 Found 828 hits with Last Name = 'matico' and Initial = 'r'
Found 828 hits with Last Name = 'matico' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598905
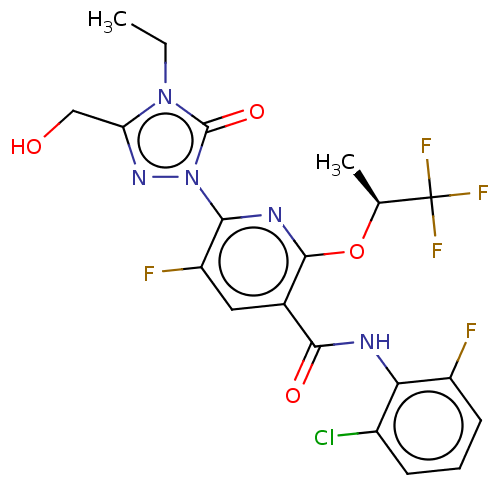
(CHEMBL5180161)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(F)cccc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598923
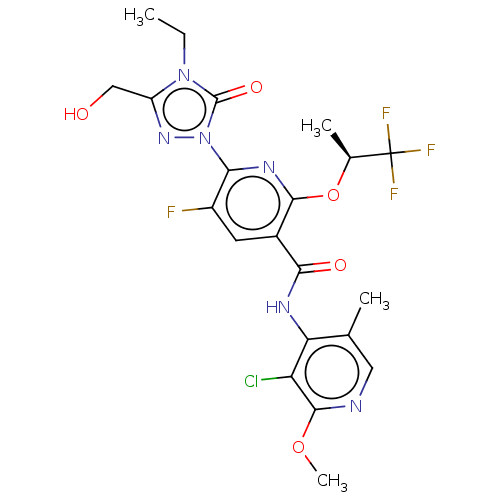
(CHEMBL5193821)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)cnc(OC)c2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598917
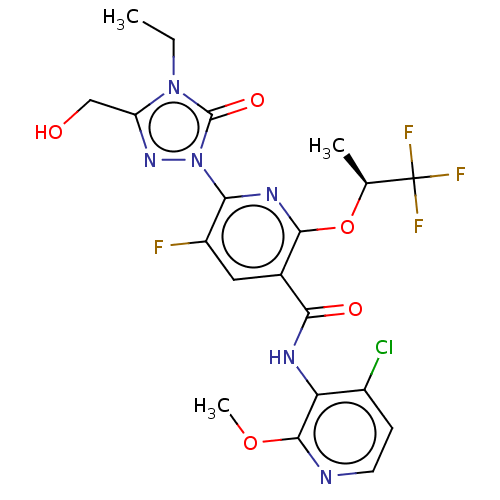
(CHEMBL5171223)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(Cl)ccnc2OC)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598902

(CHEMBL5187184)Show SMILES CCn1c(CO)nn(-c2nc(OC(C)C)c(cc2F)C(=O)Nc2c(F)cccc2Cl)c1=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598922

(CHEMBL5186161)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)cnc(OC)c2C)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598915

(CHEMBL5198030)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(Cl)ccnc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598926

(CHEMBL5206111)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)cc(OC)nc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50168737

((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...)Show SMILES C[C@@]1(O)CCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2ccc(F)cc2Cl)cc1 Show InChI InChI=1S/C20H22ClFN2O6S/c1-20(26)9-2-10-24(18(20)19(25)23-27)31(28,29)16-7-5-15(6-8-16)30-12-13-3-4-14(22)11-17(13)21/h3-8,11,18,26-27H,2,9-10,12H2,1H3,(H,23,25)/t18-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of MMP-13 using 5-FAM-TPGPLGL[Dap- (DNP)]ARRK(5-TAMRA)-amide as substrate after 45 mins |
J Med Chem 55: 7061-79 (2012)
Article DOI: 10.1021/jm300449x
BindingDB Entry DOI: 10.7270/Q2RX9D6T |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598913

(CHEMBL5193591)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)ccnc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598920

(CHEMBL5209113)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(F)cnc(OC)c2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM470454
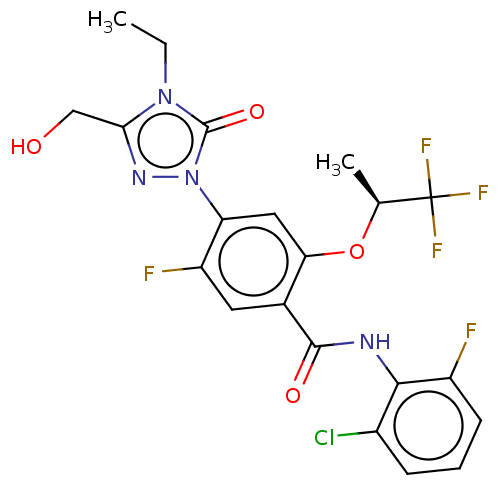
(N-(2-chloro-6-fluorophenyl)-4-[4-ethyl-3-(hydroxym...)Show SMILES CCn1c(CO)nn(-c2cc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(F)cccc2Cl)c1=O |r| Show InChI InChI=1S/C21H18ClF5N4O4/c1-3-30-17(9-32)29-31(20(30)34)15-8-16(35-10(2)21(25,26)27)11(7-14(15)24)19(33)28-18-12(22)5-4-6-13(18)23/h4-8,10,32H,3,9H2,1-2H3,(H,28,33)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50168737

