 Found 7537 hits with Last Name = 'babu' and Initial = 's'
Found 7537 hits with Last Name = 'babu' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50071565
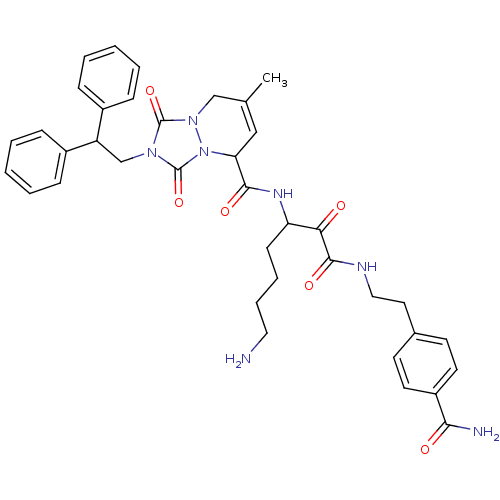
(2-(2,2-Diphenyl-ethyl)-7-methyl-1,3-dioxo-2,3,5,8-...)Show SMILES CC1=CC(C(=O)NC(CCCCN)C(=O)C(=O)NCCc2ccc(cc2)C(N)=O)n2n(C1)c(=O)n(CC(c1ccccc1)c1ccccc1)c2=O |t:1| Show InChI InChI=1S/C38H43N7O6/c1-25-22-32(35(48)42-31(14-8-9-20-39)33(46)36(49)41-21-19-26-15-17-29(18-16-26)34(40)47)45-38(51)43(37(50)44(45)23-25)24-30(27-10-4-2-5-11-27)28-12-6-3-7-13-28/h2-7,10-13,15-18,22,30-32H,8-9,14,19-21,23-24,39H2,1H3,(H2,40,47)(H,41,49)(H,42,48) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the thrombin |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169371
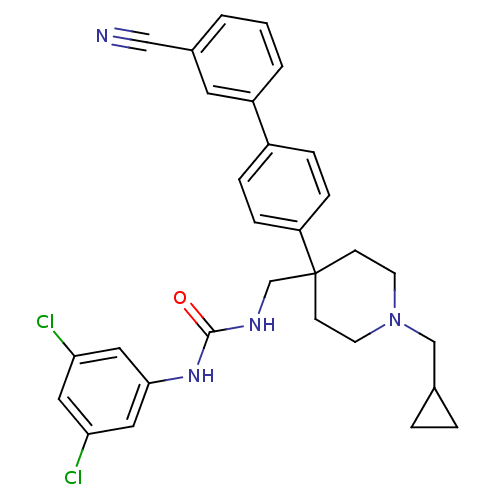
(1-[4-(3'-Cyano-biphenyl-4-yl)-1-cyclopropylmethyl-...)Show SMILES Clc1cc(Cl)cc(NC(=O)NCC2(CCN(CC3CC3)CC2)c2ccc(cc2)-c2cccc(c2)C#N)c1 Show InChI InChI=1S/C30H30Cl2N4O/c31-26-15-27(32)17-28(16-26)35-29(37)34-20-30(10-12-36(13-11-30)19-21-4-5-21)25-8-6-23(7-9-25)24-3-1-2-22(14-24)18-33/h1-3,6-9,14-17,21H,4-5,10-13,19-20H2,(H2,34,35,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50071575

(2,2-Dibutyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahydro-...)Show SMILES CCCCC1(CCCC)C(=O)N2CC(C)=CC(N2C1=O)C(=O)NC(CCCCN)C(=O)C(=O)NCCc1ccc(cc1)C(N)=O |c:14| Show InChI InChI=1S/C33H48N6O6/c1-4-6-16-33(17-7-5-2)31(44)38-21-22(3)20-26(39(38)32(33)45)29(42)37-25(10-8-9-18-34)27(40)30(43)36-19-15-23-11-13-24(14-12-23)28(35)41/h11-14,20,25-26H,4-10,15-19,21,34H2,1-3H3,(H2,35,41)(H,36,43)(H,37,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the thrombin |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50579244

(Avoralstat | BCX-4161)Show SMILES COc1cc(c(cc1C=C)C(=O)Nc1ccc(cc1)C(N)=N)-c1ccc(nc1C(O)=O)C(=O)NCC1CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of purified human plasma kallikrein assessed as inhibition constant using H-D-Pro-Phe-Arg-pNA.2HCl as substrate measured after 3 mins by m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00511
BindingDB Entry DOI: 10.7270/Q29C7288 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50071570

