 Found 1268 hits with Last Name = 'hodgson' and Initial = 'r'
Found 1268 hits with Last Name = 'hodgson' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50364330
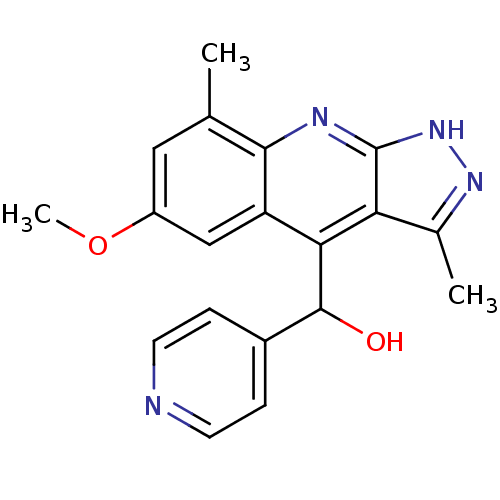
(CHEMBL1949936)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(C(O)c3ccncc3)c2c1 Show InChI InChI=1S/C19H18N4O2/c1-10-8-13(25-3)9-14-16(18(24)12-4-6-20-7-5-12)15-11(2)22-23-19(15)21-17(10)14/h4-9,18,24H,1-3H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1335-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.080
BindingDB Entry DOI: 10.7270/Q2RF5VGF |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50364327
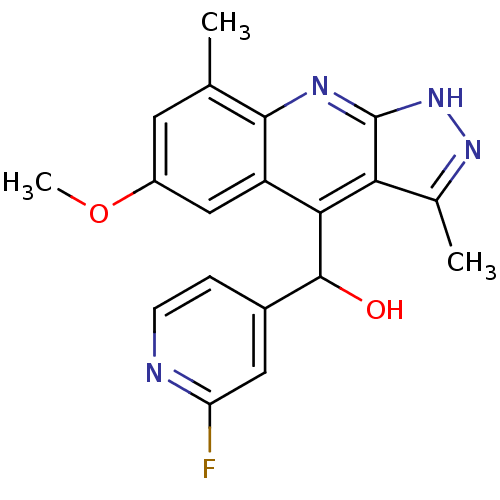
(CHEMBL1949939)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(C(O)c3ccnc(F)c3)c2c1 Show InChI InChI=1S/C19H17FN4O2/c1-9-6-12(26-3)8-13-16(18(25)11-4-5-21-14(20)7-11)15-10(2)23-24-19(15)22-17(9)13/h4-8,18,25H,1-3H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1335-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.080
BindingDB Entry DOI: 10.7270/Q2RF5VGF |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50512456
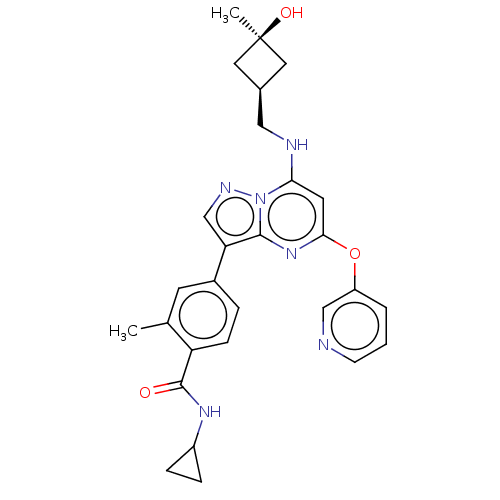
(CHEMBL4469414)Show SMILES Cc1cc(ccc1C(=O)NC1CC1)-c1cnn2c(NC[C@H]3C[C@@](C)(O)C3)cc(Oc3cccnc3)nc12 |r,wU:20.21,22.25,(48.46,-32.37,;49.53,-31.26,;49.1,-29.78,;50.17,-28.67,;51.66,-29.03,;52.09,-30.5,;51.03,-31.63,;51.46,-33.1,;50.39,-34.22,;52.95,-33.47,;54.02,-32.36,;55.49,-31.92,;54.38,-30.85,;49.74,-27.2,;50.69,-25.98,;49.82,-24.7,;48.34,-25.14,;47.04,-24.33,;47.09,-22.79,;45.78,-21.98,;44.42,-22.71,;43.98,-24.18,;42.51,-23.74,;41.01,-23.33,;41.41,-24.82,;42.95,-22.26,;45.69,-25.06,;45.64,-26.6,;44.29,-27.33,;44.25,-28.87,;42.89,-29.59,;42.85,-31.13,;44.16,-31.94,;45.52,-31.2,;45.56,-29.66,;46.95,-27.4,;48.29,-26.68,)| Show InChI InChI=1S/C28H30N6O3/c1-17-10-19(5-8-22(17)27(35)32-20-6-7-20)23-16-31-34-24(30-14-18-12-28(2,36)13-18)11-25(33-26(23)34)37-21-4-3-9-29-15-21/h3-5,8-11,15-16,18,20,30,36H,6-7,12-14H2,1-2H3,(H,32,35)/t18-,28+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant N-terminal GST-tagged and sumo-tagged human TTK (1 to 275 residues) expressed in Escherichia coli pre-incubated... |
ACS Med Chem Lett 7: 671-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00485
BindingDB Entry DOI: 10.7270/Q2K35Z46 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50512456