((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...)Show SMILES C[C@@]1(O)CCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2ccc(F)cc2Cl)cc1 Show InChI InChI=1S/C20H22ClFN2O6S/c1-20(26)9-2-10-24(18(20)19(25)23-27)31(28,29)16-7-5-15(6-8-16)30-12-13-3-4-14(22)11-17(13)21/h3-8,11,18,26-27H,2,9-10,12H2,1H3,(H,23,25)/t18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of TACE using Mca-PLAQAV-Dpa-RSSSR-NH2 as substrate preincubated 15 mins measured every 30 sec for 30 mins |
J Med Chem 55: 7061-79 (2012)
Article DOI: 10.1021/jm300449x
BindingDB Entry DOI: 10.7270/Q2RX9D6T |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598914
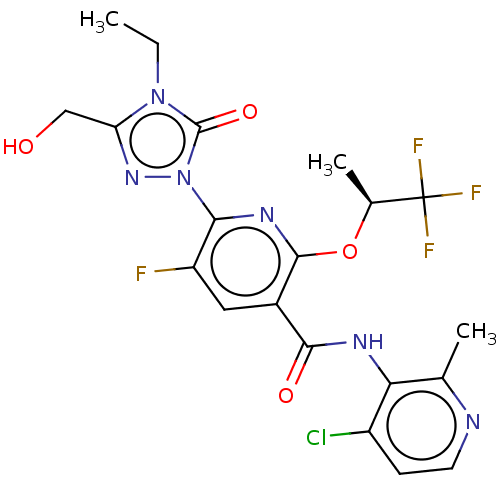
(CHEMBL5197263)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)nccc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26503
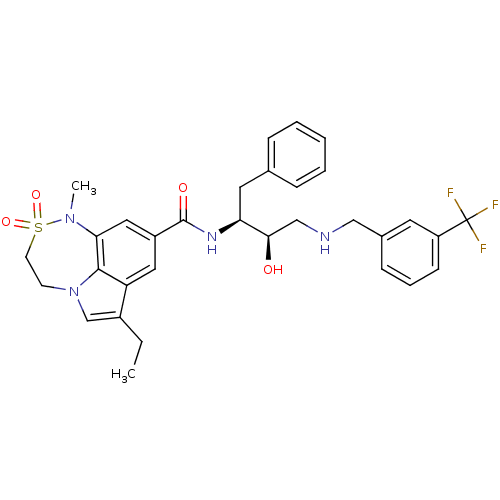
(3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trif...)Show SMILES CCc1cn2CCS(=O)(=O)N(C)c3cc(cc1c23)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C32H35F3N4O4S/c1-3-23-20-39-12-13-44(42,43)38(2)28-17-24(16-26(23)30(28)39)31(41)37-27(15-21-8-5-4-6-9-21)29(40)19-36-18-22-10-7-11-25(14-22)32(33,34)35/h4-11,14,16-17,20,27,29,36,40H,3,12-13,15,18-19H2,1-2H3,(H,37,41)/t27-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
J Med Chem 51: 3313-7 (2008)
Article DOI: 10.1021/jm800138h
BindingDB Entry DOI: 10.7270/Q2XS5SQR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26788
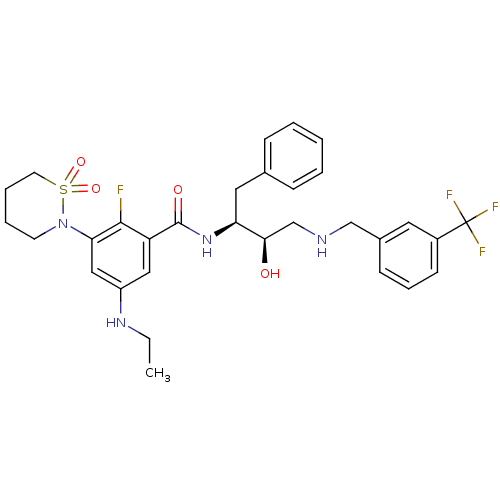
(3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-2-...)Show SMILES CCNc1cc(N2CCCCS2(=O)=O)c(F)c(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C31H36F4N4O4S/c1-2-37-24-17-25(29(32)27(18-24)39-13-6-7-14-44(39,42)43)30(41)38-26(16-21-9-4-3-5-10-21)28(40)20-36-19-22-11-8-12-23(15-22)31(33,34)35/h3-5,8-12,15,17-18,26,28,36-37,40H,2,6-7,13-14,16,19-20H2,1H3,(H,38,41)/t26-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 19: 3664-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.165
BindingDB Entry DOI: 10.7270/Q2F18X23 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26503

