

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus) | BDBM123407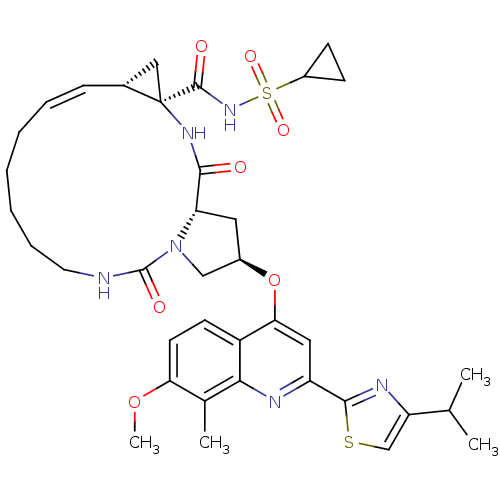 (US8741926, 91) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | -59.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123410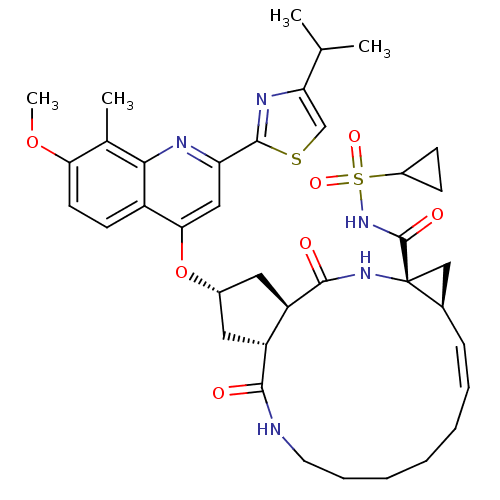 (US8741926, 82 | US8754106, 82 | US8754106, 91) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124106 (US8754106, 56) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236571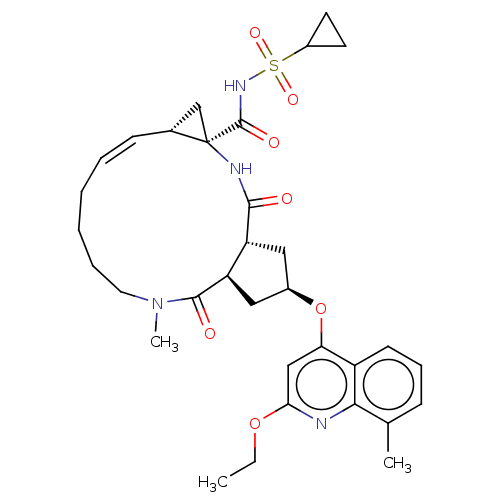 (US9365582, 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123413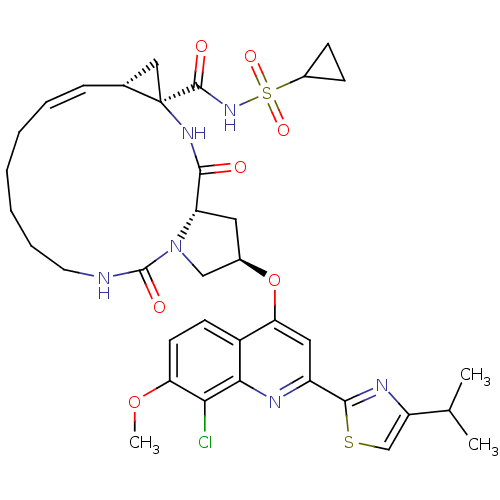 (US8741926, 94 | US8754106, 94) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236565 (US9365582, 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236566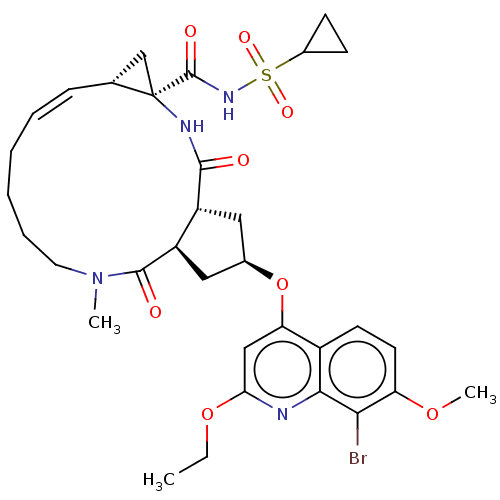 (US9365582, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123413 (US8741926, 94 | US8754106, 94) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123411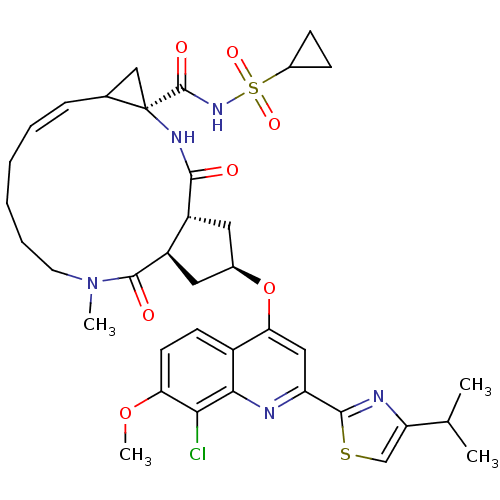 (US8741926, 56) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123415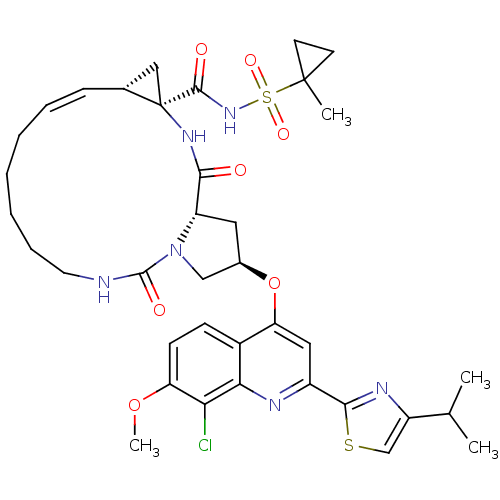 (US8741926, 95 | US8754106, 95) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123415 (US8741926, 95 | US8754106, 95) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236563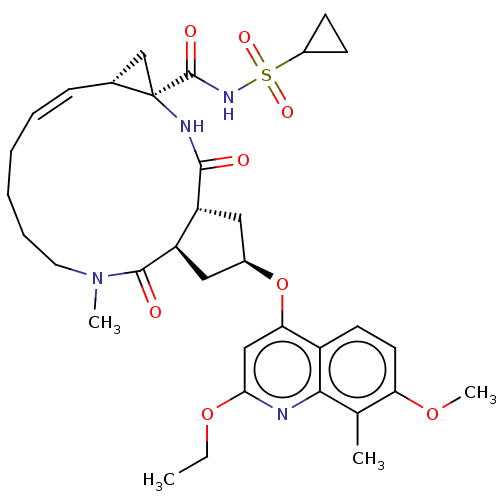 (US9365582, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236573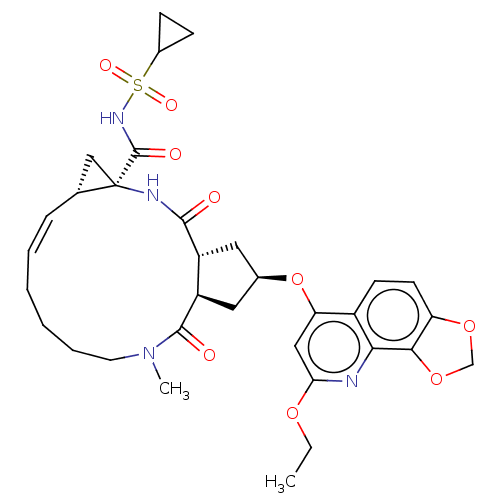 (US9365582, 15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123414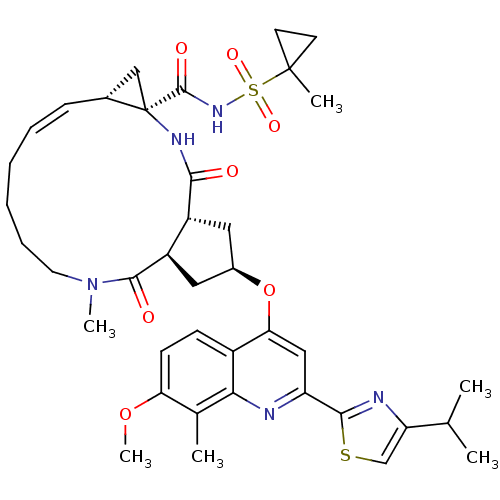 (US8741926, 48 | US8754106, 48) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.25 | -55.