(CHEMBL4469414)Show SMILES Cc1cc(ccc1C(=O)NC1CC1)-c1cnn2c(NC[C@H]3C[C@@](C)(O)C3)cc(Oc3cccnc3)nc12 |r,wU:20.21,22.25,(48.46,-32.37,;49.53,-31.26,;49.1,-29.78,;50.17,-28.67,;51.66,-29.03,;52.09,-30.5,;51.03,-31.63,;51.46,-33.1,;50.39,-34.22,;52.95,-33.47,;54.02,-32.36,;55.49,-31.92,;54.38,-30.85,;49.74,-27.2,;50.69,-25.98,;49.82,-24.7,;48.34,-25.14,;47.04,-24.33,;47.09,-22.79,;45.78,-21.98,;44.42,-22.71,;43.98,-24.18,;42.51,-23.74,;41.01,-23.33,;41.41,-24.82,;42.95,-22.26,;45.69,-25.06,;45.64,-26.6,;44.29,-27.33,;44.25,-28.87,;42.89,-29.59,;42.85,-31.13,;44.16,-31.94,;45.52,-31.2,;45.56,-29.66,;46.95,-27.4,;48.29,-26.68,)| Show InChI InChI=1S/C28H30N6O3/c1-17-10-19(5-8-22(17)27(35)32-20-6-7-20)23-16-31-34-24(30-14-18-12-28(2,36)13-18)11-25(33-26(23)34)37-21-4-3-9-29-15-21/h3-5,8-11,15-16,18,20,30,36H,6-7,12-14H2,1-2H3,(H,32,35)/t18-,28+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant N-terminal GST-tagged and sumo-tagged human TTK (1 to 275 residues) expressed in Escherichia coli pre-incubated... |
ACS Med Chem Lett 7: 671-5 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00485
BindingDB Entry DOI: 10.7270/Q2K35Z46 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073585
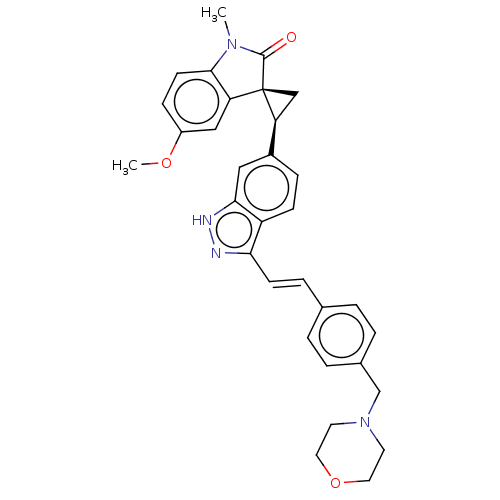
(CHEMBL3408945 | US10358436, Example A102 | US99078...)Show SMILES COc1ccc2N(C)C(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCOCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive binding to PLK4 (unknown origin) by double reciprocal plot analysis in presence of ATP |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50364339
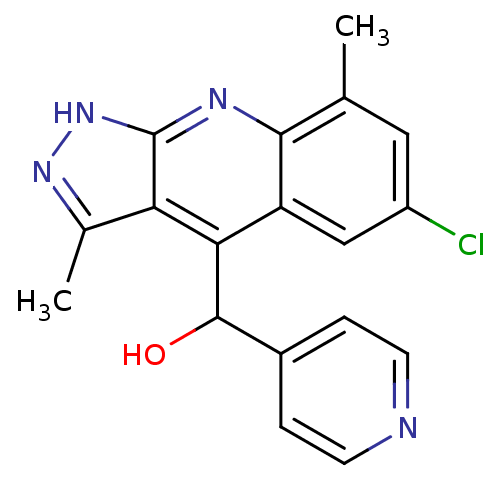
(CHEMBL1950085)Show SMILES Cc1n[nH]c2nc3c(C)cc(Cl)cc3c(C(O)c3ccncc3)c12 Show InChI InChI=1S/C18H15ClN4O/c1-9-7-12(19)8-13-15(17(24)11-3-5-20-6-4-11)14-10(2)22-23-18(14)21-16(9)13/h3-8,17,24H,1-2H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1335-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.080
BindingDB Entry DOI: 10.7270/Q2RF5VGF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50139773
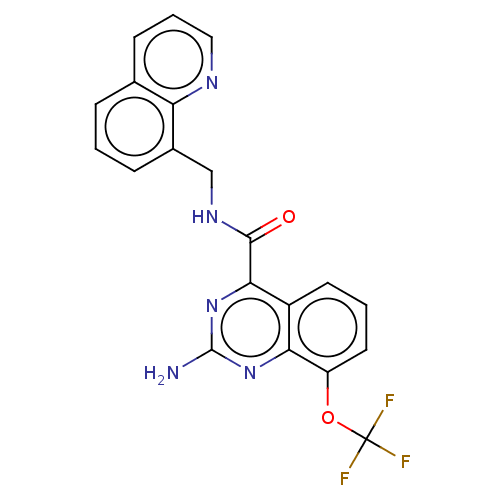
(CHEMBL3765379 | US10138212, Example 101)Show SMILES Nc1nc(C(=O)NCc2cccc3cccnc23)c2cccc(OC(F)(F)F)c2n1 Show InChI InChI=1S/C20H14F3N5O2/c21-20(22,23)30-14-8-2-7-13-16(14)27-19(24)28-17(13)18(29)26-10-12-5-1-4-11-6-3-9-25-15(11)12/h1-9H,10H2,(H,26,29)(H2,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... |
Bioorg Med Chem Lett 26: 1348-54 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.048
BindingDB Entry DOI: 10.7270/Q2S46TT3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50139771
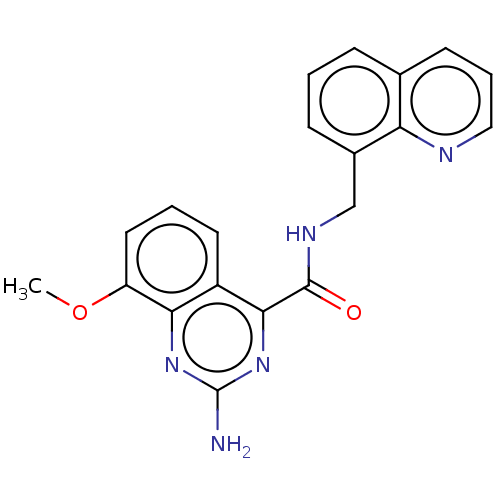
(CHEMBL3765580 | US10138212, Example 12)Show InChI InChI=1S/C20H17N5O2/c1-27-15-9-3-8-14-17(15)24-20(21)25-18(14)19(26)23-11-13-6-2-5-12-7-4-10-22-16(12)13/h2-10H,11H2,1H3,(H,23,26)(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... |
Bioorg Med Chem Lett 26: 1348-54 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.048
BindingDB Entry DOI: 10.7270/Q2S46TT3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073587

