

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50577958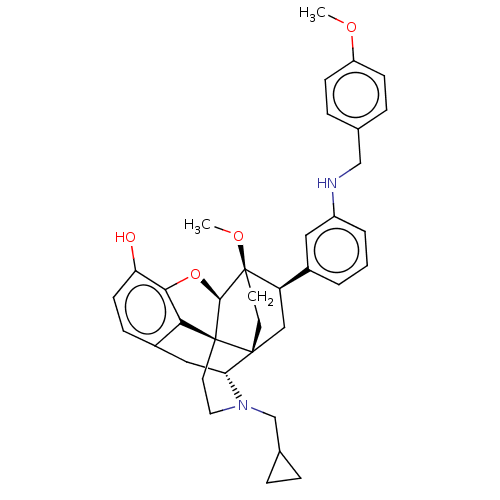 (CHEMBL4859512) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01082 BindingDB Entry DOI: 10.7270/Q2NZ8CG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50598866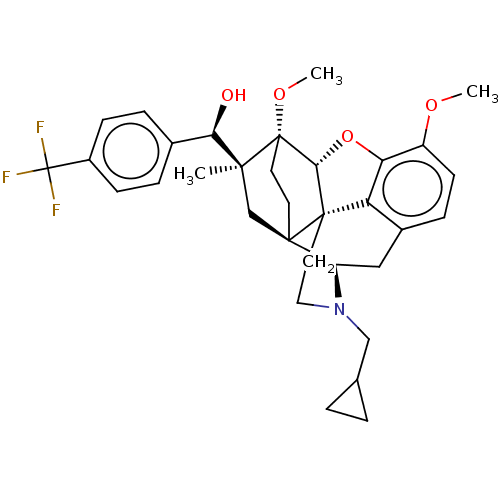 (CHEMBL5205530) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00014 BindingDB Entry DOI: 10.7270/Q2QJ7N96 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50379543 (CHEMBL2012752) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... | Bioorg Med Chem Lett 22: 2242-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.095 BindingDB Entry DOI: 10.7270/Q2MC912Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50329261 ((R)-2-(4-fluoro-3,5-dimethylphenylamino)-6-(4-fluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor assessed as proteolysis using MCA-KKVYPYPME-Dap(Dnp)-NH2 peptide substrate by FRET assay | Bioorg Med Chem Lett 20: 6850-3 (2010) Article DOI: 10.1016/j.bmcl.2010.08.058 BindingDB Entry DOI: 10.7270/Q2222V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50329261 ((R)-2-(4-fluoro-3,5-dimethylphenylamino)-6-(4-fluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor assessed as proteolysis using MCA-KKVYPYPME-Dap(Dnp)-NH2 peptide substrate after 4 hrs by FRET assay | Bioorg Med Chem Lett 21: 2030-3 (2011) Article DOI: 10.1016/j.bmcl.2011.02.010 BindingDB Entry DOI: 10.7270/Q24M94VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50598866 (CHEMBL5205530) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00014 BindingDB Entry DOI: 10.7270/Q2QJ7N96 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50577958 (CHEMBL4859512) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPDPE from rat delta opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting metho... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01082 BindingDB Entry DOI: 10.7270/Q2NZ8CG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50215536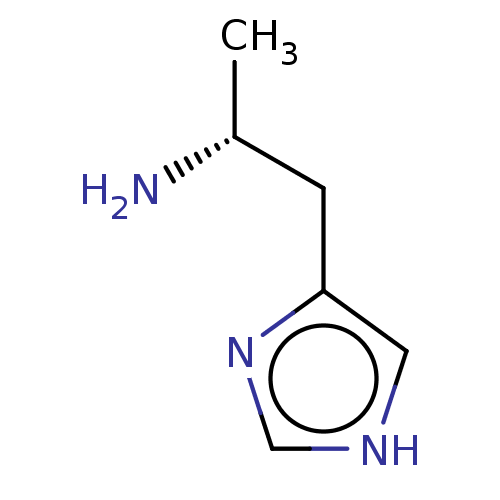 ((R)-Alpha-Methylhistamine | CHEBI:73337 | CHEMBL26...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.0851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Binding affinity towards histamine H3 receptor using [3H](R)-alpha-methylhistamine as radioligand in guinea pig cortical homogenates | Bioorg Med Chem Lett 9: 1825-30 (1999) BindingDB Entry DOI: 10.7270/Q24T6MKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50532777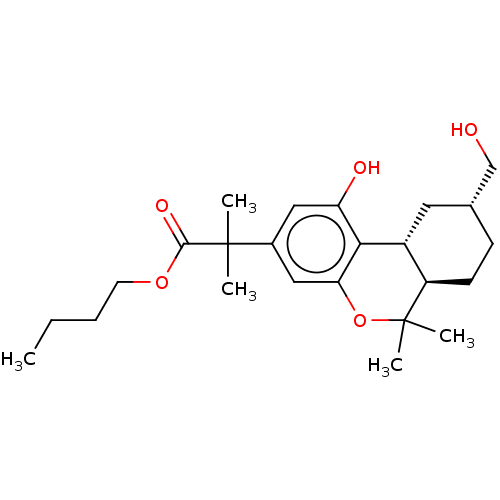 (CHEMBL4470925) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from mouse CB2 receptor expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 59: 6903-19 (2016) Article DOI: 10.1021/acs.jmedchem.6b00717 BindingDB Entry DOI: 10.7270/Q2RV0S68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50532777 (CHEMBL4470925) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from mouse CB2 receptor expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 59: 6903-19 (2016) Article DOI: 10.1021/acs.jmedchem.6b00717 BindingDB Entry DOI: 10.7270/Q2RV0S68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50532778 (CHEMBL4465566 | US10882838, Example 3.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 59: 6903-19 (2016) Article DOI: 10.1021/acs.jmedchem.6b00717 BindingDB Entry DOI: 10.7270/Q2RV0S68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50532778 (CHEMBL4465566 | US10882838, Example 3.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 59: 6903-19 (2016) Article DOI: 10.1021/acs.jmedchem.6b00717 BindingDB Entry DOI: 10.7270/Q2RV0S68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50379542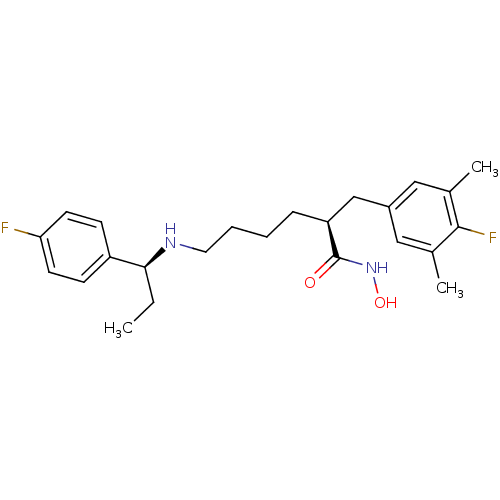 (CHEMBL2012753) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... | Bioorg Med Chem Lett 22: 2242-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.095 BindingDB Entry DOI: 