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123414 (US8741926, 48 | US8754106, 48) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123412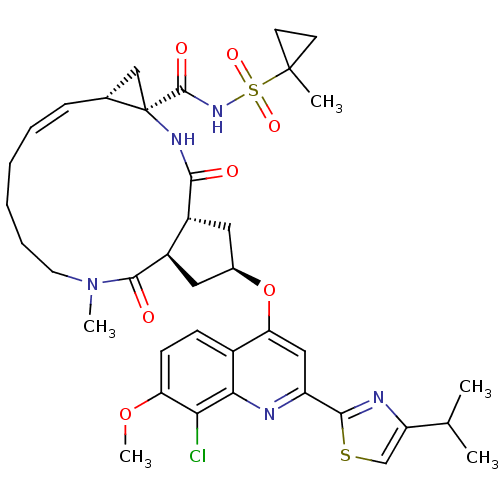 (US8741926, 57 | US8754106, 57) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123412 (US8741926, 57 | US8754106, 57) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50336504 ((2R,3aR,10Z,11aS,12aR,14aR)-N-(cyclopropylsulfonyl...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236569 (US9365582, 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50336504 ((2R,3aR,10Z,11aS,12aR,14aR)-N-(cyclopropylsulfonyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB US Patent | 0.5 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236567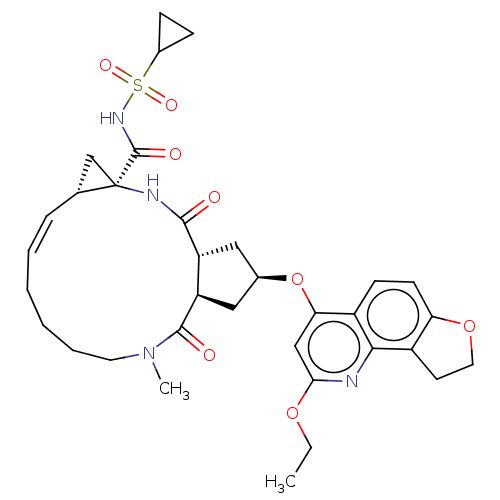 (US9365582, 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123408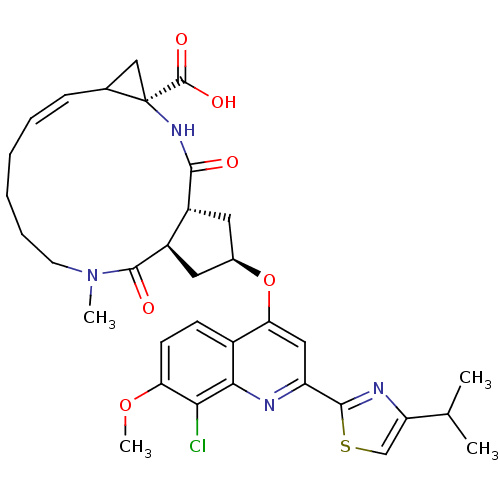 (US8741926, 55) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124105 (US8754106, 55) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236568 (US9365582, 10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123410 (US8741926, 82 | US8754106, 82 | US8754106, 91) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 9.40 | -46.