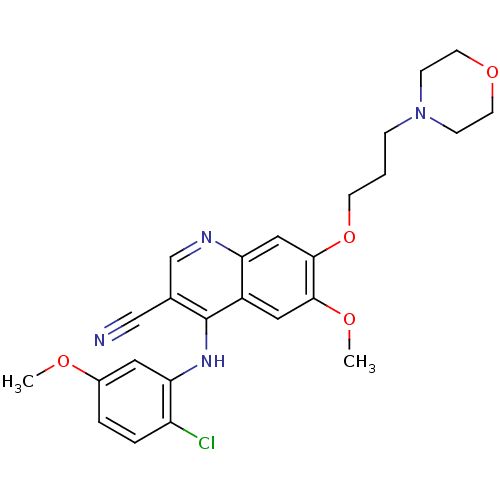 (4-Phenylamino-3-quinolinecarbonitrile deriv. 2b | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4546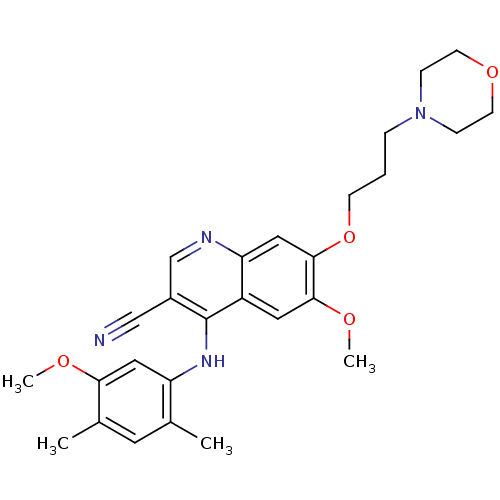 (4-Phenylamino-3-quinolinecarbonitrile deriv. 2f | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4562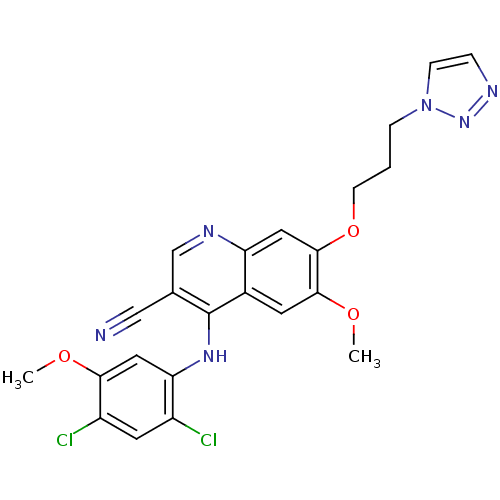 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31k |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4563 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31l |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4549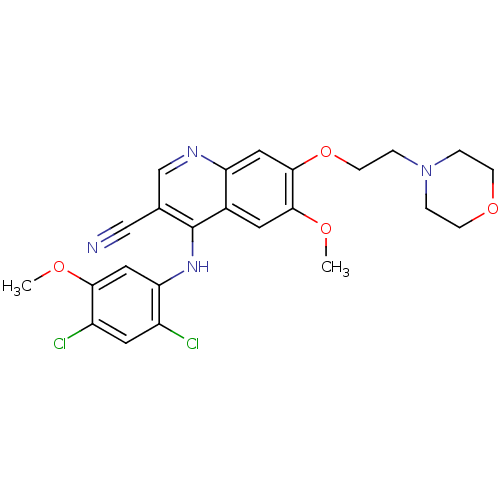 (4-Phenylamino 3-quinolinecarbonitrile deriv. 26 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4565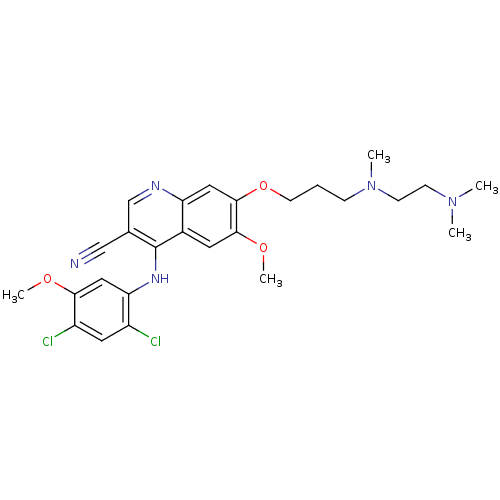 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31n |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4551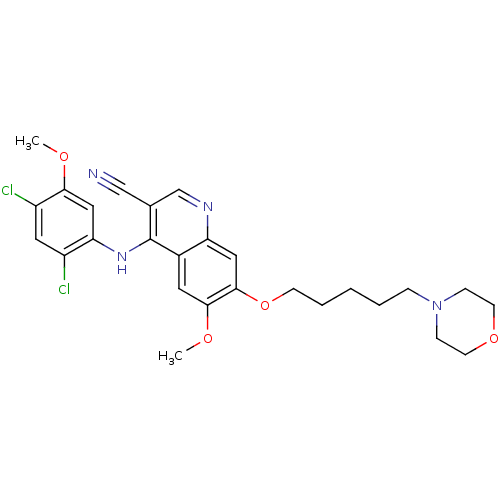 (4-Phenylamino-3-quinolinecarbonitrile deriv. 28 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM21034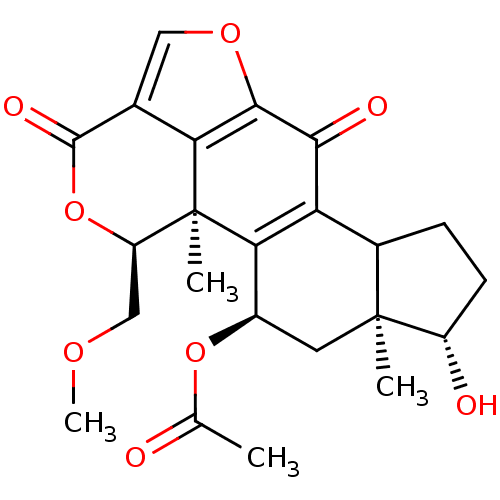 ((1R,3R,5S,6S,18S)-6-hydroxy-18-(methoxymethyl)-1,5...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Wyeth Research | Assay Description Inhibition of PI3K enzyme activity is measured in a microtiter plate-based fluorescence polarization (FP) assay. | J Med Chem 51: 1319-23 (2008) Article DOI: 10.1021/jm7012858 BindingDB Entry DOI: 10.7270/Q2NP22QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4534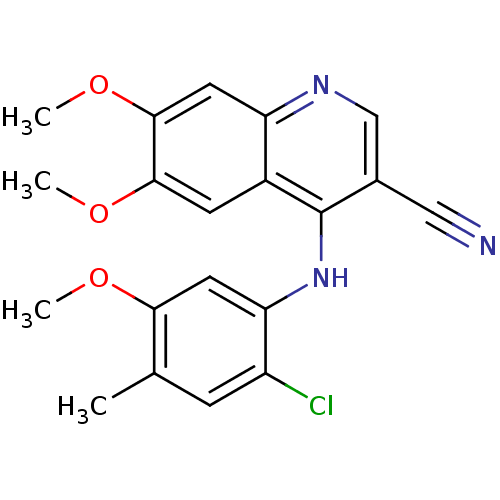 (4-Phenylamino-3-quinolinecarbonitrile deriv. 