 Found 193 hits with Last Name = 'reigan' and Initial = 'p'
Found 193 hits with Last Name = 'reigan' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079
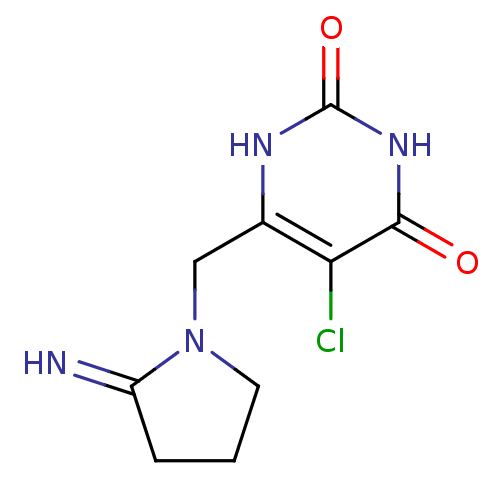
(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079

(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase by [3H]thymidine incorporation assay |
Eur J Med Chem 43: 1248-60 (2008)
Article DOI: 10.1016/j.ejmech.2007.07.015
BindingDB Entry DOI: 10.7270/Q2Z31ZDP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50105838

(FENOBAM)Show InChI InChI=1S/C11H11ClN4O2/c1-16-6-9(17)14-10(16)15-11(18)13-8-4-2-3-7(12)5-8/h2-5H,6H2,1H3,(H2,13,14,15,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by ChEMBL
| Assay Description
Displacement of MPEP from mGluR5 (unknown origin) after 3 hrs by liquid scintillation counting method |
Bioorg Med Chem 26: 4797-4803 (2018)
Article DOI: 10.1016/j.bmc.2018.08.012
BindingDB Entry DOI: 10.7270/Q2Q242TX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50105838

(FENOBAM)Show InChI InChI=1S/C11H11ClN4O2/c1-16-6-9(17)14-10(16)15-11(18)13-8-4-2-3-7(12)5-8/h2-5H,6H2,1H3,(H2,13,14,15,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by ChEMBL
| Assay Description
Displacement of MPEP from mGluR5 (unknown origin) after 3 hrs by liquid scintillation counting method |
Bioorg Med Chem 26: 4797-4803 (2018)
Article DOI: 10.1016/j.bmc.2018.08.012
BindingDB Entry DOI: 10.7270/Q2Q242TX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidine phosphorylase
(Escherichia coli) | BDBM50122770
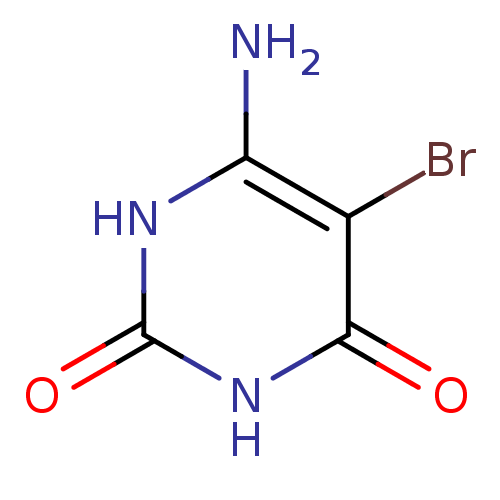
(6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...)Show InChI InChI=1S/C4H4BrN3O2/c5-1-2(6)7-4(10)8-3(1)9/h(H4,6,7,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50450061
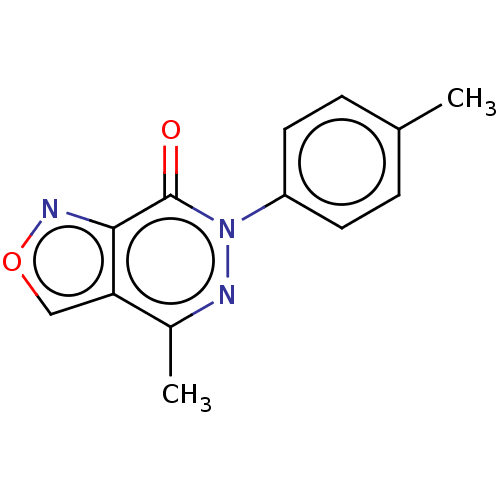
(CHEMBL4166199)Show InChI InChI=1S/C13H11N3O2/c1-8-3-5-10(6-4-8)16-13(17)12-11(7-18-15-12)9(2)14-16/h3-7H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by ChEMBL
| Assay Description
Displacement of MPEP from mGluR5 (unknown origin) after 3 hrs by liquid scintillation counting method |
Bioorg Med Chem 26: 4797-4803 (2018)
Article DOI: 10.1016/j.bmc.2018.08.012
BindingDB Entry DOI: 10.7270/Q2Q242TX |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50450078
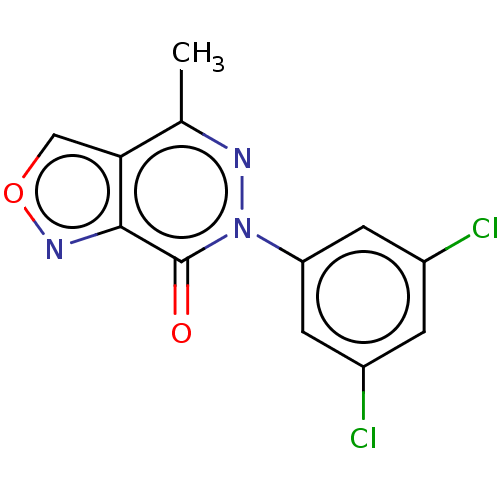
(CHEMBL4167825)Show InChI InChI=1S/C12H7Cl2N3O2/c1-6-10-5-19-16-11(10)12(18)17(15-6)9-3-7(13)2-8(14)4-9/h2-5H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by ChEMBL
| Assay Description
Displacement of MPEP from mGluR5 (unknown origin) after 3 hrs by liquid scintillation counting method |
Bioorg Med Chem 26: 4797-4803 (2018)
Article DOI: 10.1016/j.bmc.2018.08.012
BindingDB Entry DOI: 10.7270/Q2Q242TX |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50450074

