 Found 1411 hits with Last Name = 'zhao' and Initial = 'm'
Found 1411 hits with Last Name = 'zhao' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(MOUSE) | BDBM86416

(Dmt-d-Arg-Phe-A2pr-NH2)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C29H43N9O5/c1-16-11-19(39)12-17(2)20(16)14-21(31)26(41)36-22(9-6-10-35-29(33)34)27(42)37-23(13-18-7-4-3-5-8-18)28(43)38-24(15-30)25(32)40/h3-5,7-8,11-12,21-24,39H,6,9-10,13-15,30-31H2,1-2H3,(H2,32,40)(H,36,41)(H,37,42)(H,38,43)(H4,33,34,35)/t21-,22+,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0636 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50029085
(CHEBI:83405 | CHEMBL525191 | GDC-0879)Show SMILES OCCn1cc(c(n1)-c1ccncc1)-c1ccc2\C(CCc2c1)=N\O Show InChI InChI=1S/C19H18N4O2/c24-10-9-23-12-17(19(21-23)13-5-7-20-8-6-13)15-1-3-16-14(11-15)2-4-18(16)22-25/h1,3,5-8,11-12,24-25H,2,4,9-10H2/b22-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
Bioorg Med Chem 22: 6201-8 (2014)
Article DOI: 10.1016/j.bmc.2014.08.029
BindingDB Entry DOI: 10.7270/Q2RB7658 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(MOUSE) | BDBM85731
([Dmt1]DALDA)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C32H49N9O5/c1-19-15-22(42)16-20(2)23(19)18-24(34)29(44)40-26(12-8-14-38-32(36)37)30(45)41-27(17-21-9-4-3-5-10-21)31(46)39-25(28(35)43)11-6-7-13-33/h3-5,9-10,15-16,24-27,42H,6-8,11-14,17-18,33-34H2,1-2H3,(H2,35,43)(H,39,46)(H,40,44)(H,41,45)(H4,36,37,38)/t24-,25-,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM86416

(Dmt-d-Arg-Phe-A2pr-NH2)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C29H43N9O5/c1-16-11-19(39)12-17(2)20(16)14-21(31)26(41)36-22(9-6-10-35-29(33)34)27(42)37-23(13-18-7-4-3-5-8-18)28(43)38-24(15-30)25(32)40/h3-5,7-8,11-12,21-24,39H,6,9-10,13-15,30-31H2,1-2H3,(H2,32,40)(H,36,41)(H,37,42)(H,38,43)(H4,33,34,35)/t21-,22+,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM85736
(Dmt-d-Arg-Phe-Orn-NH2 | H-Dmt-D-Arg-Phe-Orn-NH2)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C31H47N9O5/c1-18-14-21(41)15-19(2)22(18)17-23(33)28(43)39-25(11-7-13-37-31(35)36)29(44)40-26(16-20-8-4-3-5-9-20)30(45)38-24(27(34)42)10-6-12-32/h3-5,8-9,14-15,23-26,41H,6-7,10-13,16-17,32-33H2,1-2H3,(H2,34,42)(H,38,45)(H,39,43)(H,40,44)(H4,35,36,37)/t23-,24-,25+,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM85736

(Dmt-d-Arg-Phe-Orn-NH2 | H-Dmt-D-Arg-Phe-Orn-NH2)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C31H47N9O5/c1-18-14-21(41)15-19(2)22(18)17-23(33)28(43)39-25(11-7-13-37-31(35)36)29(44)40-26(16-20-8-4-3-5-9-20)30(45)38-24(27(34)42)10-6-12-32/h3-5,8-9,14-15,23-26,41H,6-7,10-13,16-17,32-33H2,1-2H3,(H2,34,42)(H,38,45)(H,39,43)(H,40,44)(H4,35,36,37)/t23-,24-,25+,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM85731

([Dmt1]DALDA)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C32H49N9O5/c1-19-15-22(42)16-20(2)23(19)18-24(34)29(44)40-26(12-8-14-38-32(36)37)30(45)41-27(17-21-9-4-3-5-10-21)31(46)39-25(28(35)43)11-6-7-13-33/h3-5,9-10,15-16,24-27,42H,6-8,11-14,17-18,33-34H2,1-2H3,(H2,35,43)(H,39,46)(H,40,44)(H,41,45)(H4,36,37,38)/t24-,25-,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM85731

([Dmt1]DALDA)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C32H49N9O5/c1-19-15-22(42)16-20(2)23(19)18-24(34)29(44)40-26(12-8-14-38-32(36)37)30(45)41-27(17-21-9-4-3-5-10-21)31(46)39-25(28(35)43)11-6-7-13-33/h3-5,9-10,15-16,24-27,42H,6-8,11-14,17-18,33-34H2,1-2H3,(H2,35,43)(H,39,46)(H,40,44)(H,41,45)(H4,36,37,38)/t24-,25-,26+,27-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM86418
(Dmt-d-Ala-Phe-Phe-NH2)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C32H39N5O5/c1-19-14-24(38)15-20(2)25(19)18-26(33)31(41)35-21(3)30(40)37-28(17-23-12-8-5-9-13-23)32(42)36-27(29(34)39)16-22-10-6-4-7-11-22/h4-15,21,26-28,38H,16-18,33H2,1-3H3,(H2,34,39)(H,35,41)(H,36,42)(H,37,40)/t21-,26+,27+,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.173 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM86418