(CHEMBL3408947 | US10358436, Example A185 | US20230...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive binding to PLK4 (unknown origin) by double reciprocal plot analysis in presence of ATP |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50139748
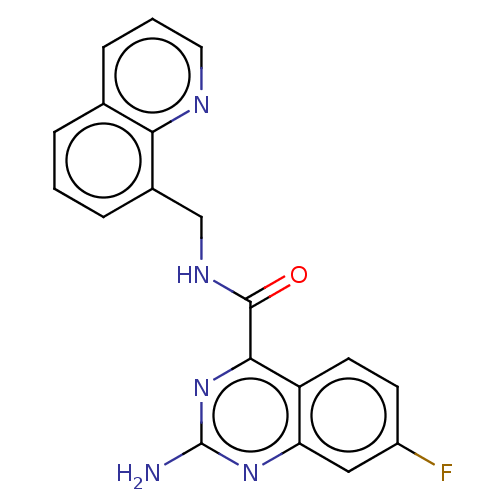
(CHEMBL3763717)Show InChI InChI=1S/C19H14FN5O/c20-13-6-7-14-15(9-13)24-19(21)25-17(14)18(26)23-10-12-4-1-3-11-5-2-8-22-16(11)12/h1-9H,10H2,(H,23,26)(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... |
Bioorg Med Chem Lett 26: 1348-54 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.048
BindingDB Entry DOI: 10.7270/Q2S46TT3 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50364340
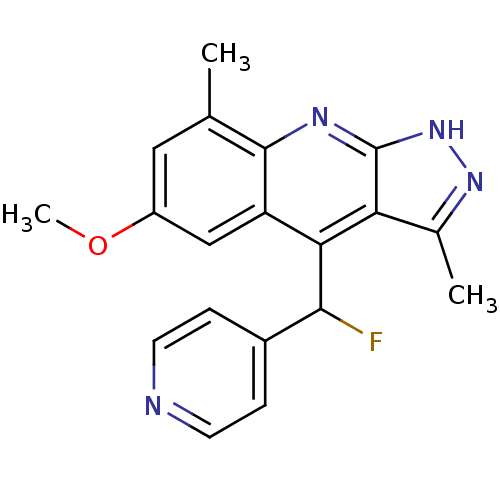
(CHEMBL1950084)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(C(F)c3ccncc3)c2c1 Show InChI InChI=1S/C19H17FN4O/c1-10-8-13(25-3)9-14-16(17(20)12-4-6-21-7-5-12)15-11(2)23-24-19(15)22-18(10)14/h4-9,17H,1-3H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1335-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.080
BindingDB Entry DOI: 10.7270/Q2RF5VGF |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50364335
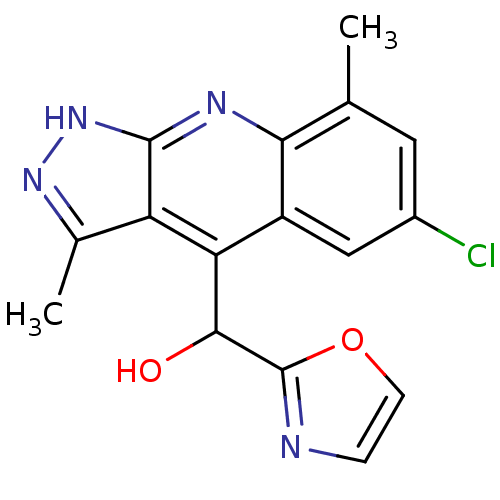
(CHEMBL1950089)Show SMILES Cc1n[nH]c2nc3c(C)cc(Cl)cc3c(C(O)c3ncco3)c12 Show InChI InChI=1S/C16H13ClN4O2/c1-7-5-9(17)6-10-12(14(22)16-18-3-4-23-16)11-8(2)20-21-15(11)19-13(7)10/h3-6,14,22H,1-2H3,(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1335-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.080
BindingDB Entry DOI: 10.7270/Q2RF5VGF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50139655
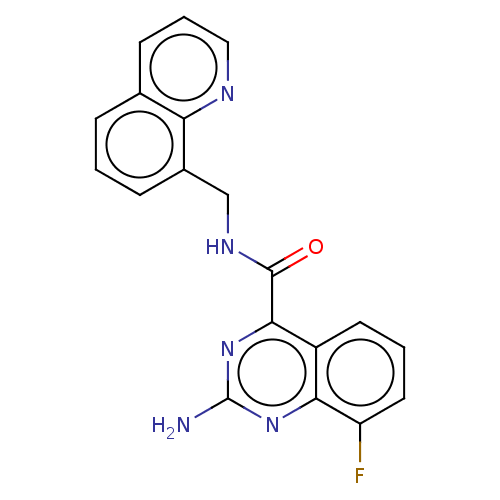
(CHEMBL3764083)Show InChI InChI=1S/C19H14FN5O/c20-14-8-2-7-13-16(14)24-19(21)25-17(13)18(26)23-10-12-5-1-4-11-6-3-9-22-15(11)12/h1-9H,10H2,(H,23,26)(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... |
Bioorg Med Chem Lett 26: 1348-54 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.048
BindingDB Entry DOI: 10.7270/Q2S46TT3 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362726
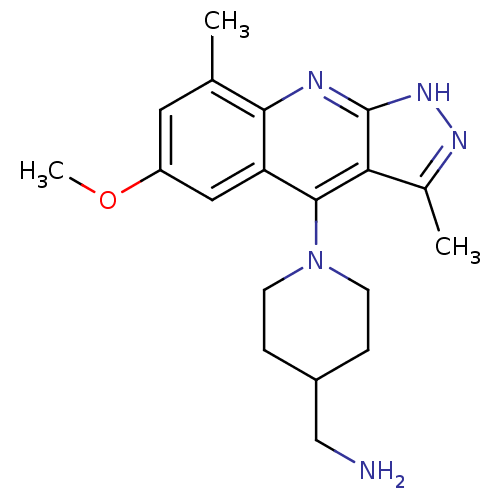
(CHEMBL1939796)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(N3CCC(CN)CC3)c2c1 Show InChI InChI=1S/C19H25N5O/c1-11-8-14(25-3)9-15-17(11)21-19-16(12(2)22-23-19)18(15)24-6-4-13(10-20)5-7-24/h8-9,13H,4-7,10,20H2,1-3H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE10A using [3H]cAMP as substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1019-22 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.127
BindingDB Entry DOI: 10.7270/Q2BV7H3J |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362037

(CHEMBL1939916)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(CN3CCOC(CO)C3)c2c1 Show InChI InChI=1S/C19H24N4O3/c1-11-6-13(25-3)7-15-16(9-23-4-5-26-14(8-23)10-24)17-12(2)21-22-19(17)20-18(11)15/h6-7,14,24H,4-5,8-10H2,1-3H3,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]cAMP from human recombinant PDE10A1 by competitive binding assay |
Bioorg Med Chem Lett 22: 235-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.023
BindingDB Entry DOI: 10.7270/Q2862GWC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50139771