(CHEMBL4171187)Show InChI InChI=1S/C13H11N3O3/c1-8-11-7-19-15-12(11)13(17)16(14-8)9-3-5-10(18-2)6-4-9/h3-7H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by ChEMBL
| Assay Description
Displacement of MPEP from mGluR5 (unknown origin) after 3 hrs by liquid scintillation counting method |
Bioorg Med Chem 26: 4797-4803 (2018)
Article DOI: 10.1016/j.bmc.2018.08.012
BindingDB Entry DOI: 10.7270/Q2Q242TX |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50450075
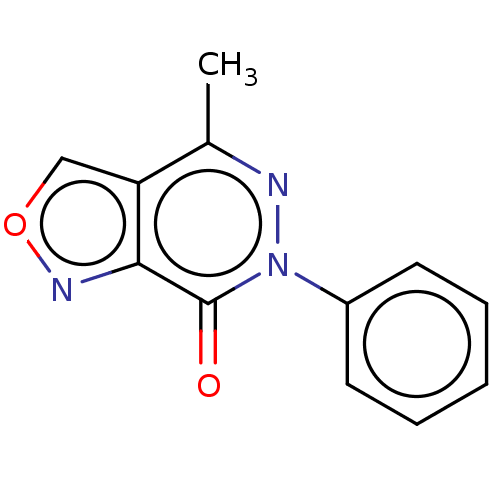
(CHEMBL4174628)Show InChI InChI=1S/C12H9N3O2/c1-8-10-7-17-14-11(10)12(16)15(13-8)9-5-3-2-4-6-9/h2-7H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by ChEMBL
| Assay Description
Displacement of MPEP from mGluR5 (unknown origin) after 3 hrs by liquid scintillation counting method |
Bioorg Med Chem 26: 4797-4803 (2018)
Article DOI: 10.1016/j.bmc.2018.08.012
BindingDB Entry DOI: 10.7270/Q2Q242TX |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50450060
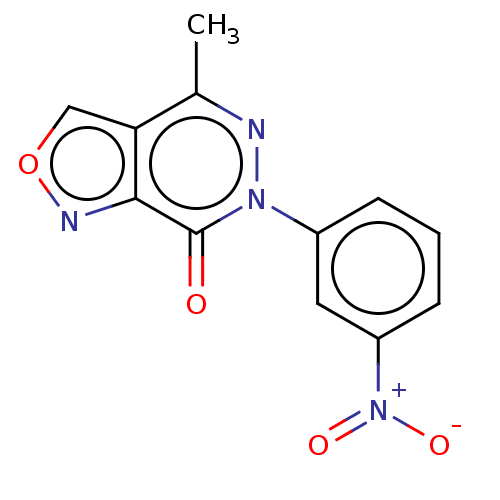
(CHEMBL4172276)Show SMILES Cc1nn(-c2cccc(c2)[N+]([O-])=O)c(=O)c2nocc12 Show InChI InChI=1S/C12H8N4O4/c1-7-10-6-20-14-11(10)12(17)15(13-7)8-3-2-4-9(5-8)16(18)19/h2-6H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by ChEMBL
| Assay Description
Displacement of MPEP from mGluR5 (unknown origin) after 3 hrs by liquid scintillation counting method |
Bioorg Med Chem 26: 4797-4803 (2018)
Article DOI: 10.1016/j.bmc.2018.08.012
BindingDB Entry DOI: 10.7270/Q2Q242TX |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50450059
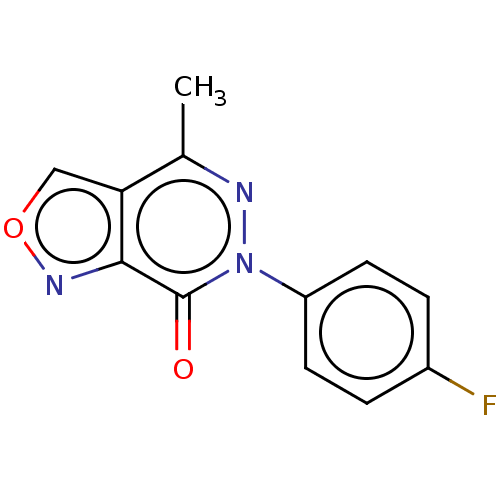