1l | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4564 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31m |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4530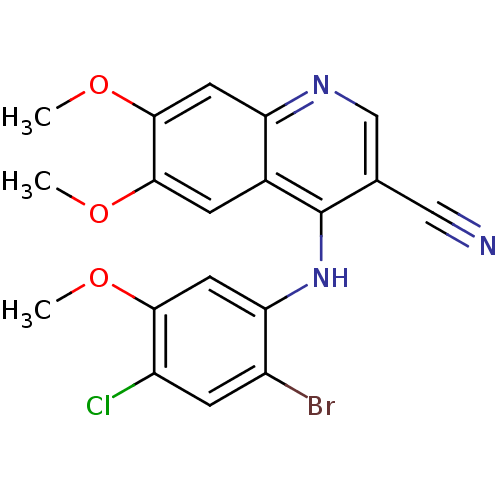 (4-Phenylamino-3-quinolinecarbonitrile deriv. 1h | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4520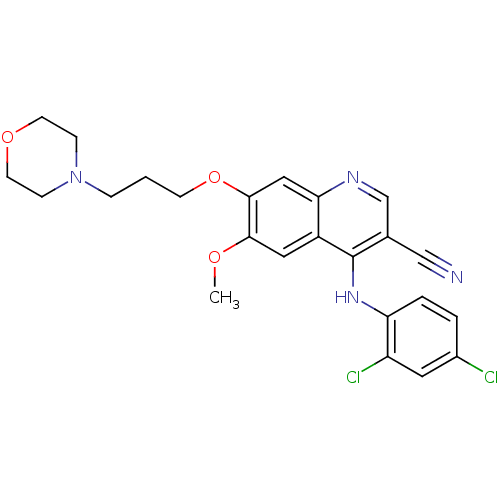 (4-Phenylamino-3-quinolinecarbonitrile deriv. 25 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4531 (4-Phenylamino-3-quinolinecarbonitrile deriv. 1i | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4525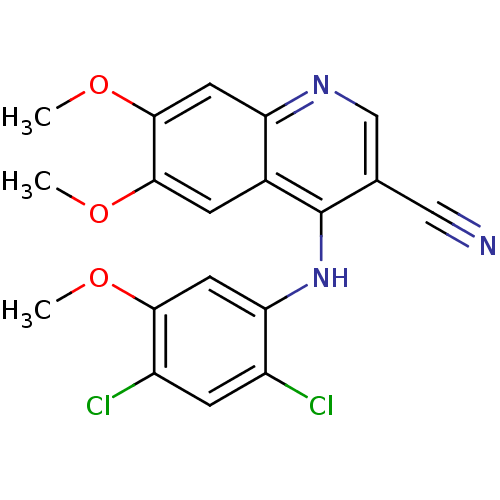 (4-Phenylamino-3-quinolinecarbonitrile deriv. 1c | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4536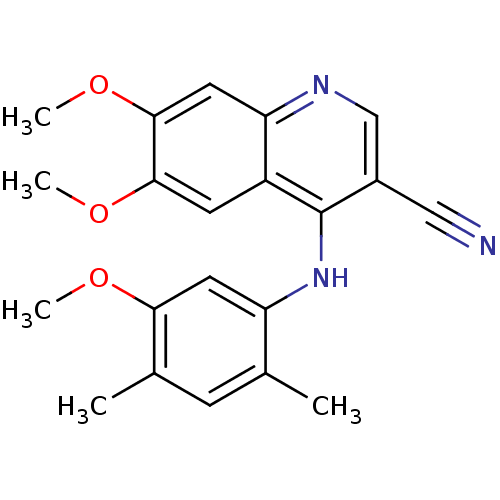 (4-Phenylamino-3-quinolinecarbonitrile deriv. 1n | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM21042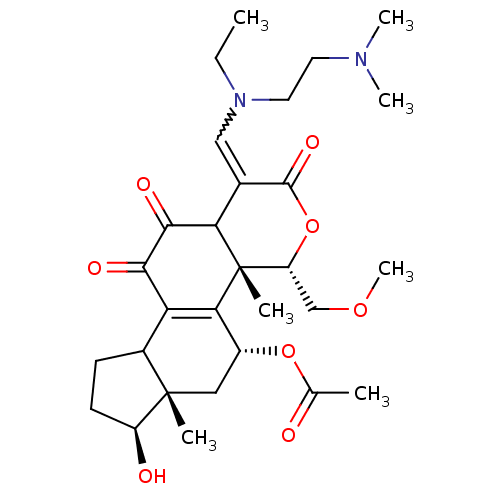 ((2R,3S,6E,14S,15S,17R)-6-({[2-(dimethylamino)ethyl...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Inhibition of PI3K enzyme activity is measured in a microtiter plate-based fluorescence polarization (FP) assay. | J Med Chem 51: 1319-23 (2008) Article DOI: 10.1021/jm7012858 BindingDB Entry DOI: 10.7270/Q2NP22QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4515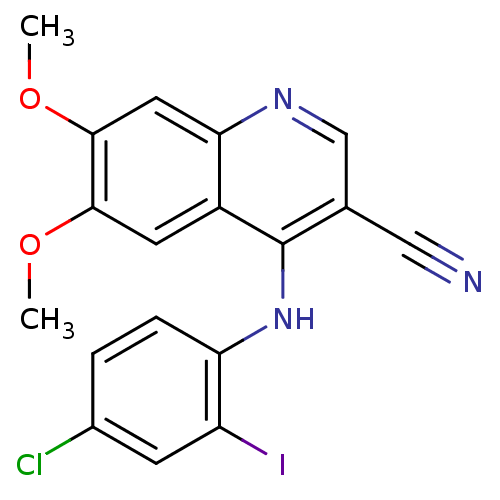 (4-Phenylamino-3-quinolinecarbonitrile deriv. 