(CHEMBL3765580 | US10138212, Example 12)Show InChI InChI=1S/C20H17N5O2/c1-27-15-9-3-8-14-17(15)24-20(21)25-18(14)19(26)23-11-13-6-2-5-12-7-4-10-22-16(12)13/h2-10H,11H2,1H3,(H,23,26)(H2,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to rat A2A adenosine receptor |
Bioorg Med Chem Lett 26: 1348-54 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.048
BindingDB Entry DOI: 10.7270/Q2S46TT3 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Arg8-vasopressin from human vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter |
Bioorg Med Chem Lett 19: 6018-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.050
BindingDB Entry DOI: 10.7270/Q2J9679W |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362722
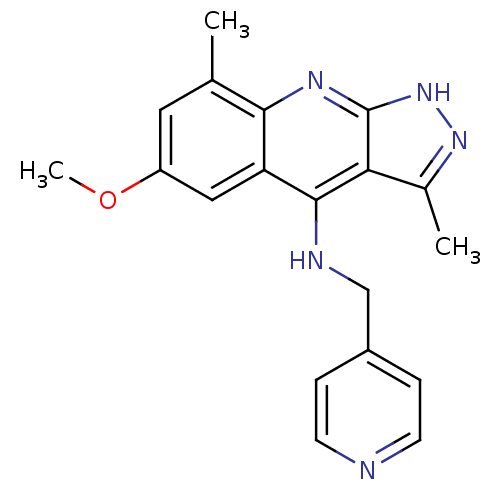
(CHEMBL1939800)Show InChI InChI=1S/C19H19N5O/c1-11-8-14(25-3)9-15-17(11)22-19-16(12(2)23-24-19)18(15)21-10-13-4-6-20-7-5-13/h4-9H,10H2,1-3H3,(H2,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE10A using [3H]cAMP as substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1019-22 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.127
BindingDB Entry DOI: 10.7270/Q2BV7H3J |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50364332

(CHEMBL1949940)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(C(O)c3ncco3)c2c1 Show InChI InChI=1S/C17H16N4O3/c1-8-6-10(23-3)7-11-13(15(22)17-18-4-5-24-17)12-9(2)20-21-16(12)19-14(8)11/h4-7,15,22H,1-3H3,(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1335-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.080
BindingDB Entry DOI: 10.7270/Q2RF5VGF |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50081537
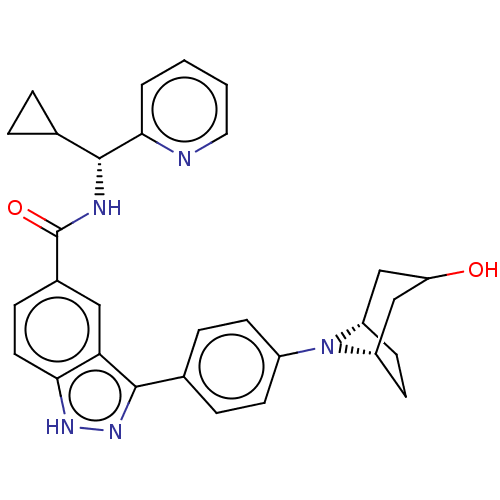
(CHEMBL3422092)Show SMILES [H][C@]12CC[C@@]([H])(CC(O)C1)N2c1ccc(cc1)-c1n[nH]c2ccc(cc12)C(=O)N[C@H](C1CC1)c1ccccn1 |r,@:7| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of amino terminal GST-fused full length human TTK using His6-SUMO-TTK-N as substrate by Lineweaver-Burk plot analysis in prese... |
J Med Chem 58: 3366-92 (2015)
Article DOI: 10.1021/jm501740a
BindingDB Entry DOI: 10.7270/Q2Q52RCN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50139765
(CHEMBL3763830 | US10138212, Example 96)Show InChI InChI=1S/C19H14BrN5O/c20-14-8-2-7-13-16(14)24-19(21)25-17(13)18(26)23-10-12-5-1-4-11-6-3-9-22-15(11)12/h1-9H,10H2,(H,23,26)(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... |
Bioorg Med Chem Lett 26: 1348-54 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.048
BindingDB Entry DOI: 10.7270/Q2S46TT3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073586
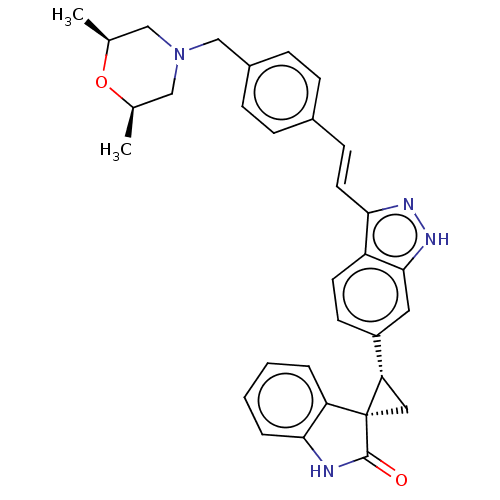
(CHEMBL3408946 | US10358436, Example A198 | US99078...)Show SMILES C[C@H]1CN(Cc2ccc(\C=C\c3n[nH]c4cc(ccc34)[C@@H]3C[C@@]33C(=O)Nc4ccccc34)cc2)C[C@@H](C)O1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive binding to PLK4 (unknown origin) by double reciprocal plot analysis in presence of ATP |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50183019
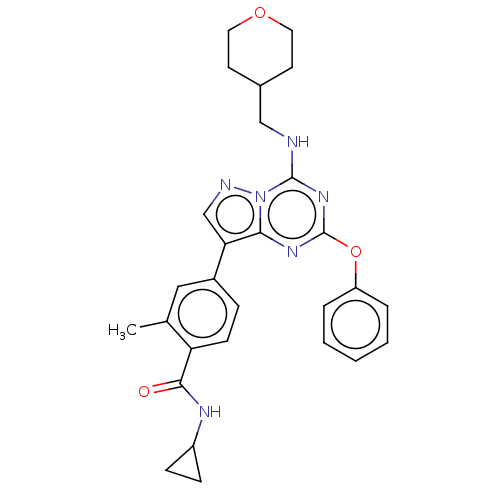
(CHEMBL3819210)Show SMILES Cc1cc(ccc1C(=O)NC1CC1)-c1cnn2c(NCC3CCOCC3)nc(Oc3ccccc3)nc12 Show InChI InChI=1S/C28H30N6O3/c1-18-15-20(7-10-23(18)26(35)31-21-8-9-21)24-17-30-34-25(24)32-28(37-22-5-3-2-4-6-22)33-27(34)29-16-19-11-13-36-14-12-19/h2-7,10,15,17,19,21H,8-9,11-14,16H2,1H3,(H,31,35)(H,29,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of TTK (unknown origin) by double reciprocal plot analysis in presence of ATP |
Bioorg Med Chem Lett 26: 3562-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.06.021
BindingDB Entry DOI: 10.7270/Q28G8NNB |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50364336