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123410 (US8741926, 82 | US8754106, 82 | US8754106, 91) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236570 (US9365582, 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236562 (US9365582, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236564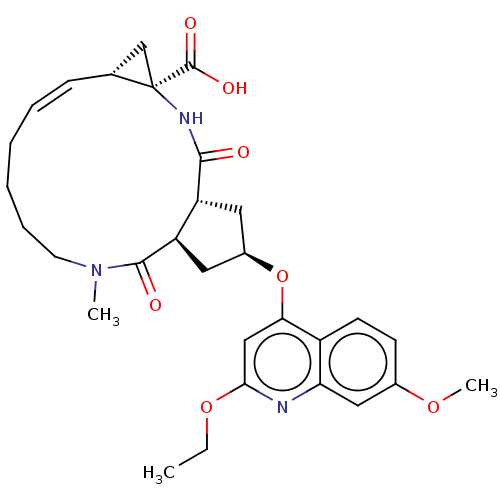 (US9365582, 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 46 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1711] (Hepatitis C virus genotype 1a (strain H77) (HCV)) | BDBM236572 (US9365582, 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 58 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Janssen Sciences Ireland UC US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US9365582 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123409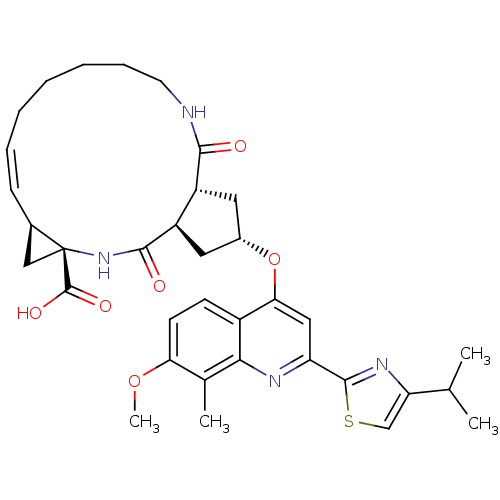 (US8741926, 81) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.00E+3 | -34.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50075135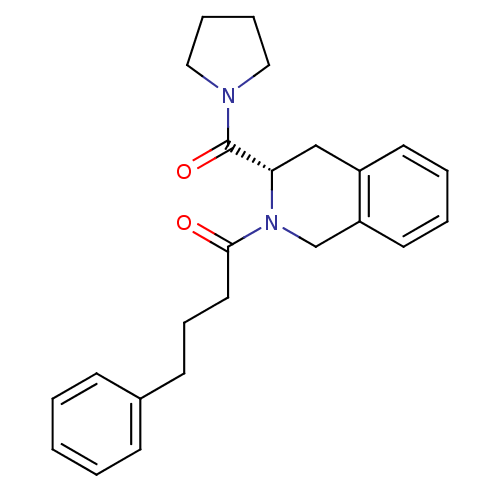 ((S)-4-phenyl-1-(3-(pyrrolidine-1-carbonyl)-3,4-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille II Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase | Bioorg Med Chem Lett 9: 437-42 (1999) BindingDB Entry DOI: 10.7270/Q2348JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50075132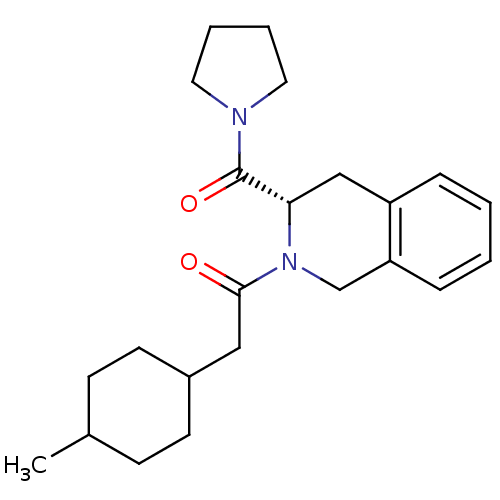 (2-(4-Methyl-cyclohexyl)-1-[(S)-3-(pyrrolidine-1-ca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille II Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase | Bioorg Med Chem Lett 9: 437-42 (1999) BindingDB Entry DOI: 10.7270/Q2348JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50075126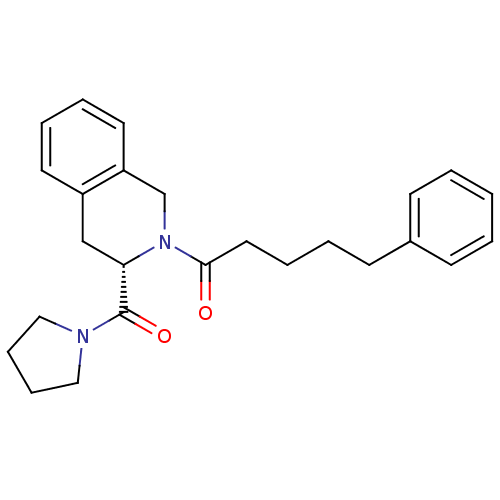 (5-Phenyl-1-[(S)-3-(pyrrolidine-1-carbonyl)-3,4-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille II Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase | Bioorg Med Chem Lett 9: 437-42 (1999) BindingDB Entry DOI: 10.7270/Q2348JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50051539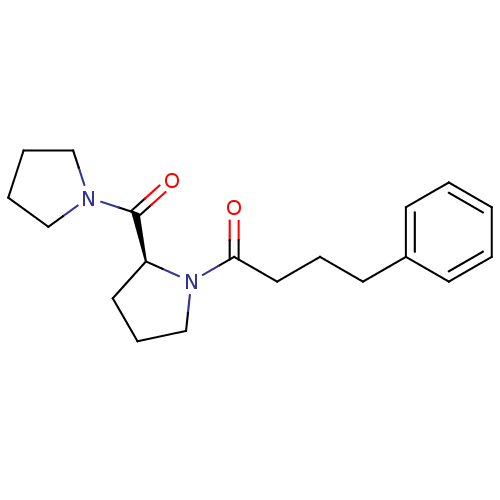 ((S)-4-phenyl-1-(2-(pyrrolidine-1-carbonyl)pyrrolid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille II Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase | Bioorg Med Chem Lett 9: 437-42 (1999) BindingDB Entry DOI: 10.7270/Q2348JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50075134 (4-(4-Chloro-2-methyl-phenoxy)-1-[(S)-3-(pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille II Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase | Bioorg Med Chem Lett 9: 437-42 (1999) BindingDB Entry DOI: 10.7270/Q2348JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50075129 (1-[(S)-3-(Pyrrolidine-1-carbonyl)-3,4-dihydro-1H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille II Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase | Bioorg Med Chem Lett 9: 437-42 (1999) BindingDB Entry DOI: 10.7270/Q2348JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50075128 (CHEMBL138674 | {6-Oxo-6-[(S)-3-(pyrrolidine-1-carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille II Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase | Bioorg Med Chem Lett 9: 437-42 (1999) BindingDB Entry DOI: 10.7270/Q2348JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50075125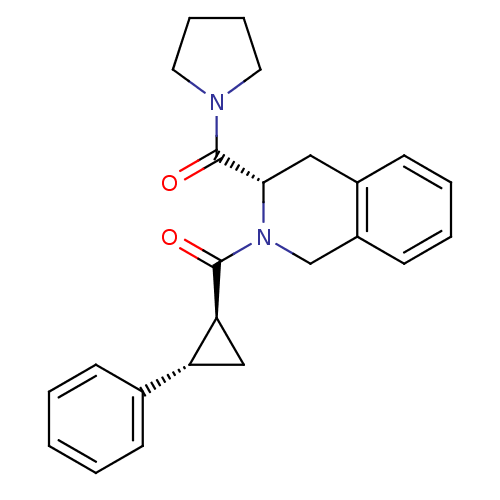 (((1S,2S)-2-Phenyl-cyclopropyl)-[(S)-3-(pyrrolidine...