

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144942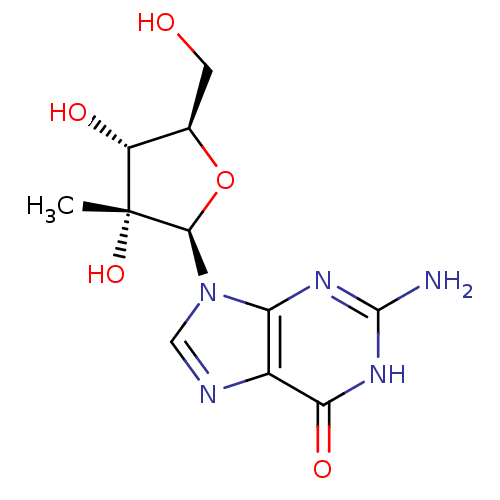 (2'-C-Me-guanosine | 2'-C-methyl-guanosine | 2'-C-m...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV NS5B-mediated RNA synthesis | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50468707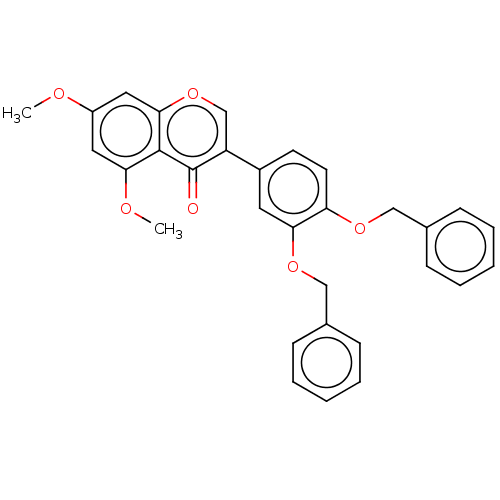 (CHEMBL4290935) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Antagonist activity at smo in SAG-treated mouse Light2 cells after 48 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144937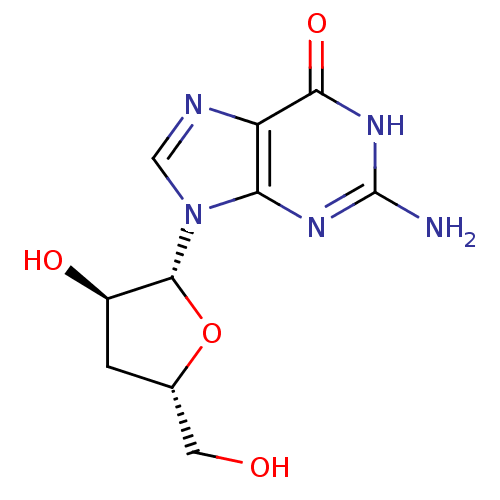 (2-Amino-9-((2R,3R,5S)-3-hydroxy-5-hydroxymethyl-te...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV NS5B-mediated RNA synthesis | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144947 (2-Amino-9-((2R,3R,4R,5R)-4-hydroxy-5-hydroxymethyl...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV NS5B-mediated RNA synthesis | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144938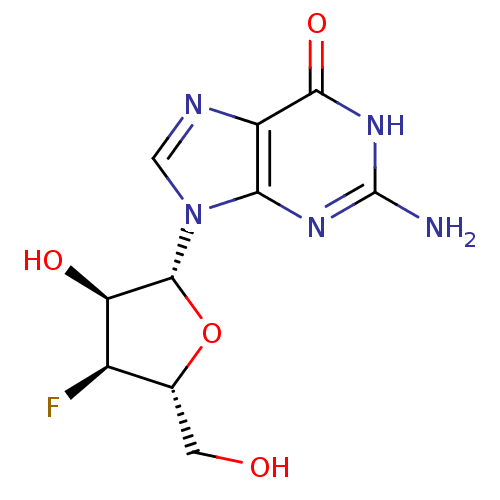 (2-Amino-9-((2R,3S,4S,5R)-4-fluoro-3-hydroxy-5-hydr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV NS5B-mediated RNA synthesis | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144948 ((2R,3R,4R,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV NS5B-mediated RNA synthesis | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50468718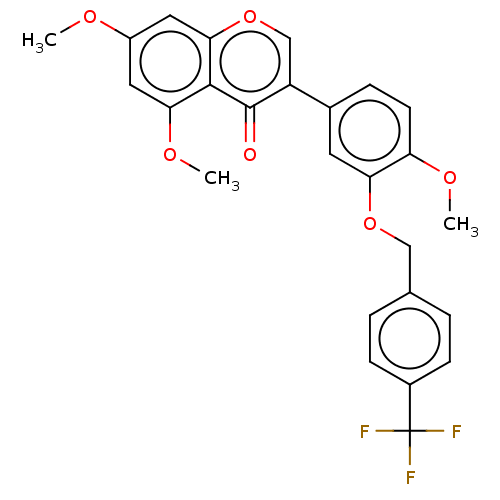 (CHEMBL4290044) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Antagonist activity at smo in SAG-treated mouse Light2 cells after 48 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144949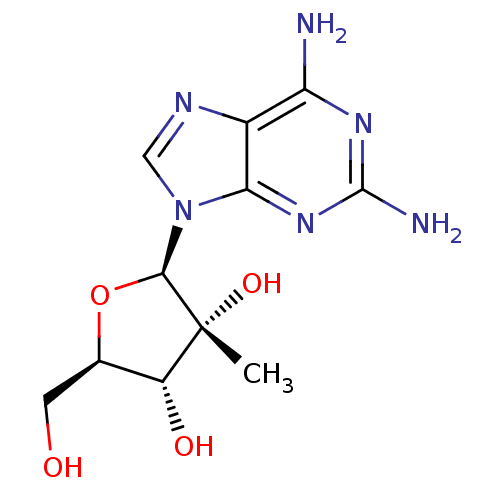 ((2R,3R,4R,5R)-2-(2,6-diamino-9H-purin-9-yl)-5-(hyd...