(8-Isobutyl-2-(4-methoxy-phenyl)-1,3-dioxo-2,3,5,8-...)Show SMILES COc1ccc(cc1)-n1c(=O)n2C(CC(C)C)C=CC(C(=O)NC(CCCCN)C(=O)C(=O)NCCc3ccc(cc3)C(N)=O)n2c1=O |c:18| Show InChI InChI=1S/C34H43N7O7/c1-21(2)20-25-13-16-28(41-34(47)39(33(46)40(25)41)24-11-14-26(48-3)15-12-24)31(44)38-27(6-4-5-18-35)29(42)32(45)37-19-17-22-7-9-23(10-8-22)30(36)43/h7-16,21,25,27-28H,4-6,17-20,35H2,1-3H3,(H2,36,43)(H,37,45)(H,38,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the trypsin enzyme |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50071573
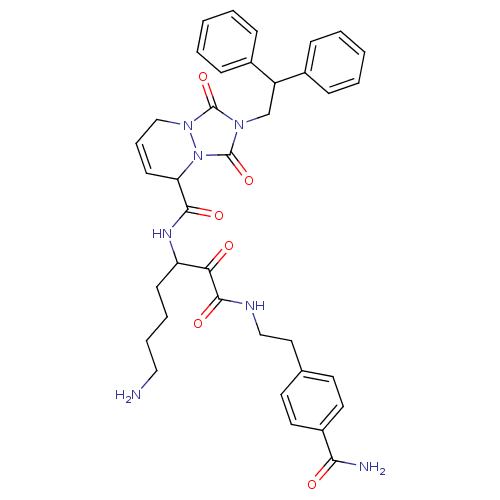
(2-(2,2-Diphenyl-ethyl)-1,3-dioxo-2,3,5,8-tetrahydr...)Show SMILES NCCCCC(NC(=O)C1C=CCn2n1c(=O)n(CC(c1ccccc1)c1ccccc1)c2=O)C(=O)C(=O)NCCc1ccc(cc1)C(N)=O |c:10| Show InChI InChI=1S/C37H41N7O6/c38-21-8-7-14-30(32(45)35(48)40-22-20-25-16-18-28(19-17-25)33(39)46)41-34(47)31-15-9-23-43-36(49)42(37(50)44(31)43)24-29(26-10-3-1-4-11-26)27-12-5-2-6-13-27/h1-6,9-13,15-19,29-31H,7-8,14,20-24,38H2,(H2,39,46)(H,40,48)(H,41,47) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the thrombin |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169377
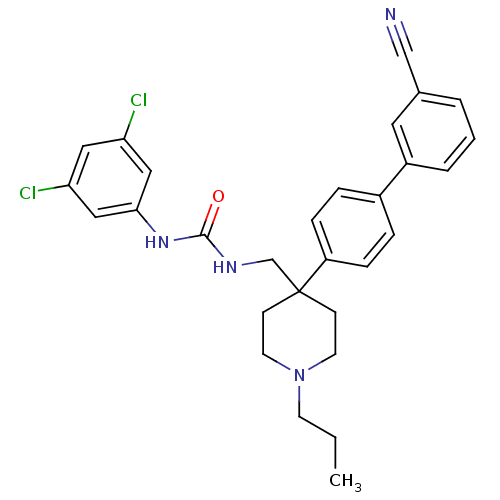
(1-[4-(3'-Cyano-biphenyl-4-yl)-1-propyl-piperidin-4...)Show SMILES CCCN1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C29H30Cl2N4O/c1-2-12-35-13-10-29(11-14-35,20-33-28(36)34-27-17-25(30)16-26(31)18-27)24-8-6-22(7-9-24)23-5-3-4-21(15-23)19-32/h3-9,15-18H,2,10-14,20H2,1H3,(H2,33,34,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169374
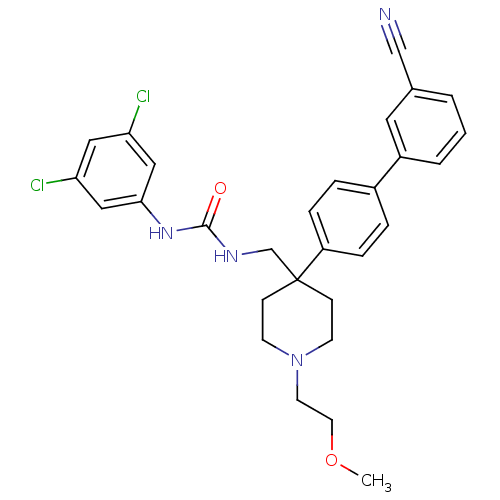
(1-[4-(3'-Cyano-biphenyl-4-yl)-1-(2-methoxy-ethyl)-...)Show SMILES COCCN1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C29H30Cl2N4O2/c1-37-14-13-35-11-9-29(10-12-35,20-33-28(36)34-27-17-25(30)16-26(31)18-27)24-7-5-22(6-8-24)23-4-2-3-21(15-23)19-32/h2-8,15-18H,9-14,20H2,1H3,(H2,33,34,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50071571
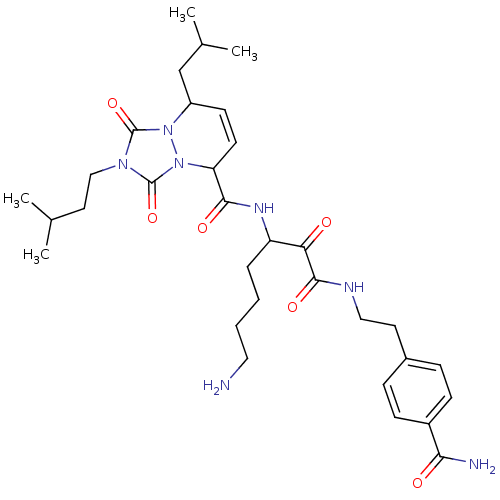
(8-Isobutyl-2-(3-methyl-butyl)-1,3-dioxo-2,3,5,8-te...)Show SMILES CC(C)CCn1c(=O)n2C(CC(C)C)C=CC(C(=O)NC(CCCCN)C(=O)C(=O)NCCc3ccc(cc3)C(N)=O)n2c1=O |c:14| Show InChI InChI=1S/C32H47N7O6/c1-20(2)15-18-37-31(44)38-24(19-21(3)4)12-13-26(39(38)32(37)45)29(42)36-25(7-5-6-16-33)27(40)30(43)35-17-14-22-8-10-23(11-9-22)28(34)41/h8-13,20-21,24-26H,5-7,14-19,33H2,1-4H3,(H2,34,41)(H,35,43)(H,36,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the trypsin enzyme |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM416926

((+)-1-(3-(aminomethyl)phenyl)-N-(5-((3-cyanophenyl...)Show SMILES NCc1cccc(c1)-n1nc(cc1C(=O)Nc1cc(ccc1F)C(NCC1CC1)c1cccc(c1)C#N)C(F)(F)F Show InChI InChI=1S/C30H26F4N6O/c31-24-10-9-22(28(37-17-18-7-8-18)21-5-1-3-19(11-21)15-35)13-25(24)38-29(41)26-14-27(30(32,33)34)39-40(26)23-6-2-4-20(12-23)16-36/h1-6,9-14,18,28,37H,7-8,16-17,36H2,(H,38,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of purified human plasma kallikrein assessed as inhibition constant using H-D-Pro-Phe-Arg-pNA.2HCl as substrate measured after 3 mins by m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00511
BindingDB Entry DOI: 10.7270/Q29C7288 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169390

(1-[4-(3'-Cyano-biphenyl-4-yl)-1-isopropyl-piperidi...)Show SMILES CC(C)N1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C29H30Cl2N4O/c1-20(2)35-12-10-29(11-13-35,19-33-28(36)34-27-16-25(30)15-26(31)17-27)24-8-6-22(7-9-24)23-5-3-4-21(14-23)18-32/h3-9,14-17,20H,10-13,19H2,1-2H3,(H2,33,34,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169389

