 Found 978 hits with Last Name = 'chopra' and Initial = 'r'
Found 978 hits with Last Name = 'chopra' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Interleukin-1 beta
(Homo sapiens (Human)) | BDBM65497
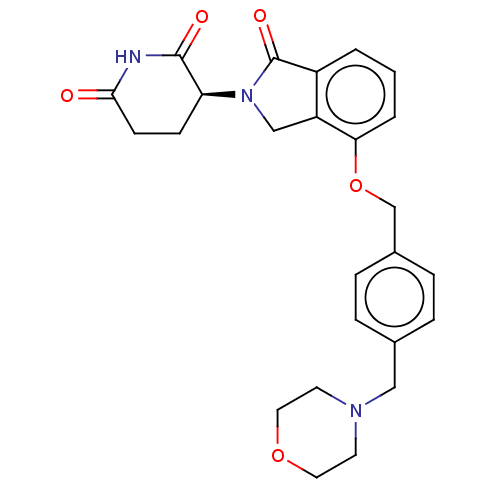
(CC-220 (Compound 6) | US9694015, 6.4S)Show SMILES O=C1N(Cc2c1cccc2OCc1ccc(CN2CCOCC2)cc1)[C@H]1CCC(=O)NC1=O Show InChI InChI=1S/C25H27N3O5/c29-23-9-8-21(24(30)26-23)28-15-20-19(25(28)31)2-1-3-22(20)33-16-18-6-4-17(5-7-18)14-27-10-12-32-13-11-27/h1-7,21H,8-16H2,(H,26,29,30)/t21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| US Patent
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | 37 |
Celgene Corporation
US Patent
| Assay Description
The Fix buffer I was warmed up to 37° C. in an incubator or water bath prior to use. The Perm Buffer III was chilled in a ⿿20° C. freezer p... |
US Patent US9694015 (2017)
BindingDB Entry DOI: 10.7270/Q2R49NZN |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM77003
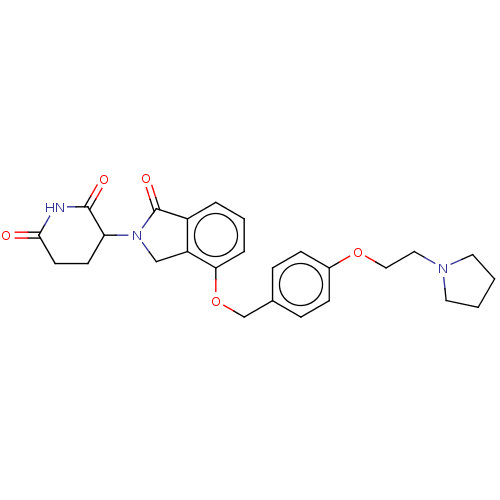
(3-(1-oxo-4-(4-(2-(pyrrolidin-1-yl)ethoxy)benzyloxy...)Show SMILES O=C1N(Cc2c1cccc2OCc1ccc(OCCN2CCCC2)cc1)C1CCC(=O)NC1=O |$;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;HN;;$| Show InChI InChI=1S/C26H29N3O5/c30-24-11-10-22(25(31)27-24)29-16-21-20(26(29)32)4-3-5-23(21)34-17-18-6-8-19(9-7-18)33-15-14-28-12-1-2-13-28/h3-9,22H,1-2,10-17H2,(H,27,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | 37 |
Celgene Corporation
US Patent
| Assay Description
The Fix buffer I was warmed up to 37° C. in an incubator or water bath prior to use. The Perm Buffer III was chilled in a ⿿20° C. freezer p... |
US Patent US9694015 (2017)
BindingDB Entry DOI: 10.7270/Q2R49NZN |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM65497

(CC-220 (Compound 6) | US9694015, 6.4S)Show SMILES O=C1N(Cc2c1cccc2OCc1ccc(CN2CCOCC2)cc1)[C@H]1CCC(=O)NC1=O Show InChI InChI=1S/C25H27N3O5/c29-23-9-8-21(24(30)26-23)28-15-20-19(25(28)31)2-1-3-22(20)33-16-18-6-4-17(5-7-18)14-27-10-12-32-13-11-27/h1-7,21H,8-16H2,(H,26,29,30)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| US Patent
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | 37 |
Celgene Corporation
US Patent
| Assay Description
The Fix buffer I was warmed up to 37° C. in an incubator or water bath prior to use. The Perm Buffer III was chilled in a ⿿20° C. freezer p... |
US Patent US9694015 (2017)
BindingDB Entry DOI: 10.7270/Q2R49NZN |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM77008
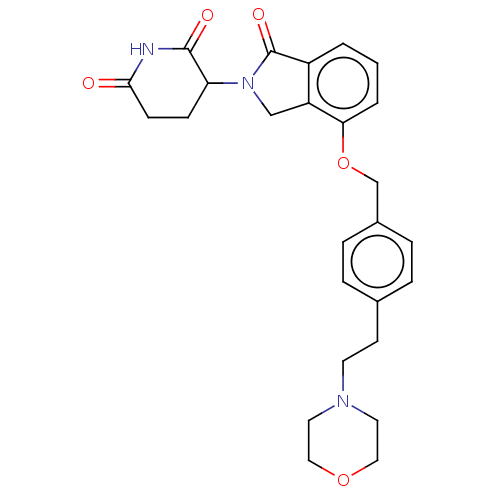
(3-{4-[4-(2-morpholin-4-yl-ethyl)-benzyloxy]-1-oxo-...)Show SMILES O=C1N(Cc2c1cccc2OCc1ccc(CCN2CCOCC2)cc1)C1CCC(=O)NC1=O Show InChI InChI=1S/C26H29N3O5/c30-24-9-8-22(25(31)27-24)29-16-21-20(26(29)32)2-1-3-23(21)34-17-19-6-4-18(5-7-19)10-11-28-12-14-33-15-13-28/h1-7,22H,8-17H2,(H,27,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | 37 |
Celgene Corporation
US Patent
| Assay Description
The Fix buffer I was warmed up to 37° C. in an incubator or water bath prior to use. The Perm Buffer III was chilled in a ⿿20° C. freezer p... |
US Patent US9694015 (2017)
BindingDB Entry DOI: 10.7270/Q2R49NZN |
More data for this
Ligand-Target Pair | |
Interleukin-1 beta
(Homo sapiens (Human)) | BDBM77001
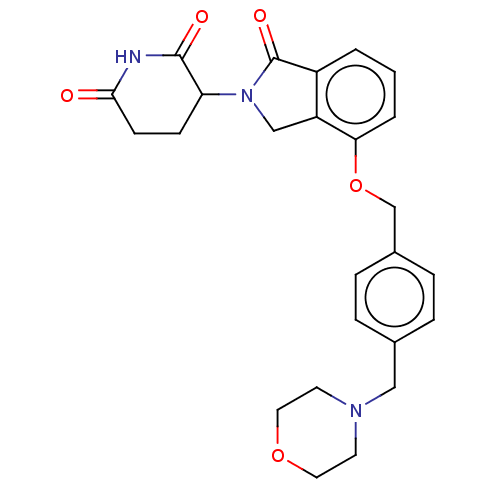
(3-(4-((4-(morpholinomethyl)benzyl)-oxy)-1-oxoisoin...)Show SMILES O=C1N(Cc2c1cccc2OCc1ccc(CN2CCOCC2)cc1)C1CCC(=O)NC1=O Show InChI InChI=1S/C25H27N3O5/c29-23-9-8-21(24(30)26-23)28-15-20-19(25(28)31)2-1-3-22(20)33-16-18-6-4-17(5-7-18)14-27-10-12-32-13-11-27/h1-7,21H,8-16H2,(H,26,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | 37 |
Celgene Corporation
US Patent
| Assay Description
The Fix buffer I was warmed up to 37° C. in an incubator or water bath prior to use. The Perm Buffer III was chilled in a ⿿20° C. freezer p... |
US Patent US9694015 (2017)
BindingDB Entry DOI: 10.7270/Q2R49NZN |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM77005
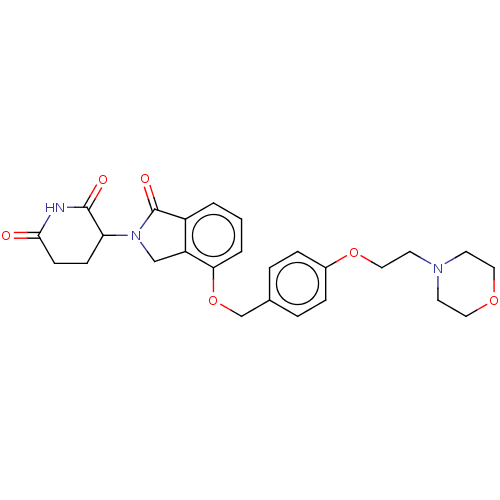
(3-(4-(4-(2-morpholin-4-yl-ethoxy)-benzyloxy)-1-oxo...)Show SMILES O=C1N(Cc2c1cccc2OCc1ccc(OCCN2CCOCC2)cc1)C1CCC(=O)NC1=O |$;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;HN;;$| Show InChI InChI=1S/C26H29N3O6/c30-24-9-8-22(25(31)27-24)29-16-21-20(26(29)32)2-1-3-23(21)35-17-18-4-6-19(7-5-18)34-15-12-28-10-13-33-14-11-28/h1-7,22H,8-17H2,(H,27,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | 37 |
Celgene Corporation
US Patent
| Assay Description
The Fix buffer I was warmed up to 37° C. in an incubator or water bath prior to use. The Perm Buffer III was chilled in a ⿿20° C. freezer p... |
US Patent US9694015 (2017)
BindingDB Entry DOI: 10.7270/Q2R49NZN |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50570849