(3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trif...)Show SMILES CCc1cn2CCS(=O)(=O)N(C)c3cc(cc1c23)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C32H35F3N4O4S/c1-3-23-20-39-12-13-44(42,43)38(2)28-17-24(16-26(23)30(28)39)31(41)37-27(15-21-8-5-4-6-9-21)29(40)19-36-18-22-10-7-11-25(14-22)32(33,34)35/h4-11,14,16-17,20,27,29,36,40H,3,12-13,15,18-19H2,1-2H3,(H,37,41)/t27-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 19: 3669-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.150
BindingDB Entry DOI: 10.7270/Q29885BR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM29782
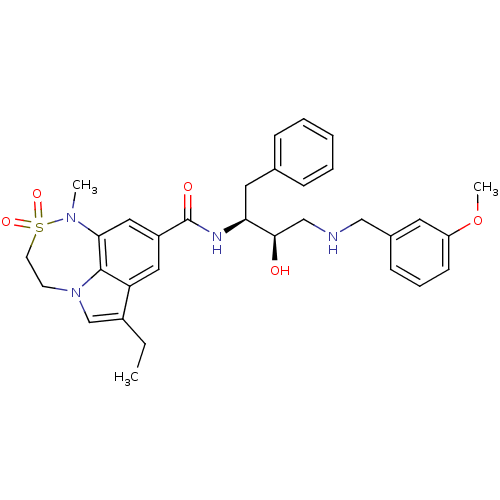
(7,6,5 tricyclic sulfonamide, 22)Show SMILES CCc1cn2CCS(=O)(=O)N(C)c3cc(cc1c23)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(OC)c1 |r| Show InChI InChI=1S/C32H38N4O5S/c1-4-24-21-36-13-14-42(39,40)35(2)29-18-25(17-27(24)31(29)36)32(38)34-28(16-22-9-6-5-7-10-22)30(37)20-33-19-23-11-8-12-26(15-23)41-3/h5-12,15,17-18,21,28,30,33,37H,4,13-14,16,19-20H2,1-3H3,(H,34,38)/t28-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 19: 3669-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.150
BindingDB Entry DOI: 10.7270/Q29885BR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26503

(3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trif...)Show SMILES CCc1cn2CCS(=O)(=O)N(C)c3cc(cc1c23)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C32H35F3N4O4S/c1-3-23-20-39-12-13-44(42,43)38(2)28-17-24(16-26(23)30(28)39)31(41)37-27(15-21-8-5-4-6-9-21)29(40)19-36-18-22-10-7-11-25(14-22)32(33,34)35/h4-11,14,16-17,20,27,29,36,40H,3,12-13,15,18-19H2,1-2H3,(H,37,41)/t27-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 19: 3674-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.149
BindingDB Entry DOI: 10.7270/Q25H7DKK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598919

(CHEMBL5176109)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(F)cncc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598916
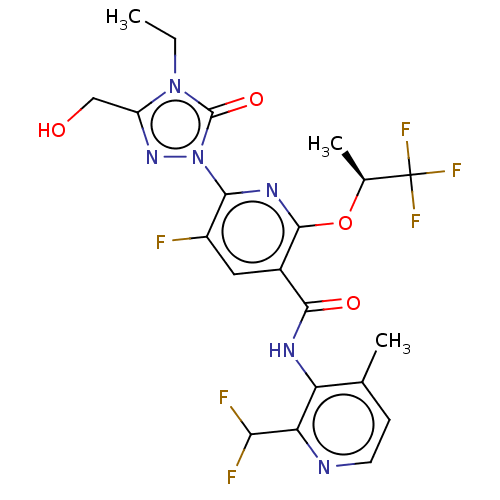
(CHEMBL5205042)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)ccnc2C(F)F)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598918
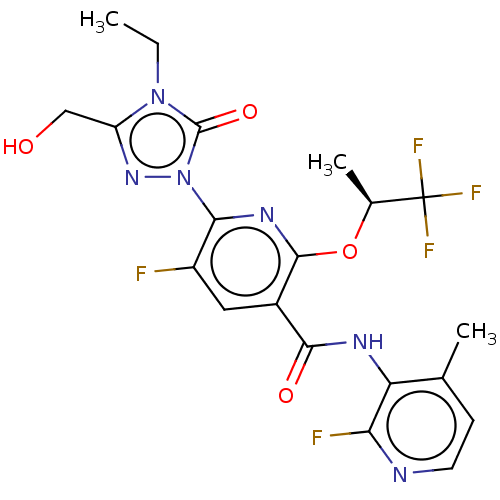
(CHEMBL5207423)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)ccnc2F)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598910
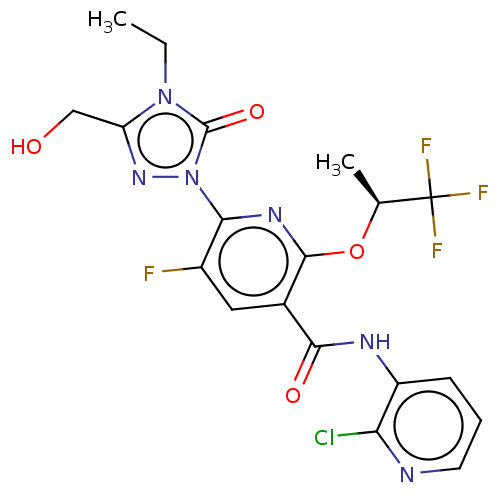
(CHEMBL5197809)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2cccnc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50322882
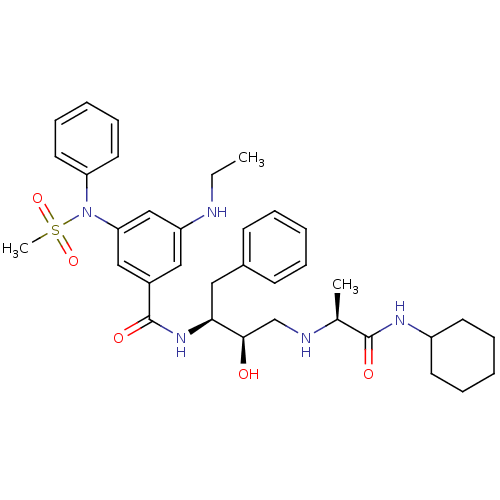
(CHEMBL1210359 | N-((1S,2R)-3-(((1S)-2-(CYCLOHEXYLA...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN[C@@H](C)C(=O)NC1CCCCC1)N(c1ccccc1)S(C)(=O)=O |r| Show InChI InChI=1S/C35H47N5O5S/c1-4-36-29-21-27(22-31(23-29)40(46(3,44)45)30-18-12-7-13-19-30)35(43)39-32(20-26-14-8-5-9-15-26)33(41)24-37-25(2)34(42)38-28-16-10-6-11-17-28/h5,7-9,12-15,18-19,21-23,25,28,32-33,36-37,41H,4,6,10-11,16-17,20,24H2,1-3H3,(H,38,42)(H,39,43)/t25-,32-,33+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 4639-44 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.111
BindingDB Entry DOI: 10.7270/Q2RB75K7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50322883
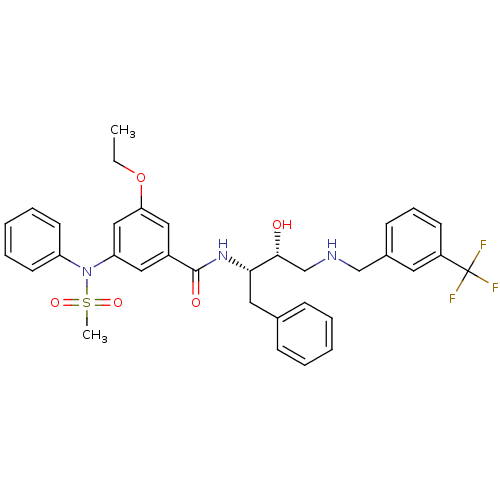
(3-ethoxy-N-((2S,3R)-3-hydroxy-1-phenyl-4-(3-(trifl...)Show SMILES CCOc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F)N(c1ccccc1)S(C)(=O)=O |r| Show InChI InChI=1S/C34H36F3N3O5S/c1-3-45-30-20-26(19-29(21-30)40(46(2,43)44)28-15-8-5-9-16-28)33(42)39-31(18-24-11-6-4-7-12-24)32(41)23-38-22-25-13-10-14-27(17-25)34(35,36)37/h4-17,19-21,31-32,38,41H,3,18,22-23H2,1-2H3,(H,39,42)/t31-,32+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 4639-44 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.111
BindingDB Entry DOI: 10.7270/Q2RB75K7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26502