1k | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM21036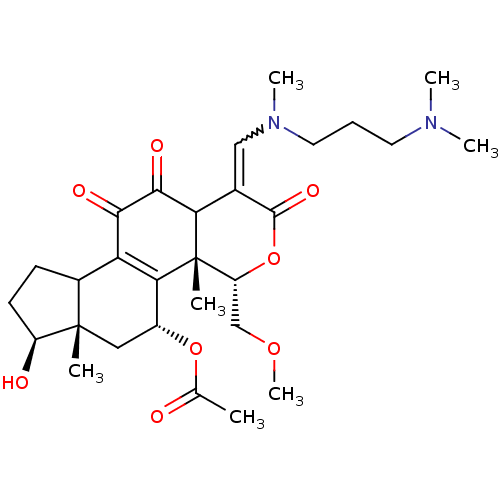 ((2R,3S,6Z,14S,15S,17R)-6-({[3-(dimethylamino)propy...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Wyeth Research | Assay Description Inhibition of PI3K enzyme activity is measured in a microtiter plate-based fluorescence polarization (FP) assay. | J Med Chem 51: 1319-23 (2008) Article DOI: 10.1021/jm7012858 BindingDB Entry DOI: 10.7270/Q2NP22QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5 (Homo sapiens (Human)) | BDBM21036 ((2R,3S,6Z,14S,15S,17R)-6-({[3-(dimethylamino)propy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Wyeth Research | Assay Description Inhibition of PI3K enzyme activity is measured in a microtiter plate-based fluorescence polarization (FP) assay. | J Med Chem 51: 1319-23 (2008) Article DOI: 10.1021/jm7012858 BindingDB Entry DOI: 10.7270/Q2NP22QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM21041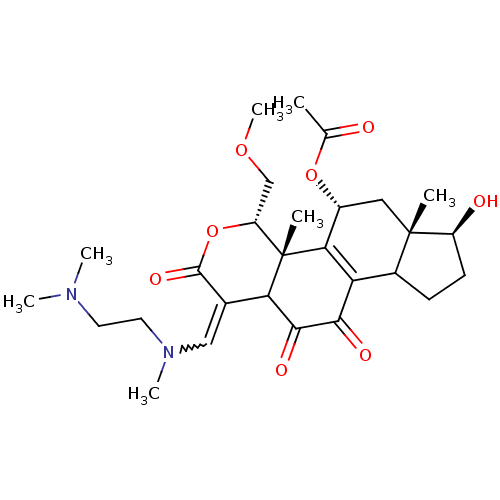 ((2R,3S,6E,14S,15S,17R)-6-({[2-(dimethylamino)ethyl...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Inhibition of PI3K enzyme activity is measured in a microtiter plate-based fluorescence polarization (FP) assay. | J Med Chem 51: 1319-23 (2008) Article DOI: 10.1021/jm7012858 BindingDB Entry DOI: 10.7270/Q2NP22QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4561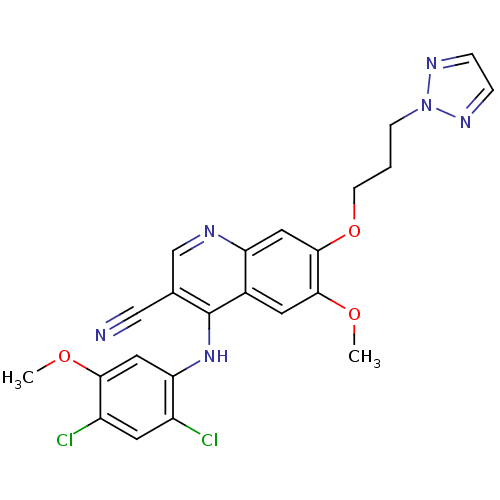 (4-Phenylamino-3-quinolinecarbonitrile deriv. 