10.7270/Q2MC912Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411339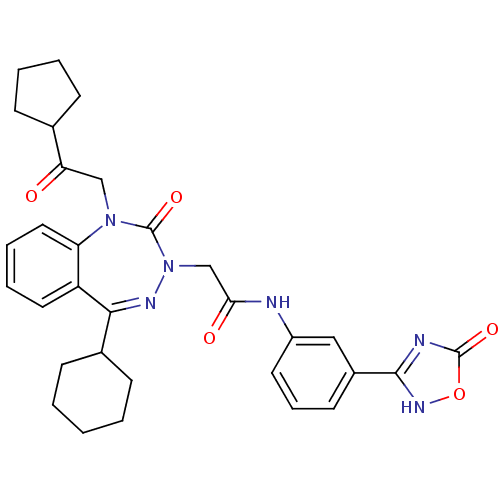 (CHEMBL227276) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50340754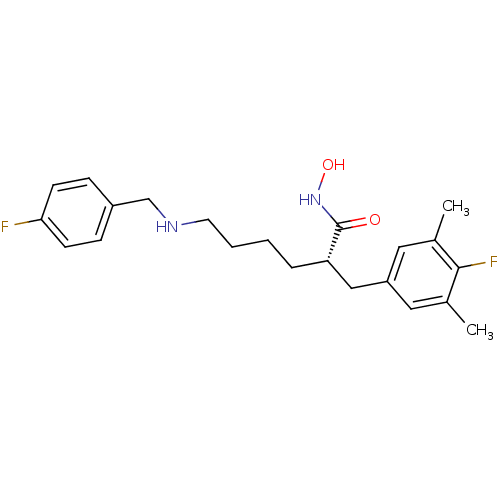 ((S)-2-(4-fluoro-3,5-dimethylbenzyl)-6-(4-fluoroben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... | Bioorg Med Chem Lett 22: 2242-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.095 BindingDB Entry DOI: 10.7270/Q2MC912Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50340754 ((S)-2-(4-fluoro-3,5-dimethylbenzyl)-6-(4-fluoroben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor assessed as proteolysis using MCA-KKVYPYPME-Dap(Dnp)-NH2 peptide substrate after 4 hrs by FRET assay | Bioorg Med Chem Lett 21: 2030-3 (2011) Article DOI: 10.1016/j.bmcl.2011.02.010 BindingDB Entry DOI: 10.7270/Q24M94VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50056102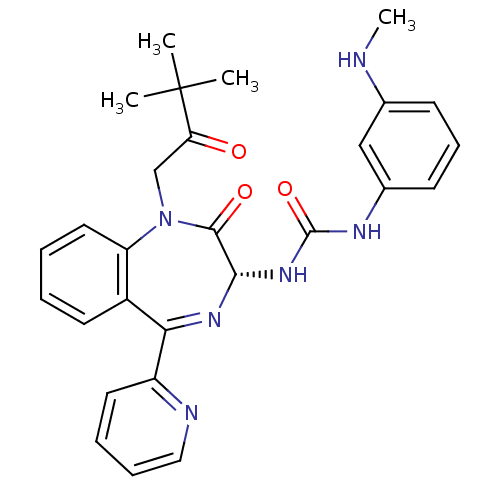 ((R)-1-(1-(3,3-dimethyl-2-oxobutyl)-2-oxo-5-(pyridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50121205 (CHEBI:18295 | Histamine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents | PubMed | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Binding affinity towards histamine H3 receptor using [3H](R)-alpha-methylhistamine as radioligand in guinea pig cortical homogenates | Bioorg Med Chem Lett 9: 1825-30 (1999) BindingDB Entry DOI: 10.7270/Q24T6MKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50215536 ((R)-Alpha-Methylhistamine | CHEBI:73337 | CHEMBL26...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Binding affinity towards histamine H3 receptor using [3H](R)-alpha-methylhistamine as radioligand in guinea pig ileum LMMP homogenates | Bioorg Med Chem Lett 9: 1825-30 (1999) BindingDB Entry DOI: 10.7270/Q24T6MKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50379536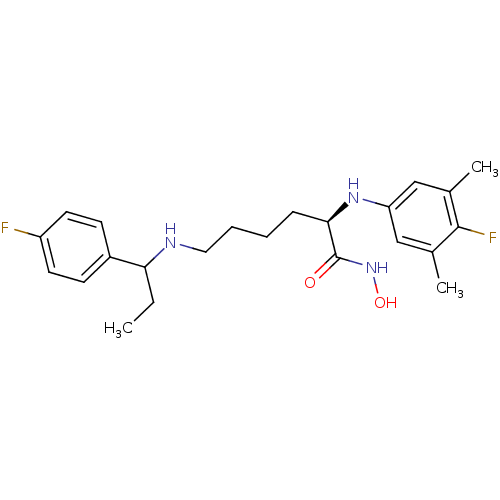 (CHEMBL2012836) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... | Bioorg