(CHEMBL4160584)Show InChI InChI=1S/C12H8FN3O2/c1-7-10-6-18-15-11(10)12(17)16(14-7)9-4-2-8(13)3-5-9/h2-6H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by ChEMBL
| Assay Description
Displacement of MPEP from mGluR5 (unknown origin) after 3 hrs by liquid scintillation counting method |
Bioorg Med Chem 26: 4797-4803 (2018)
Article DOI: 10.1016/j.bmc.2018.08.012
BindingDB Entry DOI: 10.7270/Q2Q242TX |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50450076
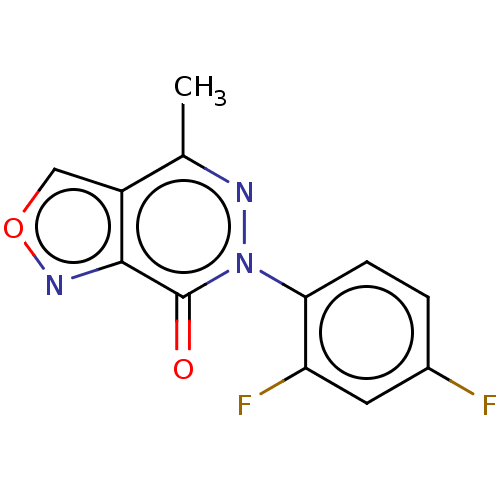
(CHEMBL4163974)Show InChI InChI=1S/C12H7F2N3O2/c1-6-8-5-19-16-11(8)12(18)17(15-6)10-3-2-7(13)4-9(10)14/h2-5H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by ChEMBL
| Assay Description
Displacement of MPEP from mGluR5 (unknown origin) after 3 hrs by liquid scintillation counting method |
Bioorg Med Chem 26: 4797-4803 (2018)
Article DOI: 10.1016/j.bmc.2018.08.012
BindingDB Entry DOI: 10.7270/Q2Q242TX |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50450077
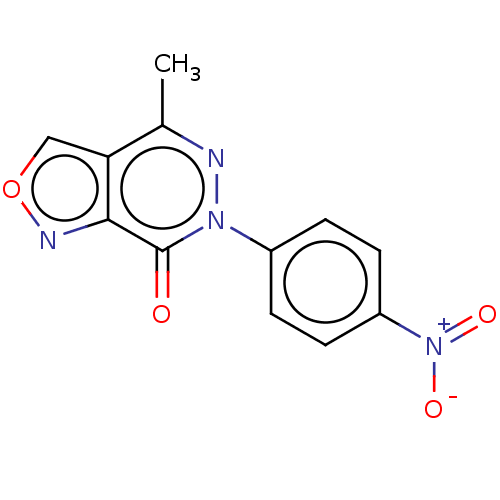
(CHEMBL4164391)Show SMILES Cc1nn(-c2ccc(cc2)[N+]([O-])=O)c(=O)c2nocc12 Show InChI InChI=1S/C12H8N4O4/c1-7-10-6-20-14-11(10)12(17)15(13-7)8-2-4-9(5-3-8)16(18)19/h2-6H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Montana
Curated by ChEMBL
| Assay Description
Displacement of MPEP from mGluR5 (unknown origin) after 3 hrs by liquid scintillation counting method |
Bioorg Med Chem 26: 4797-4803 (2018)
Article DOI: 10.1016/j.bmc.2018.08.012
BindingDB Entry DOI: 10.7270/Q2Q242TX |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50134399