31j |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM21039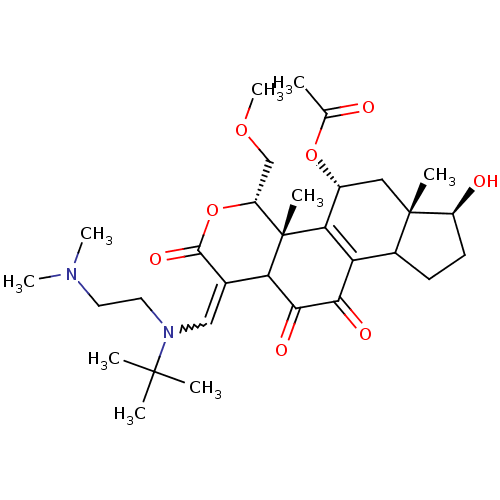 ((2R,3S,6Z,14S,15S,17R)-6-({tert-butyl[2-(dimethyla...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Wyeth Research | Assay Description Inhibition of PI3K enzyme activity is measured in a microtiter plate-based fluorescence polarization (FP) assay. | J Med Chem 51: 1319-23 (2008) Article DOI: 10.1021/jm7012858 BindingDB Entry DOI: 10.7270/Q2NP22QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM21047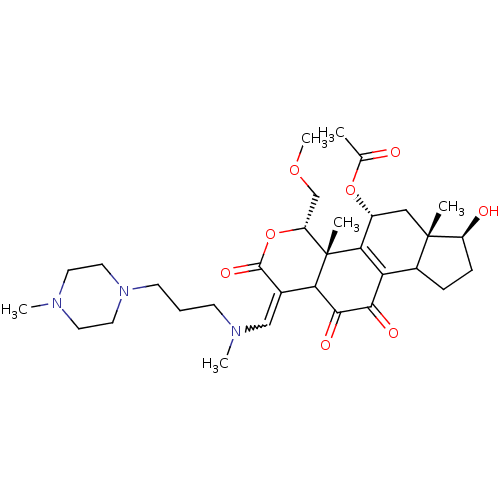 ((2R,3S,6E,14S,15S,17R)-8,14-dihydroxy-3-(methoxyme...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Inhibition of PI3K enzyme activity is measured in a microtiter plate-based fluorescence polarization (FP) assay. | J Med Chem 51: 1319-23 (2008) Article DOI: 10.1021/jm7012858 BindingDB Entry DOI: 10.7270/Q2NP22QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4524 (4-Phenylamino-3-quinolinecarbonitrile deriv. 1b | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit beta/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM21036 ((2R,3S,6Z,14S,15S,17R)-6-({[3-(dimethylamino)propy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Wyeth Research | Assay Description Inhibition of PI3K enzyme activity is measured in a microtiter plate-based fluorescence polarization (FP) assay. | J Med Chem 51: 1319-23 (2008) Article DOI: 10.1021/jm7012858 BindingDB Entry DOI: 10.7270/Q2NP22QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM21043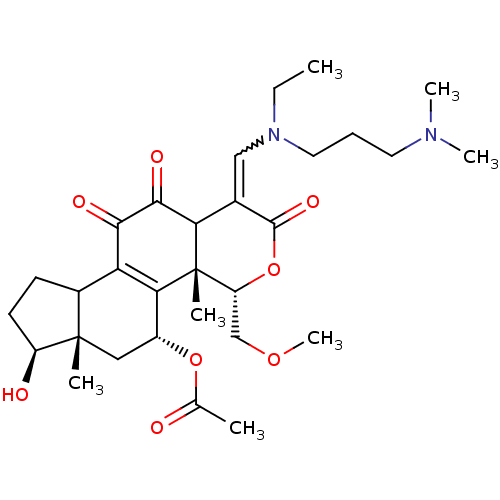 ((2R,3S,6E,14S,15S,17R)-6-({[3-(dimethylamino)propy...