

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123438 ((3,5-Dichloro-pyridin-4-yl)-(4-methyl-4-{(S)-3-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103087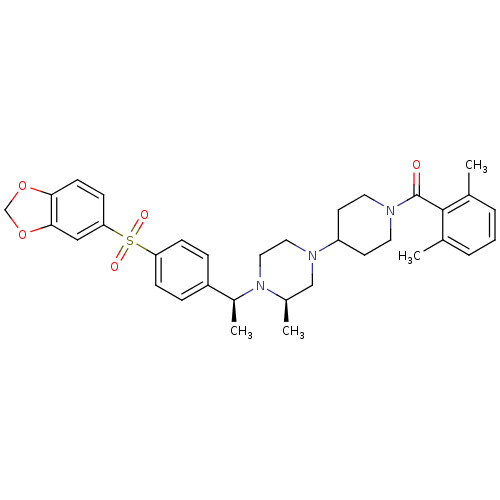 (CHEMBL305026 | [4-((R)-4-{(S)-1-[4-(Benzo[1,3]diox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Affinity for Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103078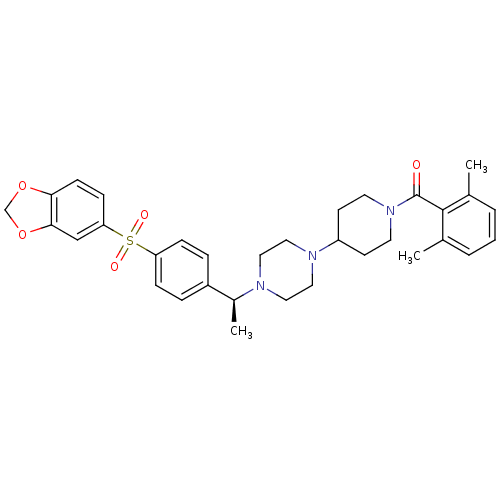 (CHEMBL67563 | [4-(4-{(S)-1-[4-(Benzo[1,3]dioxole-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Affinity for Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145684 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145681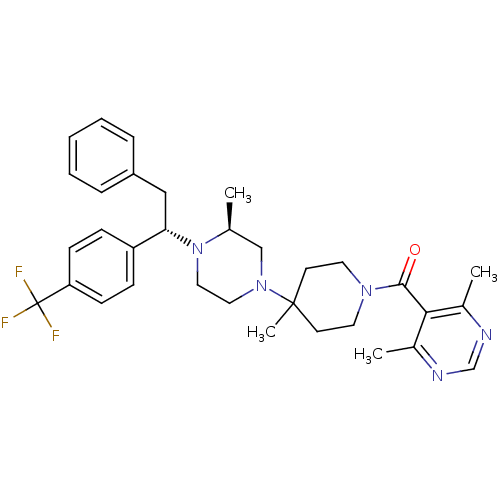 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145686 ((4-{(S)-4-[(S)-2-Cyclopropyl-1-(4-trifluoromethyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104946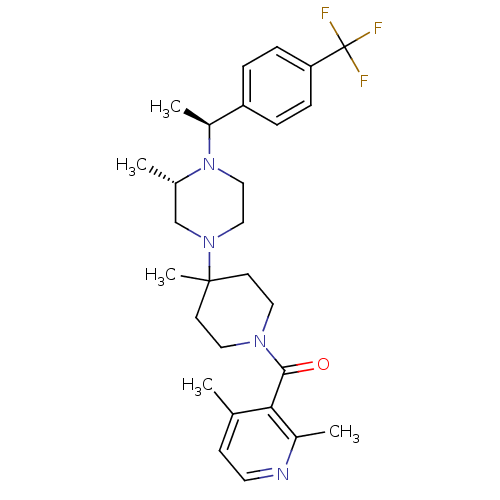 ((2,4-Dimethyl-pyridin-3-yl)-(4-methyl-4-{(S)-3-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104946 ((2,4-Dimethyl-pyridin-3-yl)-(4-methyl-4-{(S)-3-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity at C-C chemokine receptor type 5 | J Med Chem 44: 3343-6 (2001) BindingDB Entry DOI: 10.7270/Q23X85X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145685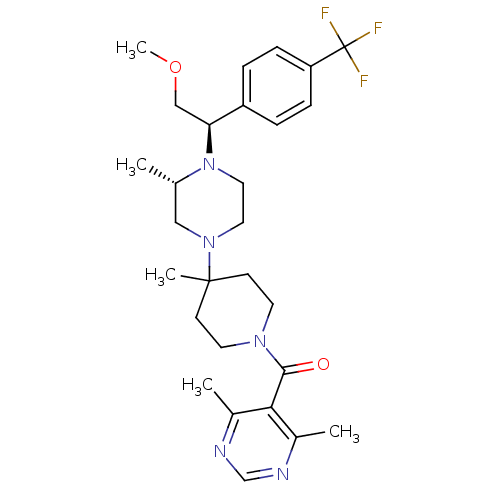 