(Dmt-d-Ala-Phe-Phe-NH2)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C32H39N5O5/c1-19-14-24(38)15-20(2)25(19)18-26(33)31(41)35-21(3)30(40)37-28(17-23-12-8-5-9-13-23)32(42)36-27(29(34)39)16-22-10-6-4-7-11-22/h4-15,21,26-28,38H,16-18,33H2,1-3H3,(H2,34,39)(H,35,41)(H,36,42)(H,37,40)/t21-,26+,27+,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM86258
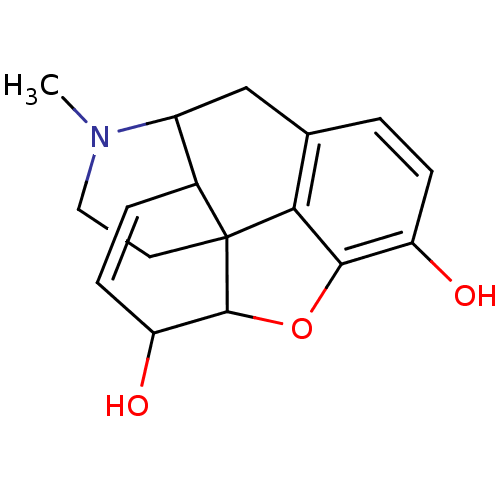
(CAS_23552-18-3 | Morphine | NSC_5980)Show SMILES CN1CCC23C4Oc5c2c(CC1C3C=CC4O)ccc5O |c:16,TLB:13:12:1.2.3:10.9.8| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86253
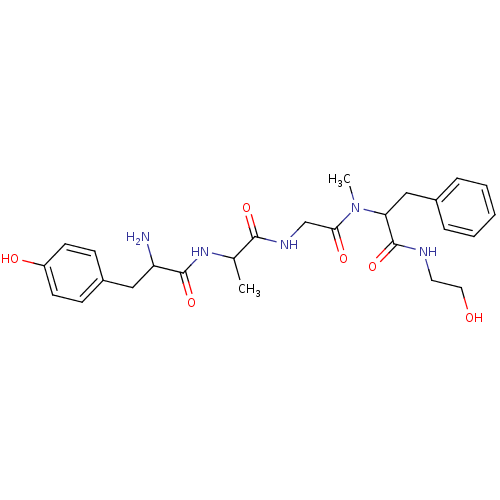
(CAS_100929-53-1 | DAMGO | NSC_104742 | US10836728,...)Show SMILES CC(NC(=O)C(N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)C(Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM86417
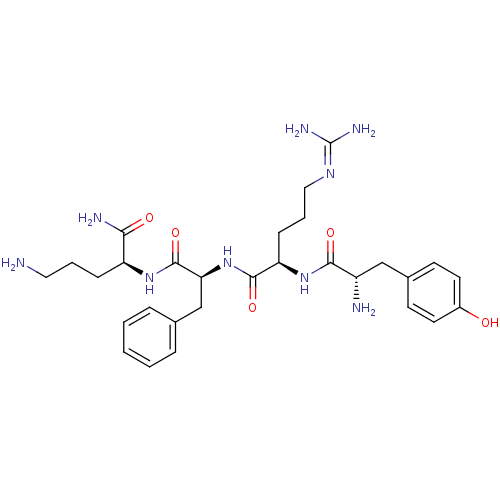
(5-amino-2-{1-[1-[1-amino-2-(4-hydroxyphenyl)ethylc...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](-[#7])=O Show InChI InChI=1S/C29H43N9O5/c30-14-4-8-22(25(32)40)36-28(43)24(17-18-6-2-1-3-7-18)38-27(42)23(9-5-15-35-29(33)34)37-26(41)21(31)16-19-10-12-20(39)13-11-19/h1-3,6-7,10-13,21-24,39H,4-5,8-9,14-17,30-31H2,(H2,32,40)(H,36,43)(H,37,41)(H,38,42)(H4,33,34,35)/t21-,22-,23+,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM86253

(CAS_100929-53-1 | DAMGO | NSC_104742 | US10836728,...)Show SMILES CC(NC(=O)C(N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)C(Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50060070
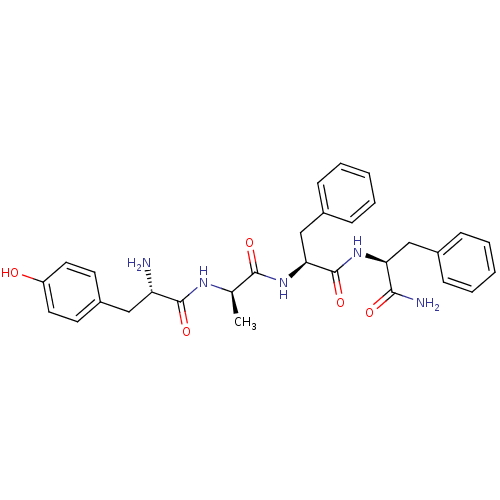
((S)-2-Amino-N-{(R)-1-[(S)-1-((S)-1-carbamoyl-2-phe...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C30H35N5O5/c1-19(33-29(39)24(31)16-22-12-14-23(36)15-13-22)28(38)35-26(18-21-10-6-3-7-11-21)30(40)34-25(27(32)37)17-20-8-4-2-5-9-20/h2-15,19,24-26,36H,16-18,31H2,1H3,(H2,32,37)(H,33,39)(H,34,40)(H,35,38)/t19-,24+,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM86419
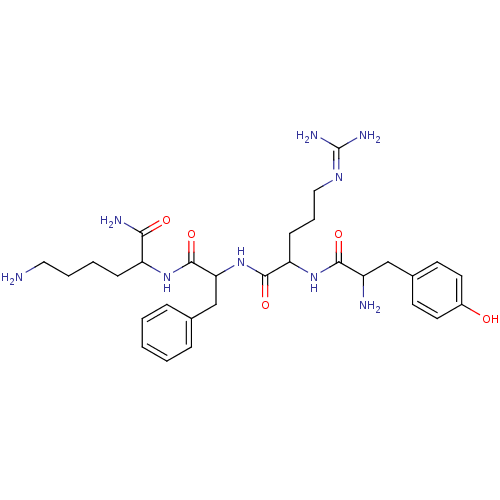
(CAS_122222 | DALDA | NSC_122222)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](-[#7])=O Show InChI InChI=1S/C30H45N9O5/c31-15-5-4-9-23(26(33)41)37-29(44)25(18-19-7-2-1-3-8-19)39-28(43)24(10-6-16-36-30(34)35)38-27(42)22(32)17-20-11-13-21(40)14-12-20/h1-3,7-8,11-14,22-25,40H,4-6,9-10,15-18,31-32H2,(H2,33,41)(H,37,44)(H,38,42)(H,39,43)(H4,34,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50266505