(CHEMBL4854733)Show SMILES COC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CP(O)(=O)[C@@H](N)CCc1ccccc1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of APN (unknown origin) using A-AMC as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128050
BindingDB Entry DOI: 10.7270/Q2445R98 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50570849

(CHEMBL4854733)Show SMILES COC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CP(O)(=O)[C@@H](N)CCc1ccccc1 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of APN (unknown origin) using A-AMC as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128050
BindingDB Entry DOI: 10.7270/Q2445R98 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16047

((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)C(C)C)[C@@H](O)C[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C41H64N8O14/c1-20(2)16-27(46-39(60)28(19-31(43)51)47-40(61)34(21(3)4)49-37(58)25(42)12-14-32(52)53)30(50)17-22(5)35(56)44-23(6)36(57)45-26(13-15-33(54)55)38(59)48-29(41(62)63)18-24-10-8-7-9-11-24/h7-11,20-23,25-30,34,50H,12-19,42H2,1-6H3,(H2,43,51)(H,44,56)(H,45,57)(H,46,60)(H,47,61)(H,48,59)(H,49,58)(H,52,53)(H,54,55)(H,62,63)/t22-,23+,25+,26+,27+,28+,29+,30+,34+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 by FRET based peptide cleavage assay |
J Med Chem 53: 1146-58 (2010)
Article DOI: 10.1021/jm901414e
BindingDB Entry DOI: 10.7270/Q2SX6DBS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM77001

(3-(4-((4-(morpholinomethyl)benzyl)-oxy)-1-oxoisoin...)Show SMILES O=C1N(Cc2c1cccc2OCc1ccc(CN2CCOCC2)cc1)C1CCC(=O)NC1=O Show InChI InChI=1S/C25H27N3O5/c29-23-9-8-21(24(30)26-23)28-15-20-19(25(28)31)2-1-3-22(20)33-16-18-6-4-17(5-7-18)14-27-10-12-32-13-11-27/h1-7,21H,8-16H2,(H,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | 37 |
Celgene Corporation
US Patent
| Assay Description
The Fix buffer I was warmed up to 37° C. in an incubator or water bath prior to use. The Perm Buffer III was chilled in a ⿿20° C. freezer p... |
US Patent US9694015 (2017)
BindingDB Entry DOI: 10.7270/Q2R49NZN |
More data for this
Ligand-Target Pair | |
Mediator of DNA damage checkpoint protein 1
(Homo sapiens (Human)) | BDBM65497

(CC-220 (Compound 6) | US9694015, 6.4S)Show SMILES O=C1N(Cc2c1cccc2OCc1ccc(CN2CCOCC2)cc1)[C@H]1CCC(=O)NC1=O Show InChI InChI=1S/C25H27N3O5/c29-23-9-8-21(24(30)26-23)28-15-20-19(25(28)31)2-1-3-22(20)33-16-18-6-4-17(5-7-18)14-27-10-12-32-13-11-27/h1-7,21H,8-16H2,(H,26,29,30)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| US Patent
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | 37 |
Celgene Corporation
US Patent
| Assay Description
The Fix buffer I was warmed up to 37° C. in an incubator or water bath prior to use. The Perm Buffer III was chilled in a ⿿20° C. freezer p... |
US Patent US9694015 (2017)
BindingDB Entry DOI: 10.7270/Q2R49NZN |
More data for this
Ligand-Target Pair | |
Granulocyte-macrophage colony-stimulating factor
(Homo sapiens (Human)) | BDBM65497

(CC-220 (Compound 6) | US9694015, 6.4S)Show SMILES O=C1N(Cc2c1cccc2OCc1ccc(CN2CCOCC2)cc1)[C@H]1CCC(=O)NC1=O Show InChI InChI=1S/C25H27N3O5/c29-23-9-8-21(24(30)26-23)28-15-20-19(25(28)31)2-1-3-22(20)33-16-18-6-4-17(5-7-18)14-27-10-12-32-13-11-27/h1-7,21H,8-16H2,(H,26,29,30)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| US Patent
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | 37 |
Celgene Corporation
US Patent
| Assay Description
The Fix buffer I was warmed up to 37° C. in an incubator or water bath prior to use. The Perm Buffer III was chilled in a ⿿20° C. freezer p... |
US Patent US9694015 (2017)
BindingDB Entry DOI: 10.7270/Q2R49NZN |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50570850

(CHEMBL4877339)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of APN (unknown origin) using A-AMC as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128050
BindingDB Entry DOI: 10.7270/Q2445R98 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50570850

(CHEMBL4877339)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of APN (unknown origin) using A-AMC as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128050
BindingDB Entry DOI: 10.7270/Q2445R98 |
More data for this
Ligand-Target Pair | |
Mediator of DNA damage checkpoint protein 1
(Homo sapiens (Human)) | BDBM77001