(CHEMBL1950088)Show SMILES Cc1n[nH]c2nc3c(C)cc(Cl)cc3c(C(O)c3ccnc(F)c3)c12 Show InChI InChI=1S/C18H14ClFN4O/c1-8-5-11(19)7-12-15(17(25)10-3-4-21-13(20)6-10)14-9(2)23-24-18(14)22-16(8)12/h3-7,17,25H,1-2H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1335-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.080
BindingDB Entry DOI: 10.7270/Q2RF5VGF |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50392835

(CHEMBL2151181)Show SMILES CC#Cc1cncc(c1)-c1cc(Cl)c(s1)[C@]1(C)CC(=O)N(C)C(N)=N1 |r,c:25| Show InChI InChI=1S/C18H17ClN4OS/c1-4-5-11-6-12(10-21-9-11)14-7-13(19)16(25-14)18(2)8-15(24)23(3)17(20)22-18/h6-7,9-10H,8H2,1-3H3,(H2,20,22)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE2 by FRET assay |
ACS Med Chem Lett 3: 897-902 (2012)
Article DOI: 10.1021/ml3001165
BindingDB Entry DOI: 10.7270/Q23779SN |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362728
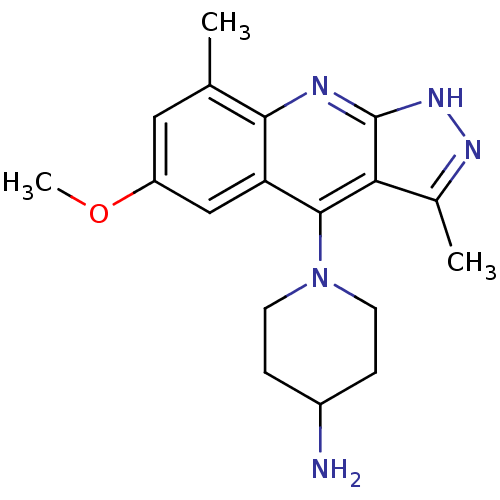
(CHEMBL1939794)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(N3CCC(N)CC3)c2c1 Show InChI InChI=1S/C18H23N5O/c1-10-8-13(24-3)9-14-16(10)20-18-15(11(2)21-22-18)17(14)23-6-4-12(19)5-7-23/h8-9,12H,4-7,19H2,1-3H3,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE10A using [3H]cAMP as substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1019-22 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.127
BindingDB Entry DOI: 10.7270/Q2BV7H3J |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362035
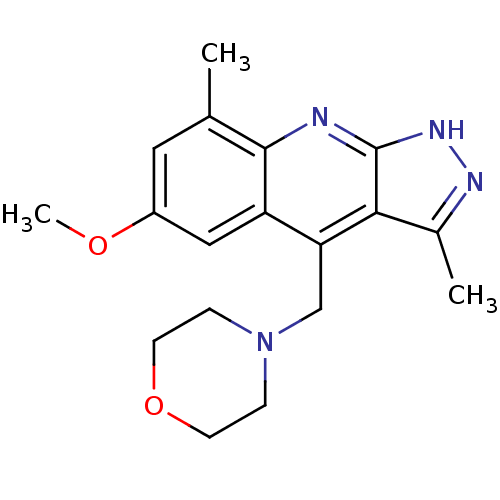
(CHEMBL1939914)Show InChI InChI=1S/C18H22N4O2/c1-11-8-13(23-3)9-14-15(10-22-4-6-24-7-5-22)16-12(2)20-21-18(16)19-17(11)14/h8-9H,4-7,10H2,1-3H3,(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]cAMP from human recombinant PDE10A1 by competitive binding assay |
Bioorg Med Chem Lett 22: 235-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.023
BindingDB Entry DOI: 10.7270/Q2862GWC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362047
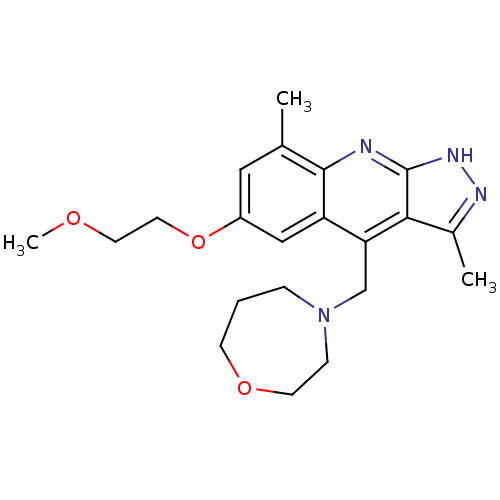
(CHEMBL1940057)Show SMILES COCCOc1cc(C)c2nc3[nH]nc(C)c3c(CN3CCCOCC3)c2c1 Show InChI InChI=1S/C21H28N4O3/c1-14-11-16(28-10-9-26-3)12-17-18(13-25-5-4-7-27-8-6-25)19-15(2)23-24-21(19)22-20(14)17/h11-12H,4-10,13H2,1-3H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]cAMP from human recombinant PDE10A1 by competitive binding assay |
Bioorg Med Chem Lett 22: 235-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.023
BindingDB Entry DOI: 10.7270/Q2862GWC |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter |
Bioorg Med Chem Lett 19: 6018-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.050
BindingDB Entry DOI: 10.7270/Q2J9679W |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50364346