(CHEMBL515526 | N-(2-(3,5-dimethyl-1H-pyrazol-1-yl)...)Show InChI InChI=1S/C17H17N5O2/c1-10-8-11(2)22(21-10)17-19-14(9-16(20-17)18-12(3)23)13-6-4-5-7-15(13)24/h4-9,24H,1-3H3,(H,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
J Med Chem 52: 709-17 (2009)
Article DOI: 10.1021/jm800908d
BindingDB Entry DOI: 10.7270/Q2PG1RKR |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM26237
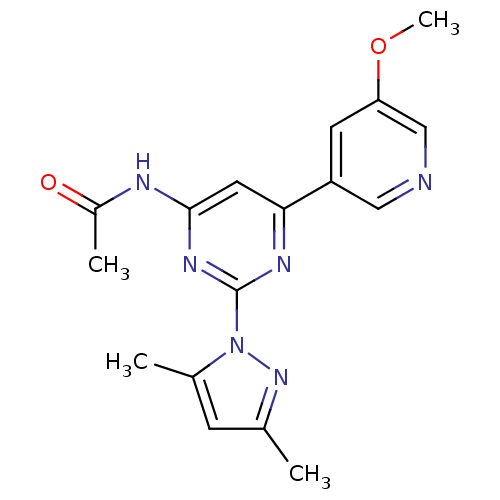
(CHEMBL458242 | N-[2-(3,5-dimethyl-1H-pyrazol-1-yl)...)Show SMILES COc1cncc(c1)-c1cc(NC(C)=O)nc(n1)-n1nc(C)cc1C Show InChI InChI=1S/C17H18N6O2/c1-10-5-11(2)23(22-10)17-20-15(7-16(21-17)19-12(3)24)13-6-14(25-4)9-18-8-13/h5-9H,1-4H3,(H,19,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
J Med Chem 52: 709-17 (2009)
Article DOI: 10.1021/jm800908d
BindingDB Entry DOI: 10.7270/Q2PG1RKR |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM86415

(Tyr-d-Arg-Phe-A2pr-NH2)Show SMILES [#7]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](-[#7])=O Show InChI InChI=1S/C27H39N9O5/c28-15-22(23(30)38)36-26(41)21(14-16-5-2-1-3-6-16)35-25(40)20(7-4-12-33-27(31)32)34-24(39)19(29)13-17-8-10-18(37)11-9-17/h1-3,5-6,8-11,19-22,37H,4,7,12-15,28-29H2,(H2,30,38)(H,34,39)(H,35,40)(H,36,41)(H4,31,32,33)/t19-,20+,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50266476
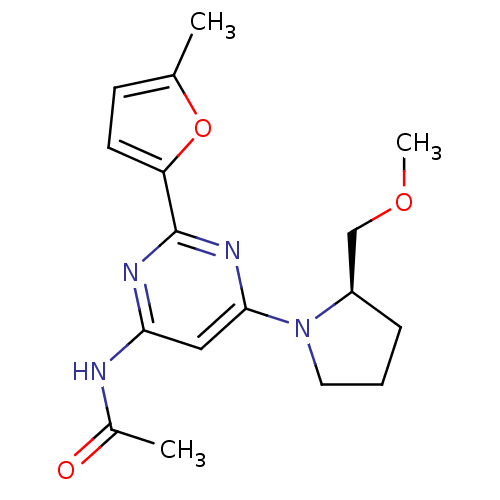
(CHEMBL508786 | N-[6-((R)-2-Methoxymethylpyrrolidin...)Show SMILES COC[C@H]1CCCN1c1cc(NC(C)=O)nc(n1)-c1ccc(C)o1 |r| Show InChI InChI=1S/C17H22N4O3/c1-11-6-7-14(24-11)17-19-15(18-12(2)22)9-16(20-17)21-8-4-5-13(21)10-23-3/h6-7,9,13H,4-5,8,10H2,1-3H3,(H,18,19,20,22)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
J Med Chem 52: 709-17 (2009)
Article DOI: 10.1021/jm800908d
BindingDB Entry DOI: 10.7270/Q2PG1RKR |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM86253