(3-(4-((4-(morpholinomethyl)benzyl)-oxy)-1-oxoisoin...)Show SMILES O=C1N(Cc2c1cccc2OCc1ccc(CN2CCOCC2)cc1)C1CCC(=O)NC1=O Show InChI InChI=1S/C25H27N3O5/c29-23-9-8-21(24(30)26-23)28-15-20-19(25(28)31)2-1-3-22(20)33-16-18-6-4-17(5-7-18)14-27-10-12-32-13-11-27/h1-7,21H,8-16H2,(H,26,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | 37 |
Celgene Corporation
US Patent
| Assay Description
The Fix buffer I was warmed up to 37° C. in an incubator or water bath prior to use. The Perm Buffer III was chilled in a ⿿20° C. freezer p... |
US Patent US9694015 (2017)
BindingDB Entry DOI: 10.7270/Q2R49NZN |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50076744

(CHEMBL3416733)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of APN (unknown origin) using A-AMC as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128050
BindingDB Entry DOI: 10.7270/Q2445R98 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50076744

(CHEMBL3416733)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of APN (unknown origin) using A-AMC as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128050
BindingDB Entry DOI: 10.7270/Q2445R98 |
More data for this
Ligand-Target Pair | |
Interleukin-6
(Homo sapiens (Human)) | BDBM65497

(CC-220 (Compound 6) | US9694015, 6.4S)Show SMILES O=C1N(Cc2c1cccc2OCc1ccc(CN2CCOCC2)cc1)[C@H]1CCC(=O)NC1=O Show InChI InChI=1S/C25H27N3O5/c29-23-9-8-21(24(30)26-23)28-15-20-19(25(28)31)2-1-3-22(20)33-16-18-6-4-17(5-7-18)14-27-10-12-32-13-11-27/h1-7,21H,8-16H2,(H,26,29,30)/t21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| US Patent
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | 37 |
Celgene Corporation
US Patent
| Assay Description
The Fix buffer I was warmed up to 37° C. in an incubator or water bath prior to use. The Perm Buffer III was chilled in a ⿿20° C. freezer p... |
US Patent US9694015 (2017)
BindingDB Entry DOI: 10.7270/Q2R49NZN |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50330271
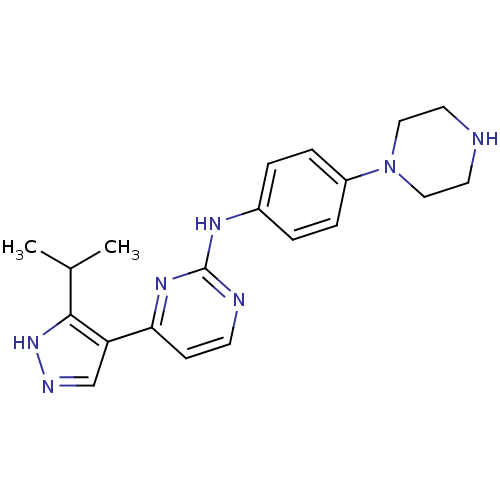
(CHEMBL1271620 | [4-(3-Isopropyl-1H-pyrazol-4-yl)-p...)Show SMILES CC(C)c1[nH]ncc1-c1ccnc(Nc2ccc(cc2)N2CCNCC2)n1 Show InChI InChI=1S/C20H25N7/c1-14(2)19-17(13-23-26-19)18-7-8-22-20(25-18)24-15-3-5-16(6-4-15)27-11-9-21-10-12-27/h3-8,13-14,21H,9-12H2,1-2H3,(H,23,26)(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin D1 expressed in baculovirus infected Sf21 cells after 60 mins by TR-FRET assay |
J Med Chem 53: 7938-57 (2010)
Article DOI: 10.1021/jm100571n
BindingDB Entry DOI: 10.7270/Q2T72HQV |
More data for this
Ligand-Target Pair | |
Interleukin-1 beta
(Homo sapiens (Human)) | BDBM77015
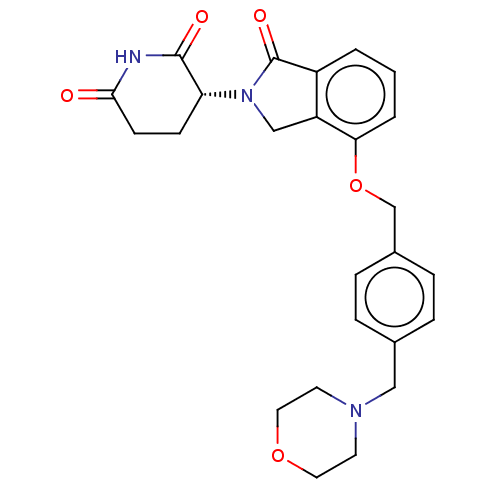
((R)-3-(4-((4-(morpholinomethyl)benzyl)-oxy)-1-oxoi...)Show SMILES O=C1N(Cc2c1cccc2OCc1ccc(CN2CCOCC2)cc1)[C@@H]1CCC(=O)NC1=O |r| Show InChI InChI=1S/C25H27N3O5/c29-23-9-8-21(24(30)26-23)28-15-20-19(25(28)31)2-1-3-22(20)33-16-18-6-4-17(5-7-18)14-27-10-12-32-13-11-27/h1-7,21H,8-16H2,(H,26,29,30)/t21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | 37 |
Celgene Corporation
US Patent
| Assay Description
The Fix buffer I was warmed up to 37° C. in an incubator or water bath prior to use. The Perm Buffer III was chilled in a ⿿20° C. freezer p... |
US Patent US9694015 (2017)
BindingDB Entry DOI: 10.7270/Q2R49NZN |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50118694
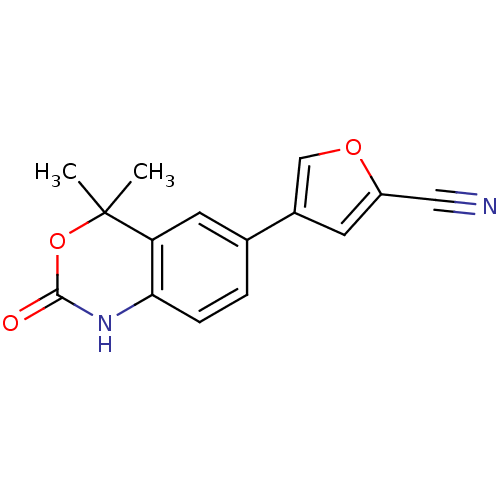
(4-(4,4-Dimethyl-2-oxo-1,4-dihydro-2H-benzo[d][1,3]...)Show InChI InChI=1S/C15H12N2O3/c1-15(2)12-6-9(10-5-11(7-16)19-8-10)3-4-13(12)17-14(18)20-15/h3-6,8H,1-2H3,(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Women's Health Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone receptor |
J Med Chem 48: 5092-5 (2005)
Article DOI: 10.1021/jm050358b
BindingDB Entry DOI: 10.7270/Q2FF3RXG |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50352863
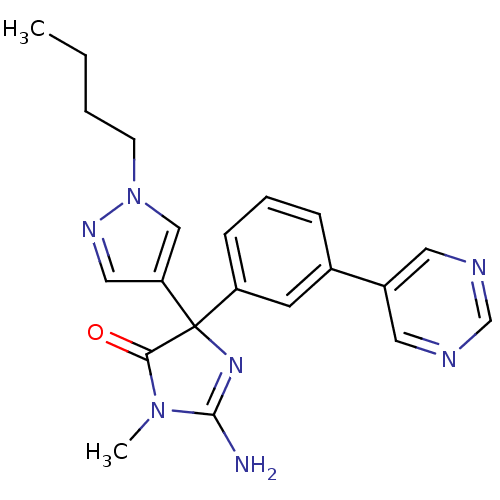
(CHEMBL1824141)Show SMILES CCCCn1cc(cn1)C1(N=C(N)N(C)C1=O)c1cccc(c1)-c1cncnc1 |t:11| Show InChI InChI=1S/C21H23N7O/c1-3-4-8-28-13-18(12-25-28)21(19(29)27(2)20(22)26-21)17-7-5-6-15(9-17)16-10-23-14-24-11-16/h5-7,9-14H,3-4,8H2,1-2H3,(H2,22,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 by FRET assay |
Bioorg Med Chem Lett 21: 5164-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.057
BindingDB Entry DOI: 10.7270/Q2WQ045T |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50570851
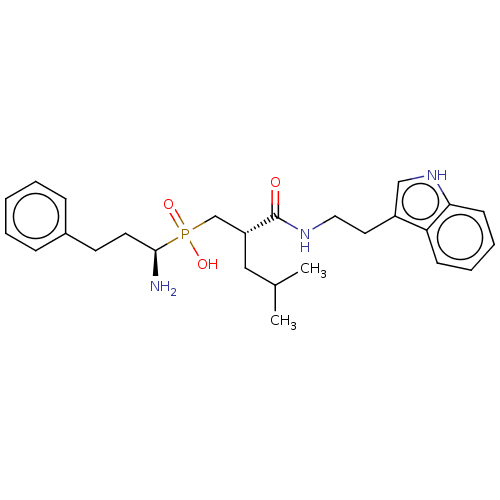
(CHEMBL4853049)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)NCCc1c[nH]c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of APN (unknown origin) using A-AMC as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128050
BindingDB Entry DOI: 10.7270/Q2445R98 |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Homo sapiens (Human)) | BDBM50570851