Med Chem Lett 22: 2242-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.095 BindingDB Entry DOI: 10.7270/Q2MC912Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411341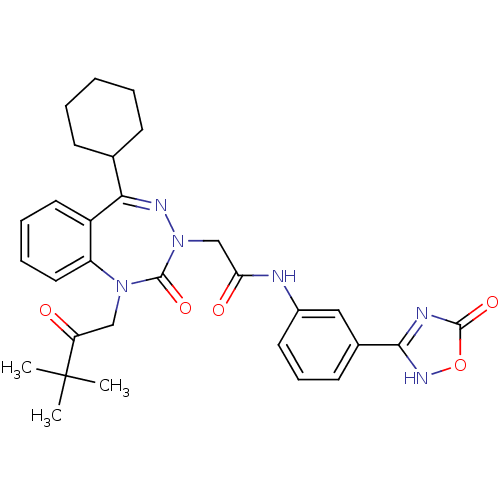 (CHEMBL226583) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50532778 (CHEMBL4465566 | US10882838, Example 3.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 59: 6903-19 (2016) Article DOI: 10.1021/acs.jmedchem.6b00717 BindingDB Entry DOI: 10.7270/Q2RV0S68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50532778 (CHEMBL4465566 | US10882838, Example 3.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from mouse CB2 receptor expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 59: 6903-19 (2016) Article DOI: 10.1021/acs.jmedchem.6b00717 BindingDB Entry DOI: 10.7270/Q2RV0S68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50067499 ((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity to mouse CB2 receptor | J Med Chem 59: 6903-19 (2016) Article DOI: 10.1021/acs.jmedchem.6b00717 BindingDB Entry DOI: 10.7270/Q2RV0S68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50532778 (CHEMBL4465566 | US10882838, Example 3.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 59: 6903-19 (2016) Article DOI: 10.1021/acs.jmedchem.6b00717 BindingDB Entry DOI: 10.7270/Q2RV0S68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50067499 ((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Binding affinity to mouse CB2 receptor | J Med Chem 59: 6903-19 (2016) Article DOI: 10.1021/acs.jmedchem.6b00717 BindingDB Entry DOI: 10.7270/Q2RV0S68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50532778 (CHEMBL4465566 | US10882838, Example 3.