(CAS_100929-53-1 | DAMGO | NSC_104742 | US10836728,...)Show SMILES CC(NC(=O)C(N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)C(Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50060070

((S)-2-Amino-N-{(R)-1-[(S)-1-((S)-1-carbamoyl-2-phe...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C30H35N5O5/c1-19(33-29(39)24(31)16-22-12-14-23(36)15-13-22)28(38)35-26(18-21-10-6-3-7-11-21)30(40)34-25(27(32)37)17-20-8-4-2-5-9-20/h2-15,19,24-26,36H,16-18,31H2,1H3,(H2,32,37)(H,33,39)(H,34,40)(H,35,38)/t19-,24+,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50266384

(CHEMBL456287 | N-[6-(3,4-Dihydro-1H-isoquinolin-2-...)Show SMILES CC(=O)Nc1cc(nc(n1)-n1nc(C)cc1C)N1CCc2ccccc2C1 Show InChI InChI=1S/C20H22N6O/c1-13-10-14(2)26(24-13)20-22-18(21-15(3)27)11-19(23-20)25-9-8-16-6-4-5-7-17(16)12-25/h4-7,10-11H,8-9,12H2,1-3H3,(H,21,22,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
J Med Chem 52: 709-17 (2009)
Article DOI: 10.1021/jm800908d
BindingDB Entry DOI: 10.7270/Q2PG1RKR |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM85731

([Dmt1]DALDA)Show SMILES [#6]-c1cc(-[#8])cc(-[#6])c1-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C32H49N9O5/c1-19-15-22(42)16-20(2)23(19)18-24(34)29(44)40-26(12-8-14-38-32(36)37)30(45)41-27(17-21-9-4-3-5-10-21)31(46)39-25(28(35)43)11-6-7-13-33/h3-5,9-10,15-16,24-27,42H,6-8,11-14,17-18,33-34H2,1-2H3,(H2,35,43)(H,39,46)(H,40,44)(H,41,45)(H4,36,37,38)/t24-,25-,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50266416

(CHEMBL458224 | N-[2-(3,5-Dimethylpyrazol-1-yl)-6-(...)Show SMILES COC[C@H]1CCCN1c1cc(NC(C)=O)nc(n1)-n1nc(C)cc1C |r| Show InChI InChI=1S/C17H24N6O2/c1-11-8-12(2)23(21-11)17-19-15(18-13(3)24)9-16(20-17)22-7-5-6-14(22)10-25-4/h8-9,14H,5-7,10H2,1-4H3,(H,18,19,20,24)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
J Med Chem 52: 709-17 (2009)
Article DOI: 10.1021/jm800908d
BindingDB Entry DOI: 10.7270/Q2PG1RKR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50432672
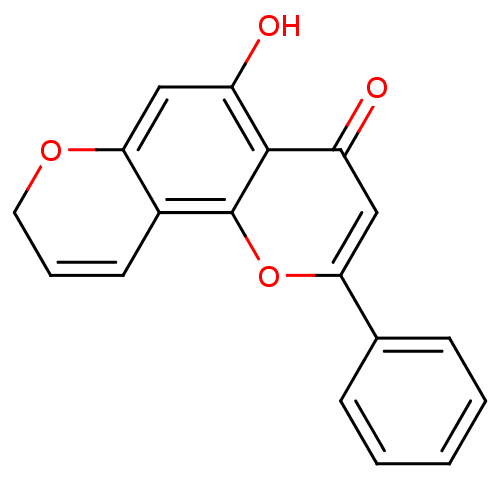
(CHEMBL2347912)Show SMILES Oc1cc2OCC=Cc2c2oc(cc(=O)c12)-c1ccccc1 |c:6| Show InChI InChI=1S/C18H12O4/c19-13-9-15(11-5-2-1-3-6-11)22-18-12-7-4-8-21-16(12)10-14(20)17(13)18/h1-7,9-10,20H,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana
Curated by ChEMBL
| Assay Description
Inhibition of human microsomal CYP1B1-dependent ethoxyresorufin-O-deethylase activity by spectrofluorimetric analysis in presence of NADPH regenerati... |
J Med Chem 58: 6481-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00494
BindingDB Entry DOI: 10.7270/Q2183895 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM86258

(CAS_23552-18-3 | Morphine | NSC_5980)Show SMILES CN1CCC23C4Oc5c2c(CC1C3C=CC4O)ccc5O |c:16,TLB:13:12:1.2.3:10.9.8| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50255246
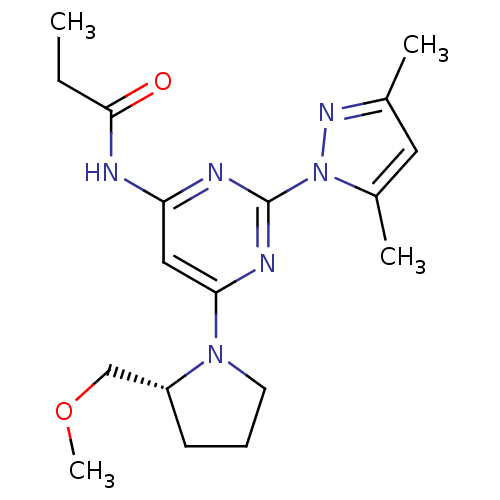
(CHEMBL469488 | N-[2-(3,5-Dimethylpyrazol-1-yl)-6-(...)Show SMILES CCC(=O)Nc1cc(nc(n1)-n1nc(C)cc1C)N1CCC[C@@H]1COC |r| Show InChI InChI=1S/C18H26N6O2/c1-5-17(25)19-15-10-16(23-8-6-7-14(23)11-26-4)21-18(20-15)24-13(3)9-12(2)22-24/h9-10,14H,5-8,11H2,1-4H3,(H,19,20,21,25)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
J Med Chem 52: 709-17 (2009)
Article DOI: 10.1021/jm800908d
BindingDB Entry DOI: 10.7270/Q2PG1RKR |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50266474