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV NS5B-mediated RNA synthesis | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50468709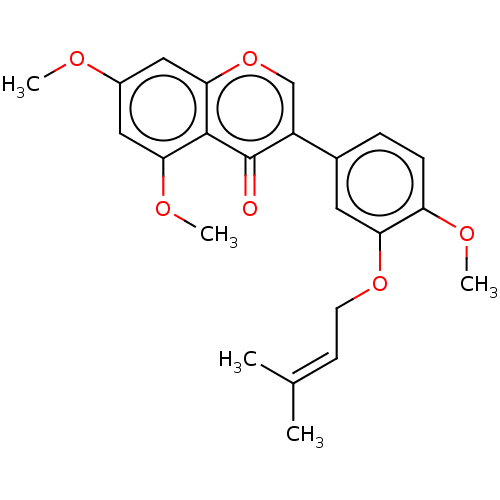 (CHEMBL4285647) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Antagonist activity at smo in SAG-treated mouse Light2 cells after 48 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50468720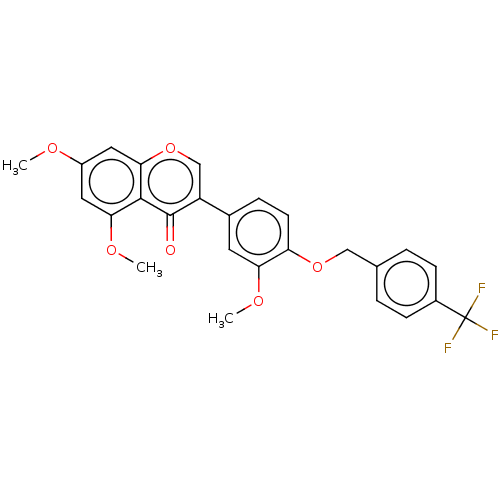 (CHEMBL4293248) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Antagonist activity at smo in SAG-treated mouse Light2 cells after 48 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50468708 (CHEMBL4291101) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Antagonist activity at smo in SAG-treated mouse Light2 cells after 48 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50468711 (CHEMBL4286606) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Antagonist activity at smo in SAG-treated mouse Light2 cells after 48 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144934 ((2R,3R,4R,5R)-2-(6-hydroxy-9H-purin-9-yl)-5-(hydro...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV NS5B-mediated RNA synthesis | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Zinc finger protein GLI1 (Mus musculus) | BDBM50468709 (CHEMBL4285647) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of Gli1 transcriptional activity in mouse embroyonic fibroblasts after 24 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50468715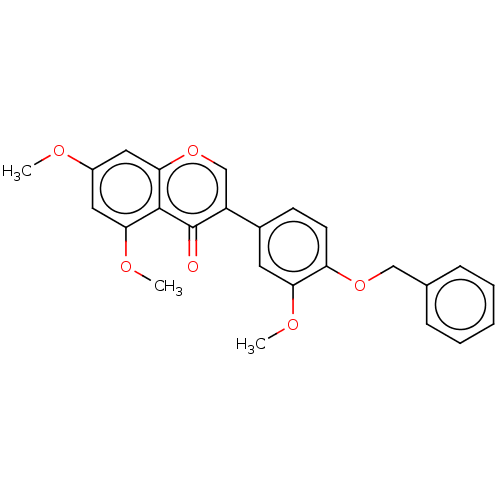 (CHEMBL4289866) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Antagonist activity at smo in SAG-treated mouse Light2 cells after 48 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50468706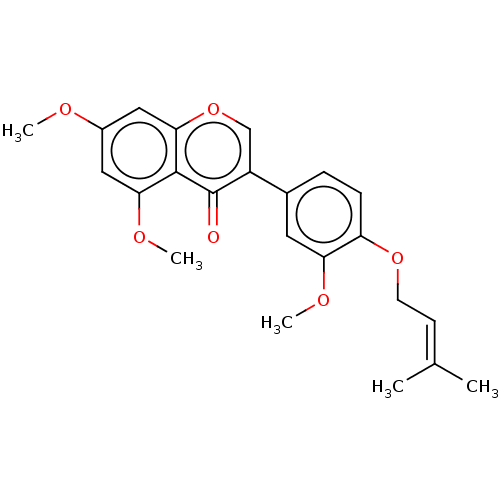 (CHEMBL4279181) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Antagonist activity at smo in SAG-treated mouse Light2 cells after 48 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50468716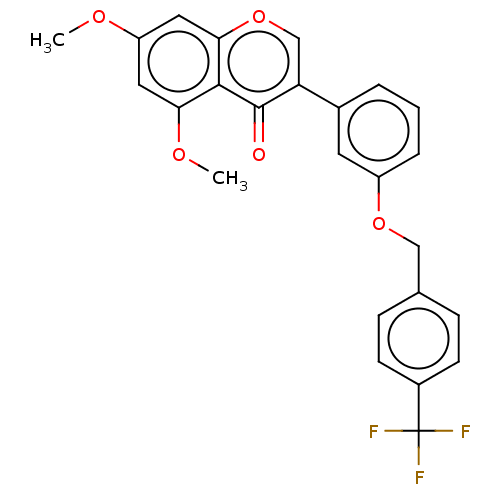 (CHEMBL4289632) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Antagonist activity