(3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C31H37F3N4O4S/c1-2-36-26-17-24(18-27(19-26)38-13-6-7-14-43(38,41)42)30(40)37-28(16-22-9-4-3-5-10-22)29(39)21-35-20-23-11-8-12-25(15-23)31(32,33)34/h3-5,8-12,15,17-19,28-29,35-36,39H,2,6-7,13-14,16,20-21H2,1H3,(H,37,40)/t28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 19: 3664-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.165
BindingDB Entry DOI: 10.7270/Q2F18X23 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26502

(3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C31H37F3N4O4S/c1-2-36-26-17-24(18-27(19-26)38-13-6-7-14-43(38,41)42)30(40)37-28(16-22-9-4-3-5-10-22)29(39)21-35-20-23-11-8-12-25(15-23)31(32,33)34/h3-5,8-12,15,17-19,28-29,35-36,39H,2,6-7,13-14,16,20-21H2,1H3,(H,37,40)/t28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26502

(3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C31H37F3N4O4S/c1-2-36-26-17-24(18-27(19-26)38-13-6-7-14-43(38,41)42)30(40)37-28(16-22-9-4-3-5-10-22)29(39)21-35-20-23-11-8-12-25(15-23)31(32,33)34/h3-5,8-12,15,17-19,28-29,35-36,39H,2,6-7,13-14,16,20-21H2,1H3,(H,37,40)/t28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
J Med Chem 51: 3313-7 (2008)
Article DOI: 10.1021/jm800138h
BindingDB Entry DOI: 10.7270/Q2XS5SQR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50168737

((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...)Show SMILES C[C@@]1(O)CCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2ccc(F)cc2Cl)cc1 Show InChI InChI=1S/C20H22ClFN2O6S/c1-20(26)9-2-10-24(18(20)19(25)23-27)31(28,29)16-7-5-15(6-8-16)30-12-13-3-4-14(22)11-17(13)21/h3-8,11,18,26-27H,2,9-10,12H2,1H3,(H,23,25)/t18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 using WAAG-3R as substrate preincubated for 15 mins measured after 1 hr |
J Med Chem 55: 7061-79 (2012)
Article DOI: 10.1021/jm300449x
BindingDB Entry DOI: 10.7270/Q2RX9D6T |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50322916
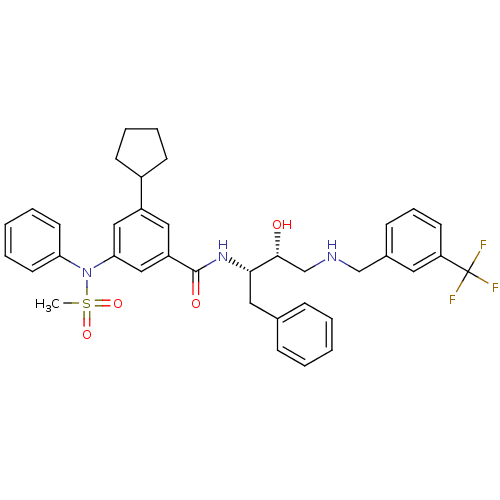
(3-cyclopentyl-N-((2S,3R)-3-hydroxy-1-phenyl-4-(3-(...)Show SMILES CS(=O)(=O)N(c1ccccc1)c1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F)C1CCCC1 |r| Show InChI InChI=1S/C37H40F3N3O4S/c1-48(46,47)43(32-17-6-3-7-18-32)33-22-29(28-14-8-9-15-28)21-30(23-33)36(45)42-34(20-26-11-4-2-5-12-26)35(44)25-41-24-27-13-10-16-31(19-27)37(38,39)40/h2-7,10-13,16-19,21-23,28,34-35,41,44H,8-9,14-15,20,24-25H2,1H3,(H,42,45)/t34-,35+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 4639-44 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.111
BindingDB Entry DOI: 10.7270/Q2RB75K7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM29796

