

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor type A (RAT) | BDBM50089269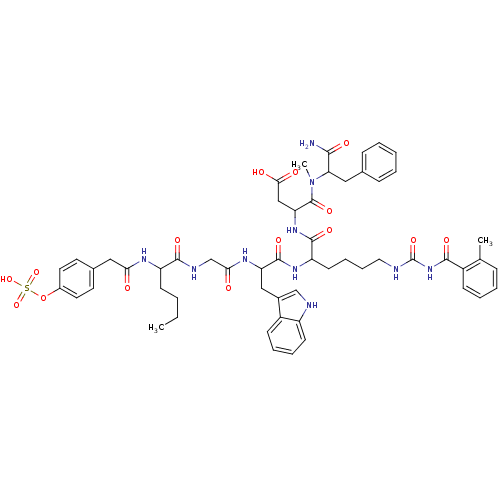 (CHEMBL276192 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061828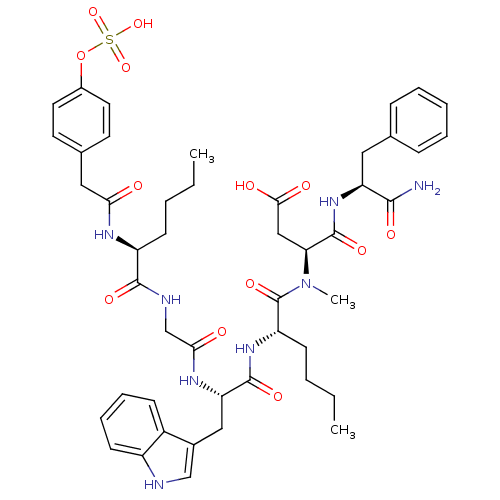 (CHEMBL76248 | CID44356929 | N-(1-Carbamoyl-2-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061828 (CHEMBL76248 | CID44356929 | N-(1-Carbamoyl-2-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061829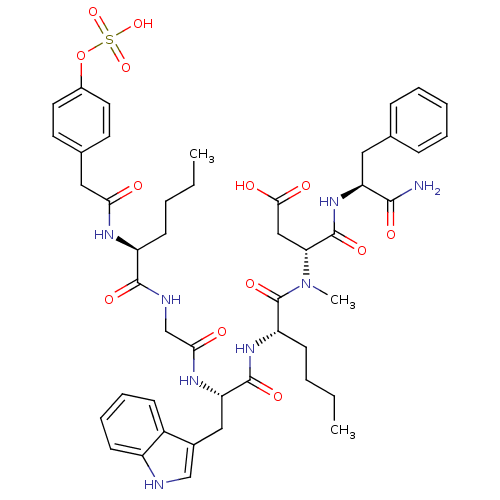 ((R)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-({(S)-2-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50089268 (CHEMBL267861 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061832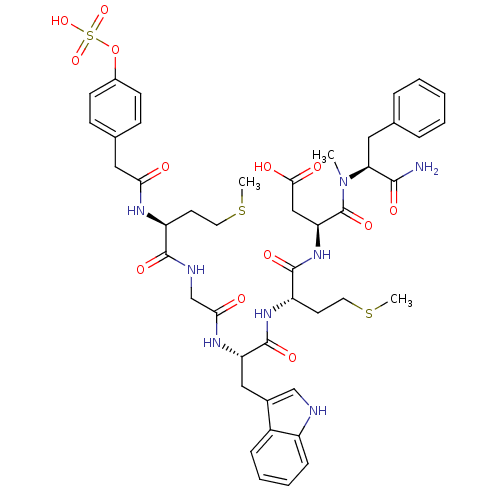 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{(S)-2-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061833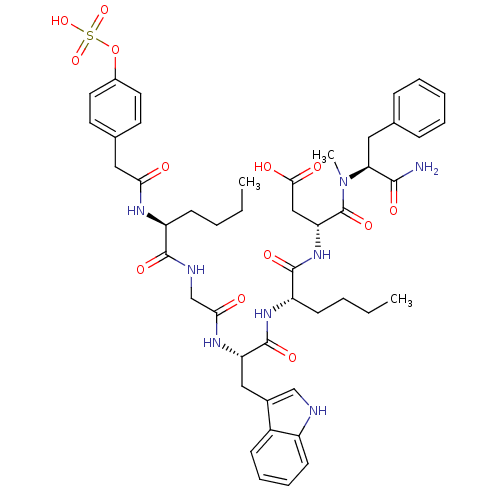 ((R)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{(S)-2-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50369326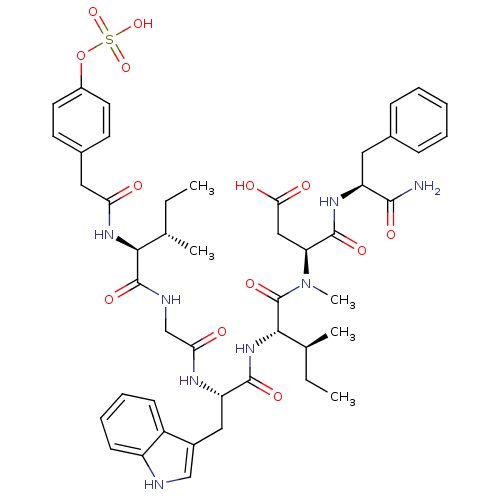 (CHEMBL1791002) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor in rat cortex synaptosomes using [125I]BH-CCK-8 as radioligand | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type B receptor using [125I]-BH-CCK-8 in rat cortex synaptosomes | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50306587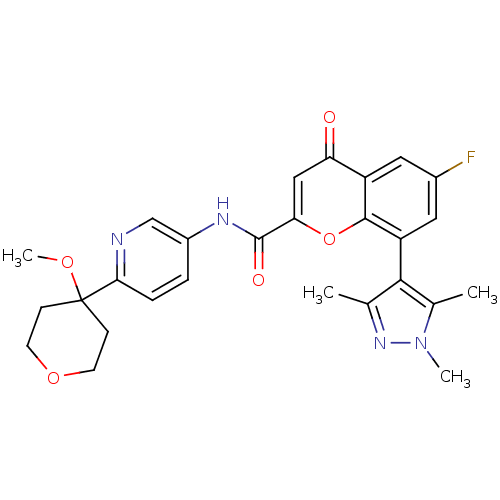 (6-Fluoro-N-(6-(4-methoxytetrahydro-2H-pyran-4-yl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to 5HT1B receptor expressed in CHO cells | J Med Chem 53: 1876-80 (2010) Article DOI: 10.1021/jm901200t BindingDB Entry DOI: 10.7270/Q25D8RZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50040237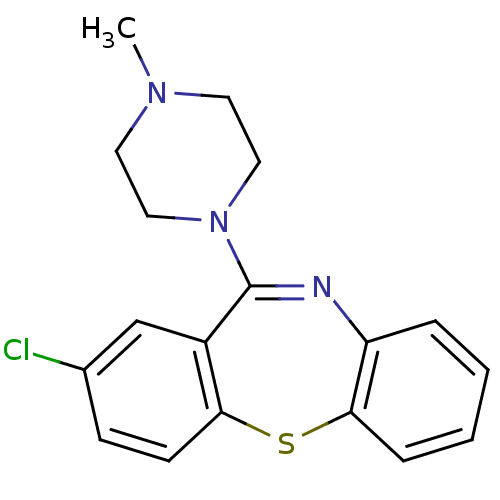 (2-Chloro-11-(4-methyl-piperazin-1-yl)-dibenzo[b,f]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca AB US Patent | Assay Description The ability of test compounds to displace 3H-raclopride at the D2s receptor can be determined on membranes from D2s-transfected CHO cells (Bmax 13 pm... | US Patent US8653257 (2014) BindingDB Entry DOI: 10.7270/Q2DR2T69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50369325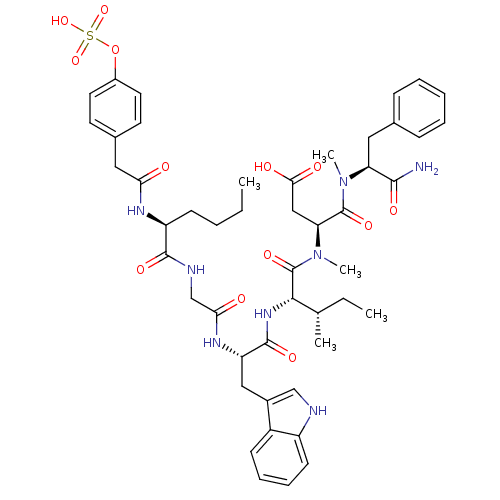 (CHEMBL1791005) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM82517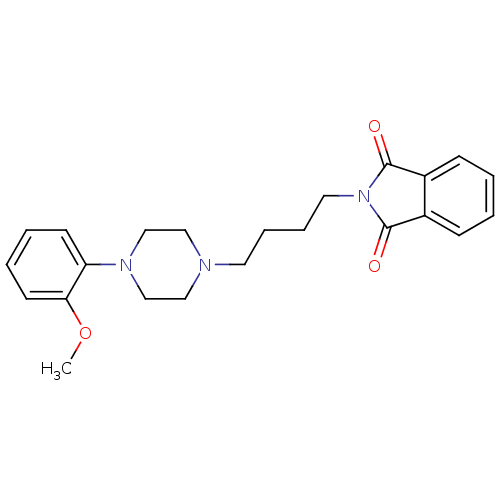 (2-{4-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-butyl}-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity of compound towards 5-hydroxytryptamine 1A receptor in rat striatal membranes by [3H]-OH-DPAT displacement. | J Med Chem 32: 1921-6 (1989) BindingDB Entry DOI: 10.7270/Q2J67FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM141314 (US8653257, 2-Chloro-11-piperazin-1-yl-dibenzo[b,f]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca AB US Patent | Assay Description The ability of test compounds to displace 3H-raclopride at the D2s receptor can be determined on membranes from D2s-transfected CHO cells (Bmax 13 pm... | US Patent US8653257 (2014) BindingDB Entry DOI: 10.7270/Q2DR2T69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50306591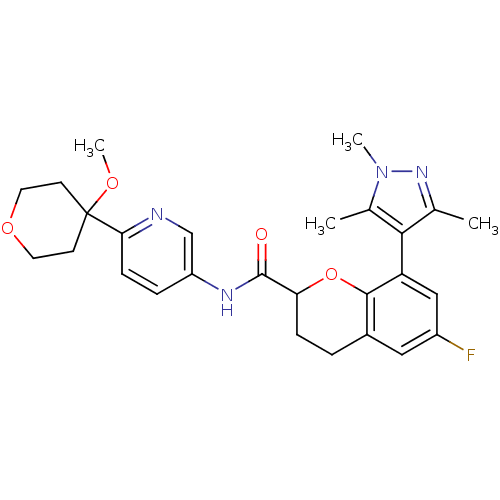 (6-Fluoro-N-(6-(4-methoxytetrahydro-2H-pyran-4-yl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to 5HT1B receptor expressed in CHO cells | J Med Chem 53: 1876-80 (2010) Article DOI: 10.1021/jm901200t BindingDB Entry DOI: 10.7270/Q25D8RZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50089276 (CHEMBL430608 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061834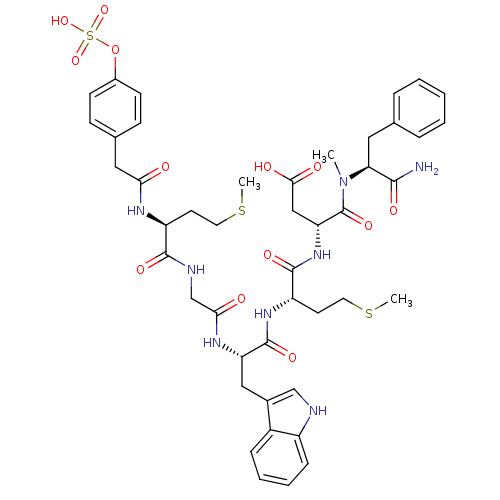 ((R)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{(S)-2-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM141315 (US8653257, 2-Fluoro-11-(4-methyl-piperazin-1-yl)-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca AB US Patent | Assay Description The ability of test compounds to displace 3H-raclopride at the D2s receptor can be determined on membranes from D2s-transfected CHO cells (Bmax 13 pm... | US Patent US8653257 (2014) BindingDB Entry DOI: 10.7270/Q2DR2T69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50061832 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{(S)-2-[(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type B receptor using [125I]-BH-CCK-8 in rat cortex synaptosomes | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50306588 (6-fluoro-8-(4-methylpiperazin-1-yl)-4-oxo-N-(4-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to 5HT1B receptor expressed in CHO cells | J Med Chem 53: 1876-80 (2010) Article DOI: 10.1021/jm901200t BindingDB Entry DOI: 10.7270/Q25D8RZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1/Beta-2/Beta-3 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM25761 (Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibitory activity against beta adrenergic receptor of rat frontal cortex homogenate using (1.0 nM) [3H]- dihydroalprenolol | J Med Chem 32: 859-63 (1989) BindingDB Entry DOI: 10.7270/Q2571CMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM82087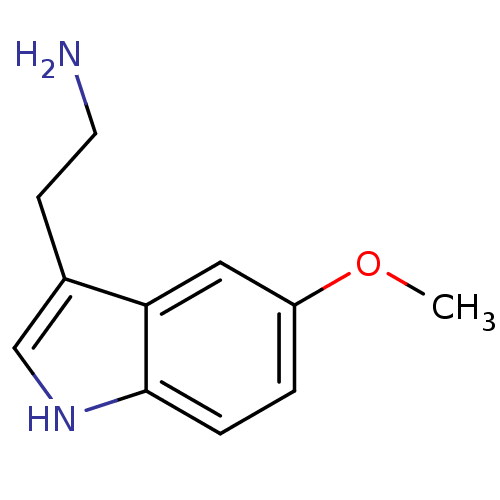 (2-(5-methoxy-1H-indol-3-yl)ethanamine | 5-MT | 5-M...