at smo in SAG-treated mouse Light2 cells after 48 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Zinc finger protein GLI1 (Mus musculus) | BDBM50468706 (CHEMBL4279181) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of Gli1 transcriptional activity in mouse embroyonic fibroblasts after 24 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Zinc finger protein GLI1 (Mus musculus) | BDBM50468718 (CHEMBL4290044) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of Gli1 transcriptional activity in mouse embroyonic fibroblasts after 24 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50468712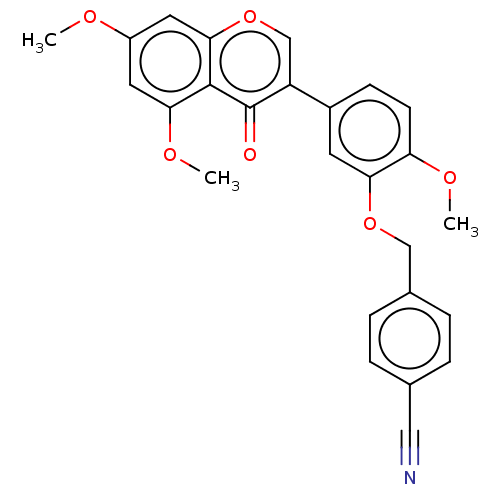 (CHEMBL4279361) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Antagonist activity at smo in SAG-treated mouse Light2 cells after 48 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Zinc finger protein GLI1 (Mus musculus) | BDBM50468711 (CHEMBL4286606) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of Gli1 transcriptional activity in mouse embroyonic fibroblasts after 24 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Zinc finger protein GLI1 (Mus musculus) | BDBM50468710 (CHEMBL4281758) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of Gli1 transcriptional activity in mouse embroyonic fibroblasts after 24 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50409930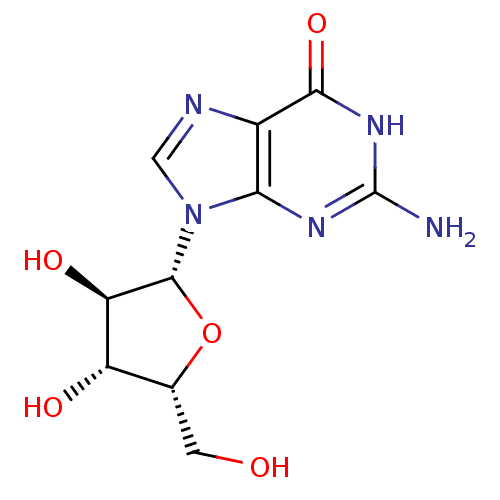 (CHEMBL2021379) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV NS5B-mediated RNA synthesis | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Zinc finger protein GLI1 (Mus musculus) | BDBM50468708 (CHEMBL4291101) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of Gli1 transcriptional activity in mouse embroyonic fibroblasts after 24 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144950 (3'-deoxyadenosine | 9-(beta-D-3'-Deoxyribofuranosy...