(sulfonamide tricyclic analogue, 7)Show SMILES CCn1cc2CCS(=O)(=O)N(C)c3cc(cc1c23)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCCC(F)(F)F |r| Show InChI InChI=1S/C27H33F3N4O4S/c1-3-34-17-19-9-12-39(37,38)33(2)22-14-20(15-23(34)25(19)22)26(36)32-21(13-18-7-5-4-6-8-18)24(35)16-31-11-10-27(28,29)30/h4-8,14-15,17,21,24,31,35H,3,9-13,16H2,1-2H3,(H,32,36)/t21-,24+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 19: 3674-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.149
BindingDB Entry DOI: 10.7270/Q25H7DKK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26788

(3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-2-...)Show SMILES CCNc1cc(N2CCCCS2(=O)=O)c(F)c(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C31H36F4N4O4S/c1-2-37-24-17-25(29(32)27(18-24)39-13-6-7-14-44(39,42)43)30(41)38-26(16-21-9-4-3-5-10-21)28(40)20-36-19-22-11-8-12-23(15-22)31(33,34)35/h3-5,8-12,15,17-18,26,28,36-37,40H,2,6-7,13-14,16,19-20H2,1H3,(H,38,41)/t26-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26786
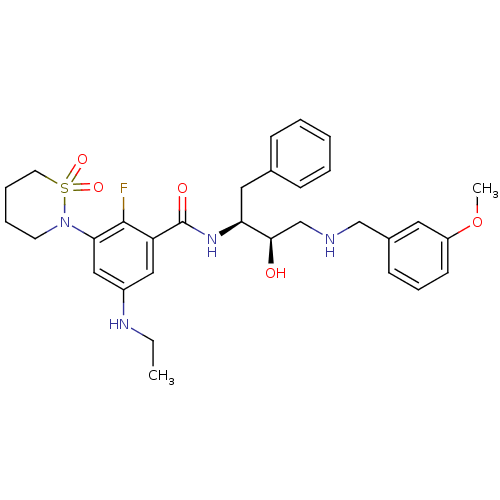
(3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-2-...)Show SMILES CCNc1cc(N2CCCCS2(=O)=O)c(F)c(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(OC)c1 |r| Show InChI InChI=1S/C31H39FN4O5S/c1-3-34-24-18-26(30(32)28(19-24)36-14-7-8-15-42(36,39)40)31(38)35-27(17-22-10-5-4-6-11-22)29(37)21-33-20-23-12-9-13-25(16-23)41-2/h4-6,9-13,16,18-19,27,29,33-34,37H,3,7-8,14-15,17,20-21H2,1-2H3,(H,35,38)/t27-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598908

(CHEMBL5193091)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2cnccc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26776
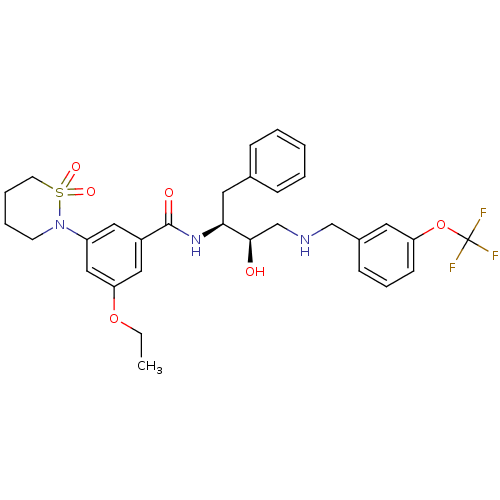
(3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-ethoxy-N-[(2S,3...)Show SMILES CCOc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(OC(F)(F)F)c1)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C31H36F3N3O6S/c1-2-42-27-18-24(17-25(19-27)37-13-6-7-14-44(37,40)41)30(39)36-28(16-22-9-4-3-5-10-22)29(38)21-35-20-23-11-8-12-26(15-23)43-31(32,33)34/h3-5,8-12,15,17-19,28-29,35,38H,2,6-7,13-14,16,20-21H2,1H3,(H,36,39)/t28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26777
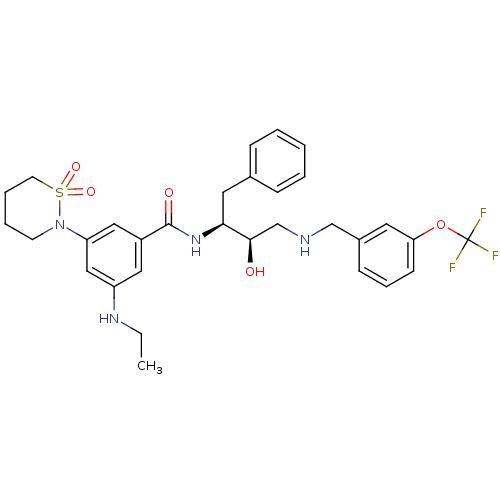
(3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(OC(F)(F)F)c1)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C31H37F3N4O5S/c1-2-36-25-17-24(18-26(19-25)38-13-6-7-14-44(38,41)42)30(40)37-28(16-22-9-4-3-5-10-22)29(39)21-35-20-23-11-8-12-27(15-23)43-31(32,33)34/h3-5,8-12,15,17-19,28-29,35-36,39H,2,6-7,13-14,16,20-21H2,1H3,(H,37,40)/t28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26782