(CHEMBL4853049)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)NCCc1c[nH]c2ccccc12 |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of APN (unknown origin) using A-AMC as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128050
BindingDB Entry DOI: 10.7270/Q2445R98 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50330281
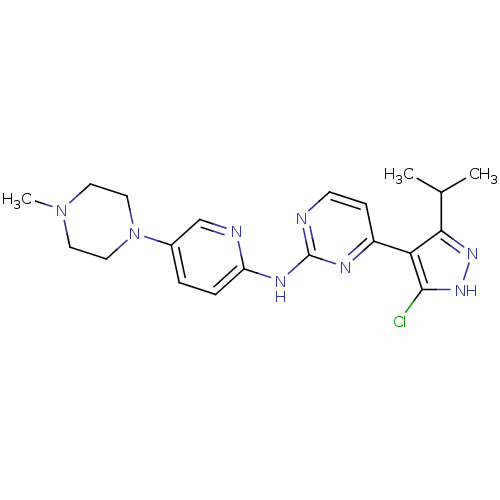
(CHEMBL1271899 | {4-[5-Chloro-3-isopropyl-1-(2-trim...)Show SMILES CC(C)c1n[nH]c(Cl)c1-c1ccnc(Nc2ccc(cn2)N2CCN(C)CC2)n1 Show InChI InChI=1S/C20H25ClN8/c1-13(2)18-17(19(21)27-26-18)15-6-7-22-20(24-15)25-16-5-4-14(12-23-16)29-10-8-28(3)9-11-29/h4-7,12-13H,8-11H2,1-3H3,(H,26,27)(H,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin D1 expressed in baculovirus infected Sf21 cells after 60 mins by TR-FRET assay |
J Med Chem 53: 7938-57 (2010)
Article DOI: 10.1021/jm100571n
BindingDB Entry DOI: 10.7270/Q2T72HQV |
More data for this
Ligand-Target Pair | |
Granulocyte-macrophage colony-stimulating factor
(Homo sapiens (Human)) | BDBM77001

(3-(4-((4-(morpholinomethyl)benzyl)-oxy)-1-oxoisoin...)Show SMILES O=C1N(Cc2c1cccc2OCc1ccc(CN2CCOCC2)cc1)C1CCC(=O)NC1=O Show InChI InChI=1S/C25H27N3O5/c29-23-9-8-21(24(30)26-23)28-15-20-19(25(28)31)2-1-3-22(20)33-16-18-6-4-17(5-7-18)14-27-10-12-32-13-11-27/h1-7,21H,8-16H2,(H,26,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | 37 |
Celgene Corporation
US Patent
| Assay Description
The Fix buffer I was warmed up to 37° C. in an incubator or water bath prior to use. The Perm Buffer III was chilled in a ⿿20° C. freezer p... |
US Patent US9694015 (2017)
BindingDB Entry DOI: 10.7270/Q2R49NZN |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor
(Homo sapiens (Human)) | BDBM77015