(CHEMBL455848 | N-[2-(3,5-Dimethylpyrazol-1-yl)-6-(...)Show SMILES COC[C@H]1C[C@@H](CN1c1cc(NC(C)=O)nc(n1)-n1nc(C)cc1C)OC |r| Show InChI InChI=1S/C18H26N6O3/c1-11-6-12(2)24(22-11)18-20-16(19-13(3)25)8-17(21-18)23-9-15(27-5)7-14(23)10-26-4/h6,8,14-15H,7,9-10H2,1-5H3,(H,19,20,21,25)/t14-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
J Med Chem 52: 709-17 (2009)
Article DOI: 10.1021/jm800908d
BindingDB Entry DOI: 10.7270/Q2PG1RKR |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50266415
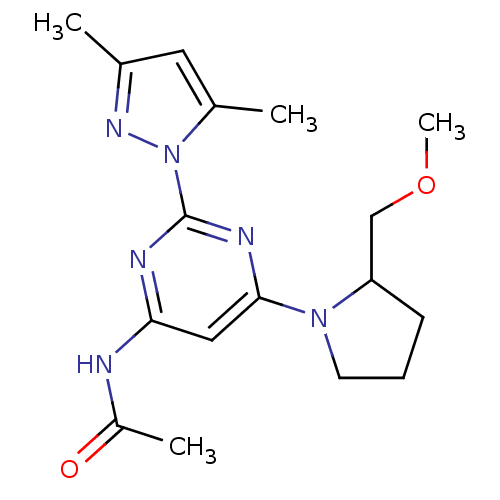
(CHEMBL516168 | N-[2-(3,5-Dimethylpyrazol-1-yl)-6-(...)Show InChI InChI=1S/C17H24N6O2/c1-11-8-12(2)23(21-11)17-19-15(18-13(3)24)9-16(20-17)22-7-5-6-14(22)10-25-4/h8-9,14H,5-7,10H2,1-4H3,(H,18,19,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
J Med Chem 52: 709-17 (2009)
Article DOI: 10.1021/jm800908d
BindingDB Entry DOI: 10.7270/Q2PG1RKR |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM86417

(5-amino-2-{1-[1-[1-amino-2-(4-hydroxyphenyl)ethylc...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](-[#7])=O Show InChI InChI=1S/C29H43N9O5/c30-14-4-8-22(25(32)40)36-28(43)24(17-18-6-2-1-3-7-18)38-27(42)23(9-5-15-35-29(33)34)37-26(41)21(31)16-19-10-12-20(39)13-11-19/h1-3,6-7,10-13,21-24,39H,4-5,8-9,14-17,30-31H2,(H2,32,40)(H,36,43)(H,37,41)(H,38,42)(H4,33,34,35)/t21-,22-,23+,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50266476

(CHEMBL508786 | N-[6-((R)-2-Methoxymethylpyrrolidin...)Show SMILES COC[C@H]1CCCN1c1cc(NC(C)=O)nc(n1)-c1ccc(C)o1 |r| Show InChI InChI=1S/C17H22N4O3/c1-11-6-7-14(24-11)17-19-15(18-12(2)22)9-16(20-17)21-8-4-5-13(21)10-23-3/h6-7,9,13H,4-5,8,10H2,1-3H3,(H,18,19,20,22)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in HEK293 cells |
J Med Chem 52: 709-17 (2009)
Article DOI: 10.1021/jm800908d
BindingDB Entry DOI: 10.7270/Q2PG1RKR |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50266381
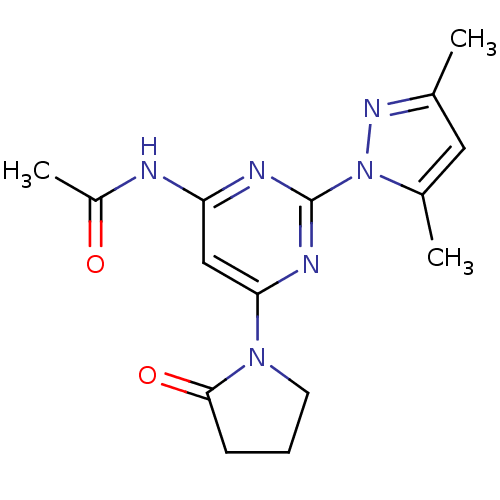
(CHEMBL515992 | N-[2-(3,5-Dimethylpyrazol-1-yl)-6-(...)Show InChI InChI=1S/C15H18N6O2/c1-9-7-10(2)21(19-9)15-17-12(16-11(3)22)8-13(18-15)20-6-4-5-14(20)23/h7-8H,4-6H2,1-3H3,(H,16,17,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
J Med Chem 52: 709-17 (2009)
Article DOI: 10.1021/jm800908d
BindingDB Entry DOI: 10.7270/Q2PG1RKR |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM86415