(N-[(2S,3R)-1-(3,5-difluorophenyl)-3-hydroxy-4-{[(3...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNCc1cccc(OC)c1)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C31H38F2N4O5S/c1-3-35-26-15-23(16-27(18-26)37-9-4-5-10-43(37,40)41)31(39)36-29(14-22-11-24(32)17-25(33)12-22)30(38)20-34-19-21-7-6-8-28(13-21)42-2/h6-8,11-13,15-18,29-30,34-35,38H,3-5,9-10,14,19-20H2,1-2H3,(H,36,39)/t29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM29815

(sulfonamide tricyclic analogue, 28)Show SMILES CCn1cc2CCS(=O)(=O)N(C)c3cc(cc1c23)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNc1ccccc1 |r| Show InChI InChI=1S/C30H34N4O4S/c1-3-34-20-22-14-15-39(37,38)33(2)26-17-23(18-27(34)29(22)26)30(36)32-25(16-21-10-6-4-7-11-21)28(35)19-31-24-12-8-5-9-13-24/h4-13,17-18,20,25,28,31,35H,3,14-16,19H2,1-2H3,(H,32,36)/t25-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 19: 3674-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.149
BindingDB Entry DOI: 10.7270/Q25H7DKK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50322884
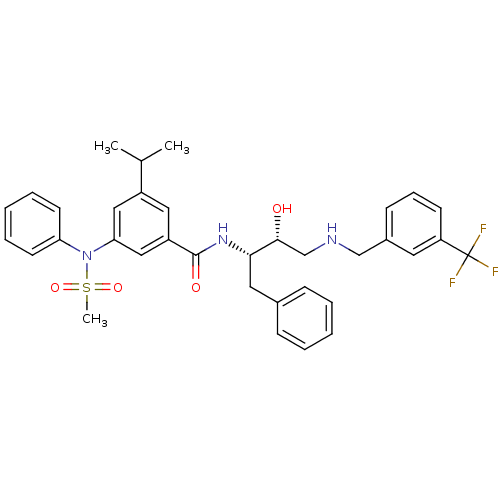
(CHEMBL1210361 | N-((2S,3R)-3-hydroxy-1-phenyl-4-(3...)Show SMILES CC(C)c1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F)N(c1ccccc1)S(C)(=O)=O |r| Show InChI InChI=1S/C35H38F3N3O4S/c1-24(2)27-19-28(21-31(20-27)41(46(3,44)45)30-15-8-5-9-16-30)34(43)40-32(18-25-11-6-4-7-12-25)33(42)23-39-22-26-13-10-14-29(17-26)35(36,37)38/h4-17,19-21,24,32-33,39,42H,18,22-23H2,1-3H3,(H,40,43)/t32-,33+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 4639-44 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.111
BindingDB Entry DOI: 10.7270/Q2RB75K7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26774

(3-(1,1-dioxo-1,2-thiazinan-2-yl)-N-[(2S,3R)-3-hydr...)Show SMILES CCCc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C32H38F3N3O4S/c1-2-9-24-16-26(20-28(18-24)38-14-6-7-15-43(38,41)42)31(40)37-29(19-23-10-4-3-5-11-23)30(39)22-36-21-25-12-8-13-27(17-25)32(33,34)35/h3-5,8,10-13,16-18,20,29-30,36,39H,2,6-7,9,14-15,19,21-22H2,1H3,(H,37,40)/t29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM29789

(7,6,5 tricyclic sulfonamide, 35)Show SMILES CCN1c2cc(cc3c(CC)cn(CCS1(=O)=O)c23)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC1CCOCC1 |r| Show InChI InChI=1S/C30H40N4O5S/c1-3-22-20-33-12-15-40(37,38)34(4-2)27-18-23(17-25(22)29(27)33)30(36)32-26(16-21-8-6-5-7-9-21)28(35)19-31-24-10-13-39-14-11-24/h5-9,17-18,20,24,26,28,31,35H,3-4,10-16,19H2,1-2H3,(H,32,36)/t26-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 19: 3669-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.150
BindingDB Entry DOI: 10.7270/Q29885BR |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26502