((R)-3-(4-((4-(morpholinomethyl)benzyl)-oxy)-1-oxoi...)Show SMILES O=C1N(Cc2c1cccc2OCc1ccc(CN2CCOCC2)cc1)[C@@H]1CCC(=O)NC1=O |r| Show InChI InChI=1S/C25H27N3O5/c29-23-9-8-21(24(30)26-23)28-15-20-19(25(28)31)2-1-3-22(20)33-16-18-6-4-17(5-7-18)14-27-10-12-32-13-11-27/h1-7,21H,8-16H2,(H,26,29,30)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | 37 |
Celgene Corporation
US Patent
| Assay Description
The Fix buffer I was warmed up to 37° C. in an incubator or water bath prior to use. The Perm Buffer III was chilled in a ⿿20° C. freezer p... |
US Patent US9694015 (2017)
BindingDB Entry DOI: 10.7270/Q2R49NZN |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50303747
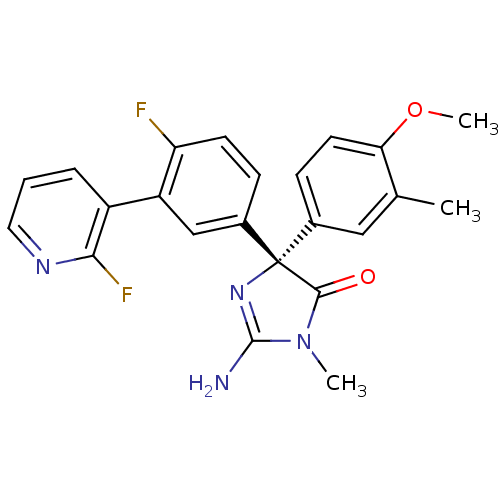
((4S)-2-amino-4-(4-fluoro-3-(2-fluoropyridin-3-yl)p...)Show SMILES COc1ccc(cc1C)[C@]1(N=C(N)N(C)C1=O)c1ccc(F)c(c1)-c1cccnc1F |r,t:11| Show InChI InChI=1S/C23H20F2N4O2/c1-13-11-14(7-9-19(13)31-3)23(21(30)29(2)22(26)28-23)15-6-8-18(24)17(12-15)16-5-4-10-27-20(16)25/h4-12H,1-3H3,(H2,26,28)/t23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 by FRET based peptide cleavage assay |
J Med Chem 53: 1146-58 (2010)
Article DOI: 10.1021/jm901414e
BindingDB Entry DOI: 10.7270/Q2SX6DBS |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50352854
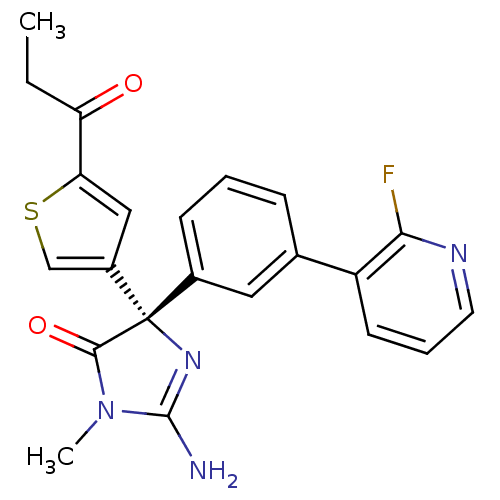
(CHEMBL1824361)Show SMILES CCC(=O)c1cc(cs1)[C@@]1(N=C(N)N(C)C1=O)c1cccc(c1)-c1cccnc1F |r,t:11| Show InChI InChI=1S/C22H19FN4O2S/c1-3-17(28)18-11-15(12-30-18)22(20(29)27(2)21(24)26-22)14-7-4-6-13(10-14)16-8-5-9-25-19(16)23/h4-12H,3H2,1-2H3,(H2,24,26)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 by FRET assay |
Bioorg Med Chem Lett 21: 5164-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.057
BindingDB Entry DOI: 10.7270/Q2WQ045T |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50352875
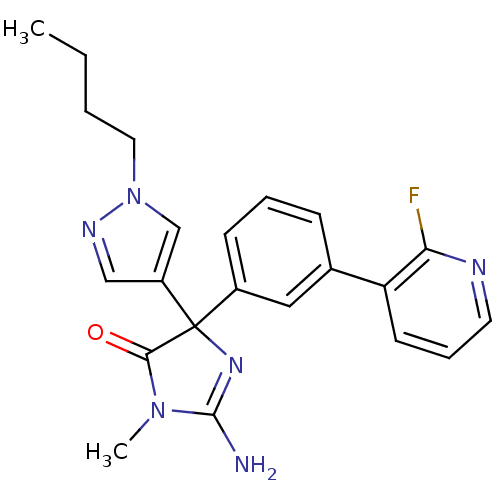
(CHEMBL1824344)Show SMILES CCCCn1cc(cn1)C1(N=C(N)N(C)C1=O)c1cccc(c1)-c1cccnc1F |t:11| Show InChI InChI=1S/C22H23FN6O/c1-3-4-11-29-14-17(13-26-29)22(20(30)28(2)21(24)27-22)16-8-5-7-15(12-16)18-9-6-10-25-19(18)23/h5-10,12-14H,3-4,11H2,1-2H3,(H2,24,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 by FRET assay |
Bioorg Med Chem Lett 21: 5164-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.057
BindingDB Entry DOI: 10.7270/Q2WQ045T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50330261
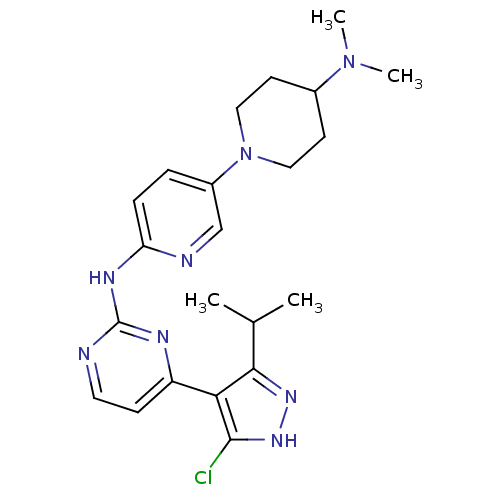
(4-(5-Chloro-3-isopropyl-1H-pyrazol-4-yl)-N-(5-(4-(...)Show SMILES CC(C)c1n[nH]c(Cl)c1-c1ccnc(Nc2ccc(cn2)N2CCC(CC2)N(C)C)n1 Show InChI InChI=1S/C22H29ClN8/c1-14(2)20-19(21(23)29-28-20)17-7-10-24-22(26-17)27-18-6-5-16(13-25-18)31-11-8-15(9-12-31)30(3)4/h5-7,10,13-15H,8-9,11-12H2,1-4H3,(H,28,29)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin D1 expressed in baculovirus infected Sf21 cells after 60 mins by TR-FRET assay |
J Med Chem 53: 7938-57 (2010)
Article DOI: 10.1021/jm100571n
BindingDB Entry DOI: 10.7270/Q2T72HQV |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50310150
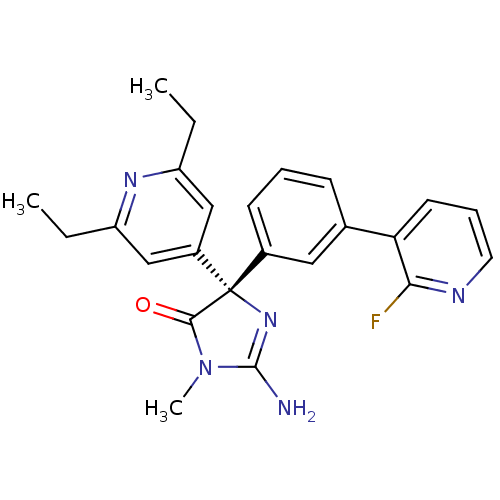
((S)-2-amino-4-(2,6-diethylpyridin-4-yl)-4-(3-(2-fl...)Show SMILES CCc1cc(cc(CC)n1)[C@@]1(N=C(N)N(C)C1=O)c1cccc(c1)-c1cccnc1F |r,t:12| Show InChI InChI=1S/C24H24FN5O/c1-4-18-13-17(14-19(5-2)28-18)24(22(31)30(3)23(26)29-24)16-9-6-8-15(12-16)20-10-7-11-27-21(20)25/h6-14H,4-5H2,1-3H3,(H2,26,29)/t24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 by FRET assay |
Bioorg Med Chem 18: 630-9 (2010)
Article DOI: 10.1016/j.bmc.2009.12.007
BindingDB Entry DOI: 10.7270/Q2DV1KT4 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50310150

((S)-2-amino-4-(2,6-diethylpyridin-4-yl)-4-(3-(2-fl...)Show SMILES CCc1cc(cc(CC)n1)[C@@]1(N=C(N)N(C)C1=O)c1cccc(c1)-c1cccnc1F |r,t:12| Show InChI InChI=1S/C24H24FN5O/c1-4-18-13-17(14-19(5-2)28-18)24(22(31)30(3)23(26)29-24)16-9-6-8-15(12-16)20-10-7-11-27-21(20)25/h6-14H,4-5H2,1-3H3,(H2,26,29)/t24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 by FRET assay |
Bioorg Med Chem 18: 630-9 (2010)
Article DOI: 10.1016/j.bmc.2009.12.007
BindingDB Entry DOI: 10.7270/Q2DV1KT4 |
More data for this
Ligand-Target Pair | |
Interleukin-6
(Homo sapiens (Human)) | BDBM77001