(1-[4-(3'-Cyano-biphenyl-4-yl)-1-cyclopentyl-piperi...)Show SMILES Clc1cc(Cl)cc(NC(=O)NCC2(CCN(CC2)C2CCCC2)c2ccc(cc2)-c2cccc(c2)C#N)c1 Show InChI InChI=1S/C31H32Cl2N4O/c32-26-17-27(33)19-28(18-26)36-30(38)35-21-31(12-14-37(15-13-31)29-6-1-2-7-29)25-10-8-23(9-11-25)24-5-3-4-22(16-24)20-34/h3-5,8-11,16-19,29H,1-2,6-7,12-15,21H2,(H2,35,36,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317048
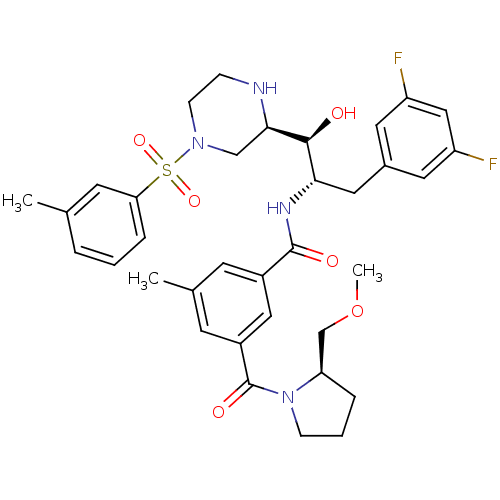
(CHEMBL1097342 | N-((1S,2S)-3-(3,5-difluorophenyl)-...)Show SMILES COC[C@H]1CCCN1C(=O)c1cc(C)cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)[C@H]1CN(CCN1)S(=O)(=O)c1cccc(C)c1 |r| Show InChI InChI=1S/C35H42F2N4O6S/c1-22-6-4-8-30(14-22)48(45,46)40-11-9-38-32(20-40)33(42)31(17-24-15-27(36)19-28(37)16-24)39-34(43)25-12-23(2)13-26(18-25)35(44)41-10-5-7-29(41)21-47-3/h4,6,8,12-16,18-19,29,31-33,38,42H,5,7,9-11,17,20-21H2,1-3H3,(H,39,43)/t29-,31+,32-,33+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition oh BACE1 |
Bioorg Med Chem Lett 20: 2837-42 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.050
BindingDB Entry DOI: 10.7270/Q2BK1CH7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169393
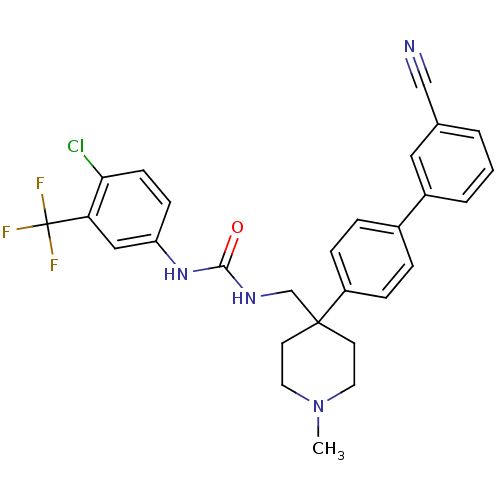
(1-(4-Chloro-3-trifluoromethyl-phenyl)-3-[4-(3'-cya...)Show SMILES CN1CCC(CNC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C28H26ClF3N4O/c1-36-13-11-27(12-14-36,22-7-5-20(6-8-22)21-4-2-3-19(15-21)17-33)18-34-26(37)35-23-9-10-25(29)24(16-23)28(30,31)32/h2-10,15-16H,11-14,18H2,1H3,(H2,34,35,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317060
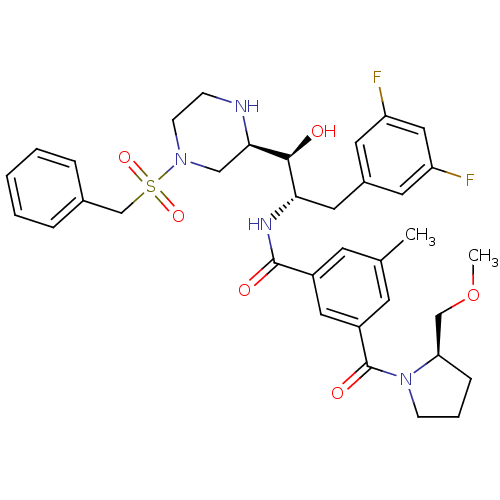
(CHEMBL1097353 | N-((1S,2S)-1-((R)-4-(benzylsulfony...)Show SMILES COC[C@H]1CCCN1C(=O)c1cc(C)cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)[C@H]1CN(CCN1)S(=O)(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C35H42F2N4O6S/c1-23-13-26(18-27(14-23)35(44)41-11-6-9-30(41)21-47-2)34(43)39-31(17-25-15-28(36)19-29(37)16-25)33(42)32-20-40(12-10-38-32)48(45,46)22-24-7-4-3-5-8-24/h3-5,7-8,13-16,18-19,30-33,38,42H,6,9-12,17,20-22H2,1-2H3,(H,39,43)/t30-,31+,32-,33+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition oh BACE1 |
Bioorg Med Chem Lett 20: 2837-42 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.050
BindingDB Entry DOI: 10.7270/Q2BK1CH7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317027
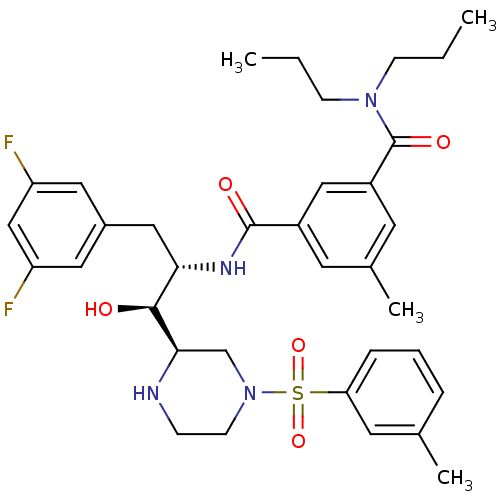
(CHEMBL1097321 | N1-((1S,2S)-3-(3,5-difluorophenyl)...)Show SMILES CCCN(CCC)C(=O)c1cc(C)cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)[C@H]1CN(CCN1)S(=O)(=O)c1cccc(C)c1 |r| Show InChI InChI=1S/C35H44F2N4O5S/c1-5-11-40(12-6-2)35(44)27-15-24(4)14-26(20-27)34(43)39-31(19-25-17-28(36)21-29(37)18-25)33(42)32-22-41(13-10-38-32)47(45,46)30-9-7-8-23(3)16-30/h7-9,14-18,20-21,31-33,38,42H,5-6,10-13,19,22H2,1-4H3,(H,39,43)/t31-,32+,33-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition oh BACE1 |
Bioorg Med Chem Lett 20: 2837-42 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.050
BindingDB Entry DOI: 10.7270/Q2BK1CH7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317051
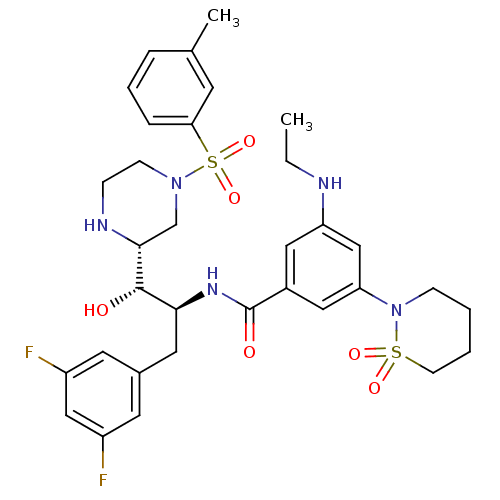
(CHEMBL1097345 | N-[(1S,2S)-3-(3,5-difluorophenyl)-...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)[C@H]1CN(CCN1)S(=O)(=O)c1cccc(C)c1)N1CCCCS1(=O)=O |r| Show InChI InChI=1S/C33H41F2N5O6S2/c1-3-36-27-17-24(18-28(20-27)40-10-4-5-12-47(40,43)44)33(42)38-30(16-23-14-25(34)19-26(35)15-23)32(41)31-21-39(11-9-37-31)48(45,46)29-8-6-7-22(2)13-29/h6-8,13-15,17-20,30-32,36-37,41H,3-5,9-12,16,21H2,1-2H3,(H,38,42)/t30-,31+,32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition oh BACE1 |
Bioorg Med Chem Lett 20: 2837-42 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.050
BindingDB Entry DOI: 10.7270/Q2BK1CH7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317052
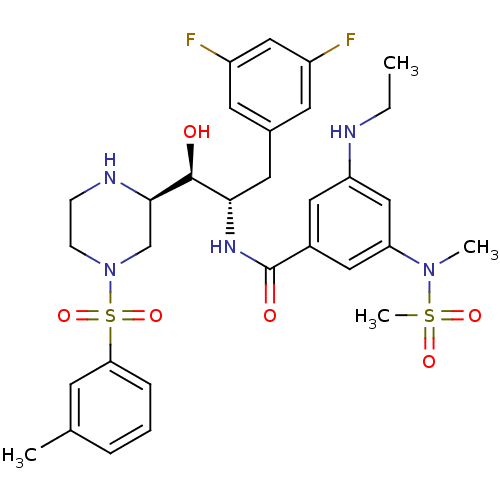