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity of compound towards 5-hydroxytryptamine 1A receptor in rat striatal membranes by [3H]-OH-DPAT displacement. | J Med Chem 32: 1921-6 (1989) BindingDB Entry DOI: 10.7270/Q2J67FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50018459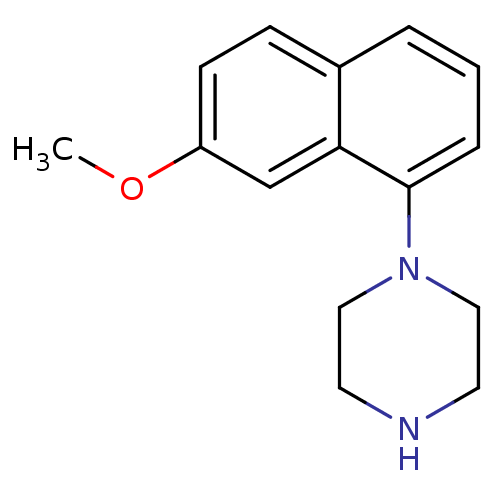 (1-(7-Methoxy-naphthalen-1-yl)-piperazine | 4-(7-Me...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity of compound towards 5-hydroxytryptamine 1A receptor in rat striatal membranes by [3H]-OH-DPAT displacement. | J Med Chem 32: 1921-6 (1989) BindingDB Entry DOI: 10.7270/Q2J67FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50089275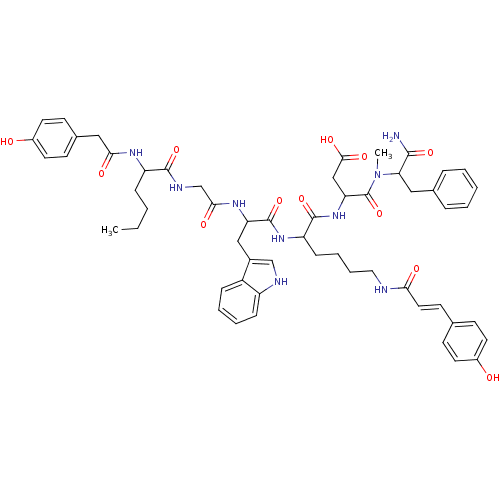 (CHEMBL384303 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50089277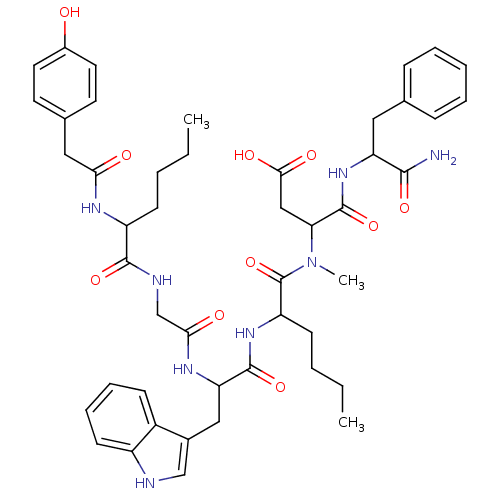 (CHEMBL311187 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-({...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50018456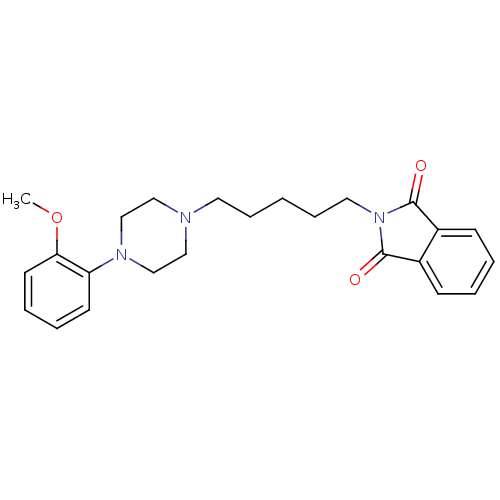 (2-{5-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-pentyl}...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity of compound towards 5-HT 1A receptor by measuring ability to displace [3H]-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat stria... | J Med Chem 32: 1921-6 (1989) BindingDB Entry DOI: 10.7270/Q2J67FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50018452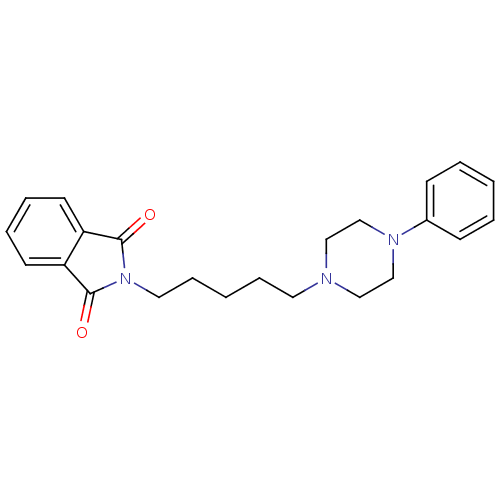 (2-[5-(4-Phenyl-piperazin-1-yl)-pentyl]-isoindole-1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity of compound towards 5-hydroxytryptamine 1A receptor in rat striatal membranes by [3H]-OH-DPAT displacement. | J Med Chem 32: 1921-6 (1989) BindingDB Entry DOI: 10.7270/Q2J67FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50306590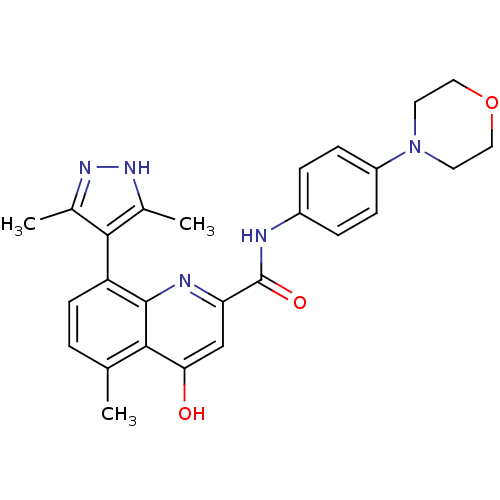 (5-Methyl-4-oxo-8-(1H-pyrazol-4-yl)-1,4-dihydroquin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to 5HT1B receptor expressed in CHO cells | J Med Chem 53: 1876-80 (2010) Article DOI: 10.1021/jm901200t BindingDB Entry DOI: 10.7270/Q25D8RZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM30707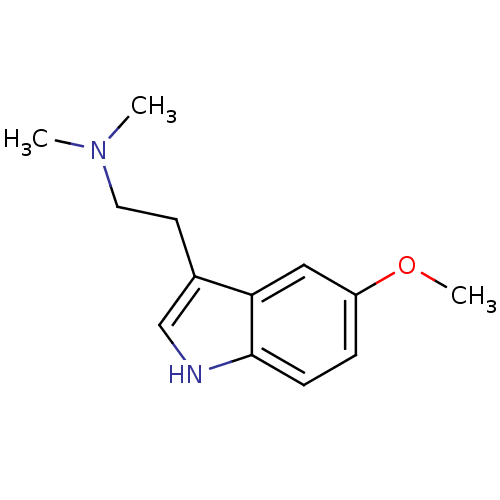 (2-(5-methoxy-1H-indol-3-yl)-N,N-dimethyl-ethanamin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity of compound towards 5-hydroxytryptamine 1A receptor in rat striatal membranes by [3H]-OH-DPAT displacement. | J Med Chem 32: 1921-6 (1989) BindingDB Entry DOI: 10.7270/Q2J67FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50018458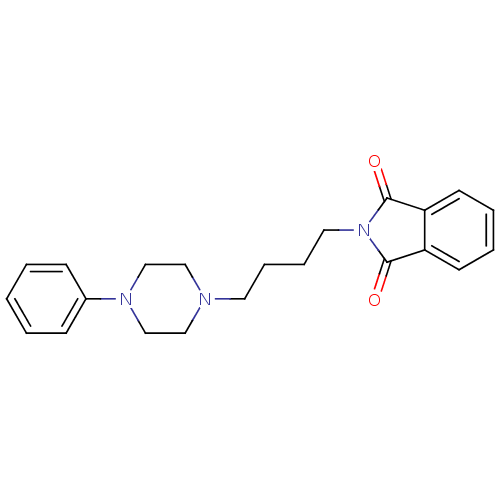 (2-[4-(4-Phenyl-piperazin-1-yl)-butyl]-isoindole-1,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity of compound towards 5-hydroxytryptamine 1A receptor in rat striatal membranes by [3H]-OH-DPAT displacement. | J Med Chem 32: 1921-6 (1989) BindingDB Entry DOI: 10.7270/Q2J67FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50018449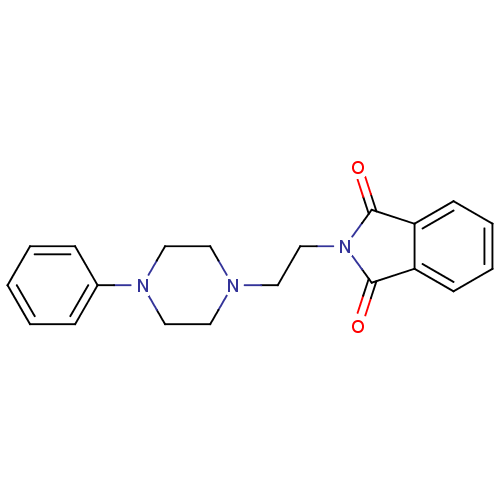 (2-[2-(4-Phenyl-piperazin-1-yl)-ethyl]-isoindole-1,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity of compound towards 5-hydroxytryptamine 1A receptor in rat striatal membranes by [3H]-OH-DPAT displacement. | J Med Chem 32: 1921-6 (1989) BindingDB Entry DOI: 10.7270/Q2J67FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM141312 (US8653257, 2-Fluoro-11-piperazin-1-yl-dibenzo[b,f]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca AB US Patent | Assay Description The ability of test compounds to displace 3H-raclopride at the D2s receptor can be