(CHEMBL1950083)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(C(=O)c3ccncc3)c2c1 Show InChI InChI=1S/C19H16N4O2/c1-10-8-13(25-3)9-14-16(18(24)12-4-6-20-7-5-12)15-11(2)22-23-19(15)21-17(10)14/h4-9H,1-3H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1335-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.080
BindingDB Entry DOI: 10.7270/Q2RF5VGF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50139653
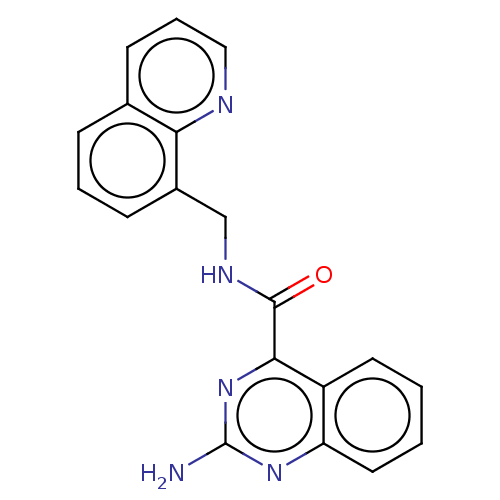
(CHEMBL3765818)Show InChI InChI=1S/C19H15N5O/c20-19-23-15-9-2-1-8-14(15)17(24-19)18(25)22-11-13-6-3-5-12-7-4-10-21-16(12)13/h1-10H,11H2,(H,22,25)(H2,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... |
Bioorg Med Chem Lett 26: 1348-54 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.048
BindingDB Entry DOI: 10.7270/Q2S46TT3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50139830
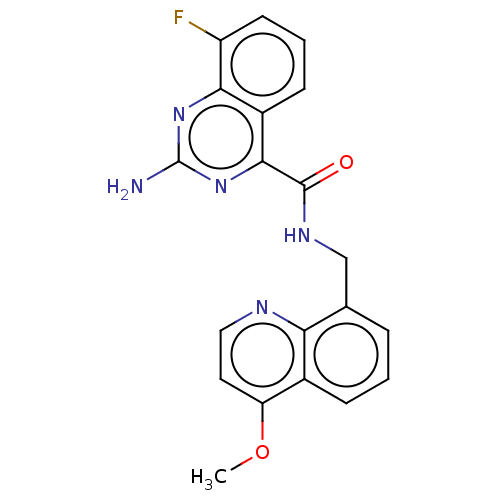
(CHEMBL3763374)Show SMILES COc1ccnc2c(CNC(=O)c3nc(N)nc4c(F)cccc34)cccc12 Show InChI InChI=1S/C20H16FN5O2/c1-28-15-8-9-23-16-11(4-2-5-12(15)16)10-24-19(27)18-13-6-3-7-14(21)17(13)25-20(22)26-18/h2-9H,10H2,1H3,(H,24,27)(H2,22,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to rat A2A adenosine receptor |
Bioorg Med Chem Lett 26: 1348-54 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.048
BindingDB Entry DOI: 10.7270/Q2S46TT3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50139655

(CHEMBL3764083)Show InChI InChI=1S/C19H14FN5O/c20-14-8-2-7-13-16(14)24-19(21)25-17(13)18(26)23-10-12-5-1-4-11-6-3-9-22-15(11)12/h1-9H,10H2,(H,23,26)(H2,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to rat A2A adenosine receptor |
Bioorg Med Chem Lett 26: 1348-54 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.048
BindingDB Entry DOI: 10.7270/Q2S46TT3 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50380636

(CHEMBL2017060)Show SMILES COc1cc2CCn3cnc(-c4cnc(s4)C(=O)N4CCCOCC4)c3-c2cc1OC Show InChI InChI=1S/C22H24N4O4S/c1-28-16-10-14-4-6-26-13-24-19(20(26)15(14)11-17(16)29-2)18-12-23-21(31-18)22(27)25-5-3-8-30-9-7-25/h10-13H,3-9H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A assessed as inhibition of [3H]cAMP hydrolysis by scintillation proximity assay |
Bioorg Med Chem Lett 22: 2585-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.113
BindingDB Entry DOI: 10.7270/Q2319WX5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50392835

(CHEMBL2151181)Show SMILES CC#Cc1cncc(c1)-c1cc(Cl)c(s1)[C@]1(C)CC(=O)N(C)C(N)=N1 |r,c:25| Show InChI InChI=1S/C18H17ClN4OS/c1-4-5-11-6-12(10-21-9-11)14-7-13(19)16(25-14)18(2)8-15(24)23(3)17(20)22-18/h6-7,9-10H,8H2,1-3H3,(H2,20,22)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 by FRET assay |
ACS Med Chem Lett 3: 897-902 (2012)
Article DOI: 10.1021/ml3001165
BindingDB Entry DOI: 10.7270/Q23779SN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50139817
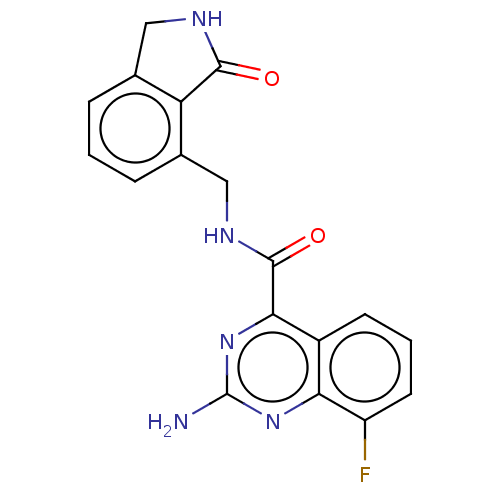
(CHEMBL3765164)Show SMILES Nc1nc(C(=O)NCc2cccc3CNC(=O)c23)c2cccc(F)c2n1 Show InChI InChI=1S/C18H14FN5O2/c19-12-6-2-5-11-14(12)23-18(20)24-15(11)17(26)22-8-10-4-1-3-9-7-21-16(25)13(9)10/h1-6H,7-8H2,(H,21,25)(H,22,26)(H2,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... |
Bioorg Med Chem Lett 26: 1348-54 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.048
BindingDB Entry DOI: 10.7270/Q2S46TT3 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50364344
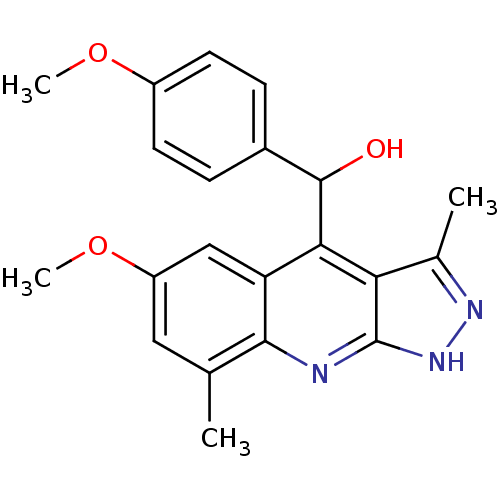
(CHEMBL1950078)Show SMILES COc1ccc(cc1)C(O)c1c2c(C)n[nH]c2nc2c(C)cc(OC)cc12 Show InChI InChI=1S/C21H21N3O3/c1-11-9-15(27-4)10-16-18(17-12(2)23-24-21(17)22-19(11)16)20(25)13-5-7-14(26-3)8-6-13/h5-10,20,25H,1-4H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1335-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.080
BindingDB Entry DOI: 10.7270/Q2RF5VGF |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362046