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Inhibition of PI3K enzyme activity is measured in a microtiter plate-based fluorescence polarization (FP) assay. | J Med Chem 51: 1319-23 (2008) Article DOI: 10.1021/jm7012858 BindingDB Entry DOI: 10.7270/Q2NP22QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit beta/4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM21036 ((2R,3S,6Z,14S,15S,17R)-6-({[3-(dimethylamino)propy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Wyeth Research | Assay Description Inhibition of PI3K enzyme activity is measured in a microtiter plate-based fluorescence polarization (FP) assay. | J Med Chem 51: 1319-23 (2008) Article DOI: 10.1021/jm7012858 BindingDB Entry DOI: 10.7270/Q2NP22QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM21049 ((2R,3S,6E,14S,15S,17R)-8,14-dihydroxy-3-(methoxyme...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Inhibition of PI3K enzyme activity is measured in a microtiter plate-based fluorescence polarization (FP) assay. | J Med Chem 51: 1319-23 (2008) Article DOI: 10.1021/jm7012858 BindingDB Entry DOI: 10.7270/Q2NP22QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM21046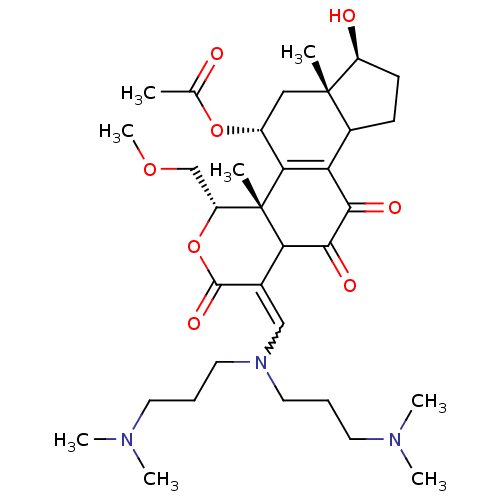 ((2R,3S,6E,14S,15S,17R)-6-({bis[3-(dimethylamino)pr...