determined on membranes from D2s-transfected CHO cells (Bmax 13 pm... | US Patent US8653257 (2014) BindingDB Entry DOI: 10.7270/Q2DR2T69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50306589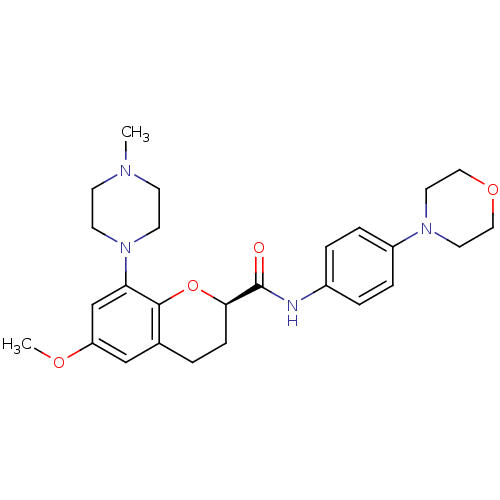 ((R)-6-methoxy-8-(4-methylpiperazin-1-yl)-N-(4-morp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to 5HT1B receptor expressed in CHO cells | J Med Chem 53: 1876-80 (2010) Article DOI: 10.1021/jm901200t BindingDB Entry DOI: 10.7270/Q25D8RZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50246936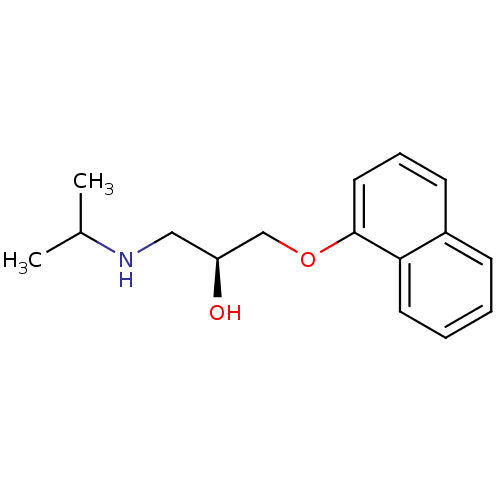 ((-)-(S)-Propranolol | 1-(ISOPROPYLAMINO)-3-(1-NAPH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1B receptor of rat striatal membrane homogenate using [3H]5-HT as the radioligand. | J Med Chem 32: 859-63 (1989) BindingDB Entry DOI: 10.7270/Q2571CMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50089272 (CHEMBL312359 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061830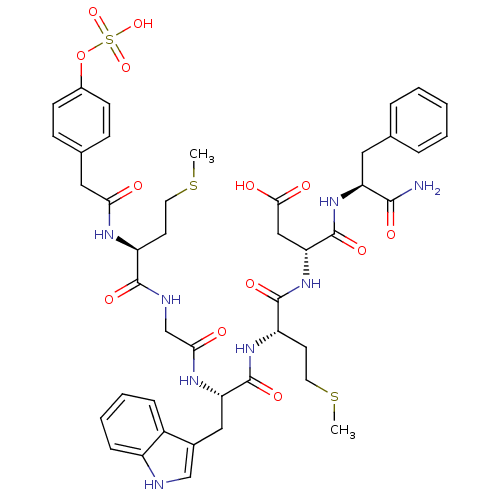 ((R)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{(S)-2-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50018457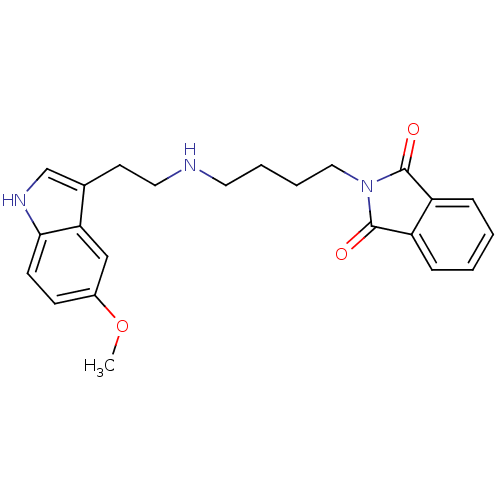 (2-{4-[2-(5-Methoxy-1H-indol-3-yl)-ethylamino]-buty...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity of compound towards 5-hydroxytryptamine 1A receptor in rat striatal membranes by [3H]-OH-DPAT displacement. | J Med Chem 32: 1921-6 (1989) BindingDB Entry DOI: 10.7270/Q2J67FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50018451 (2-{4-[3-(Naphthalen-1-yloxy)-propylamino]-butyl}-i...