(CHEMBL1940056)Show InChI InChI=1S/C18H22N4O2/c1-11-8-13(23)9-14-15(10-22-4-3-6-24-7-5-22)16-12(2)20-21-18(16)19-17(11)14/h8-9,23H,3-7,10H2,1-2H3,(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]cAMP from human recombinant PDE10A1 by competitive binding assay |
Bioorg Med Chem Lett 22: 235-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.023
BindingDB Entry DOI: 10.7270/Q2862GWC |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50364345
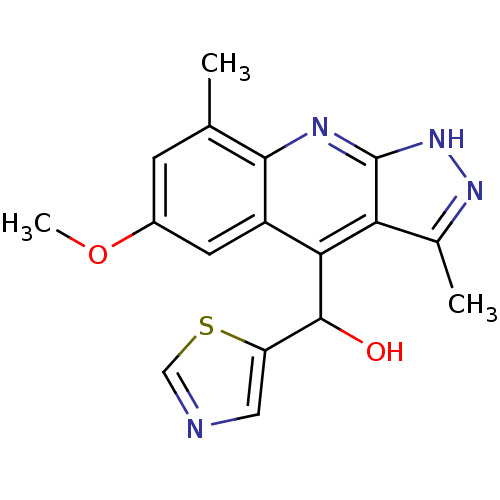
(CHEMBL1949942)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(C(O)c3cncs3)c2c1 Show InChI InChI=1S/C17H16N4O2S/c1-8-4-10(23-3)5-11-14(16(22)12-6-18-7-24-12)13-9(2)20-21-17(13)19-15(8)11/h4-7,16,22H,1-3H3,(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1335-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.080
BindingDB Entry DOI: 10.7270/Q2RF5VGF |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50364342

(CHEMBL1950081)Show SMILES COc1ccc(cc1)C(C#N)c1c2c(C)n[nH]c2nc2c(C)cc(OC)cc12 Show InChI InChI=1S/C22H20N4O2/c1-12-9-16(28-4)10-17-20(19-13(2)25-26-22(19)24-21(12)17)18(11-23)14-5-7-15(27-3)8-6-14/h5-10,18H,1-4H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1335-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.080
BindingDB Entry DOI: 10.7270/Q2RF5VGF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50139767
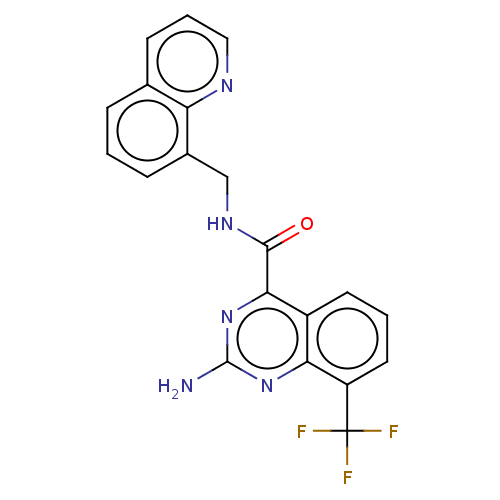
(CHEMBL3763199 | US10138212, Example 92)Show SMILES Nc1nc(C(=O)NCc2cccc3cccnc23)c2cccc(c2n1)C(F)(F)F Show InChI InChI=1S/C20H14F3N5O/c21-20(22,23)14-8-2-7-13-16(14)27-19(24)28-17(13)18(29)26-10-12-5-1-4-11-6-3-9-25-15(11)12/h1-9H,10H2,(H,26,29)(H2,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... |
Bioorg Med Chem Lett 26: 1348-54 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.048
BindingDB Entry DOI: 10.7270/Q2S46TT3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50139838
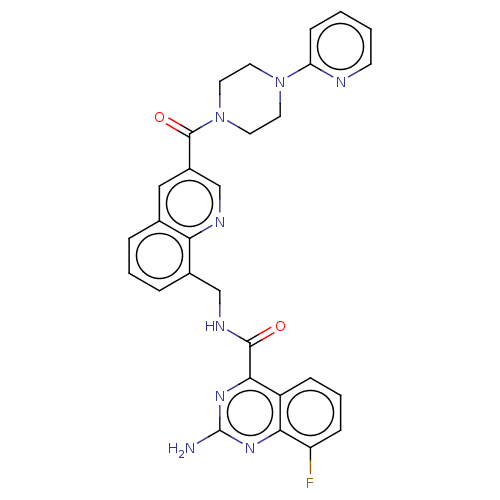
(CHEMBL3763514)Show SMILES Nc1nc(C(=O)NCc2cccc3cc(cnc23)C(=O)N2CCN(CC2)c2ccccn2)c2cccc(F)c2n1 Show InChI InChI=1S/C29H25FN8O2/c30-22-8-4-7-21-25(22)35-29(31)36-26(21)27(39)34-16-19-6-3-5-18-15-20(17-33-24(18)19)28(40)38-13-11-37(12-14-38)23-9-1-2-10-32-23/h1-10,15,17H,11-14,16H2,(H,34,39)(H2,31,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... |
Bioorg Med Chem Lett 26: 1348-54 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.048
BindingDB Entry DOI: 10.7270/Q2S46TT3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50139773

(CHEMBL3765379 | US10138212, Example 101)Show SMILES Nc1nc(C(=O)NCc2cccc3cccnc23)c2cccc(OC(F)(F)F)c2n1 Show InChI InChI=1S/C20H14F3N5O2/c21-20(22,23)30-14-8-2-7-13-16(14)27-19(24)28-17(13)18(29)26-10-12-5-1-4-11-6-3-9-25-15(11)12/h1-9H,10H2,(H,26,29)(H2,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to rat A2A adenosine receptor |
Bioorg Med Chem Lett 26: 1348-54 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.048
BindingDB Entry DOI: 10.7270/Q2S46TT3 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50139752
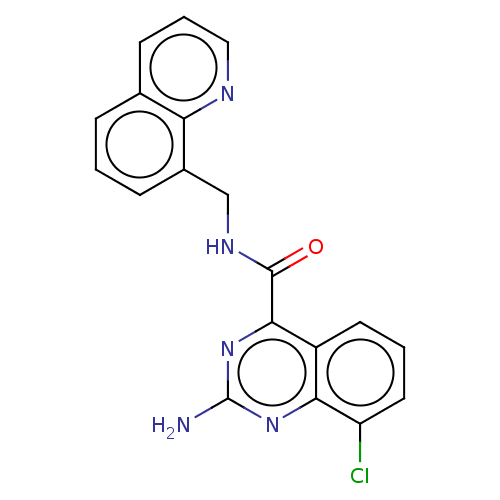
(CHEMBL3764434 | US10138212, Example 91)Show InChI InChI=1S/C19H14ClN5O/c20-14-8-2-7-13-16(14)24-19(21)25-17(13)18(26)23-10-12-5-1-4-11-6-3-9-22-15(11)12/h1-9H,10H2,(H,23,26)(H2,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to rat A2A adenosine receptor |
Bioorg Med Chem Lett 26: 1348-54 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.048
BindingDB Entry DOI: 10.7270/Q2S46TT3 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362036