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV NS5B-mediated RNA synthesis | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Zinc finger protein GLI1 (Mus musculus) | BDBM50468716 (CHEMBL4289632) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of Gli1 transcriptional activity in mouse embroyonic fibroblasts after 24 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144933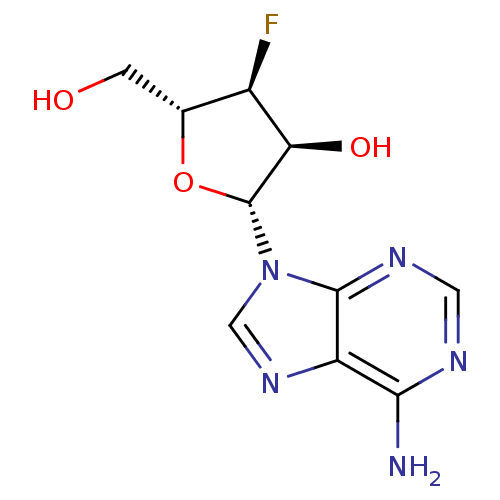 ((2R,3S,4S,5R)-4-Fluoro-5-hydroxymethyl-2-(6-methyl...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV NS5B-mediated RNA synthesis | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50468713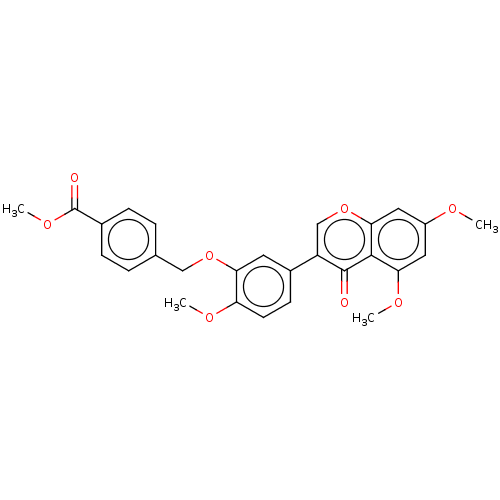 (CHEMBL4293424) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Antagonist activity at smo in SAG-treated mouse Light2 cells after 48 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50468719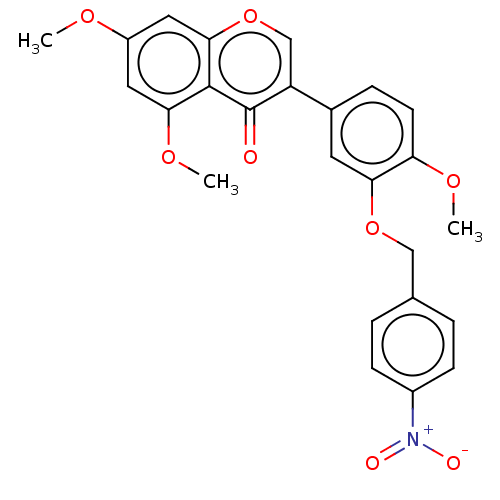 (CHEMBL4278297) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Antagonist activity at smo in SAG-treated mouse Light2 cells after 48 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50468710 (CHEMBL4281758) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Antagonist activity at smo in SAG-treated mouse Light2 cells after 48 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50468714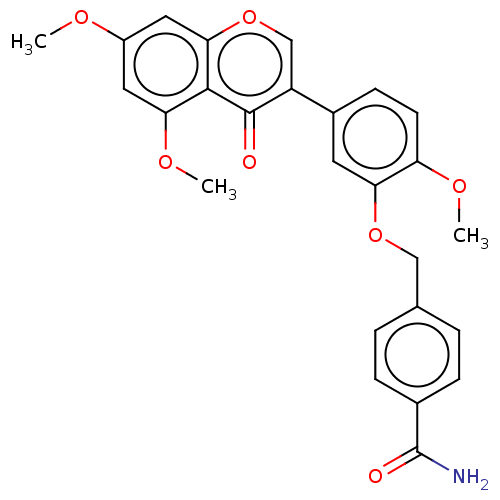 (CHEMBL4282816) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Antagonist activity at smo in SAG-treated mouse Light2 cells after 48 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Mus musculus) | BDBM50468717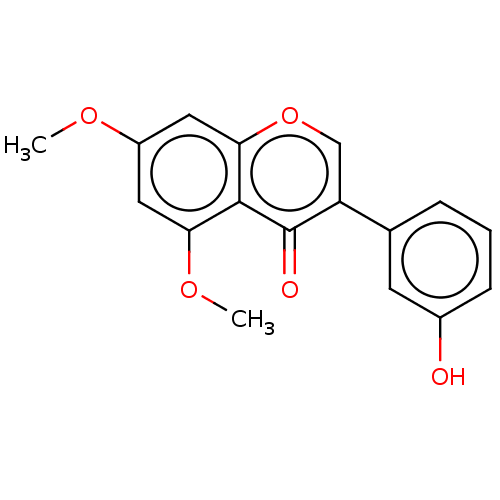 (CHEMBL4286204) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Antagonist activity at smo in SAG-treated mouse Light2 cells after 48 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Zinc finger protein GLI1 (Mus musculus) | BDBM50468715 (CHEMBL4289866) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of