(CHEMBL1097346 | N-((1S,2S)-3-(3,5-difluorophenyl)-...)Show SMILES CCNc1cc(cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)[C@H]1CN(CCN1)S(=O)(=O)c1cccc(C)c1)N(C)S(C)(=O)=O |r| Show InChI InChI=1S/C31H39F2N5O6S2/c1-5-34-25-15-22(16-26(18-25)37(3)45(4,41)42)31(40)36-28(14-21-12-23(32)17-24(33)13-21)30(39)29-19-38(10-9-35-29)46(43,44)27-8-6-7-20(2)11-27/h6-8,11-13,15-18,28-30,34-35,39H,5,9-10,14,19H2,1-4H3,(H,36,40)/t28-,29+,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition oh BACE1 |
Bioorg Med Chem Lett 20: 2837-42 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.050
BindingDB Entry DOI: 10.7270/Q2BK1CH7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317055
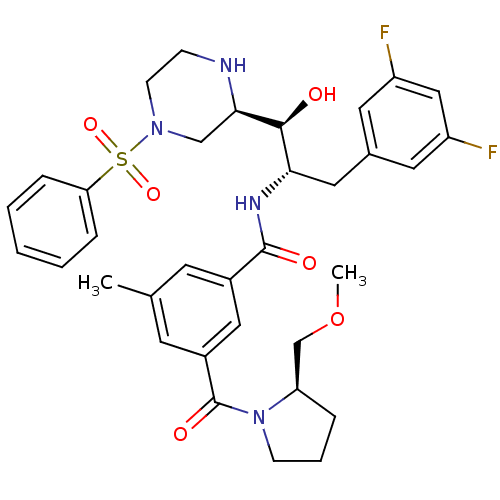
(CHEMBL1097348 | N-((1S,2S)-3-(3,5-difluorophenyl)-...)Show SMILES COC[C@H]1CCCN1C(=O)c1cc(C)cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)[C@H]1CN(CCN1)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C34H40F2N4O6S/c1-22-13-24(18-25(14-22)34(43)40-11-6-7-28(40)21-46-2)33(42)38-30(17-23-15-26(35)19-27(36)16-23)32(41)31-20-39(12-10-37-31)47(44,45)29-8-4-3-5-9-29/h3-5,8-9,13-16,18-19,28,30-32,37,41H,6-7,10-12,17,20-21H2,1-2H3,(H,38,42)/t28-,30+,31-,32+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition oh BACE1 |
Bioorg Med Chem Lett 20: 2837-42 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.050
BindingDB Entry DOI: 10.7270/Q2BK1CH7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317056
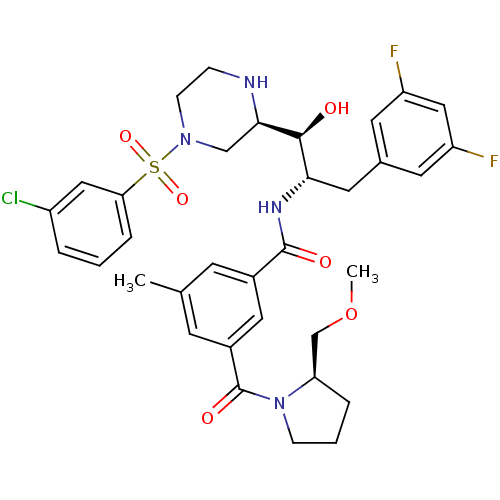
(CHEMBL1097349 | N-((1S,2S)-1-((R)-4-(3-chloropheny...)Show SMILES COC[C@H]1CCCN1C(=O)c1cc(C)cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)[C@H]1CN(CCN1)S(=O)(=O)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C34H39ClF2N4O6S/c1-21-11-23(16-24(12-21)34(44)41-9-4-6-28(41)20-47-2)33(43)39-30(15-22-13-26(36)18-27(37)14-22)32(42)31-19-40(10-8-38-31)48(45,46)29-7-3-5-25(35)17-29/h3,5,7,11-14,16-18,28,30-32,38,42H,4,6,8-10,15,19-20H2,1-2H3,(H,39,43)/t28-,30+,31-,32+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition oh BACE1 |
Bioorg Med Chem Lett 20: 2837-42 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.050
BindingDB Entry DOI: 10.7270/Q2BK1CH7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317058
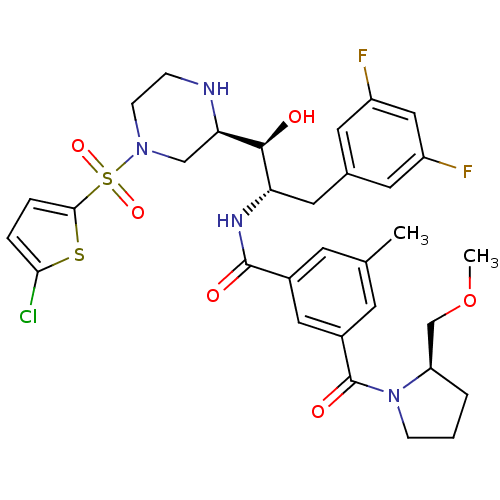
(CHEMBL1097351 | N-((1S,2S)-1-((R)-4-(5-chlorothiop...)Show SMILES COC[C@H]1CCCN1C(=O)c1cc(C)cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)[C@H]1CN(CCN1)S(=O)(=O)c1ccc(Cl)s1 |r| Show InChI InChI=1S/C32H37ClF2N4O6S2/c1-19-10-21(15-22(11-19)32(42)39-8-3-4-25(39)18-45-2)31(41)37-26(14-20-12-23(34)16-24(35)13-20)30(40)27-17-38(9-7-36-27)47(43,44)29-6-5-28(33)46-29/h5-6,10-13,15-16,25-27,30,36,40H,3-4,7-9,14,17-18H2,1-2H3,(H,37,41)/t25-,26+,27-,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition oh BACE1 |
Bioorg Med Chem Lett 20: 2837-42 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.050
BindingDB Entry DOI: 10.7270/Q2BK1CH7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317050
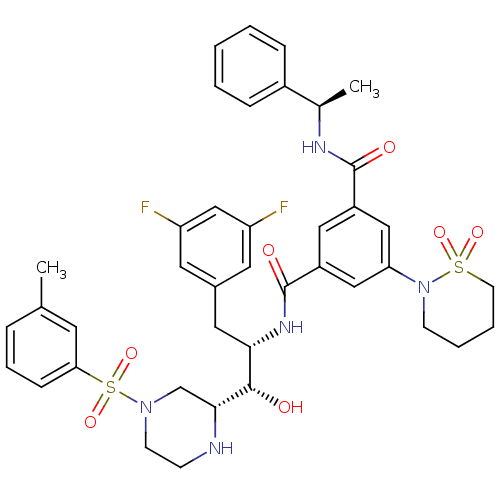
(1-N-[(1S,2S)-3-(3,5-difluorophenyl)-1-hydroxy-1-[(...)Show SMILES C[C@@H](NC(=O)c1cc(cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)[C@H]1CN(CCN1)S(=O)(=O)c1cccc(C)c1)N1CCCCS1(=O)=O)c1ccccc1 |r| Show InChI InChI=1S/C40H45F2N5O7S2/c1-26-9-8-12-35(17-26)56(53,54)46-15-13-43-37(25-46)38(48)36(20-28-18-32(41)24-33(42)19-28)45-40(50)31-21-30(39(49)44-27(2)29-10-4-3-5-11-29)22-34(23-31)47-14-6-7-16-55(47,51)52/h3-5,8-12,17-19,21-24,27,36-38,43,48H,6-7,13-16,20,25H2,1-2H3,(H,44,49)(H,45,50)/t27-,36+,37-,38+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition oh BACE1 |
Bioorg Med Chem Lett 20: 2837-42 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.050
BindingDB Entry DOI: 10.7270/Q2BK1CH7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50392835