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity of compound towards 5-hydroxytryptamine 1A receptor in rat striatal membranes by [3H]-OH-DPAT displacement. | J Med Chem 32: 1921-6 (1989) BindingDB Entry DOI: 10.7270/Q2J67FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50061971 (CHEMBL138958 | Methyl-[2-(naphthalen-1-yloxy)-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Virginia/Virginia Commonwealth University Curated by ChEMBL | Assay Description Agonist activity to the human recombinant 5-hydroxytryptamine 1B receptor | J Med Chem 40: 4415-9 (1998) Article DOI: 10.1021/jm970507t BindingDB Entry DOI: 10.7270/Q2Z038TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50061971 (CHEMBL138958 | Methyl-[2-(naphthalen-1-yloxy)-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Virginia/Virginia Commonwealth University Curated by ChEMBL | Assay Description Compound was tested for its agonist activity against human 5-hydroxytryptamine 1D receptor alpha for comparison purpose. | J Med Chem 40: 4415-9 (1998) Article DOI: 10.1021/jm970507t BindingDB Entry DOI: 10.7270/Q2Z038TH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50018463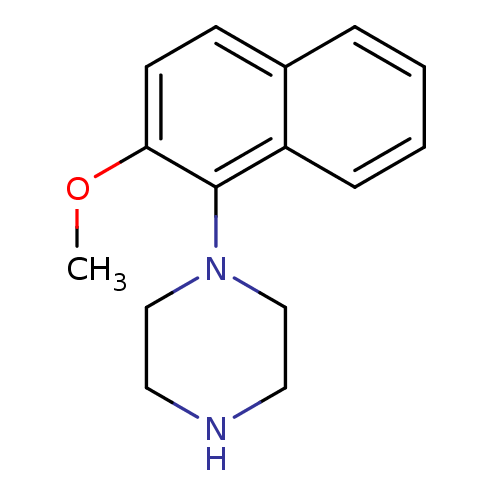 (1-(2-Methoxy-naphthalen-1-yl)-piperazine | CHEMBL2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity of compound towards 5-hydroxytryptamine 1A receptor in rat striatal membranes by [3H]-OH-DPAT displacement. | J Med Chem 32: 1921-6 (1989) BindingDB Entry DOI: 10.7270/Q2J67FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50089275 (CHEMBL384303 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor in rat cortex synaptosomes using [125I]BH-CCK-8 as radioligand | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM141313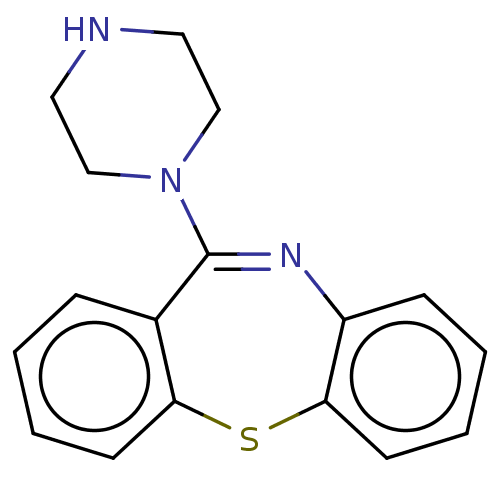 (US8653257, 11-Piperazin-1-yl-dibenzo[b,f][1,4]thia...