(Tyr-d-Arg-Phe-A2pr-NH2)Show SMILES [#7]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](-[#7])=O Show InChI InChI=1S/C27H39N9O5/c28-15-22(23(30)38)36-26(41)21(14-16-5-2-1-3-6-16)35-25(40)20(7-4-12-33-27(31)32)34-24(39)19(29)13-17-8-10-18(37)11-9-17/h1-3,5-6,8-11,19-22,37H,4,7,12-15,28-29H2,(H2,30,38)(H,34,39)(H,35,40)(H,36,41)(H4,31,32,33)/t19-,20+,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM86419

(CAS_122222 | DALDA | NSC_122222)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](-[#7])=O Show InChI InChI=1S/C30H45N9O5/c31-15-5-4-9-23(26(33)41)37-29(44)25(18-19-7-2-1-3-8-19)39-28(43)24(10-6-16-36-30(34)35)38-27(42)22(32)17-20-11-13-21(40)14-12-20/h1-3,7-8,11-14,22-25,40H,4-6,9-10,15-18,31-32H2,(H2,33,41)(H,37,44)(H,38,42)(H,39,43)(H4,34,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 307: 947-54 (2003)
Article DOI: 10.1124/jpet.103.054775
BindingDB Entry DOI: 10.7270/Q24748FX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50432672

(CHEMBL2347912)Show SMILES Oc1cc2OCC=Cc2c2oc(cc(=O)c12)-c1ccccc1 |c:6| Show InChI InChI=1S/C18H12O4/c19-13-9-15(11-5-2-1-3-6-11)22-18-12-7-4-8-21-16(12)10-14(20)17(13)18/h1-7,9-10,20H,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana
Curated by ChEMBL
| Assay Description
Inhibition of human microsomal CYP1A2-dependent methoxyresorufin-O-demethylase activity by spectrofluorimetric analysis in presence of NADPH regenera... |
J Med Chem 58: 6481-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00494
BindingDB Entry DOI: 10.7270/Q2183895 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50266504
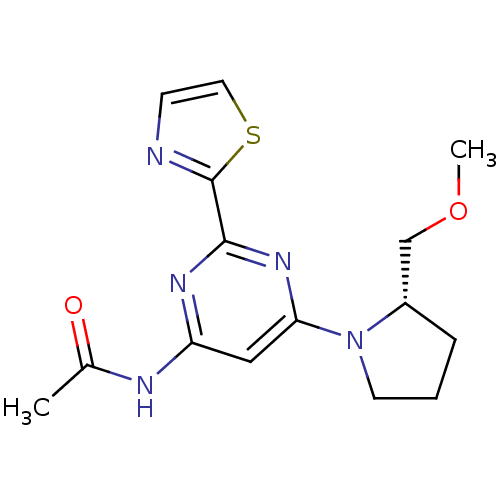
(CHEMBL456069 | N-[6-((S)-2-Methoxymethylpyrrolidin...)Show SMILES COC[C@@H]1CCCN1c1cc(NC(C)=O)nc(n1)-c1nccs1 |r| Show InChI InChI=1S/C15H19N5O2S/c1-10(21)17-12-8-13(20-6-3-4-11(20)9-22-2)19-14(18-12)15-16-5-7-23-15/h5,7-8,11H,3-4,6,9H2,1-2H3,(H,17,18,19,21)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
J Med Chem 52: 709-17 (2009)
Article DOI: 10.1021/jm800908d
BindingDB Entry DOI: 10.7270/Q2PG1RKR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana
Curated by ChEMBL
| Assay Description
Inhibition of human microsomal CYP1B1-dependent ethoxyresorufin-O-deethylase activity by spectrofluorimetric analysis in presence of NADPH regenerati... |
J Med Chem 58: 6481-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00494
BindingDB Entry DOI: 10.7270/Q2183895 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cruzipain
(Trypanosoma cruzi) | BDBM50229128
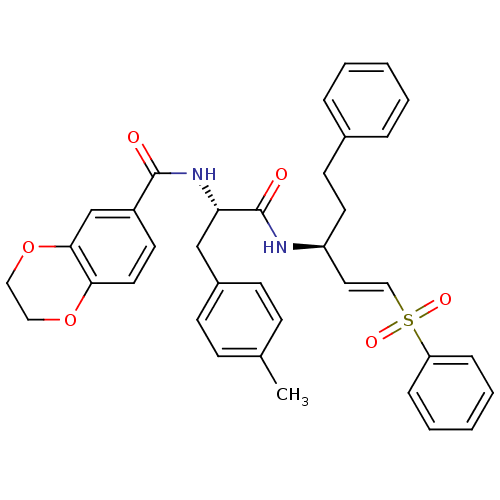
(CHEMBL401401 | N-((S)-1-oxo-1-((S)-5-phenyl-1-(phe...)Show SMILES Cc1ccc(C[C@H](NC(=O)c2ccc3OCCOc3c2)C(=O)N[C@@H](CCc2ccccc2)\C=C\S(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C36H36N2O6S/c1-26-12-14-28(15-13-26)24-32(38-35(39)29-17-19-33-34(25-29)44-22-21-43-33)36(40)37-30(18-16-27-8-4-2-5-9-27)20-23-45(41,42)31-10-6-3-7-11-31/h2-15,17,19-20,23,25,30,32H,16,18,21-22,24H2,1H3,(H,37,40)(H,38,39)/b23-20+/t30-,32-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
Bioorg Med Chem Lett 18: 624-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.070
BindingDB Entry DOI: 10.7270/Q2DN44SC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50266416