(CHEMBL2151181)Show SMILES CC#Cc1cncc(c1)-c1cc(Cl)c(s1)[C@]1(C)CC(=O)N(C)C(N)=N1 |r,c:25| Show InChI InChI=1S/C18H17ClN4OS/c1-4-5-11-6-12(10-21-9-11)14-7-13(19)16(25-14)18(2)8-15(24)23(3)17(20)22-18/h6-7,9-10H,8H2,1-3H3,(H2,20,22)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE2 by FRET assay |
ACS Med Chem Lett 3: 897-902 (2012)
Article DOI: 10.1021/ml3001165
BindingDB Entry DOI: 10.7270/Q23779SN |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169382

(1-[4-(3'-Cyano-biphenyl-4-yl)-1-cyclohexyl-piperid...)Show SMILES Clc1cc(Cl)cc(NC(=O)NCC2(CCN(CC2)C2CCCCC2)c2ccc(cc2)-c2cccc(c2)C#N)c1 Show InChI InChI=1S/C32H34Cl2N4O/c33-27-18-28(34)20-29(19-27)37-31(39)36-22-32(13-15-38(16-14-32)30-7-2-1-3-8-30)26-11-9-24(10-12-26)25-6-4-5-23(17-25)21-35/h4-6,9-12,17-20,30H,1-3,7-8,13-16,22H2,(H2,36,37,39) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169376

(1-[4-(3'-Cyano-biphenyl-4-yl)-1-methyl-piperidin-4...)Show SMILES CN1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C27H26Cl2N4O/c1-33-11-9-27(10-12-33,18-31-26(34)32-25-15-23(28)14-24(29)16-25)22-7-5-20(6-8-22)21-4-2-3-19(13-21)17-30/h2-8,13-16H,9-12,18H2,1H3,(H2,31,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169396

(1-[4-(3'-Cyano-biphenyl-4-yl)-1-methyl-piperidin-4...)Show SMILES CN1CCC(CNC(=O)Nc2ccc(F)c(F)c2)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C27H26F2N4O/c1-33-13-11-27(12-14-33,18-31-26(34)32-23-9-10-24(28)25(29)16-23)22-7-5-20(6-8-22)21-4-2-3-19(15-21)17-30/h2-10,15-16H,11-14,18H2,1H3,(H2,31,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169386
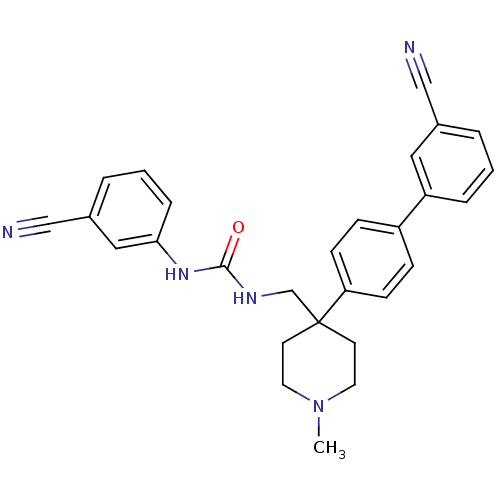
(1-[4-(3'-Cyano-biphenyl-4-yl)-1-methyl-piperidin-4...)Show SMILES CN1CCC(CNC(=O)Nc2cccc(c2)C#N)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C28H27N5O/c1-33-14-12-28(13-15-33,20-31-27(34)32-26-7-3-5-22(17-26)19-30)25-10-8-23(9-11-25)24-6-2-4-21(16-24)18-29/h2-11,16-17H,12-15,20H2,1H3,(H2,31,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50071568

(2-Amino-2-benzyl-7-methyl-1,3-dioxo-2,3,5,8-tetrah...)Show SMILES CC1=CC(N2N(C1)C(=O)C(N)(Cc1ccccc1)C2=O)C(=O)NC(CCCCN)C(=O)C(=O)NCc1ccc(cc1)C(N)=O |t:1| Show InChI InChI=1S/C31H37N7O6/c1-19-15-24(38-30(44)31(34,29(43)37(38)18-19)16-20-7-3-2-4-8-20)27(41)36-23(9-5-6-14-32)25(39)28(42)35-17-21-10-12-22(13-11-21)26(33)40/h2-4,7-8,10-13,15,23-24H,5-6,9,14,16-18,32,34H2,1H3,(H2,33,40)(H,35,42)(H,36,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the thrombin |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase
(Escherichia coli (strain K12)) | BDBM50183258
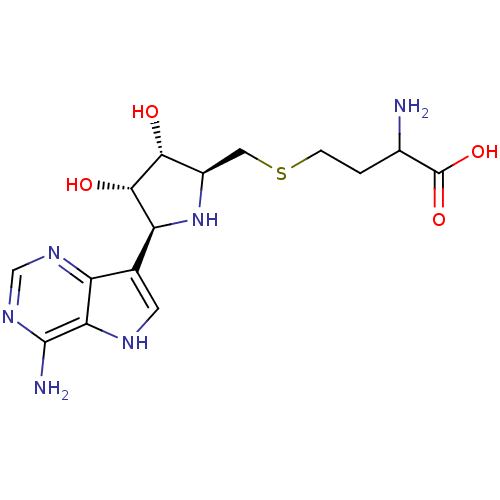
(2-amino-4-[5-(4-amino-5H-pyrrolo[3,2-d]pyrimidin-7...)Show SMILES NC(CCSC[C@H]1N[C@H]([C@H](O)[C@@H]1O)c1c[nH]c2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C15H22N6O4S/c16-7(15(24)25)1-2-26-4-8-12(22)13(23)10(21-8)6-3-18-11-9(6)19-5-20-14(11)17/h3,5,7-8,10,12-13,18,21-23H,1-2,4,16H2,(H,24,25)(H2,17,19,20)/t7?,8-,10+,12-,13+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli MTAN |
Bioorg Med Chem Lett 16: 2662-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.029
BindingDB Entry DOI: 10.7270/Q2RJ4J20 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50392835