(3-(4-((4-(morpholinomethyl)benzyl)-oxy)-1-oxoisoin...)Show SMILES O=C1N(Cc2c1cccc2OCc1ccc(CN2CCOCC2)cc1)C1CCC(=O)NC1=O Show InChI InChI=1S/C25H27N3O5/c29-23-9-8-21(24(30)26-23)28-15-20-19(25(28)31)2-1-3-22(20)33-16-18-6-4-17(5-7-18)14-27-10-12-32-13-11-27/h1-7,21H,8-16H2,(H,26,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | 37 |
Celgene Corporation
US Patent
| Assay Description
The Fix buffer I was warmed up to 37° C. in an incubator or water bath prior to use. The Perm Buffer III was chilled in a ⿿20° C. freezer p... |
US Patent US9694015 (2017)
BindingDB Entry DOI: 10.7270/Q2R49NZN |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50352852
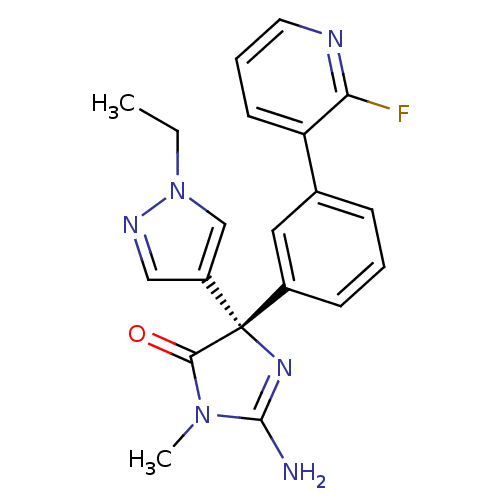
(CHEMBL1824145)Show SMILES CCn1cc(cn1)[C@@]1(N=C(N)N(C)C1=O)c1cccc(c1)-c1cccnc1F |r,t:9| Show InChI InChI=1S/C20H19FN6O/c1-3-27-12-15(11-24-27)20(18(28)26(2)19(22)25-20)14-7-4-6-13(10-14)16-8-5-9-23-17(16)21/h4-12H,3H2,1-2H3,(H2,22,25)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 by FRET assay |
Bioorg Med Chem Lett 21: 5164-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.057
BindingDB Entry DOI: 10.7270/Q2WQ045T |
More data for this
Ligand-Target Pair | |
Endoplasmic reticulum aminopeptidase 2
(Homo sapiens (Human)) | BDBM50076744

(CHEMBL3416733)Show SMILES CC(C)C[C@H](CP(O)(=O)[C@@H](N)CCc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ERAP2 using R-AMC as substrate incubated for 5 to 10 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128050
BindingDB Entry DOI: 10.7270/Q2445R98 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50330267
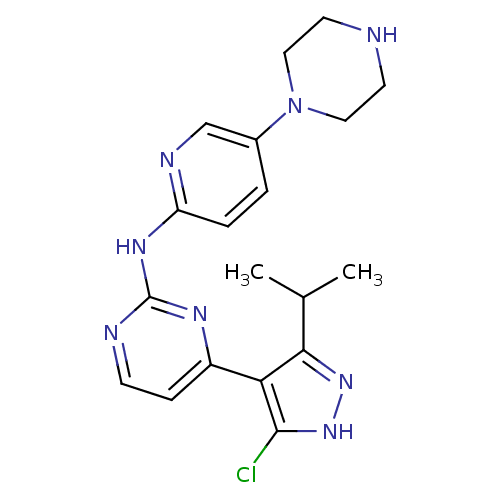
(4-[5-chloro-3-(1-methylethyl)-1H-pyrazol-4-yl]-N-(...)Show SMILES CC(C)c1n[nH]c(Cl)c1-c1ccnc(Nc2ccc(cn2)N2CCNCC2)n1 Show InChI InChI=1S/C19H23ClN8/c1-12(2)17-16(18(20)27-26-17)14-5-6-22-19(24-14)25-15-4-3-13(11-23-15)28-9-7-21-8-10-28/h3-6,11-12,21H,7-10H2,1-2H3,(H,26,27)(H,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin D1 expressed in baculovirus infected Sf21 cells after 60 mins by TR-FRET assay |
J Med Chem 53: 7938-57 (2010)
Article DOI: 10.1021/jm100571n
BindingDB Entry DOI: 10.7270/Q2T72HQV |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50330284
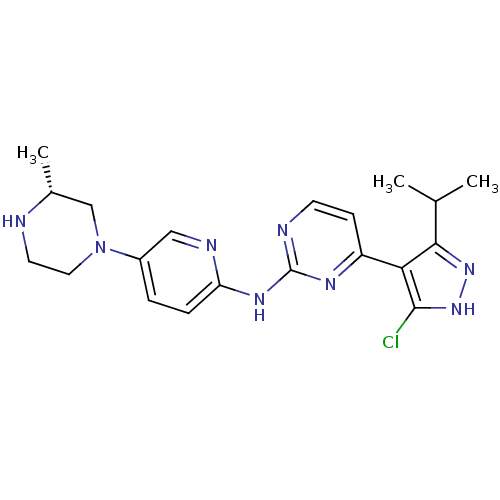
(CHEMBL1272010 | [4-(5-Chloro-3-isopropyl-1H-pyrazo...)Show SMILES CC(C)c1n[nH]c(Cl)c1-c1ccnc(Nc2ccc(cn2)N2CCN[C@H](C)C2)n1 |r| Show InChI InChI=1S/C20H25ClN8/c1-12(2)18-17(19(21)28-27-18)15-6-7-23-20(25-15)26-16-5-4-14(10-24-16)29-9-8-22-13(3)11-29/h4-7,10,12-13,22H,8-9,11H2,1-3H3,(H,27,28)(H,23,24,25,26)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin D1 expressed in baculovirus infected Sf21 cells after 60 mins by TR-FRET assay |
J Med Chem 53: 7938-57 (2010)
Article DOI: 10.1021/jm100571n
BindingDB Entry DOI: 10.7270/Q2T72HQV |
More data for this
Ligand-Target Pair | |
Mediator of DNA damage checkpoint protein 1
(Homo sapiens (Human)) | BDBM77015