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca AB US Patent | Assay Description The ability of test compounds to displace 3H-raclopride at the D2s receptor can be determined on membranes from D2s-transfected CHO cells (Bmax 13 pm... | US Patent US8653257 (2014) BindingDB Entry DOI: 10.7270/Q2DR2T69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50016913 (Butyl-ethyl-[2-(naphthalen-1-yloxy)-ethyl]-amine |...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1A receptor of rat hippocampal tissue using [3H]OH-DPAT as radioligand. | J Med Chem 32: 859-63 (1989) BindingDB Entry DOI: 10.7270/Q2571CMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50018468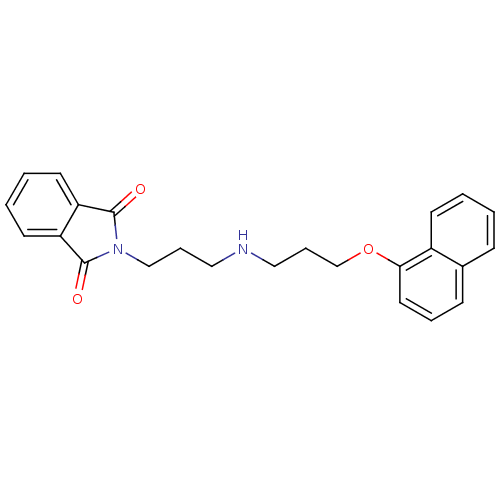 (2-{3-[3-(Naphthalen-1-yloxy)-propylamino]-propyl}-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity of compound towards 5-HT 1A receptor by measuring ability to displace [3H]-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat stria... | J Med Chem 32: 1921-6 (1989) BindingDB Entry DOI: 10.7270/Q2J67FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50016909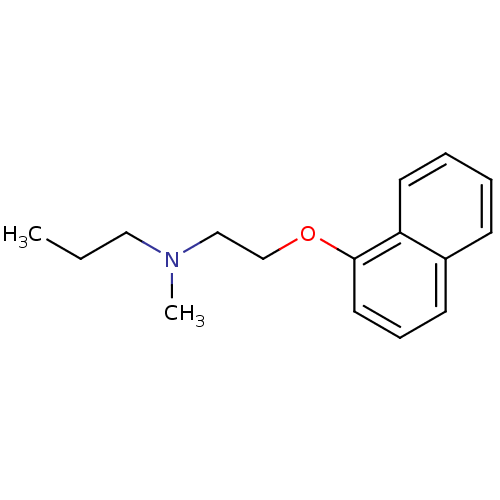 (CHEMBL140417 | Methyl-[2-(naphthalen-1-yloxy)-ethy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1A receptor of rat hippocampal tissue using [3H]OH-DPAT as radioligand. | J Med Chem 32: 859-63 (1989) BindingDB Entry DOI: 10.7270/Q2571CMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50018454 (2-[3-(8-Methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity of compound towards 5-hydroxytryptamine 1A receptor in rat striatal membranes by [3H]-OH-DPAT displacement. | J Med Chem 32: 1921-6 (1989) BindingDB Entry DOI: 10.7270/Q2J67FWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 157 total ) | Next | Last >> |