(3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C31H37F3N4O4S/c1-2-36-26-17-24(18-27(19-26)38-13-6-7-14-43(38,41)42)30(40)37-28(16-22-9-4-3-5-10-22)29(39)21-35-20-23-11-8-12-25(15-23)31(32,33)34/h3-5,8-12,15,17-19,28-29,35-36,39H,2,6-7,13-14,16,20-21H2,1H3,(H,37,40)/t28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 19: 3664-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.165
BindingDB Entry DOI: 10.7270/Q2F18X23 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26506
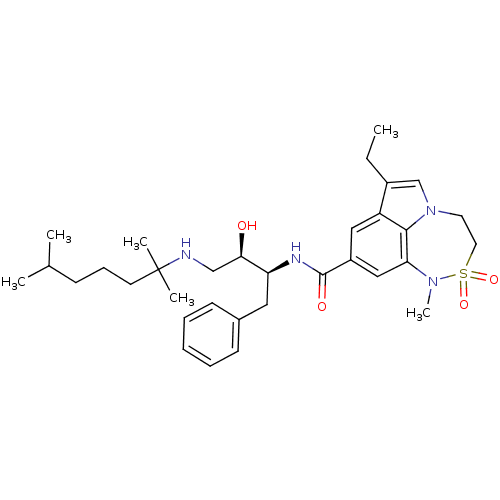
(BMCL193669 Compound 24 | N-[(2S,3R)-4-[(2,6-dimeth...)Show SMILES CCc1cn2CCS(=O)(=O)N(C)c3cc(cc1c23)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC(C)(C)CCCC(C)C |r| Show InChI InChI=1S/C33H48N4O4S/c1-7-25-22-37-16-17-42(40,41)36(6)29-20-26(19-27(25)31(29)37)32(39)35-28(18-24-13-9-8-10-14-24)30(38)21-34-33(4,5)15-11-12-23(2)3/h8-10,13-14,19-20,22-23,28,30,34,38H,7,11-12,15-18,21H2,1-6H3,(H,35,39)/t28-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
J Med Chem 51: 3313-7 (2008)
Article DOI: 10.1021/jm800138h
BindingDB Entry DOI: 10.7270/Q2XS5SQR |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM29794

(sulfonamide tricyclic analogue, 5)Show SMILES CCn1cc2CCS(=O)(=O)N(C)c3cc(cc1c23)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC1CCOCC1 |r| Show InChI InChI=1S/C29H38N4O5S/c1-3-33-19-21-11-14-39(36,37)32(2)25-16-22(17-26(33)28(21)25)29(35)31-24(15-20-7-5-4-6-8-20)27(34)18-30-23-9-12-38-13-10-23/h4-8,16-17,19,23-24,27,30,34H,3,9-15,18H2,1-2H3,(H,31,35)/t24-,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 19: 3674-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.149
BindingDB Entry DOI: 10.7270/Q25H7DKK |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26506

(BMCL193669 Compound 24 | N-[(2S,3R)-4-[(2,6-dimeth...)Show SMILES CCc1cn2CCS(=O)(=O)N(C)c3cc(cc1c23)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC(C)(C)CCCC(C)C |r| Show InChI InChI=1S/C33H48N4O4S/c1-7-25-22-37-16-17-42(40,41)36(6)29-20-26(19-27(25)31(29)37)32(39)35-28(18-24-13-9-8-10-14-24)30(38)21-34-33(4,5)15-11-12-23(2)3/h8-10,13-14,19-20,22-23,28,30,34,38H,7,11-12,15-18,21H2,1-6H3,(H,35,39)/t28-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 19: 3669-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.150
BindingDB Entry DOI: 10.7270/Q29885BR |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598907
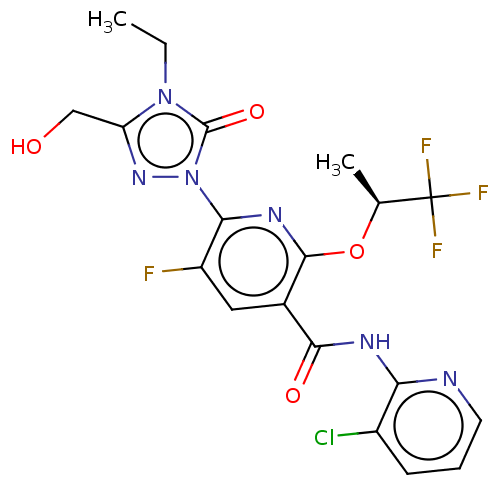
(CHEMBL5178023)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2ncccc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598912