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-{(S)-4-[(R)-2-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123436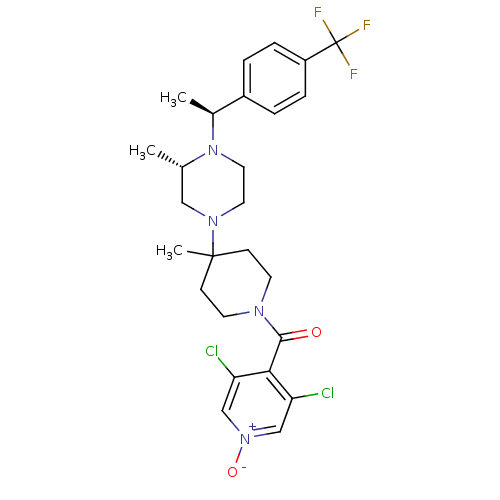 ((3,5-Dichloro-1-oxy-pyridin-4-yl)-(4-methyl-4-{(S)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123435 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104950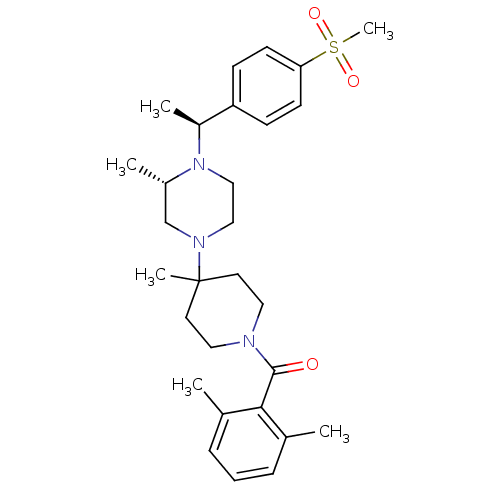 ((2,6-Dimethyl-phenyl)-(4-{(S)-4-[(S)-1-(4-methanes...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards C-C chemokine receptor type 5 | J Med Chem 44: 3343-6 (2001) BindingDB Entry DOI: 10.7270/Q23X85X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123435 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability of compound to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104952 ((2,6-Dimethyl-phenyl)-(4-{(S)-4-[(S)-1-(4-iodo-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards C-C chemokine receptor type 5 | J Med Chem 44: 3343-6 (2001) BindingDB Entry DOI: 10.7270/Q23X85X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104944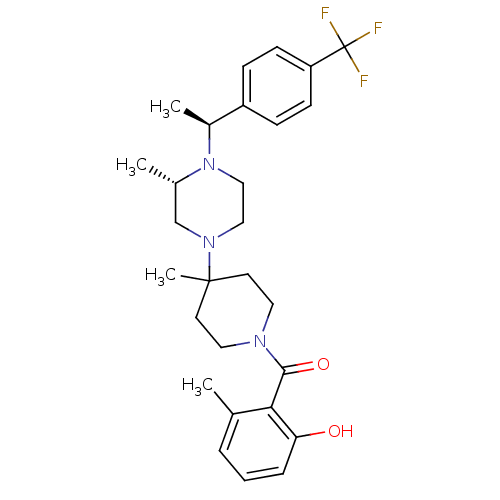 ((2-Hydroxy-6-methyl-phenyl)-(4-methyl-4-{(S)-3-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards C-C chemokine receptor type 5 | J Med Chem 44: 3343-6 (2001) BindingDB Entry DOI: 10.7270/Q23X85X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104953 ((2-Amino-6-chloro-phenyl)-(4-{(S)-4-[(S)-1-(4-iodo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards C-C chemokine receptor type 5 | J Med Chem 44: 3343-6 (2001) BindingDB Entry DOI: 10.7270/Q23X85X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104958 ((2-Amino-6-chloro-phenyl)-(4-{(S)-4-[(S)-1-(4-meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards C-C chemokine receptor type 5 | J Med Chem 44: 3343-6 (2001) BindingDB Entry DOI: 10.7270/Q23X85X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104955 ((2-Amino-6-chloro-phenyl)-(4-methyl-4-{(S)-3-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards C-C chemokine receptor type 5 | J Med Chem 44: 3343-6 (2001) BindingDB Entry DOI: 10.7270/Q23X85X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123445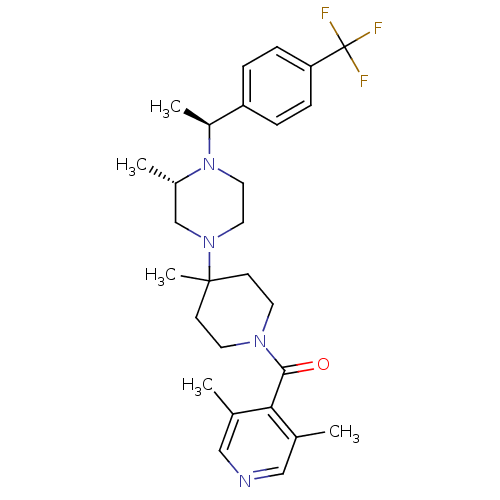 ((3,5-Dimethyl-pyridin-4-yl)-(4-methyl-4-{(S)-3-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50145682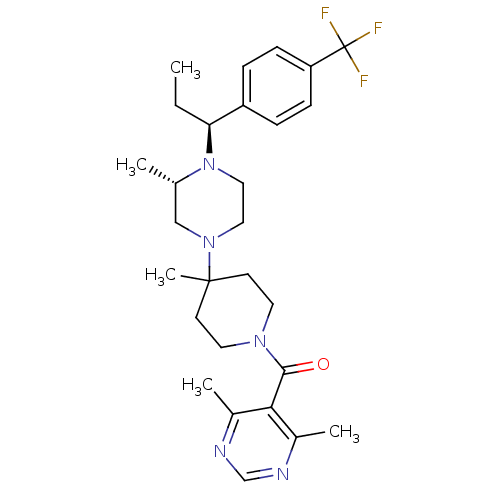 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against C-C chemokine receptor type 5 | J Med Chem 47: 2405-8 (2004) Article DOI: 10.1021/jm0304515 BindingDB Entry DOI: 10.7270/Q26W99H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104954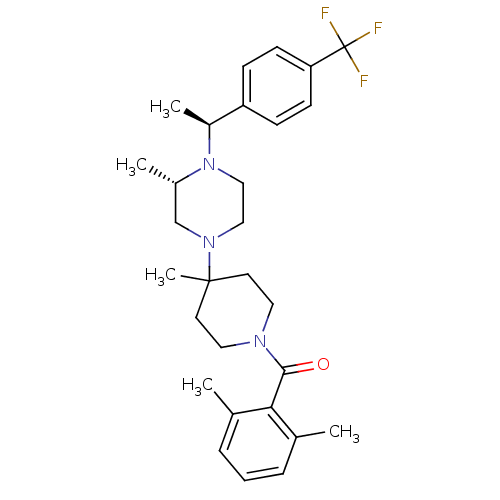 ((2,6-Dimethyl-phenyl)-(4-methyl-4-{(S)-3-methyl-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards C-C chemokine receptor type 5 | J Med Chem 44: 3343-6 (2001) BindingDB Entry DOI: 