(1,7-Dihydro-pyrrolo[2,3-d]pyrimidine-2,4-dione | 7...)Show InChI InChI=1S/C6H5N3O2/c10-5-3-1-2-7-4(3)8-6(11)9-5/h1-2H,(H3,7,8,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of bovine xanthine oxidase using hypoxanthine as a substrate |
Eur J Med Chem 43: 1248-60 (2008)
Article DOI: 10.1016/j.ejmech.2007.07.015
BindingDB Entry DOI: 10.7270/Q2Z31ZDP |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50134399

(1,7-Dihydro-pyrrolo[2,3-d]pyrimidine-2,4-dione | 7...)Show InChI InChI=1S/C6H5N3O2/c10-5-3-1-2-7-4(3)8-6(11)9-5/h1-2H,(H3,7,8,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of bovine xanthine oxidase using xanthine as a substrate |
Eur J Med Chem 43: 1248-60 (2008)
Article DOI: 10.1016/j.ejmech.2007.07.015
BindingDB Entry DOI: 10.7270/Q2Z31ZDP |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122764
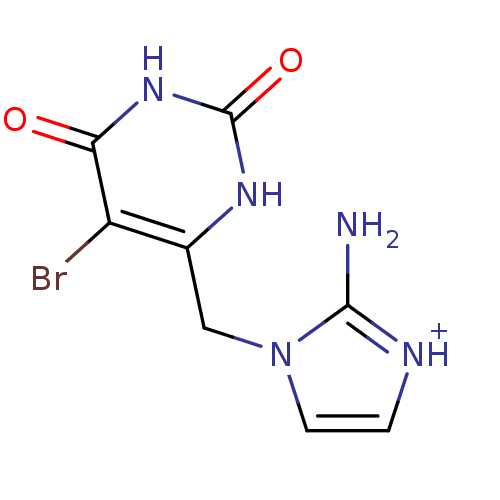
(2-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-py...)Show InChI InChI=1S/C8H8BrN5O2/c9-5-4(12-8(16)13-6(5)15)3-14-2-1-11-7(14)10/h1-2H,3H2,(H2,10,11)(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase |
J Med Chem 46: 207-9 (2003)
Article DOI: 10.1021/jm020964w
BindingDB Entry DOI: 10.7270/Q24X574R |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM25017
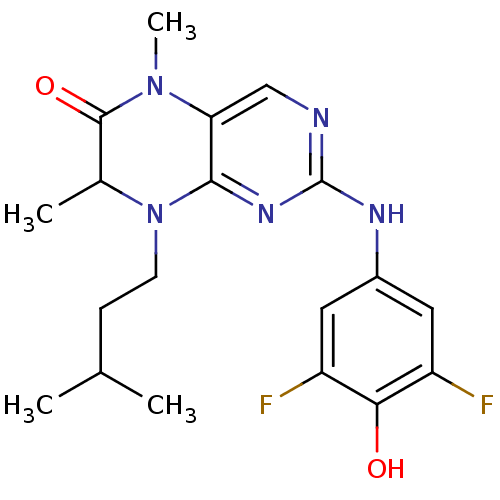
(2-[(3,5-difluoro-4-hydroxyphenyl)amino]-5,7-dimeth...)Show SMILES CC(C)CCN1C(C)C(=O)N(C)c2cnc(Nc3cc(F)c(O)c(F)c3)nc12 Show InChI InChI=1S/C19H23F2N5O2/c1-10(2)5-6-26-11(3)18(28)25(4)15-9-22-19(24-17(15)26)23-12-7-13(20)16(27)14(21)8-12/h7-11,27H,5-6H2,1-4H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Anschutz Medical Campus
Curated by ChEMBL
| Assay Description
Inhibition of RSK1 (unknown origin) |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115303
BindingDB Entry DOI: 10.7270/Q2XW4PCP |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-2
(Homo sapiens (Human)) | BDBM50111384
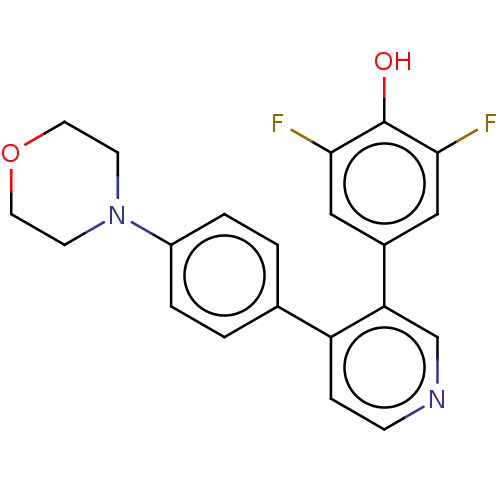
(CHEMBL3604794)Show SMILES Oc1c(F)cc(cc1F)-c1cnccc1-c1ccc(cc1)N1CCOCC1 Show InChI InChI=1S/C21H18F2N2O2/c22-19-11-15(12-20(23)21(19)26)18-13-24-6-5-17(18)14-1-3-16(4-2-14)25-7-9-27-10-8-25/h1-6,11-13,26H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Anschutz Medical Campus
Curated by ChEMBL
| Assay Description
Inhibition of RSK3 (unknown origin) |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115303
BindingDB Entry DOI: 10.7270/Q2XW4PCP |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM25017

(2-[(3,5-difluoro-4-hydroxyphenyl)amino]-5,7-dimeth...)Show SMILES CC(C)CCN1C(C)C(=O)N(C)c2cnc(Nc3cc(F)c(O)c(F)c3)nc12 Show InChI InChI=1S/C19H23F2N5O2/c1-10(2)5-6-26-11(3)18(28)25(4)15-9-22-19(24-17(15)26)23-12-7-13(20)16(27)14(21)8-12/h7-11,27H,5-6H2,1-4H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Anschutz Medical Campus
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 (unknown origin) |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115303
BindingDB Entry DOI: 10.7270/Q2XW4PCP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50111384