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Inhibition of PI3K enzyme activity is measured in a microtiter plate-based fluorescence polarization (FP) assay. | J Med Chem 51: 1319-23 (2008) Article DOI: 10.1021/jm7012858 BindingDB Entry DOI: 10.7270/Q2NP22QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM21045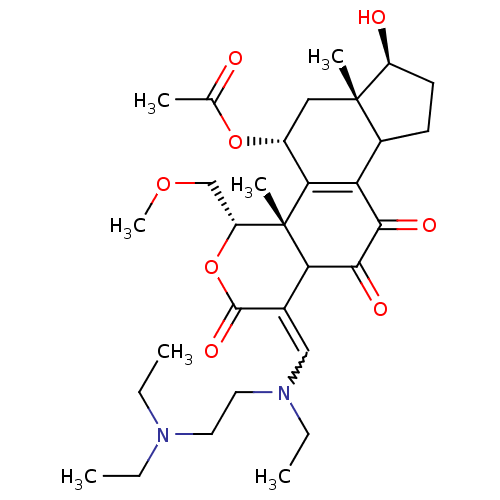 ((2R,3S,6E,14S,15S,17R)-6-({[2-(diethylamino)ethyl]...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Inhibition of PI3K enzyme activity is measured in a microtiter plate-based fluorescence polarization (FP) assay. | J Med Chem 51: 1319-23 (2008) Article DOI: 10.1021/jm7012858 BindingDB Entry DOI: 10.7270/Q2NP22QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM21048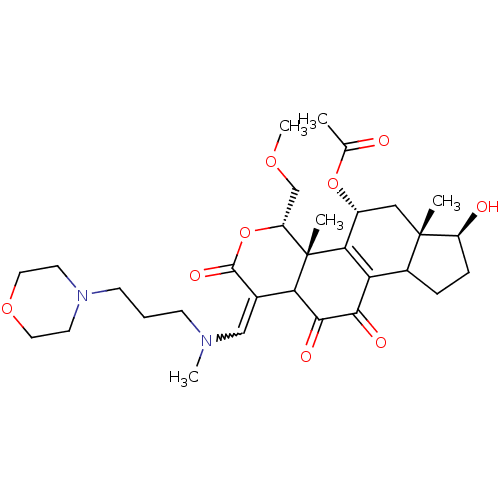 ((2R,3S,6E,14S,15S,17R)-8,14-dihydroxy-3-(methoxyme...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Inhibition of PI3K enzyme activity is measured in a microtiter plate-based fluorescence polarization (FP) assay. | J Med Chem 51: 1319-23 (2008) Article DOI: 10.1021/jm7012858 BindingDB Entry DOI: 10.7270/Q2NP22QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4548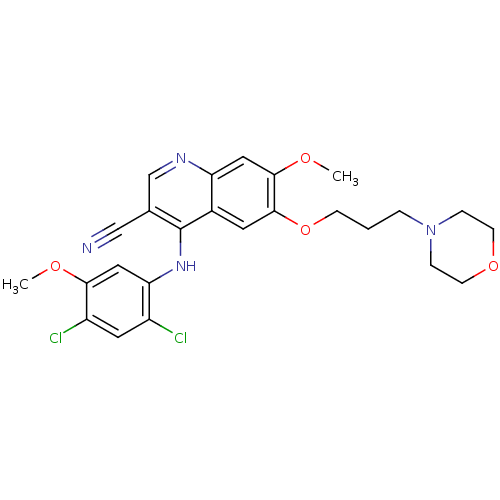 (4-Phenylamino-3-quinolinecarbonitrile deriv. 18 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4492 (4-[(2,4-Dichlorophenyl)amino]-6,7-dimethoxy-3-quin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Wyeth-Ayerst Research | Assay Description Src kinase activity was measured in an ELISA format. IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the