10.7270/Q23X85X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104956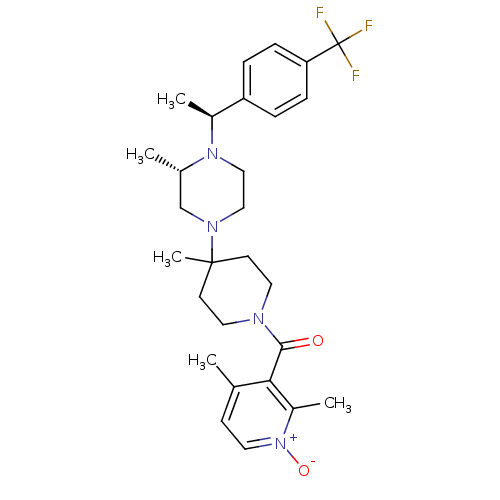 ((2,4-Dimethyl-1-oxy-pyridin-3-yl)-(4-methyl-4-{(S)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity at C-C chemokine receptor type 5 | J Med Chem 44: 3343-6 (2001) BindingDB Entry DOI: 10.7270/Q23X85X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104956 ((2,4-Dimethyl-1-oxy-pyridin-3-yl)-(4-methyl-4-{(S)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104949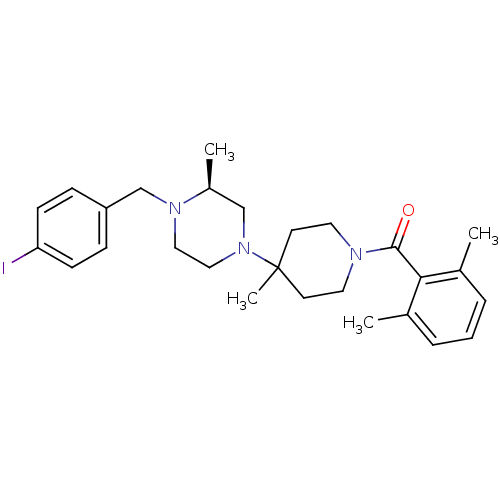 ((2,6-Dimethyl-phenyl)-{4-[(S)-4-(4-iodo-benzyl)-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards C-C chemokine receptor type 5 | J Med Chem 44: 3343-6 (2001) BindingDB Entry DOI: 10.7270/Q23X85X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123442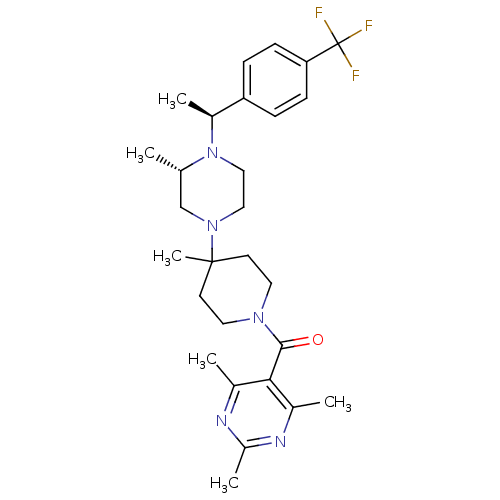 ((4-Methyl-4-{(S)-3-methyl-4-[(S)-1-(4-trifluoromet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123444 ((4,6-Dimethyl-2-trifluoromethyl-pyrimidin-5-yl)-(4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123446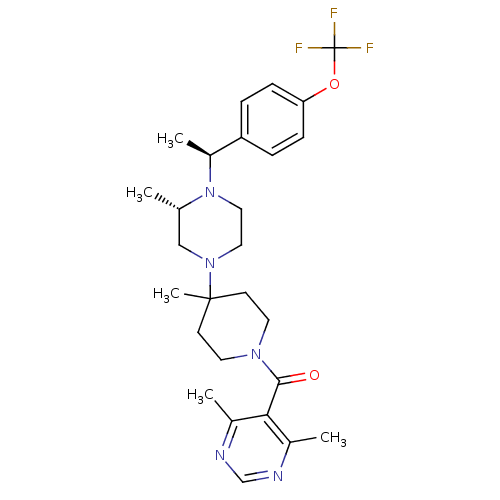 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-methyl-4-{(S)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123437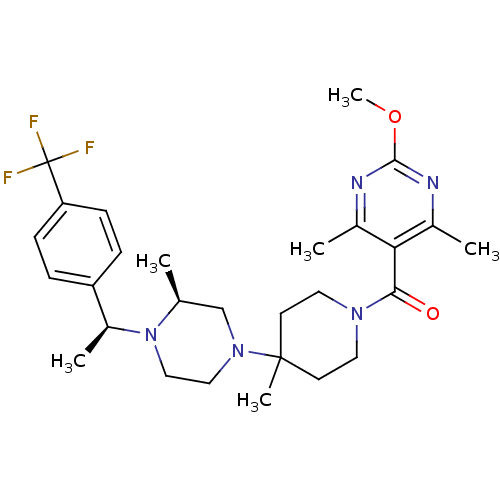 ((2-Methoxy-4,6-dimethyl-pyrimidin-5-yl)-(4-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123443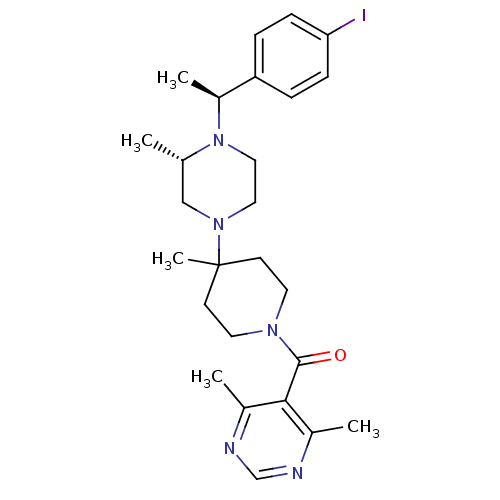 ((4,6-Dimethyl-pyrimidin-5-yl)-(4-{(S)-4-[(S)-1-(4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123439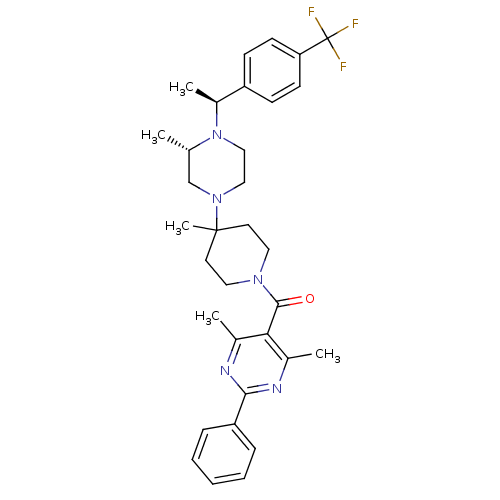 ((4,6-Dimethyl-2-phenyl-pyrimidin-5-yl)-(4-methyl-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104951 ((2-Hydroxy-6-methyl-phenyl)-(4-{(S)-4-[(S)-1-(4-io...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards C-C chemokine receptor type 5 | J Med Chem 44: 3343-6 (2001) BindingDB Entry DOI: 10.7270/Q23X85X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104959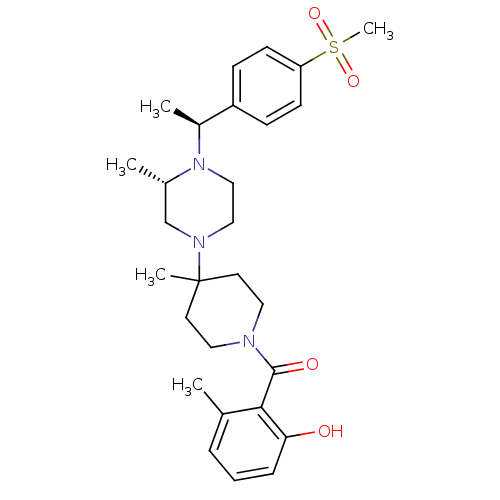 ((2-Hydroxy-6-methyl-phenyl)-(4-{(S)-4-[(S)-1-(4-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards C-C chemokine receptor type 5 | J Med Chem 44: 3343-6 (2001) BindingDB Entry DOI: 10.7270/Q23X85X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103083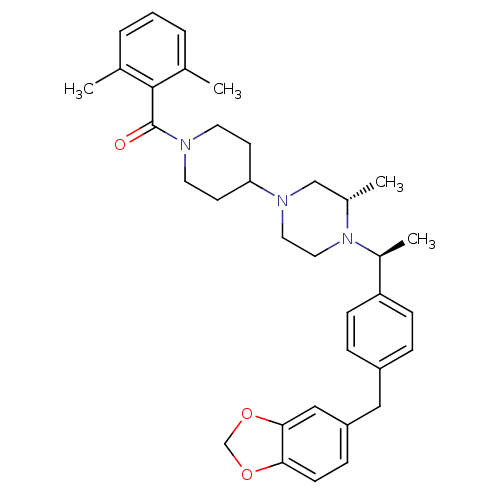 ((4-{(S)-4-[(S)-1-(4-Benzo[1,3]dioxol-5-ylmethyl-ph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled RANTES binding to C-C chemokine receptor type 5 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123447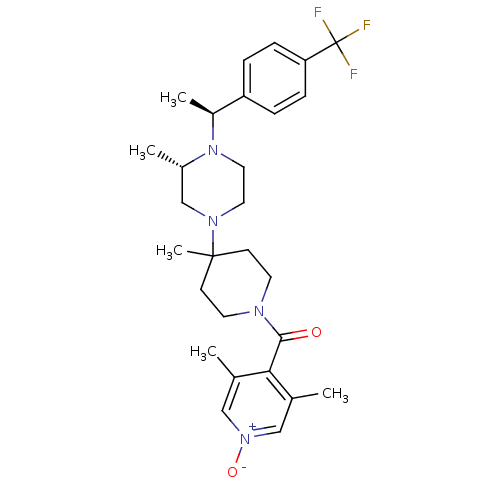 ((3,5-Dimethyl-1-oxy-pyridin-4-yl)-(4-methyl-4-{(S)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104957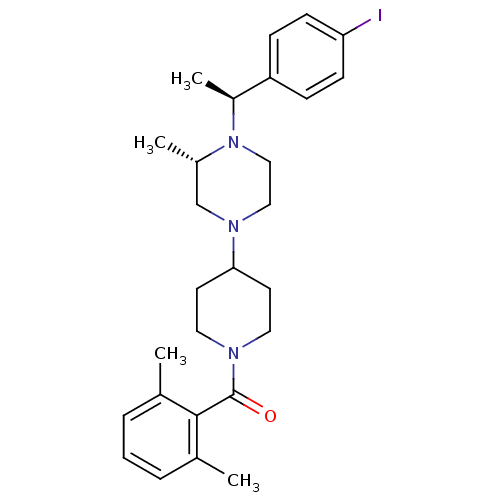 ((2,6-Dimethyl-phenyl)-(4-{(S)-4-[(S)-1-(4-iodo-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards C-C chemokine receptor type 5 | J Med Chem 44: 3343-6 (2001) BindingDB Entry DOI: 10.7270/Q23X85X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123440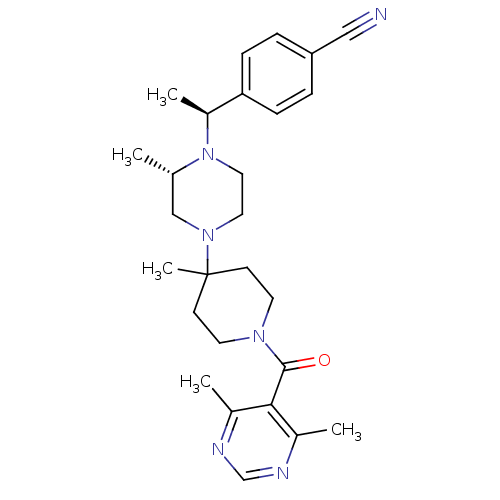 (4-((S)-1-{(S)-4-[1-(4,6-Dimethyl-pyrimidine-5-carb...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104947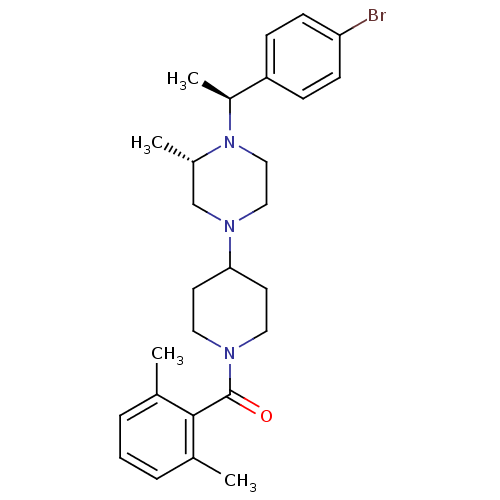 ((4-{4-[1-(4-Bromo-phenyl)-ethyl]-3-methyl-piperazi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards C-C chemokine receptor type 5 | J Med Chem 44: 3343-6 (2001) BindingDB Entry DOI: 10.7270/Q23X85X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103088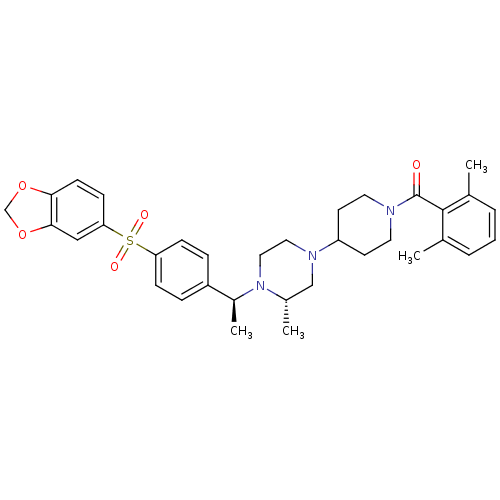 (CHEMBL70010 | [4-((S)-4-{(S)-1-[4-(Benzo[1,3]dioxo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled RANTES binding to C-C chemokine receptor type 5 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104961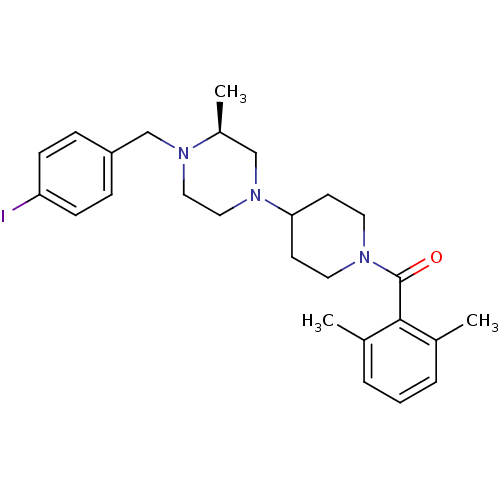 ((2,6-Dimethyl-phenyl)-{4-[(S)-4-(4-iodo-benzyl)-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards C-C chemokine receptor type 5 | J Med Chem 44: 3343-6 (2001) BindingDB Entry DOI: 10.7270/Q23X85X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103091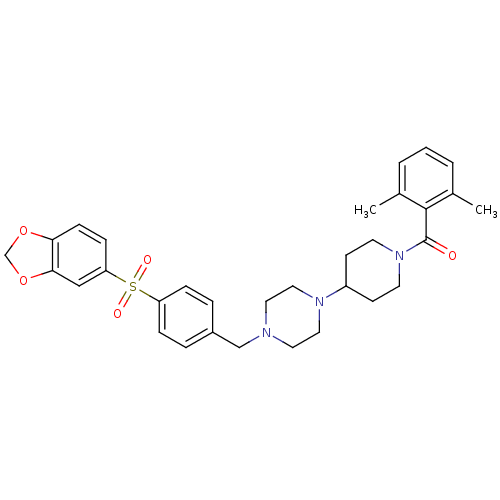 ((4-{4-[4-(Benzo[1,3]dioxole-5-sulfonyl)-benzyl]-pi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Affinity for Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103080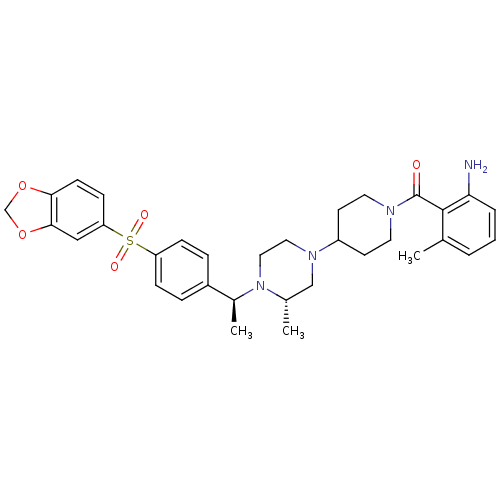 ((2-Amino-6-methyl-phenyl)-[4-((S)-4-{(S)-1-[4-(ben...