(CHEMBL3604794)Show SMILES Oc1c(F)cc(cc1F)-c1cnccc1-c1ccc(cc1)N1CCOCC1 Show InChI InChI=1S/C21H18F2N2O2/c22-19-11-15(12-20(23)21(19)26)18-13-24-6-5-17(18)14-1-3-16(4-2-14)25-7-9-27-10-8-25/h1-6,11-13,26H,7-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Anschutz Medical Campus
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 (unknown origin) |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115303
BindingDB Entry DOI: 10.7270/Q2XW4PCP |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM50111384

(CHEMBL3604794)Show SMILES Oc1c(F)cc(cc1F)-c1cnccc1-c1ccc(cc1)N1CCOCC1 Show InChI InChI=1S/C21H18F2N2O2/c22-19-11-15(12-20(23)21(19)26)18-13-24-6-5-17(18)14-1-3-16(4-2-14)25-7-9-27-10-8-25/h1-6,11-13,26H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Anschutz Medical Campus
Curated by ChEMBL
| Assay Description
Inhibition of RSK1 (unknown origin) |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115303
BindingDB Entry DOI: 10.7270/Q2XW4PCP |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-2
(Homo sapiens (Human)) | BDBM25017

(2-[(3,5-difluoro-4-hydroxyphenyl)amino]-5,7-dimeth...)Show SMILES CC(C)CCN1C(C)C(=O)N(C)c2cnc(Nc3cc(F)c(O)c(F)c3)nc12 Show InChI InChI=1S/C19H23F2N5O2/c1-10(2)5-6-26-11(3)18(28)25(4)15-9-22-19(24-17(15)26)23-12-7-13(20)16(27)14(21)8-12/h7-11,27H,5-6H2,1-4H3,(H,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Anschutz Medical Campus
Curated by ChEMBL
| Assay Description
Inhibition of RSK3 (unknown origin) |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115303
BindingDB Entry DOI: 10.7270/Q2XW4PCP |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-6
(Homo sapiens (Human)) | BDBM25017

(2-[(3,5-difluoro-4-hydroxyphenyl)amino]-5,7-dimeth...)Show SMILES CC(C)CCN1C(C)C(=O)N(C)c2cnc(Nc3cc(F)c(O)c(F)c3)nc12 Show InChI InChI=1S/C19H23F2N5O2/c1-10(2)5-6-26-11(3)18(28)25(4)15-9-22-19(24-17(15)26)23-12-7-13(20)16(27)14(21)8-12/h7-11,27H,5-6H2,1-4H3,(H,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Anschutz Medical Campus
Curated by ChEMBL
| Assay Description
Inhibition of RSK4 (unknown origin) using KEAKEKRQEQIAKRRRLSSLRASTSKSGGSQK a substrate by [32P] gammaATP assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115303
BindingDB Entry DOI: 10.7270/Q2XW4PCP |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM642525
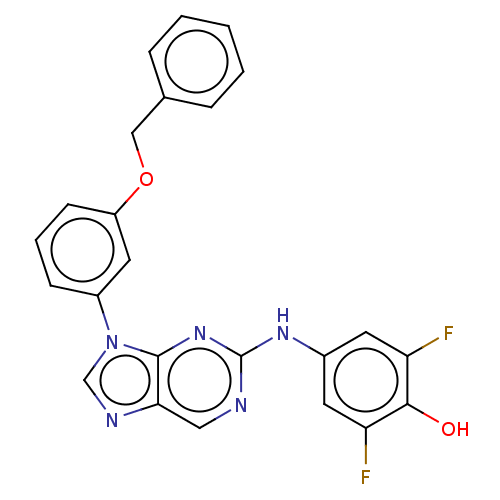
(US11851436, Compound A1-8)Show SMILES Oc1c(F)cc(Nc2ncc3ncn(-c4cccc(OCc5ccccc5)c4)c3n2)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 15.7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50568111
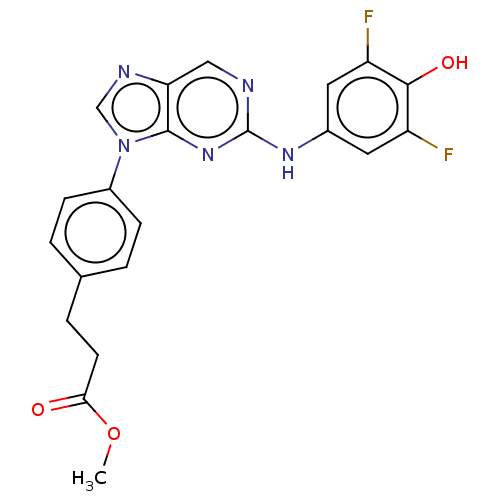
(CHEMBL4878141 | US11851436, Compound A1-12)Show SMILES COC(=O)CCc1ccc(cc1)-n1cnc2cnc(Nc3cc(F)c(O)c(F)c3)nc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of RSK2 (unknown origin) using Ulight-rpS6 as substrate incubated for 1 hr in presence of ATP by TR FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116220
BindingDB Entry DOI: 10.7270/Q2H70KKB |
More data for this
Ligand-Target Pair | |
5'-AMP-activated protein kinase catalytic subunit alpha-2
(Homo sapiens (Human)) | BDBM50564453