((R)-3-(4-((4-(morpholinomethyl)benzyl)-oxy)-1-oxoi...)Show SMILES O=C1N(Cc2c1cccc2OCc1ccc(CN2CCOCC2)cc1)[C@@H]1CCC(=O)NC1=O |r| Show InChI InChI=1S/C25H27N3O5/c29-23-9-8-21(24(30)26-23)28-15-20-19(25(28)31)2-1-3-22(20)33-16-18-6-4-17(5-7-18)14-27-10-12-32-13-11-27/h1-7,21H,8-16H2,(H,26,29,30)/t21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | 37 |
Celgene Corporation
US Patent
| Assay Description
The Fix buffer I was warmed up to 37° C. in an incubator or water bath prior to use. The Perm Buffer III was chilled in a ⿿20° C. freezer p... |
US Patent US9694015 (2017)
BindingDB Entry DOI: 10.7270/Q2R49NZN |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50330269
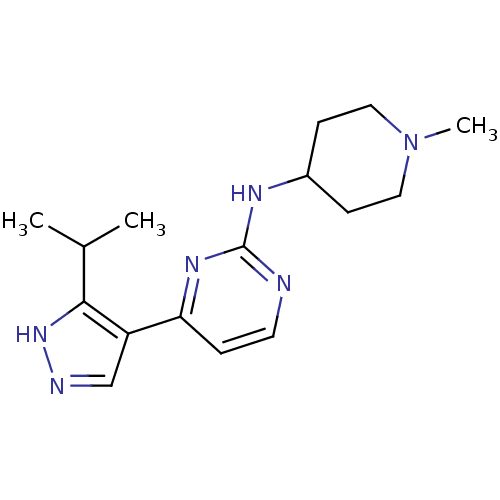
(4-[3-(1-methylethyl)-1H-pyrazol-4-yl]-N-(1-methylp...)Show InChI InChI=1S/C16H24N6/c1-11(2)15-13(10-18-21-15)14-4-7-17-16(20-14)19-12-5-8-22(3)9-6-12/h4,7,10-12H,5-6,8-9H2,1-3H3,(H,18,21)(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin D1 expressed in baculovirus infected Sf21 cells after 60 mins by TR-FRET assay |
J Med Chem 53: 7938-57 (2010)
Article DOI: 10.1021/jm100571n
BindingDB Entry DOI: 10.7270/Q2T72HQV |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50330268
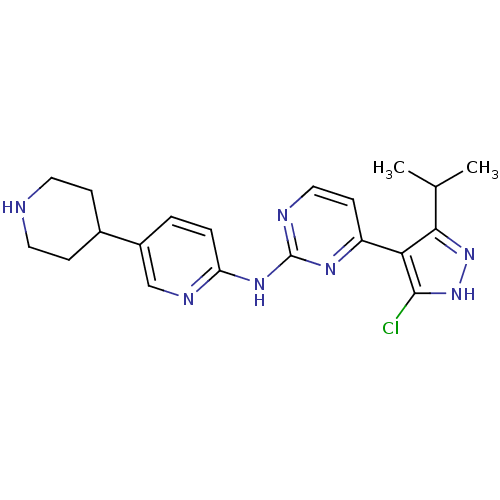
(4-(5-Chloro-3-isopropyl-1H-pyrazol-4-yl)-N-(5-(pip...)Show SMILES CC(C)c1n[nH]c(Cl)c1-c1ccnc(Nc2ccc(cn2)C2CCNCC2)n1 Show InChI InChI=1S/C20H24ClN7/c1-12(2)18-17(19(21)28-27-18)15-7-10-23-20(25-15)26-16-4-3-14(11-24-16)13-5-8-22-9-6-13/h3-4,7,10-13,22H,5-6,8-9H2,1-2H3,(H,27,28)(H,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin D1 expressed in baculovirus infected Sf21 cells after 60 mins by TR-FRET assay |
J Med Chem 53: 7938-57 (2010)
Article DOI: 10.1021/jm100571n
BindingDB Entry DOI: 10.7270/Q2T72HQV |
More data for this
Ligand-Target Pair | |
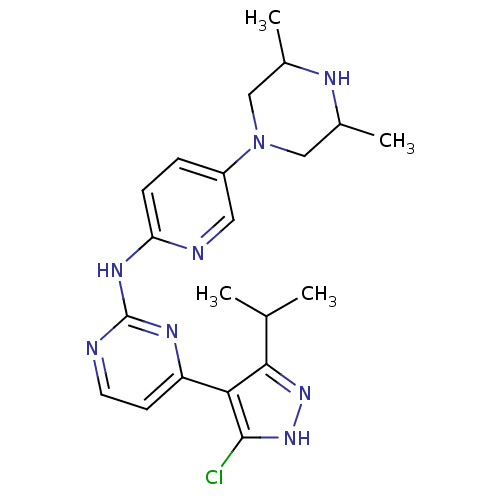
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50330286
(CHEMBL1272065 | [4-(5-Chloro-3-isopropyl-1H-pyrazo...)Show SMILES CC(C)c1n[nH]c(Cl)c1-c1ccnc(Nc2ccc(cn2)N2CC(C)NC(C)C2)n1 Show InChI InChI=1S/C21H27ClN8/c1-12(2)19-18(20(22)29-28-19)16-7-8-23-21(26-16)27-17-6-5-15(9-24-17)30-10-13(3)25-14(4)11-30/h5-9,12-14,25H,10-11H2,1-4H3,(H,28,29)(H,23,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin D1 expressed in baculovirus infected Sf21 cells after 60 mins by TR-FRET assay |
J Med Chem 53: 7938-57 (2010)
Article DOI: 10.1021/jm100571n
BindingDB Entry DOI: 10.7270/Q2T72HQV |
More data for this
Ligand-Target Pair | |
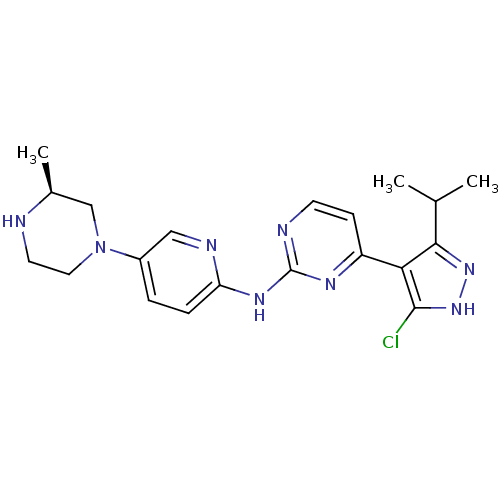
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50330283
(CHEMBL1271954 | [4-(5-Chloro-3-isopropyl-1H-pyrazo...)Show SMILES CC(C)c1n[nH]c(Cl)c1-c1ccnc(Nc2ccc(cn2)N2CCN[C@@H](C)C2)n1 |r| Show InChI InChI=1S/C20H25ClN8/c1-12(2)18-17(19(21)28-27-18)15-6-7-23-20(25-15)26-16-5-4-14(10-24-16)29-9-8-22-13(3)11-29/h4-7,10,12-13,22H,8-9,11H2,1-3H3,(H,27,28)(H,23,24,25,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin D1 expressed in baculovirus infected Sf21 cells after 60 mins by TR-FRET assay |
J Med Chem 53: 7938-57 (2010)
Article DOI: 10.1021/jm100571n
BindingDB Entry DOI: 10.7270/Q2T72HQV |
More data for this
Ligand-Target Pair | |
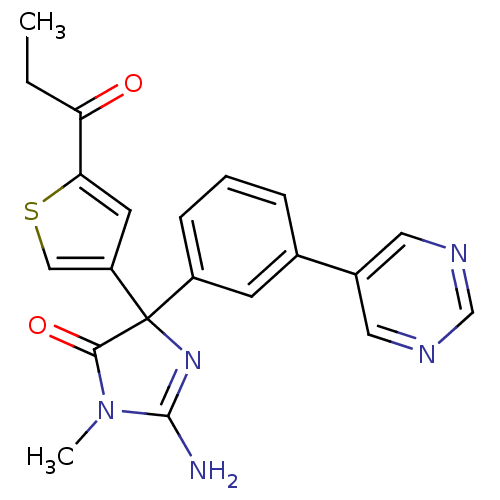
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50352884
(CHEMBL1824353)Show SMILES CCC(=O)c1cc(cs1)C1(N=C(N)N(C)C1=O)c1cccc(c1)-c1cncnc1 |t:11| Show InChI InChI=1S/C21H19N5O2S/c1-3-17(27)18-8-16(11-29-18)21(19(28)26(2)20(22)25-21)15-6-4-5-13(7-15)14-9-23-12-24-10-14/h4-12H,3H2,1-2H3,(H2,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 by FRET assay |
Bioorg Med Chem Lett 21: 5164-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.057
BindingDB Entry DOI: 10.7270/Q2WQ045T |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50118696
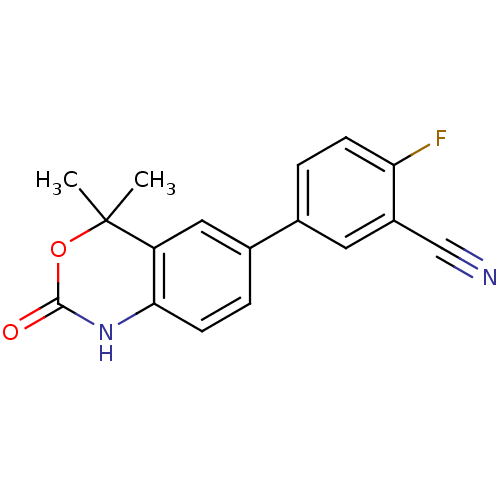
(5-(4,4-Dimethyl-2-oxo-1,4-dihydro-2H-benzo[d][1,3]...)Show InChI InChI=1S/C17H13FN2O2/c1-17(2)13-8-11(4-6-15(13)20-16(21)22-17)10-3-5-14(18)12(7-10)9-19/h3-8H,1-2H3,(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Women's Health Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human progesterone receptor |
J Med Chem 48: 5092-5 (2005)
Article DOI: 10.1021/jm050358b
BindingDB Entry DOI: 10.7270/Q2FF3RXG |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50352849
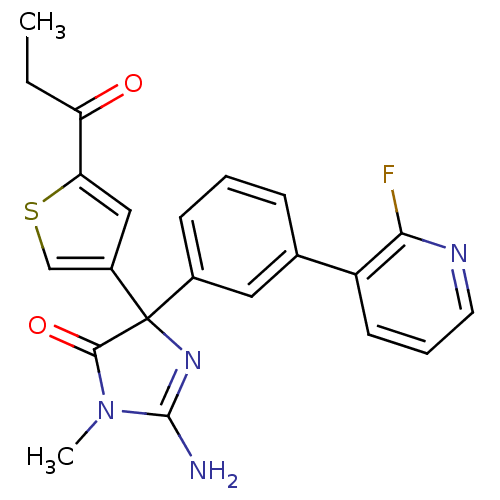
(CHEMBL1824360)Show SMILES CCC(=O)c1cc(cs1)C1(N=C(N)N(C)C1=O)c1cccc(c1)-c1cccnc1F |t:11| Show InChI InChI=1S/C22H19FN4O2S/c1-3-17(28)18-11-15(12-30-18)22(20(29)27(2)21(24)26-22)14-7-4-6-13(10-14)16-8-5-9-25-19(16)23/h4-12H,3H2,1-2H3,(H2,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 by FRET assay |
Bioorg Med Chem Lett 21: 5164-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.057
BindingDB Entry DOI: 10.7270/Q2WQ045T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50330285
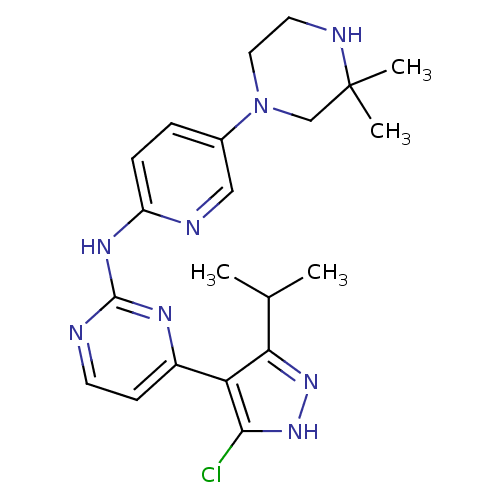
(CHEMBL1272011 | [4-(5-Chloro-3-isopropyl-1H-pyrazo...)Show SMILES CC(C)c1n[nH]c(Cl)c1-c1ccnc(Nc2ccc(cn2)N2CCNC(C)(C)C2)n1 Show InChI InChI=1S/C21H27ClN8/c1-13(2)18-17(19(22)29-28-18)15-7-8-23-20(26-15)27-16-6-5-14(11-24-16)30-10-9-25-21(3,4)12-30/h5-8,11,13,25H,9-10,12H2,1-4H3,(H,28,29)(H,23,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin D1 expressed in baculovirus infected Sf21 cells after 60 mins by TR-FRET assay |
J Med Chem 53: 7938-57 (2010)
Article DOI: 10.1021/jm100571n
BindingDB Entry DOI: 10.7270/Q2T72HQV |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50352872