(CHEMBL1939915)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(CN3CCOC(C)C3)c2c1 Show InChI InChI=1S/C19H24N4O2/c1-11-7-14(24-4)8-15-16(10-23-5-6-25-12(2)9-23)17-13(3)21-22-19(17)20-18(11)15/h7-8,12H,5-6,9-10H2,1-4H3,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]cAMP from human recombinant PDE10A1 by competitive binding assay |
Bioorg Med Chem Lett 22: 235-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.023
BindingDB Entry DOI: 10.7270/Q2862GWC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50139752

(CHEMBL3764434 | US10138212, Example 91)Show InChI InChI=1S/C19H14ClN5O/c20-14-8-2-7-13-16(14)24-19(21)25-17(13)18(26)23-10-12-5-1-4-11-6-3-9-22-15(11)12/h1-9H,10H2,(H,23,26)(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... |
Bioorg Med Chem Lett 26: 1348-54 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.048
BindingDB Entry DOI: 10.7270/Q2S46TT3 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50364333
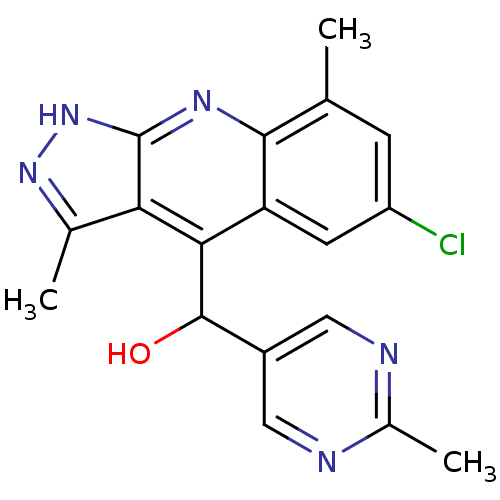
(CHEMBL1950091)Show SMILES Cc1n[nH]c2nc3c(C)cc(Cl)cc3c(C(O)c3cnc(C)nc3)c12 Show InChI InChI=1S/C18H16ClN5O/c1-8-4-12(19)5-13-15(17(25)11-6-20-10(3)21-7-11)14-9(2)23-24-18(14)22-16(8)13/h4-7,17,25H,1-3H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE10A assessed as hydrolysis of cAMP to AMP using [3H]cAMP substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1335-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.080
BindingDB Entry DOI: 10.7270/Q2RF5VGF |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362033

(CHEMBL1939912)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(CN3CC[C@@H](O)C3)c2c1 |r| Show InChI InChI=1S/C18H22N4O2/c1-10-6-13(24-3)7-14-15(9-22-5-4-12(23)8-22)16-11(2)20-21-18(16)19-17(10)14/h6-7,12,23H,4-5,8-9H2,1-3H3,(H,19,20,21)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]cAMP from human recombinant PDE10A1 by competitive binding assay |
Bioorg Med Chem Lett 22: 235-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.023
BindingDB Entry DOI: 10.7270/Q2862GWC |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50362725
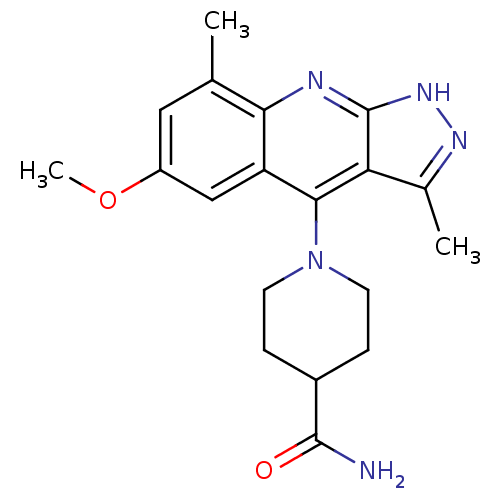
(CHEMBL1939797)Show SMILES COc1cc(C)c2nc3[nH]nc(C)c3c(N3CCC(CC3)C(N)=O)c2c1 Show InChI InChI=1S/C19H23N5O2/c1-10-8-13(26-3)9-14-16(10)21-19-15(11(2)22-23-19)17(14)24-6-4-12(5-7-24)18(20)25/h8-9,12H,4-7H2,1-3H3,(H2,20,25)(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE10A using [3H]cAMP as substrate by scintillation proximity assay |
Bioorg Med Chem Lett 22: 1019-22 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.127
BindingDB Entry DOI: 10.7270/Q2BV7H3J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50139766
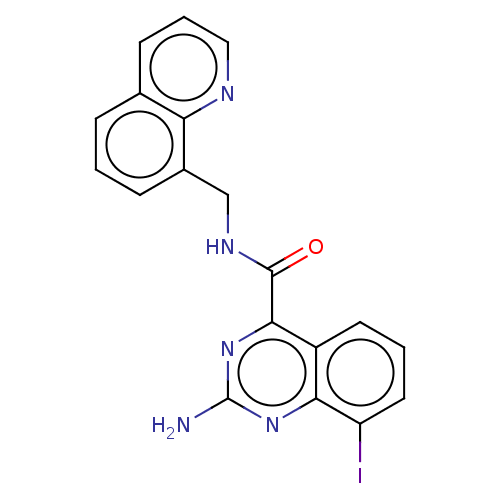
(CHEMBL3764070)Show InChI InChI=1S/C19H14IN5O/c20-14-8-2-7-13-16(14)24-19(21)25-17(13)18(26)23-10-12-5-1-4-11-6-3-9-22-15(11)12/h1-9H,10H2,(H,23,26)(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... |
Bioorg Med Chem Lett 26: 1348-54 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.048
BindingDB Entry DOI: 10.7270/Q2S46TT3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data