(CHEMBL4794096)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(cc23)C#N)c1C | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human His6-tagged AMPK alpha2 using ULight CRBtide as substrate incubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112316
BindingDB Entry DOI: 10.7270/Q2348Q5W |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM642526
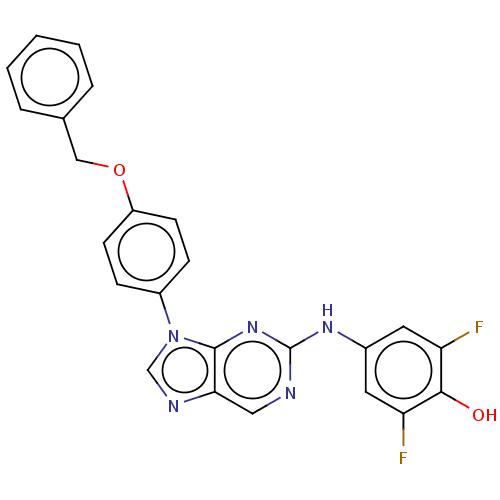
(US11851436, Compound A1-9)Show SMILES Oc1c(F)cc(Nc2ncc3ncn(-c4ccc(OCc5ccccc5)cc4)c3n2)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 17.8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-2
(Homo sapiens (Human)) | BDBM25017

(2-[(3,5-difluoro-4-hydroxyphenyl)amino]-5,7-dimeth...)Show SMILES CC(C)CCN1C(C)C(=O)N(C)c2cnc(Nc3cc(F)c(O)c(F)c3)nc12 Show InChI InChI=1S/C19H23F2N5O2/c1-10(2)5-6-26-11(3)18(28)25(4)15-9-22-19(24-17(15)26)23-12-7-13(20)16(27)14(21)8-12/h7-11,27H,5-6H2,1-4H3,(H,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Anschutz Medical Campus
Curated by ChEMBL
| Assay Description
Inhibition of RSK3 (unknown origin) using KEAKEKRQEQIAKRRRLSSLRASTSKSGGSQK a substrate by [32P] gammaATP assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115303
BindingDB Entry DOI: 10.7270/Q2XW4PCP |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50568112
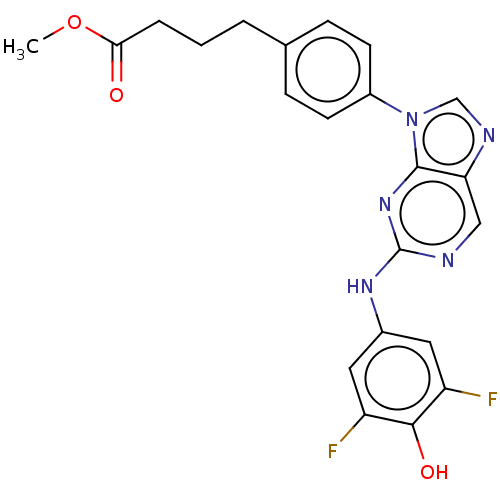
(CHEMBL4851452)Show SMILES COC(=O)CCCc1ccc(cc1)-n1cnc2cnc(Nc3cc(F)c(O)c(F)c3)nc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of RSK2 (unknown origin) using Ulight-rpS6 as substrate incubated for 1 hr in presence of ATP by TR FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116220
BindingDB Entry DOI: 10.7270/Q2H70KKB |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50568106
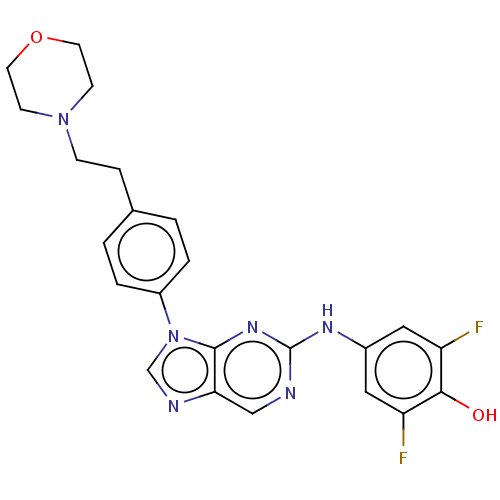
(CHEMBL4849660 | US11851436, Compound A1-4)Show SMILES Oc1c(F)cc(Nc2ncc3ncn(-c4ccc(CCN5CCOCC5)cc4)c3n2)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of RSK2 (unknown origin) using Ulight-rpS6 as substrate incubated for 1 hr in presence of ATP by TR FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116220
BindingDB Entry DOI: 10.7270/Q2H70KKB |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50568105
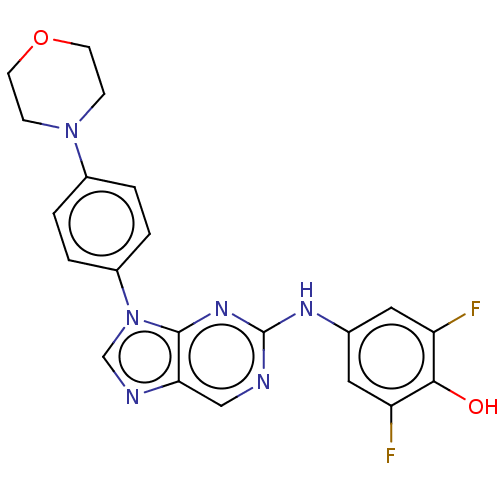
(CHEMBL4858455 | US11851436, Compound A1-3)Show SMILES Oc1c(F)cc(Nc2ncc3ncn(-c4ccc(cc4)N4CCOCC4)c3n2)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 18.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122764