(CHEMBL458224 | N-[2-(3,5-Dimethylpyrazol-1-yl)-6-(...)Show SMILES COC[C@H]1CCCN1c1cc(NC(C)=O)nc(n1)-n1nc(C)cc1C |r| Show InChI InChI=1S/C17H24N6O2/c1-11-8-12(2)23(21-11)17-19-15(18-13(3)24)9-16(20-17)22-7-5-6-14(22)10-25-4/h8-9,14H,5-7,10H2,1-4H3,(H,18,19,20,24)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Inhibition of human adenosine A3 receptor |
J Med Chem 52: 709-17 (2009)
Article DOI: 10.1021/jm800908d
BindingDB Entry DOI: 10.7270/Q2PG1RKR |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50255188

(CHEMBL480444 | [2-(3,5-Dimethylpyrazol-1-yl)-6-((R...)Show SMILES COC[C@H]1CCCN1c1cc(NC(=O)OC)nc(n1)-n1nc(C)cc1C |r| Show InChI InChI=1S/C17H24N6O3/c1-11-8-12(2)23(21-11)16-18-14(19-17(24)26-4)9-15(20-16)22-7-5-6-13(22)10-25-3/h8-9,13H,5-7,10H2,1-4H3,(H,18,19,20,24)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
J Med Chem 52: 709-17 (2009)
Article DOI: 10.1021/jm800908d
BindingDB Entry DOI: 10.7270/Q2PG1RKR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana
Curated by ChEMBL
| Assay Description
Inhibition of human microsomal CYP1A2-dependent methoxyresorufin-O-demethylase activity by spectrofluorimetric analysis in presence of NADPH regenera... |
J Med Chem 58: 6481-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00494
BindingDB Entry DOI: 10.7270/Q2183895 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50255185
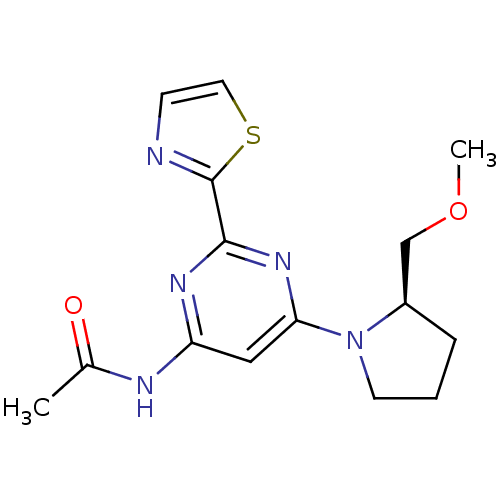
(CHEMBL480443 | N-[6-((R)-2-Methoxymethylpyrrolidin...)Show SMILES COC[C@H]1CCCN1c1cc(NC(C)=O)nc(n1)-c1nccs1 |r| Show InChI InChI=1S/C15H19N5O2S/c1-10(21)17-12-8-13(20-6-3-4-11(20)9-22-2)19-14(18-12)15-16-5-7-23-15/h5,7-8,11H,3-4,6,9H2,1-2H3,(H,17,18,19,21)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
J Med Chem 52: 709-17 (2009)
Article DOI: 10.1021/jm800908d
BindingDB Entry DOI: 10.7270/Q2PG1RKR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50553786

(CHEMBL4789106)Show SMILES CC1(CN)CCN(CC1)c1cnc(Sc2cccc(Cl)c2Cl)c(N)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114106
BindingDB Entry DOI: 10.7270/Q26977NN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50266475

(CHEMBL455849 | N-[2-(3,5-Dimethylpyrazol-1-yl)-6-(...)Show SMILES COC[C@H]1C[C@H](CN1c1cc(NC(C)=O)nc(n1)-n1nc(C)cc1C)OC |r| Show InChI InChI=1S/C18H26N6O3/c1-11-6-12(2)24(22-11)18-20-16(19-13(3)25)8-17(21-18)23-9-15(27-5)7-14(23)10-26-4/h6,8,14-15H,7,9-10H2,1-5H3,(H,19,20,21,25)/t14-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
J Med Chem 52: 709-17 (2009)
Article DOI: 10.1021/jm800908d
BindingDB Entry DOI: 10.7270/Q2PG1RKR |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50266441