(CHEMBL1824341)Show SMILES CC(C)CCn1cc(cn1)C1(N=C(N)N(C)C1=O)c1cccc(c1)-c1cccnc1F |t:12| Show InChI InChI=1S/C23H25FN6O/c1-15(2)9-11-30-14-18(13-27-30)23(21(31)29(3)22(25)28-23)17-7-4-6-16(12-17)19-8-5-10-26-20(19)24/h4-8,10,12-15H,9,11H2,1-3H3,(H2,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 by FRET assay |
Bioorg Med Chem Lett 21: 5164-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.057
BindingDB Entry DOI: 10.7270/Q2WQ045T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50330282
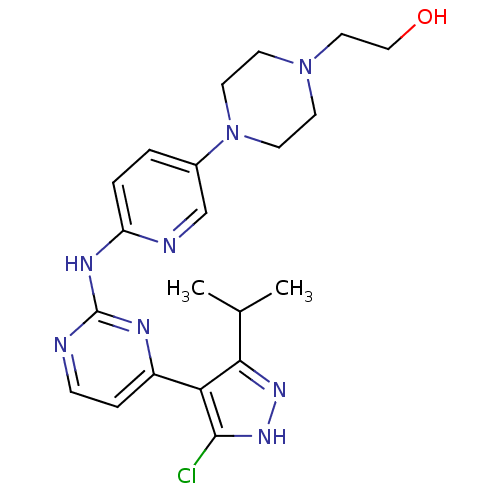
(2-(4-{6-[4-(5-Chloro-3-isopropyl-1H-pyrazol-4-yl)-...)Show SMILES CC(C)c1n[nH]c(Cl)c1-c1ccnc(Nc2ccc(cn2)N2CCN(CCO)CC2)n1 Show InChI InChI=1S/C21H27ClN8O/c1-14(2)19-18(20(22)28-27-19)16-5-6-23-21(25-16)26-17-4-3-15(13-24-17)30-9-7-29(8-10-30)11-12-31/h3-6,13-14,31H,7-12H2,1-2H3,(H,27,28)(H,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human CDK4/cyclin D1 expressed in baculovirus infected Sf21 cells after 60 mins by TR-FRET assay |
J Med Chem 53: 7938-57 (2010)
Article DOI: 10.1021/jm100571n
BindingDB Entry DOI: 10.7270/Q2T72HQV |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50352872

(CHEMBL1824341)Show SMILES CC(C)CCn1cc(cn1)C1(N=C(N)N(C)C1=O)c1cccc(c1)-c1cccnc1F |t:12| Show InChI InChI=1S/C23H25FN6O/c1-15(2)9-11-30-14-18(13-27-30)23(21(31)29(3)22(25)28-23)17-7-4-6-16(12-17)19-8-5-10-26-20(19)24/h4-8,10,12-15H,9,11H2,1-3H3,(H2,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 by FRET assay |
Bioorg Med Chem Lett 21: 5164-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.057
BindingDB Entry DOI: 10.7270/Q2WQ045T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data