(2-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-py...)Show InChI InChI=1S/C8H8BrN5O2/c9-5-4(12-8(16)13-6(5)15)3-14-2-1-11-7(14)10/h1-2H,3H2,(H2,10,11)(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM20079

(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against thymidine phosphorylase of Escherichia coli |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122764

(2-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-py...)Show InChI InChI=1S/C8H8BrN5O2/c9-5-4(12-8(16)13-6(5)15)3-14-2-1-11-7(14)10/h1-2H,3H2,(H2,10,11)(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase |
Eur J Med Chem 43: 1248-60 (2008)
Article DOI: 10.1016/j.ejmech.2007.07.015
BindingDB Entry DOI: 10.7270/Q2Z31ZDP |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM20079

(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of Thymidine phosphorylase from Escherichia coli |
Bioorg Med Chem Lett 14: 5247-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.036
BindingDB Entry DOI: 10.7270/Q2PR7VGP |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079

(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase |
J Med Chem 46: 207-9 (2003)
Article DOI: 10.1021/jm020964w
BindingDB Entry DOI: 10.7270/Q24X574R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50122771
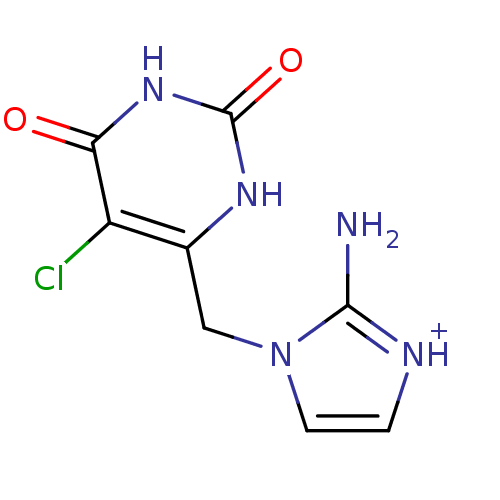
(2-Amino-1-(5-chloro-2,6-dioxo-1,2,3,6-tetrahydro-p...)Show InChI InChI=1S/C8H8ClN5O2/c9-5-4(12-8(16)13-6(5)15)3-14-2-1-11-7(14)10/h1-2H,3H2,(H2,10,11)(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against recombinant purified E. coli Thymidine Phosphorylase |
J Med Chem 46: 207-9 (2003)
Article DOI: 10.1021/jm020964w
BindingDB Entry DOI: 10.7270/Q24X574R |
More data for this
Ligand-Target Pair | |
5'-AMP-activated protein kinase catalytic subunit alpha-2
(Homo sapiens (Human)) | BDBM50564457

(CHEMBL4800500)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(CCO)cc23)c1C | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human His6-tagged AMPK alpha2 using ULight CRBtide as substrate incubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112316
BindingDB Entry DOI: 10.7270/Q2348Q5W |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM25017

(2-[(3,5-difluoro-4-hydroxyphenyl)amino]-5,7-dimeth...)Show SMILES CC(C)CCN1C(C)C(=O)N(C)c2cnc(Nc3cc(F)c(O)c(F)c3)nc12 Show InChI InChI=1S/C19H23F2N5O2/c1-10(2)5-6-26-11(3)18(28)25(4)15-9-22-19(24-17(15)26)23-12-7-13(20)16(27)14(21)8-12/h7-11,27H,5-6H2,1-4H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 22.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM25017

(2-[(3,5-difluoro-4-hydroxyphenyl)amino]-5,7-dimeth...)Show SMILES CC(C)CCN1C(C)C(=O)N(C)c2cnc(Nc3cc(F)c(O)c(F)c3)nc12 Show InChI InChI=1S/C19H23F2N5O2/c1-10(2)5-6-26-11(3)18(28)25(4)15-9-22-19(24-17(15)26)23-12-7-13(20)16(27)14(21)8-12/h7-11,27H,5-6H2,1-4H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of RSK2 (unknown origin) using Ulight-rpS6 as substrate incubated for 1 hr in presence of ATP by TR FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116220
BindingDB Entry DOI: 10.7270/Q2H70KKB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079