(CHEMBL2151181)Show SMILES CC#Cc1cncc(c1)-c1cc(Cl)c(s1)[C@]1(C)CC(=O)N(C)C(N)=N1 |r,c:25| Show InChI InChI=1S/C18H17ClN4OS/c1-4-5-11-6-12(10-21-9-11)14-7-13(19)16(25-14)18(2)8-15(24)23(3)17(20)22-18/h6-7,9-10H,8H2,1-3H3,(H2,20,22)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 by FRET assay |
ACS Med Chem Lett 3: 897-902 (2012)
Article DOI: 10.1021/ml3001165
BindingDB Entry DOI: 10.7270/Q23779SN |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50071567
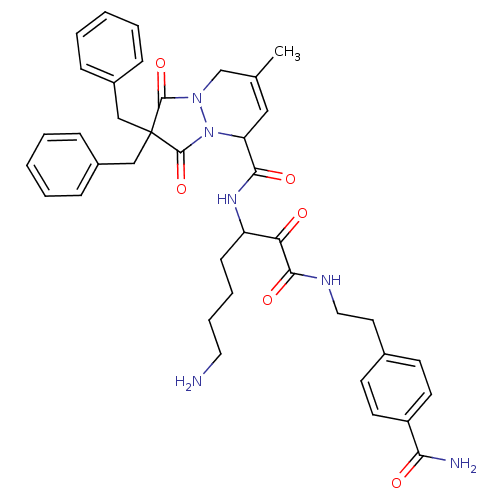
(2,2-Dibenzyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahydro...)Show SMILES CC1=CC(N2N(C1)C(=O)C(Cc1ccccc1)(Cc1ccccc1)C2=O)C(=O)NC(CCCCN)C(=O)C(=O)NCCc1ccc(cc1)C(N)=O |t:1| Show InChI InChI=1S/C39H44N6O6/c1-26-22-32(35(48)43-31(14-8-9-20-40)33(46)36(49)42-21-19-27-15-17-30(18-16-27)34(41)47)45-38(51)39(37(50)44(45)25-26,23-28-10-4-2-5-11-28)24-29-12-6-3-7-13-29/h2-7,10-13,15-18,22,31-32H,8-9,14,19-21,23-25,40H2,1H3,(H2,41,47)(H,42,49)(H,43,48) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the thrombin |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317059
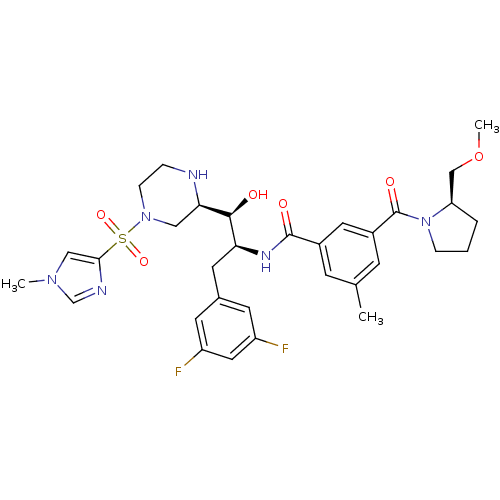
(CHEMBL1097352 | N-((1S,2S)-3-(3,5-difluorophenyl)-...)Show SMILES COC[C@H]1CCCN1C(=O)c1cc(C)cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)[C@H]1CN(CCN1)S(=O)(=O)c1cn(C)cn1 |r| Show InChI InChI=1S/C32H40F2N6O6S/c1-20-9-22(14-23(10-20)32(43)40-7-4-5-26(40)18-46-3)31(42)37-27(13-21-11-24(33)15-25(34)12-21)30(41)28-16-39(8-6-35-28)47(44,45)29-17-38(2)19-36-29/h9-12,14-15,17,19,26-28,30,35,41H,4-8,13,16,18H2,1-3H3,(H,37,42)/t26-,27+,28-,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition oh BACE1 |
Bioorg Med Chem Lett 20: 2837-42 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.050
BindingDB Entry DOI: 10.7270/Q2BK1CH7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317057
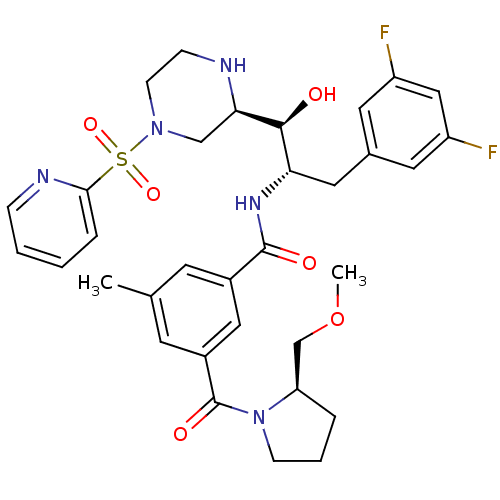
(CHEMBL1097350 | N-((1S,2S)-3-(3,5-difluorophenyl)-...)Show SMILES COC[C@H]1CCCN1C(=O)c1cc(C)cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)[C@H]1CN(CCN1)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C33H39F2N5O6S/c1-21-12-23(17-24(13-21)33(43)40-10-5-6-27(40)20-46-2)32(42)38-28(16-22-14-25(34)18-26(35)15-22)31(41)29-19-39(11-9-36-29)47(44,45)30-7-3-4-8-37-30/h3-4,7-8,12-15,17-18,27-29,31,36,41H,5-6,9-11,16,19-20H2,1-2H3,(H,38,42)/t27-,28+,29-,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition oh BACE1 |
Bioorg Med Chem Lett 20: 2837-42 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.050
BindingDB Entry DOI: 10.7270/Q2BK1CH7 |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50271627

(CHEMBL507651 | N'-[(1S,2S)-2-[(4S)-1-benzyl-5-oxoi...)Show SMILES CCCN(CCC)C(=O)c1cc(C)cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)[C@@H]1NCN(Cc2ccccc2)C1=O |r| Show InChI InChI=1S/C34H40F2N4O4/c1-4-11-39(12-5-2)33(43)26-14-22(3)13-25(18-26)32(42)38-29(17-24-15-27(35)19-28(36)16-24)31(41)30-34(44)40(21-37-30)20-23-9-7-6-8-10-23/h6-10,13-16,18-19,29-31,37,41H,4-5,11-12,17,20-21H2,1-3H3,(H,38,42)/t29-,30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin E |
Bioorg Med Chem Lett 18: 3236-41 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.050
BindingDB Entry DOI: 10.7270/Q2PZ58MR |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169387

(CHEMBL366703 | N-(4'-{4-[3-(3,5-Dichloro-phenyl)-u...)Show SMILES CN1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccc(NC(C)=O)c1 Show InChI InChI=1S/C28H30Cl2N4O2/c1-19(35)32-25-5-3-4-21(14-25)20-6-8-22(9-7-20)28(10-12-34(2)13-11-28)18-31-27(36)33-26-16-23(29)15-24(30)17-26/h3-9,14-17H,10-13,18H2,1-2H3,(H,32,35)(H2,31,33,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169388

(1-(3,5-Dichloro-phenyl)-3-[1-methanesulfonyl-4-(4-...)Show SMILES CS(=O)(=O)N1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccnc1 Show InChI InChI=1S/C25H26Cl2N4O3S/c1-35(33,34)31-11-8-25(9-12-31,17-29-24(32)30-23-14-21(26)13-22(27)15-23)20-6-4-18(5-7-20)19-3-2-10-28-16-19/h2-7,10,13-16H,8-9,11-12,17H2,1H3,(H2,29,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169398
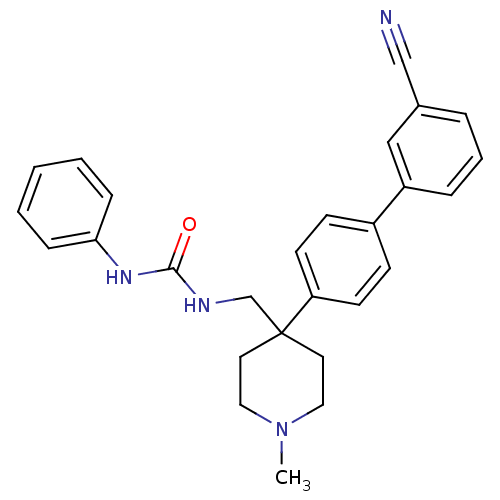
(1-[4-(3'-Cyano-biphenyl-4-yl)-1-methyl-piperidin-4...)Show SMILES CN1CCC(CNC(=O)Nc2ccccc2)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C27H28N4O/c1-31-16-14-27(15-17-31,20-29-26(32)30-25-8-3-2-4-9-25)24-12-10-22(11-13-24)23-7-5-6-21(18-23)19-28/h2-13,18H,14-17,20H2,1H3,(H2,29,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza B virus (B/Lee/40)) | BDBM4707
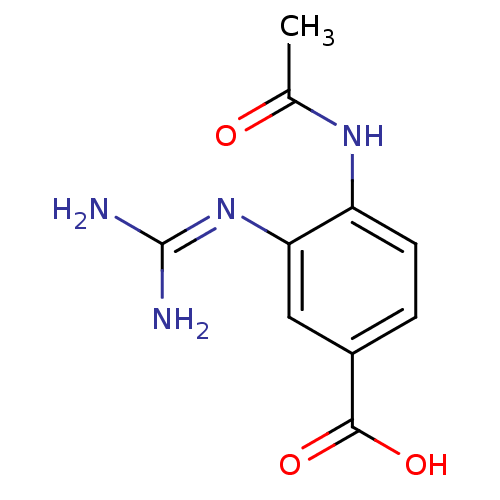
(3-(2,2-diaminoimino)-4-methylcarboxamidobenzoate |...)Show SMILES [#6]-[#6](=O)-[#7]-c1ccc(cc1\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O Show InChI InChI=1S/C10H12N4O3/c1-5(15)13-7-3-2-6(9(16)17)4-8(7)14-10(11)12/h2-4H,1H3,(H,13,15)(H,16,17)(H4,11,12,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against B/Lee/40 Influenza B Neuraminidase. |
J Med Chem 42: 2332-43 (1999)
Article DOI: 10.1021/jm980707k
BindingDB Entry DOI: 10.7270/Q25B01NJ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169370
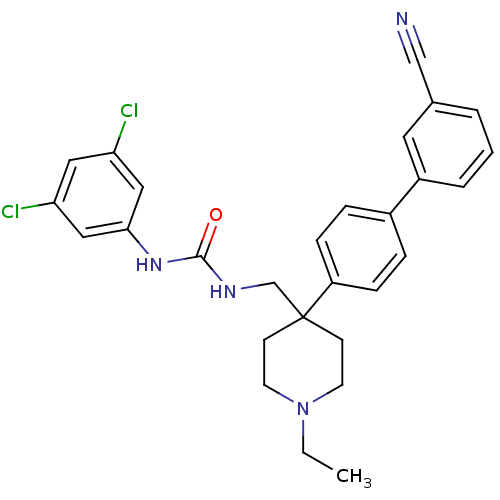
(1-[4-(3'-Cyano-biphenyl-4-yl)-1-ethyl-piperidin-4-...)Show SMILES CCN1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C28H28Cl2N4O/c1-2-34-12-10-28(11-13-34,19-32-27(35)33-26-16-24(29)15-25(30)17-26)23-8-6-21(7-9-23)22-5-3-4-20(14-22)18-31/h3-9,14-17H,2,10-13,19H2,1H3,(H2,32,33,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169392