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability of the compound to inhibit [125I]-labeled RANTES binding to C-C chemokine receptor type 5 expressed in membrane preparation of CHO cells | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103085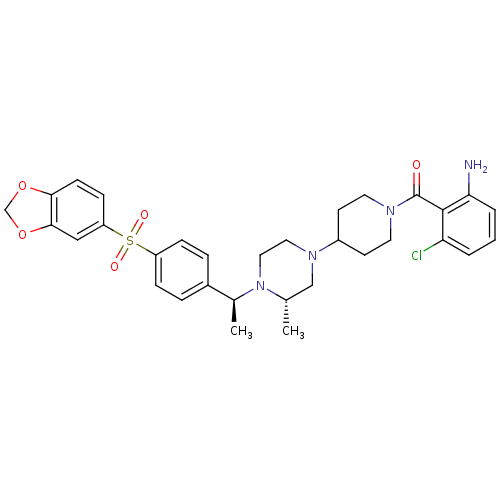 ((2-Amino-6-chloro-phenyl)-[4-((S)-4-{(S)-1-[4-(ben...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled RANTES binding to C-C chemokine receptor type 5 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103090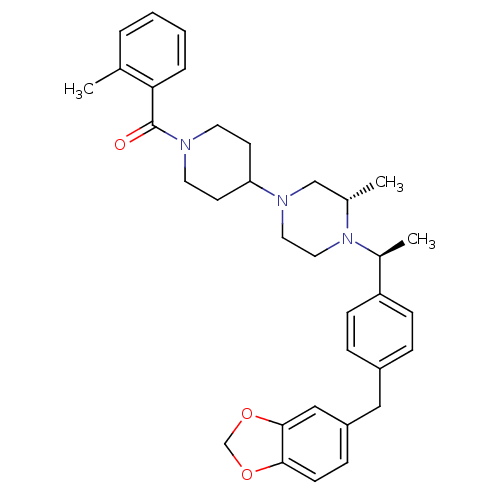 ((4-{(S)-4-[(S)-1-(4-Benzo[1,3]dioxol-5-ylmethyl-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Affinity for Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50123441 ((2-Amino-4,6-dimethyl-pyrimidin-5-yl)-(4-methyl-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit [125I]-labeled RANTES binding to the CCR5 receptor expressed in membrane preparations from CHO cells | Bioorg Med Chem Lett 13: 567-71 (2003) BindingDB Entry DOI: 10.7270/Q28K78G6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103089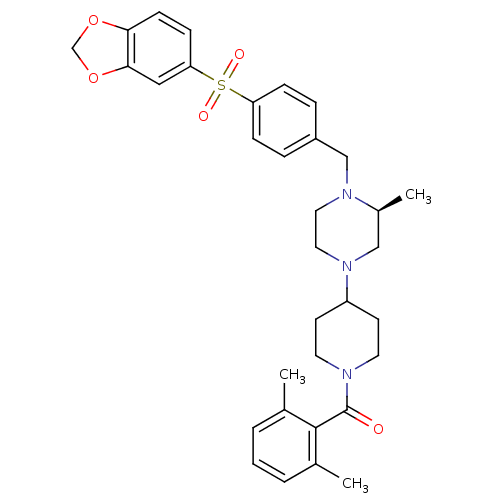 ((4-{(S)-4-[4-(Benzo[1,3]dioxole-5-sulfonyl)-benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Affinity for Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103084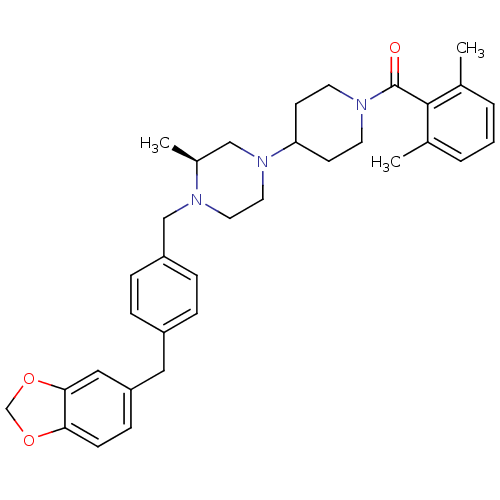 (CHEMBL66623 | {4-[(S)-4-(4-Benzo[1,3]dioxol-5-ylme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Affinity for Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103084 (CHEMBL66623 | {4-[(S)-4-(4-Benzo[1,3]dioxol-5-ylme...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled RANTES binding to C-C chemokine receptor type 5 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103086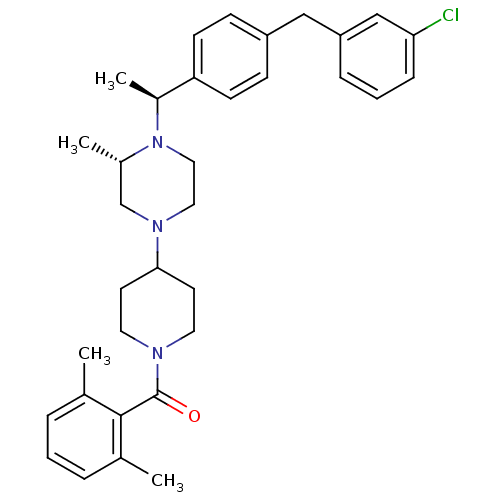 (CHEMBL413972 | [4-((S)-4-{(S)-1-[4-(3-Chloro-benzy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled RANTES binding to C-C chemokine receptor type 5 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50103079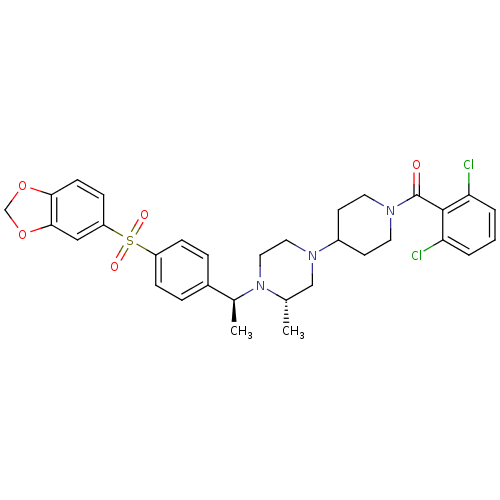 (CHEMBL303503 | [4-((S)-4-{(S)-1-[4-(Benzo[1,3]diox...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-labeled RANTES binding to C-C chemokine receptor type 5 | Bioorg Med Chem Lett 11: 2143-6 (2001) BindingDB Entry DOI: 10.7270/Q2VQ320K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104960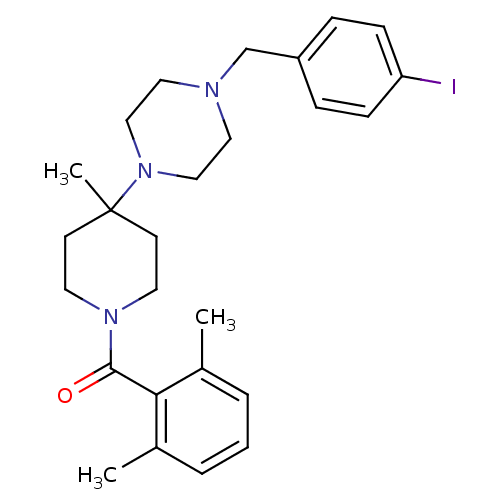 ((2,6-Dimethyl-phenyl)-{4-[4-(4-iodo-benzyl)-pipera...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards C-C chemokine receptor type 5 | J Med Chem 44: 3343-6 (2001) BindingDB Entry DOI: 10.7270/Q23X85X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 323 total ) | Next | Last >> |