(CHEMBL515344 | N-[2-(3,5-Dimethylpyrazol-1-yl)-6-(...)Show SMILES CC(=O)Nc1cc(nc(n1)-n1nc(C)cc1C)N1CCC[C@H]1CO |r| Show InChI InChI=1S/C16H22N6O2/c1-10-7-11(2)22(20-10)16-18-14(17-12(3)24)8-15(19-16)21-6-4-5-13(21)9-23/h7-8,13,23H,4-6,9H2,1-3H3,(H,17,18,19,24)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
J Med Chem 52: 709-17 (2009)
Article DOI: 10.1021/jm800908d
BindingDB Entry DOI: 10.7270/Q2PG1RKR |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50266412
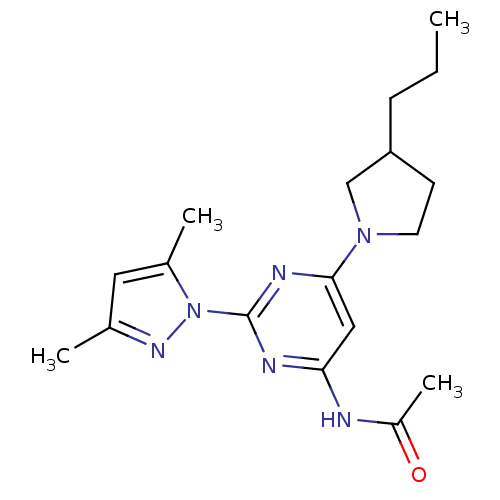
(CHEMBL456051 | N-[2-(3,5-Dimethylpyrazol-1-yl)-6-(...)Show SMILES CCCC1CCN(C1)c1cc(NC(C)=O)nc(n1)-n1nc(C)cc1C Show InChI InChI=1S/C18H26N6O/c1-5-6-15-7-8-23(11-15)17-10-16(19-14(4)25)20-18(21-17)24-13(3)9-12(2)22-24/h9-10,15H,5-8,11H2,1-4H3,(H,19,20,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
J Med Chem 52: 709-17 (2009)
Article DOI: 10.1021/jm800908d
BindingDB Entry DOI: 10.7270/Q2PG1RKR |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50266503
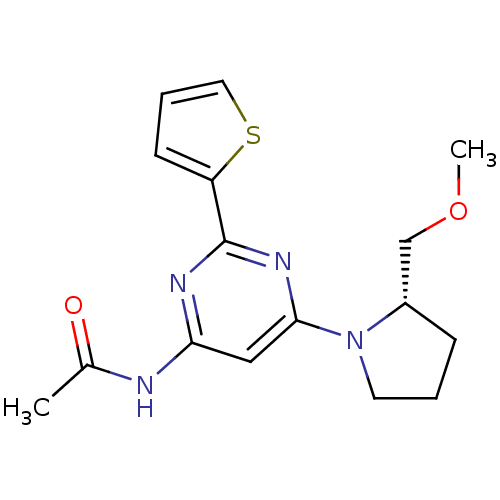
(CHEMBL456068 | N-[6-((S)-2-Methoxymethylpyrrolidin...)Show SMILES COC[C@@H]1CCCN1c1cc(NC(C)=O)nc(n1)-c1cccs1 |r| Show InChI InChI=1S/C16H20N4O2S/c1-11(21)17-14-9-15(20-7-3-5-12(20)10-22-2)19-16(18-14)13-6-4-8-23-13/h4,6,8-9,12H,3,5,7,10H2,1-2H3,(H,17,18,19,21)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
J Med Chem 52: 709-17 (2009)
Article DOI: 10.1021/jm800908d
BindingDB Entry DOI: 10.7270/Q2PG1RKR |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50266385
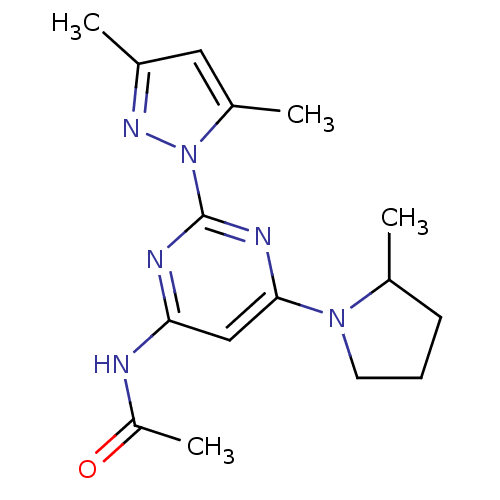
(CHEMBL456073 | N-[2-(3,5-Dimethylpyrazol-1-yl)-6-(...)Show InChI InChI=1S/C16H22N6O/c1-10-8-12(3)22(20-10)16-18-14(17-13(4)23)9-15(19-16)21-7-5-6-11(21)2/h8-9,11H,5-7H2,1-4H3,(H,17,18,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells |
J Med Chem 52: 709-17 (2009)
Article DOI: 10.1021/jm800908d
BindingDB Entry DOI: 10.7270/Q2PG1RKR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50113259
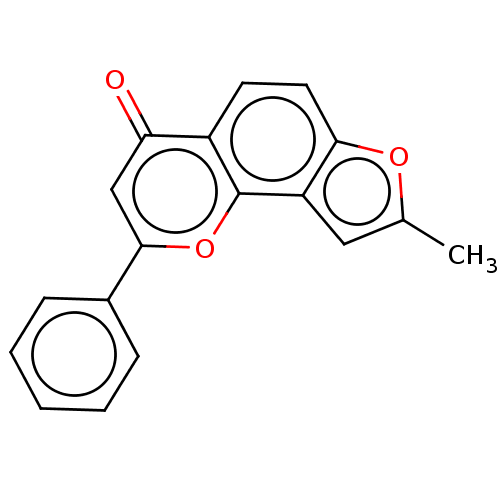
(CHEMBL3601433)Show InChI InChI=1S/C18H12O3/c1-11-9-14-16(20-11)8-7-13-15(19)10-17(21-18(13)14)12-5-3-2-4-6-12/h2-10H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana
Curated by ChEMBL
| Assay Description
Inhibition of human microsomal CYP1A2-dependent methoxyresorufin-O-demethylase activity by spectrofluorimetric analysis in presence of NADPH regenera... |
J Med Chem 58: 6481-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00494
BindingDB Entry DOI: 10.7270/Q2183895 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data