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from mouse CB2 receptor expressed in HEK293 cell membranes by scintillation counting method | J Med Chem 59: 6903-19 (2016) Article DOI: 10.1021/acs.jmedchem.6b00717 BindingDB Entry DOI: 10.7270/Q2RV0S68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50379541 (CHEMBL2012832) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... | Bioorg Med Chem Lett 22: 2242-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.095 BindingDB Entry DOI: 10.7270/Q2MC912Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50329265 ((R)-2-(4-fluoro-3,5-dimethylphenylamino)-6-(4-fluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor assessed as proteolysis using MCA-KKVYPYPME-Dap(Dnp)-NH2 peptide substrate by FRET assay | Bioorg Med Chem Lett 20: 6850-3 (2010) Article DOI: 10.1016/j.bmcl.2010.08.058 BindingDB Entry DOI: 10.7270/Q2222V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50329265 ((R)-2-(4-fluoro-3,5-dimethylphenylamino)-6-(4-fluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... | Bioorg Med Chem Lett 22: 2242-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.095 BindingDB Entry DOI: 10.7270/Q2MC912Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50329265 ((R)-2-(4-fluoro-3,5-dimethylphenylamino)-6-(4-fluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor assessed as proteolysis using MCA-KKVYPYPME-Dap(Dnp)-NH2 peptide substrate after 4 hrs by FRET assay | Bioorg Med Chem Lett 21: 2030-3 (2011) Article DOI: 10.1016/j.bmcl.2011.02.010 BindingDB Entry DOI: 10.7270/Q24M94VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50002875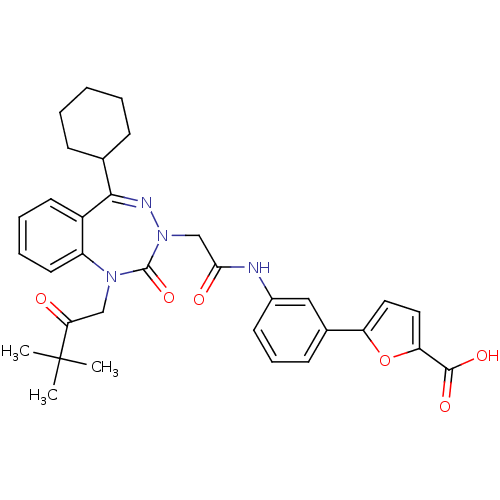 (CHEMBL388144) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50340755 ((S)-2-(4-fluoro-3-methylbenzyl)-6-(4-fluorobenzyla...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor assessed as proteolysis using MCA-KKVYPYPME-Dap(Dnp)-NH2 peptide substrate after 4 hrs by FRET assay | Bioorg Med Chem Lett 21: 2030-3 (2011) Article DOI: 10.1016/j.bmcl.2011.02.010 BindingDB Entry DOI: 10.7270/Q24M94VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50379533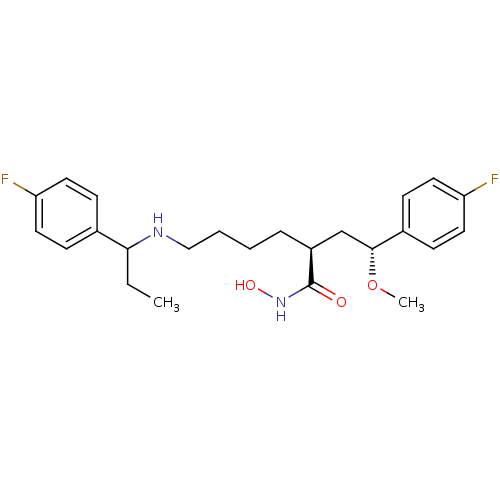 (CHEMBL2012838) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... | Bioorg Med Chem Lett 22: 2242-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.095 BindingDB Entry DOI: 10.7270/Q2MC912Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411340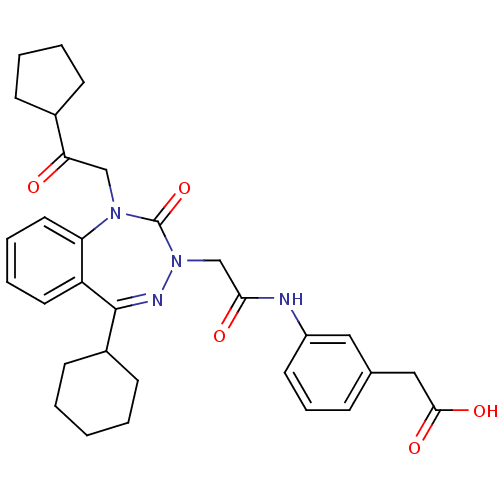 (CHEMBL387948) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50577958 (CHEMBL4859512) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.294 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01082 BindingDB Entry DOI: 10.7270/Q2NZ8CG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50329270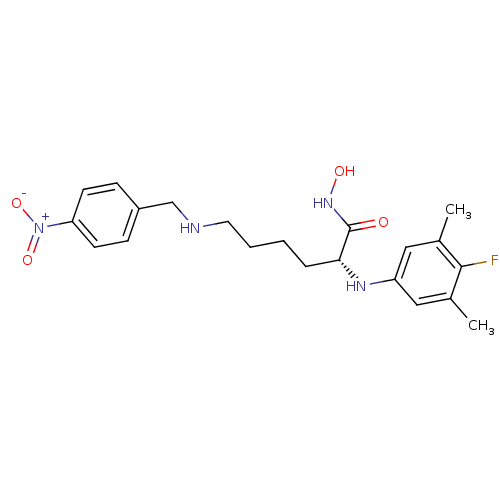 ((R)-2-(4-fluoro-3,5-dimethylphenylamino)-N-hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor assessed as proteolysis using MCA-KKVYPYPME-Dap(Dnp)-NH2 peptide substrate by FRET assay | Bioorg Med Chem Lett 20: 6850-3 (2010) Article DOI: 10.1016/j.bmcl.2010.08.058 BindingDB Entry DOI: 10.7270/Q2222V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411344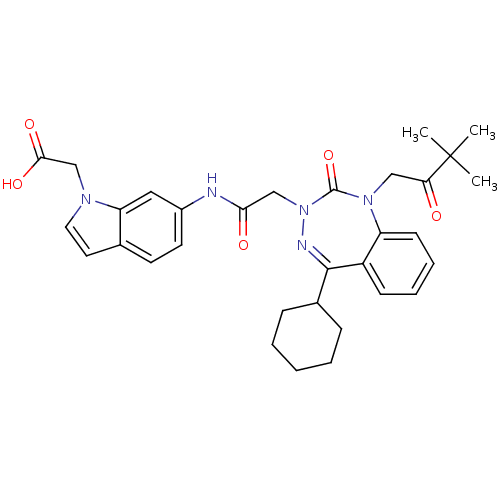 (CHEMBL389711) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50379540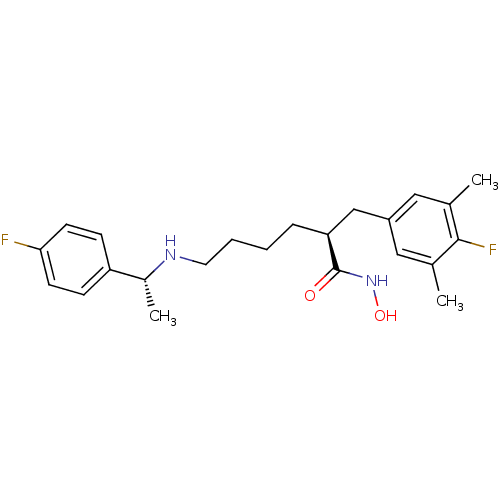 (CHEMBL2012750) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... | Bioorg Med Chem Lett 22: 2242-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.095 BindingDB Entry DOI: 10.7270/Q2MC912Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582411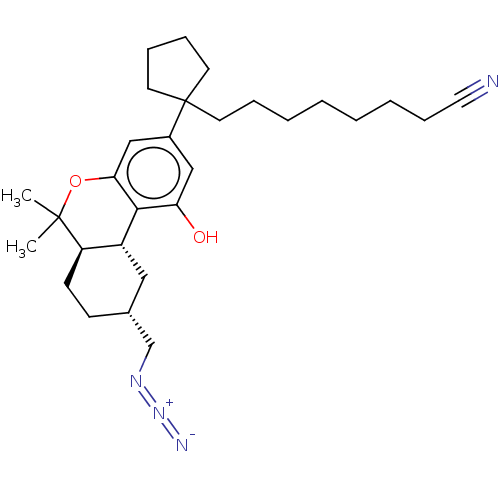 (CHEMBL5084325) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50582411 (CHEMBL5084325) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from human CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50379535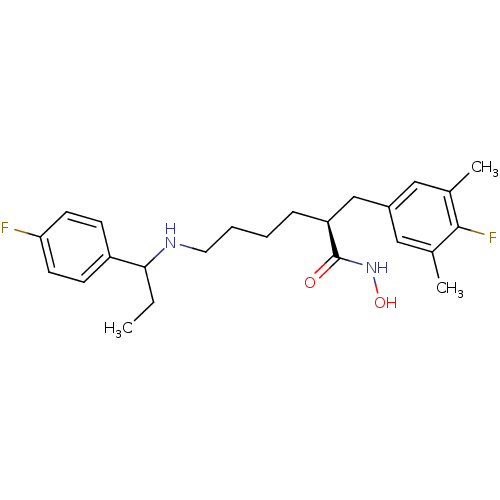 (CHEMBL2010824) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of N-terminal 6-histine tagged Bacillus anthracis LF 263-C terminal catalytic domain using MCA-KKVYPYPME-Dap(Dnp)-NH2 