tra... | J Med Chem 44: 3965-77 (2001) Article DOI: 10.1021/jm0102250 BindingDB Entry DOI: 10.7270/Q2TD9VHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM21065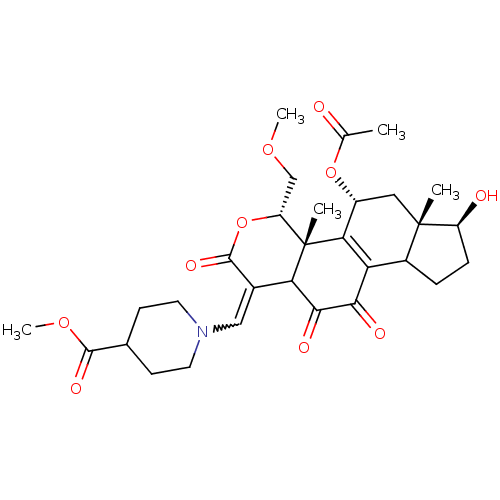 (17-Hydroxywortmannin, S25 | methyl 1-{[(2R,3S,6E,1...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Inhibition of PI3K enzyme activity is measured in a microtiter plate-based fluorescence polarization (FP) assay. | J Med Chem 51: 1319-23 (2008) Article DOI: 10.1021/jm7012858 BindingDB Entry DOI: 10.7270/Q2NP22QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM21066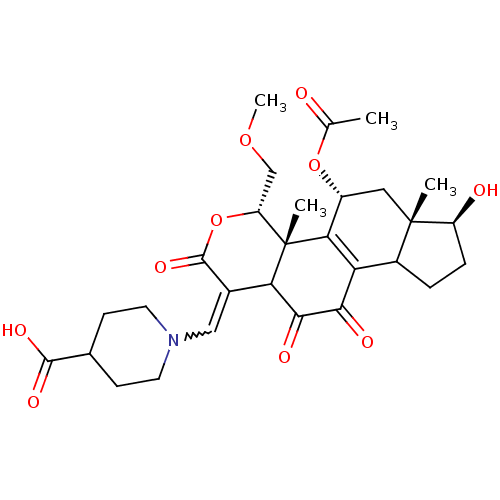 (1-{[(2R,3S,6E,14S,15S,17R)-17-(acetyloxy)-8,14-dih...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Inhibition of PI3K enzyme activity is measured in a microtiter plate-based fluorescence polarization (FP) assay. | J Med Chem 51: 1319-23 (2008) Article DOI: 10.1021/jm7012858 BindingDB Entry DOI: 10.7270/Q2NP22QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM21068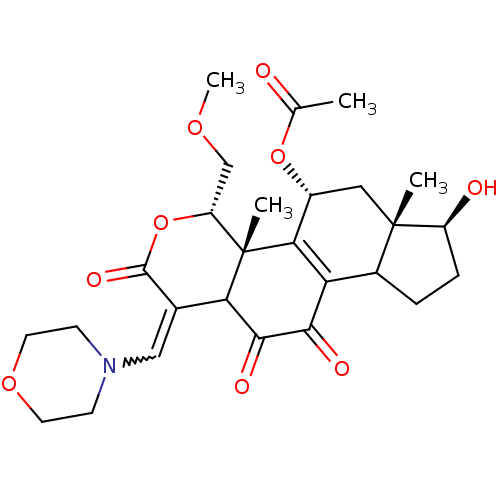 ((2R,3S,6E,14S,15S,17R)-8,14-dihydroxy-3-(methoxyme...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Inhibition of PI3K enzyme activity is measured in a microtiter plate-based fluorescence polarization (FP) assay. | J Med Chem 51: 1319-23 (2008) Article DOI: 10.1021/jm7012858 BindingDB Entry DOI: 10.7270/Q2NP22QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 151 total ) | Next | Last >> |