Gli1 transcriptional activity in mouse embroyonic fibroblasts after 24 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144939 ((2R,3R,4R,5R)-2-(2-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV NS5B-mediated RNA synthesis | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144945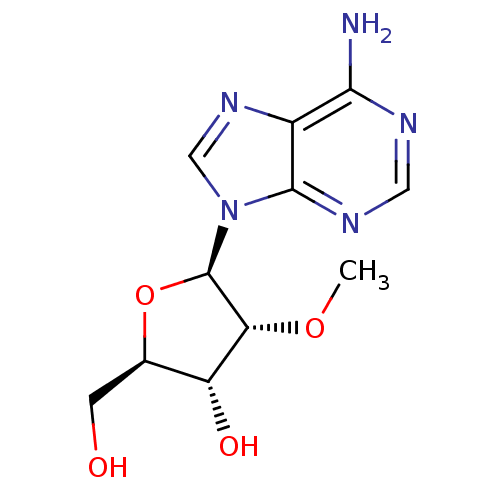 ((2R,3R,4R,5R)-2-Hydroxymethyl-4-methoxy-5-(6-methy...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV NS5B-mediated RNA synthesis | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144936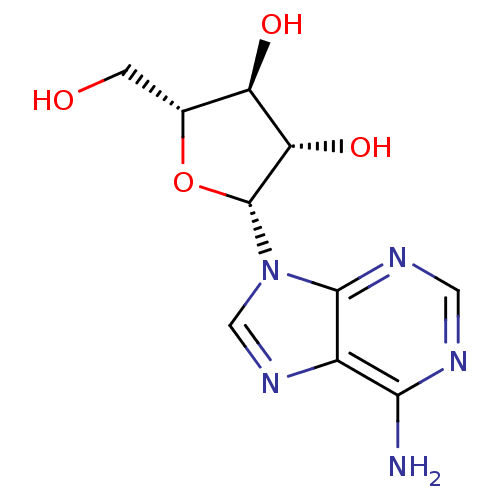 (CHEMBL1090 | VIDARABINE | adenine arabinoside) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV NS5B-mediated RNA synthesis | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50408410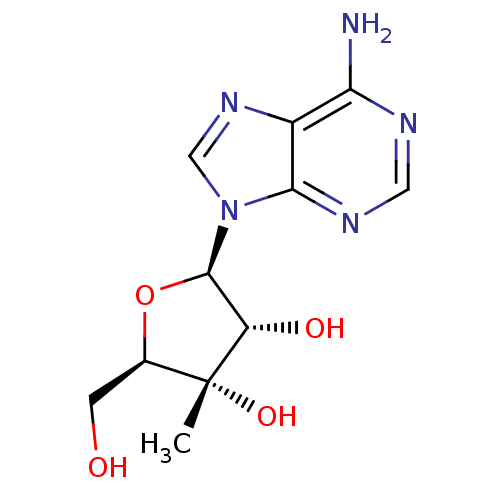 (CHEMBL480143) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV NS5B-mediated RNA synthesis | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144935 ((2R,3R,4R,5R)-2-(6-amino-9H-purin-9-yl)-3-ethyl-5-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV NS5B-mediated RNA synthesis | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50422565 (CHEMBL2311161) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV NS5B-mediated RNA synthesis | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144932 ((2R,3R,4R,5R)-4-Fluoro-2-hydroxymethyl-5-(6-methyl...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV NS5B-mediated RNA synthesis | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144941 ((2R,3R,4R,5R)-5-(6-amino-9H-purin-9-yl)-2-(hydroxy...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV NS5B-mediated RNA synthesis | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144940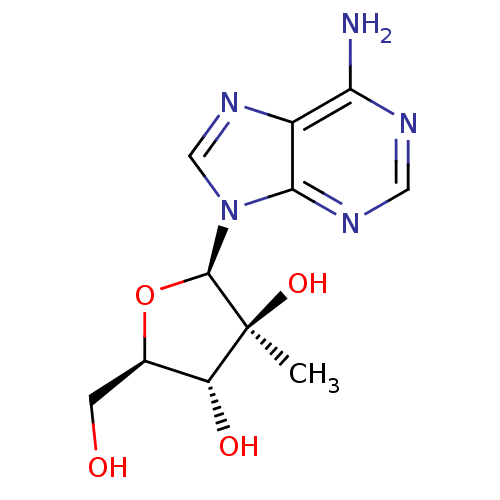 ((2R,3S,4R,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV NS5B-mediated RNA synthesis | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Zinc finger protein GLI1 (Mus musculus) | BDBM50468720 (CHEMBL4293248) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of Gli1 transcriptional activity in mouse embroyonic fibroblasts after 24 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Zinc finger protein GLI1 (Mus musculus) | BDBM50468707 (CHEMBL4290935) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of Gli1 transcriptional activity in mouse embroyonic fibroblasts after 24 hrs by renilla luciferase reporter gene assay | Eur J Med Chem 156: 554-562 (2018) Article DOI: 10.1016/j.ejmech.2018.07.017 BindingDB Entry DOI: 10.7270/Q2HD7ZB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144932 ((2R,3R,4R,5R)-4-Fluoro-2-hydroxymethyl-5-(6-methyl...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV RNA replication | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144930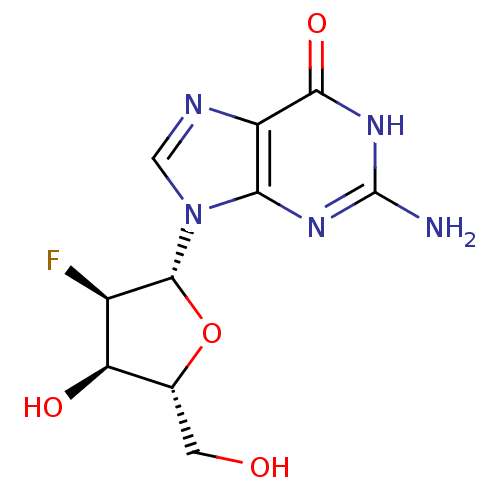 (2-Amino-9-((2R,3R,4R,5R)-3-fluoro-4-hydroxy-5-hydr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV RNA replication | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144933 ((2R,3S,4S,5R)-4-Fluoro-5-hydroxymethyl-2-(6-methyl...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV RNA replication | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144936 (CHEMBL1090 | VIDARABINE | adenine arabinoside) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV RNA replication | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144940 ((2R,3S,4R,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV RNA replication | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50144941 ((2R,3R,4R,5R)-5-(6-amino-9H-purin-9-yl)-2-(hydroxy...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HCV RNA replication | J Med Chem 47: 2283-95 (2004) Article DOI: 10.1021/jm030424e BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 63 total ) | Next | Last >> |