as substrate after 4... | Bioorg Med Chem Lett 22: 2242-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.095 BindingDB Entry DOI: 10.7270/Q2MC912Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411342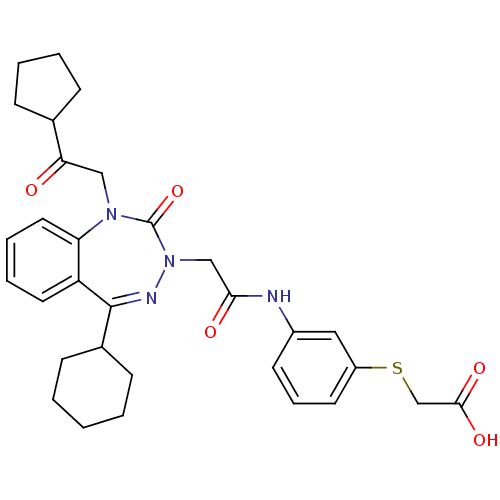 (CHEMBL227333) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50411336 (CHEMBL227330) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50340756 ((S)-6-(4-chlorobenzylamino)-2-(4-fluoro-3-methylbe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor assessed as proteolysis using MCA-KKVYPYPME-Dap(Dnp)-NH2 peptide substrate after 4 hrs by FRET assay | Bioorg Med Chem Lett 21: 2030-3 (2011) Article DOI: 10.1016/j.bmcl.2011.02.010 BindingDB Entry DOI: 10.7270/Q24M94VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50002880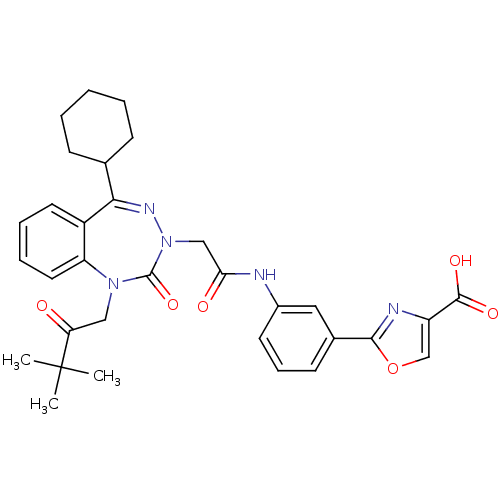 (CHEMBL437736) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [3H]BH-CCK-8S from human recombinant CCK2 receptor expressed in NIH3T3 cells | J Med Chem 50: 3101-12 (2007) Checked by Author Article DOI: 10.1021/jm070139l BindingDB Entry DOI: 10.7270/Q2QJ7JHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM50340757 ((S)-2-(4-fluoro-3,5-dimethylbenzyl)-6-(4-fluoro-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PanThera Biopharma, LLC Curated by ChEMBL | Assay Description Inhibition of Bacillus anthracis lethal factor assessed as proteolysis using MCA-KKVYPYPME-Dap(Dnp)-NH2 peptide substrate after 4 hrs by FRET assay | Bioorg Med Chem Lett 21: 2030-3 (2011) Article DOI: 10.1016/j.bmcl.2011.02.010 BindingDB Entry DOI: 10.7270/Q24M94VB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582403 (CHEMBL5085420) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582400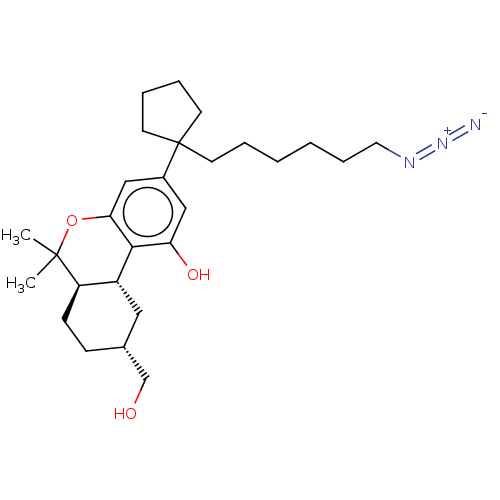 (CHEMBL5077315) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582405 (CHEMBL5074603) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5251 total ) | Next | Last >> |