(CHEMBL5170313)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2cc(C)cnc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50322886
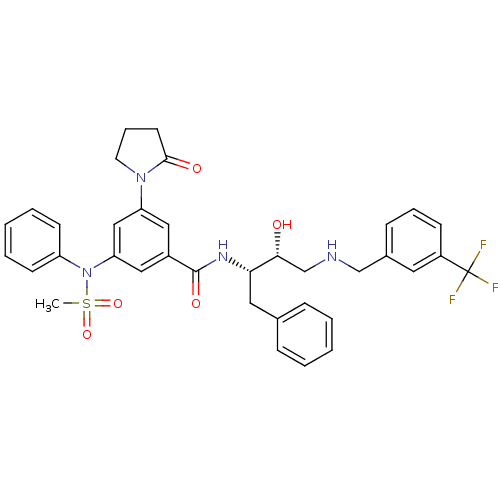
(CHEMBL1210363 | N-((2S,3R)-3-hydroxy-1-phenyl-4-(3...)Show SMILES CS(=O)(=O)N(c1ccccc1)c1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F)N1CCCC1=O |r| Show InChI InChI=1S/C36H37F3N4O5S/c1-49(47,48)43(29-14-6-3-7-15-29)31-21-27(20-30(22-31)42-17-9-16-34(42)45)35(46)41-32(19-25-10-4-2-5-11-25)33(44)24-40-23-26-12-8-13-28(18-26)36(37,38)39/h2-8,10-15,18,20-22,32-33,40,44H,9,16-17,19,23-24H2,1H3,(H,41,46)/t32-,33+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 4639-44 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.111
BindingDB Entry DOI: 10.7270/Q2RB75K7 |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50122453
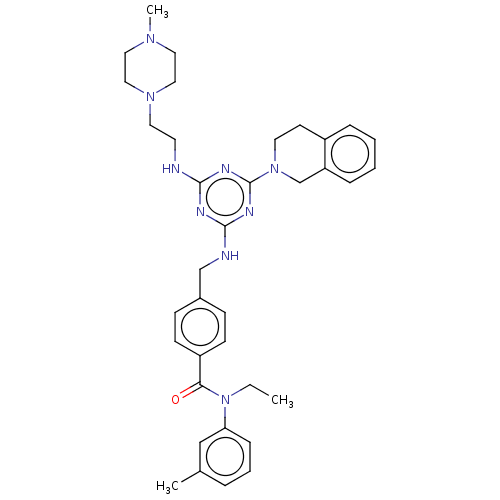
(CHEMBL3622491)Show SMILES CCN(C(=O)c1ccc(CNc2nc(NCCN3CCN(C)CC3)nc(n2)N2CCc3ccccc3C2)cc1)c1cccc(C)c1 Show InChI InChI=1S/C36H45N9O/c1-4-45(32-11-7-8-27(2)24-32)33(46)30-14-12-28(13-15-30)25-38-35-39-34(37-17-19-43-22-20-42(3)21-23-43)40-36(41-35)44-18-16-29-9-5-6-10-31(29)26-44/h5-15,24H,4,16-23,25-26H2,1-3H3,(H2,37,38,39,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ADAMTS4 (213 to 575 amino acid residues) using WAAG-3R as substrate preincubated for 15 mins followed by substrate ad... |
ACS Med Chem Lett 6: 888-93 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00138
BindingDB Entry DOI: 10.7270/Q2416ZWQ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM29770
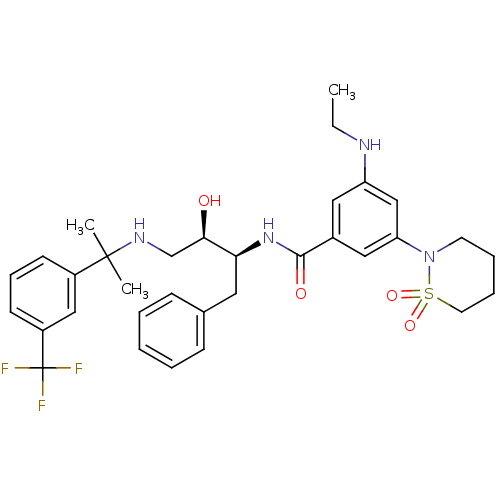
(hydroxyethylamine derivative, 25)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNC(C)(C)c1cccc(c1)C(F)(F)F)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C33H41F3N4O4S/c1-4-37-27-18-24(19-28(21-27)40-15-8-9-16-45(40,43)44)31(42)39-29(17-23-11-6-5-7-12-23)30(41)22-38-32(2,3)25-13-10-14-26(20-25)33(34,35)36/h5-7,10-14,18-21,29-30,37-38,41H,4,8-9,15-17,22H2,1-3H3,(H,39,42)/t29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 19: 3664-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.165
BindingDB Entry DOI: 10.7270/Q2F18X23 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM26773
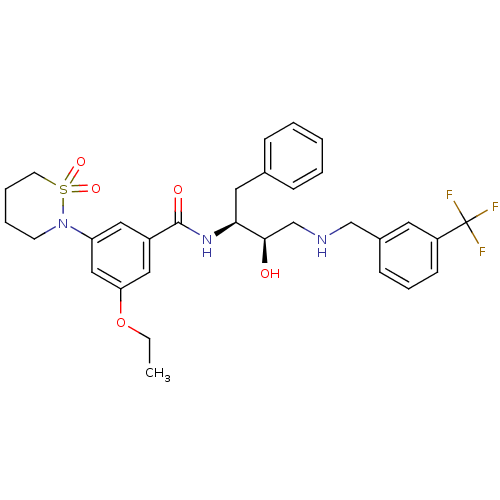
(3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-ethoxy-N-[(2S,3...)Show SMILES CCOc1cc(cc(c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CNCc1cccc(c1)C(F)(F)F)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C31H36F3N3O5S/c1-2-42-27-18-24(17-26(19-27)37-13-6-7-14-43(37,40)41)30(39)36-28(16-22-9-4-3-5-10-22)29(38)21-35-20-23-11-8-12-25(15-23)31(32,33)34/h3-5,8-12,15,17-19,28-29,35,38H,2,6-7,13-14,16,20-21H2,1H3,(H,36,39)/t28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK
| Assay Description
Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... |
Bioorg Med Chem Lett 18: 1022-6 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.020
BindingDB Entry DOI: 10.7270/Q2MC8XBP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data