(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM25017

(2-[(3,5-difluoro-4-hydroxyphenyl)amino]-5,7-dimeth...)Show SMILES CC(C)CCN1C(C)C(=O)N(C)c2cnc(Nc3cc(F)c(O)c(F)c3)nc12 Show InChI InChI=1S/C19H23F2N5O2/c1-10(2)5-6-26-11(3)18(28)25(4)15-9-22-19(24-17(15)26)23-12-7-13(20)16(27)14(21)8-12/h7-11,27H,5-6H2,1-4H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Anschutz Medical Campus
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 (unknown origin) using KEAKEKRQEQIAKRRRLSSLRASTSKSGGSQK a substrate by [32P] gammaATP assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115303
BindingDB Entry DOI: 10.7270/Q2XW4PCP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50568104

(CHEMBL4858257 | US11851436, Compound A1-2) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 24.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50568105

(CHEMBL4858455 | US11851436, Compound A1-3)Show SMILES Oc1c(F)cc(Nc2ncc3ncn(-c4ccc(cc4)N4CCOCC4)c3n2)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of RSK2 (unknown origin) using Ulight-rpS6 as substrate incubated for 1 hr in presence of ATP by TR FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116220
BindingDB Entry DOI: 10.7270/Q2H70KKB |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50568110
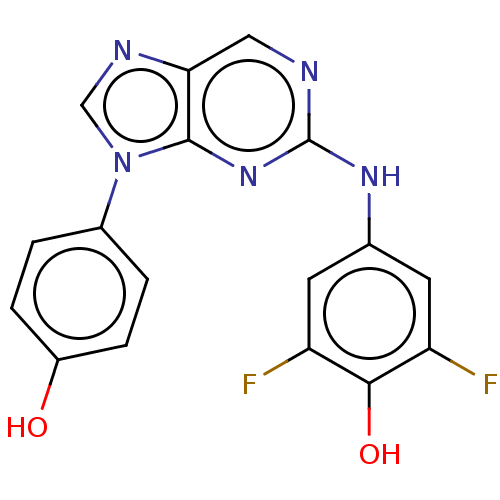
(CHEMBL4865383 | US11851436, Compound A1-11)Show SMILES Oc1ccc(cc1)-n1cnc2cnc(Nc3cc(F)c(O)c(F)c3)nc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 25.7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50568114
(CHEMBL4857399)Show SMILES Oc1c(F)cc(Nc2ncc3ccn(-c4ccc(cc4)N4CCOCC4)c3n2)cc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of RSK2 (unknown origin) using Ulight-rpS6 as substrate incubated for 1 hr in presence of ATP by TR FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116220
BindingDB Entry DOI: 10.7270/Q2H70KKB |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM25017

(2-[(3,5-difluoro-4-hydroxyphenyl)amino]-5,7-dimeth...)Show SMILES CC(C)CCN1C(C)C(=O)N(C)c2cnc(Nc3cc(F)c(O)c(F)c3)nc12 Show InChI InChI=1S/C19H23F2N5O2/c1-10(2)5-6-26-11(3)18(28)25(4)15-9-22-19(24-17(15)26)23-12-7-13(20)16(27)14(21)8-12/h7-11,27H,5-6H2,1-4H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Anschutz Medical Campus
Curated by ChEMBL
| Assay Description
Inhibition of RSK1 (unknown origin) using KEAKEKRQEQIAKRRRLSSLRASTSKSGGSQK a substrate by [32P] gammaATP assay |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115303
BindingDB Entry DOI: 10.7270/Q2XW4PCP |
More data for this
Ligand-Target Pair | |
5'-AMP-activated protein kinase catalytic subunit alpha-2
(Homo sapiens (Human)) | BDBM50564458
(CHEMBL4788376)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(CCC#N)cc23)c1C | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human His6-tagged AMPK alpha2 using ULight CRBtide as substrate incubated for 1 hr followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112316
BindingDB Entry DOI: 10.7270/Q2348Q5W |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50568111

(CHEMBL4878141 | US11851436, Compound A1-12)Show SMILES COC(=O)CCc1ccc(cc1)-n1cnc2cnc(Nc3cc(F)c(O)c(F)c3)nc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 34.7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50568115
(CHEMBL4864190)Show SMILES CN1CCN(CC1)c1ccc(cc1)-n1ccc2cnc(Nc3cc(F)c(O)c(F)c3)nc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of RSK2 (unknown origin) using Ulight-rpS6 as substrate incubated for 1 hr in presence of ATP by TR FRET assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116220
BindingDB Entry DOI: 10.7270/Q2H70KKB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data