(1-[4-(3'-Chloro-biphenyl-4-yl)-1-methyl-piperidin-...)Show SMILES CN1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccc(Cl)c1 Show InChI InChI=1S/C26H26Cl3N3O/c1-32-11-9-26(10-12-32,17-30-25(33)31-24-15-22(28)14-23(29)16-24)20-7-5-18(6-8-20)19-3-2-4-21(27)13-19/h2-8,13-16H,9-12,17H2,1H3,(H2,30,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317025
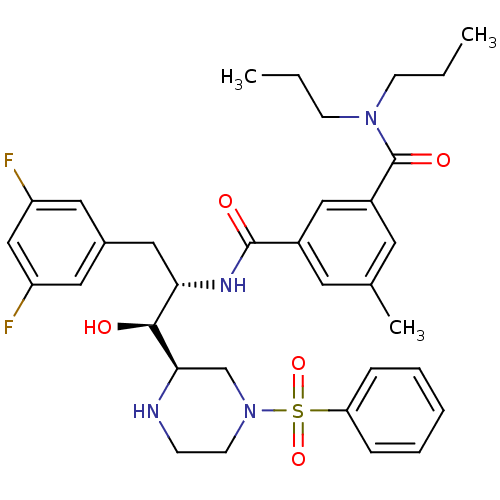
(CHEMBL1097319 | N'-{(1S,2S)-1-(3,5-difluorobenzyl)...)Show SMILES CCCN(CCC)C(=O)c1cc(C)cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)[C@H]1CN(CCN1)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C34H42F2N4O5S/c1-4-12-39(13-5-2)34(43)26-16-23(3)15-25(20-26)33(42)38-30(19-24-17-27(35)21-28(36)18-24)32(41)31-22-40(14-11-37-31)46(44,45)29-9-7-6-8-10-29/h6-10,15-18,20-21,30-32,37,41H,4-5,11-14,19,22H2,1-3H3,(H,38,42)/t30-,31+,32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition oh BACE1 |
Bioorg Med Chem Lett 20: 2837-42 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.050
BindingDB Entry DOI: 10.7270/Q2BK1CH7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50271626
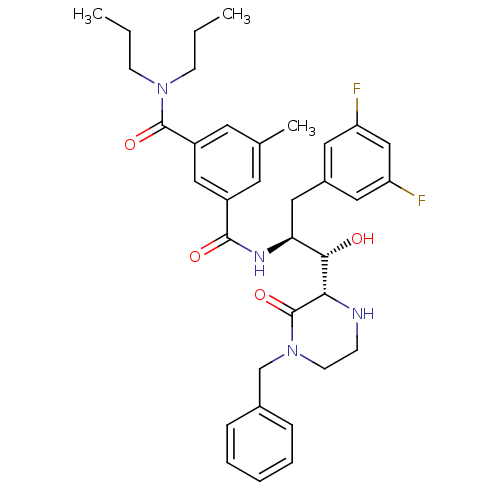
(CHEMBL509210 | N'-[(1S,2S)-2-[(2S)-4-benzyl-3-oxop...)Show SMILES CCCN(CCC)C(=O)c1cc(C)cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)[C@@H]1NCCN(Cc2ccccc2)C1=O |r| Show InChI InChI=1S/C35H42F2N4O4/c1-4-12-40(13-5-2)34(44)27-16-23(3)15-26(20-27)33(43)39-30(19-25-17-28(36)21-29(37)18-25)32(42)31-35(45)41(14-11-38-31)22-24-9-7-6-8-10-24/h6-10,15-18,20-21,30-32,38,42H,4-5,11-14,19,22H2,1-3H3,(H,39,43)/t30-,31-,32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition oh BACE1 |
Bioorg Med Chem Lett 20: 2837-42 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.050
BindingDB Entry DOI: 10.7270/Q2BK1CH7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317049
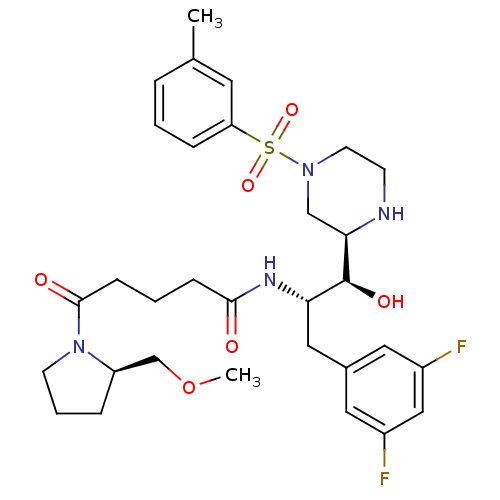
(CHEMBL1097343 | N-((1S,2S)-3-(3,5-difluorophenyl)-...)Show SMILES COC[C@H]1CCCN1C(=O)CCCC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)[C@H]1CN(CCN1)S(=O)(=O)c1cccc(C)c1 |r| Show InChI InChI=1S/C31H42F2N4O6S/c1-21-6-3-8-26(14-21)44(41,42)36-13-11-34-28(19-36)31(40)27(17-22-15-23(32)18-24(33)16-22)35-29(38)9-4-10-30(39)37-12-5-7-25(37)20-43-2/h3,6,8,14-16,18,25,27-28,31,34,40H,4-5,7,9-13,17,19-20H2,1-2H3,(H,35,38)/t25-,27+,28-,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition oh BACE1 |
Bioorg Med Chem Lett 20: 2837-42 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.050
BindingDB Entry DOI: 10.7270/Q2BK1CH7 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317037
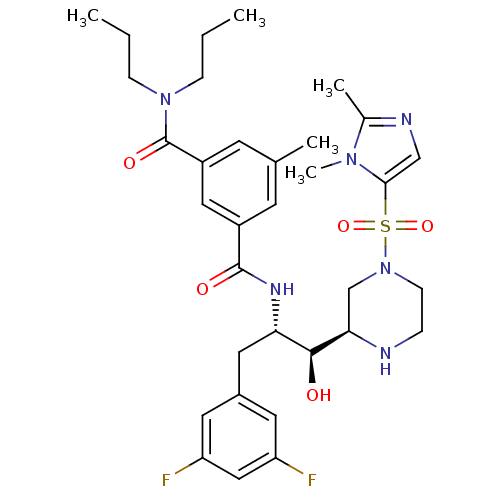
(CHEMBL1097331 | N1-((1S,2S)-3-(3,5-difluorophenyl)...)Show SMILES CCCN(CCC)C(=O)c1cc(C)cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)[C@H]1CN(CCN1)S(=O)(=O)c1cnc(C)n1C |r| Show InChI InChI=1S/C33H44F2N6O5S/c1-6-9-40(10-7-2)33(44)25-13-21(3)12-24(17-25)32(43)38-28(16-23-14-26(34)18-27(35)15-23)31(42)29-20-41(11-8-36-29)47(45,46)30-19-37-22(4)39(30)5/h12-15,17-19,28-29,31,36,42H,6-11,16,20H2,1-5H3,(H,38,43)/t28-,29+,31-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition oh BACE1 |
Bioorg Med Chem Lett 20: 2837-42 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.050
BindingDB Entry DOI: 10.7270/Q2BK1CH7 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169391

(1-(3,5-Dichloro-phenyl)-3-[1-methyl-4-(4-pyridin-3...)Show SMILES CN1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccnc1 Show InChI InChI=1S/C25H26Cl2N4O/c1-31-11-8-25(9-12-31,17-29-24(32)30-23-14-21(26)13-22(27)15-23)20-6-4-18(5-7-20)19-3-2-10-28-16-19/h2-7,10,13-16H,8-9,11-12,17H2,1H3,(H2,29,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50071566
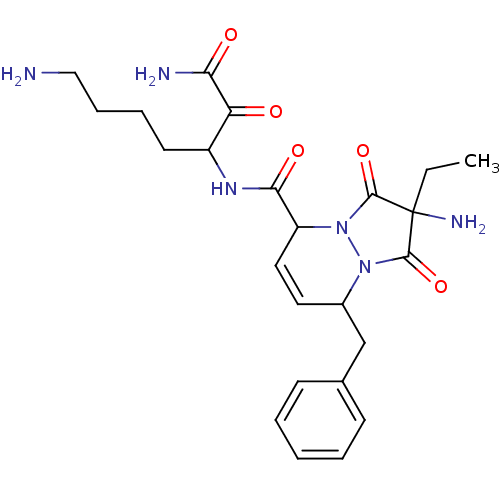
(2-Amino-8-benzyl-2-ethyl-1,3-dioxo-2,3,5,8-tetrahy...)Show SMILES CCC1(N)C(=O)N2C(Cc3ccccc3)C=CC(N2C1=O)C(=O)NC(CCCCN)C(=O)C(N)=O |c:16| Show InChI InChI=1S/C24H32N6O5/c1-2-24(27)22(34)29-16(14-15-8-4-3-5-9-15)11-12-18(30(29)23(24)35)21(33)28-17(10-6-7-13-25)19(31)20(26)32/h3-5,8-9,11-12,16-18H,2,6-7,10,13-14,25,27H2,1H3,(H2,26,32)(H,28,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the thrombin |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317026
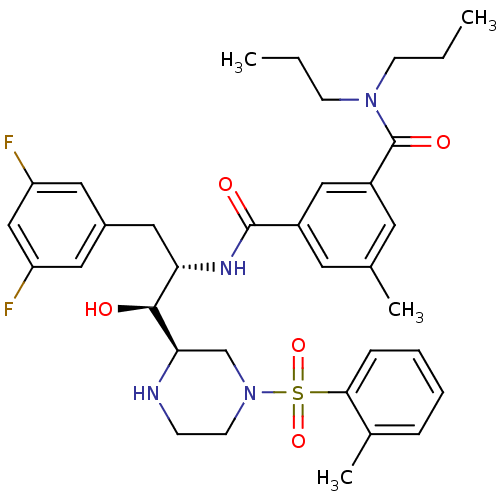
(CHEMBL1097320 | N1-((1S,2S)-3-(3,5-difluorophenyl)...)Show SMILES CCCN(CCC)C(=O)c1cc(C)cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)[C@H]1CN(CCN1)S(=O)(=O)c1ccccc1C |r| Show InChI InChI=1S/C35H44F2N4O5S/c1-5-12-40(13-6-2)35(44)27-16-23(3)15-26(20-27)34(43)39-30(19-25-17-28(36)21-29(37)18-25)33(42)31-22-41(14-11-38-31)47(45,46)32-10-8-7-9-24(32)4/h7-10,15-18,20-21,30-31,33,38,42H,5-6,11-14,19,22H2,1-4H3,(H,39,43)/t30-,31+,33-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition oh BACE1 |
Bioorg Med Chem Lett 20: 2837-42 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.050
BindingDB Entry DOI: 10.7270/Q2BK1CH7 |
More data for this
Ligand-Target Pair | |
Purine nucleoside phosphorylase
(Mus musculus) | BDBM50039549
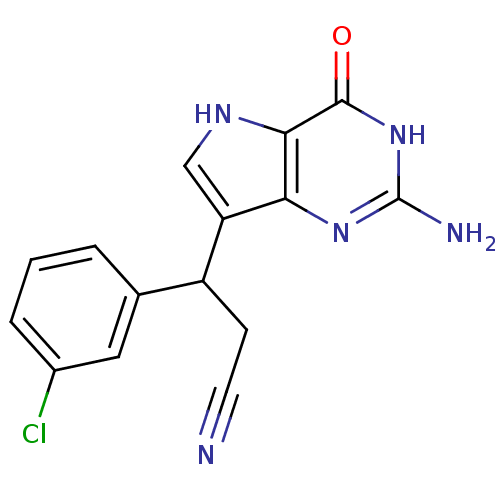
(3-(2-Amino-4-oxo-4,5-dihydro-3H-pyrrolo[3,2-d]pyri...)Show SMILES Nc1nc2c(c[nH]c2c(=O)[nH]1)C(CC#N)c1cccc(Cl)c1 Show InChI InChI=1S/C15H12ClN5O/c16-9-3-1-2-8(6-9)10(4-5-17)11-7-19-13-12(11)20-15(18)21-14(13)22/h1-3,6-7,10,19H,4H2,(H3,18,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioCryst Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against Purine nucleoside Phosphorylase from calf spleen |
J Med Chem 36: 3771-83 (1994)
BindingDB Entry DOI: 10.7270/Q23J3DKS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50071574

(2,2-Diisobutyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahyd...)Show SMILES CC(C)CC1(CC(C)C)C(=O)N2CC(C)=CC(N2C1=O)C(=O)NC(CCCCN)C(=O)C(=O)NCCc1ccc(cc1)C(N)=O |c:14| Show InChI InChI=1S/C33H48N6O6/c1-20(2)17-33(18-21(3)4)31(44)38-19-22(5)16-26(39(38)32(33)45)29(42)37-25(8-6-7-14-34)27(40)30(43)36-15-13-23-9-11-24(12-10-23)28(35)41/h9-12,16,20-21,25-26H,6-8,13-15,17-19,34H2,1-5H3,(H2,35,41)(H,36,43)(H,37,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the thrombin |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50317048

(CHEMBL1097342 | N-((1S,2S)-3-(3,5-difluorophenyl)-...)Show SMILES COC[C@H]1CCCN1C(=O)c1cc(C)cc(c1)C(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)[C@H]1CN(CCN1)S(=O)(=O)c1cccc(C)c1 |r| Show InChI InChI=1S/C35H42F2N4O6S/c1-22-6-4-8-30(14-22)48(45,46)40-11-9-38-32(20-40)33(42)31(17-24-15-27(36)19-28(37)16-24)39-34(43)25-12-23(2)13-26(18-25)35(44)41-10-5-7-29(41)21-47-3/h4,6,8,12-16,18-19,29,31-33,38,42H,5,7,9-11,17,20-21H2,1-3H3,(H,39,43)/t29-,31+,32-,33+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition oh BACE2 |
Bioorg Med Chem Lett 20: 2837-42 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.050
BindingDB Entry DOI: 10.7270/Q2BK1CH7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data