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille II Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase | Bioorg Med Chem Lett 9: 437-42 (1999) BindingDB Entry DOI: 10.7270/Q2348JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50075130 (1-[(S)-3-(Pyrrolidine-1-carbonyl)-3,4-dihydro-1H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille II Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase | Bioorg Med Chem Lett 9: 437-42 (1999) BindingDB Entry DOI: 10.7270/Q2348JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [2420-3010] (Hepatitis C virus genotype 1b (isolate Con1) (HCV)) | BDBM243470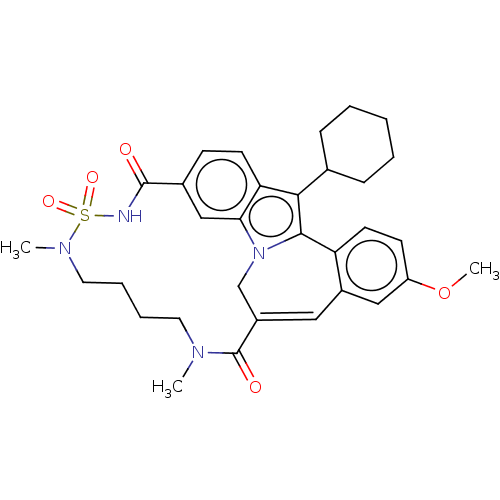 (US9427440, 18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen Sciences Ireland UC US Patent | Assay Description IC50 con1b were determined according to the method as described previously (Pauwels et al, 2007, J Virol 81:6909-19) using a primer-dependent transcr... | US Patent US9427440 (2016) BindingDB Entry DOI: 10.7270/Q2DN43Z6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein [2420-3010] (Hepatitis C virus genotype 1b (isolate Con1) (HCV)) | BDBM243471 (US9427440, 19) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen Sciences Ireland UC US Patent | Assay Description IC50 con1b were determined according to the method as described previously (Pauwels et al, 2007, J Virol 81:6909-19) using a primer-dependent transcr... | US Patent US9427440 (2016) BindingDB Entry DOI: 10.7270/Q2DN43Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50075133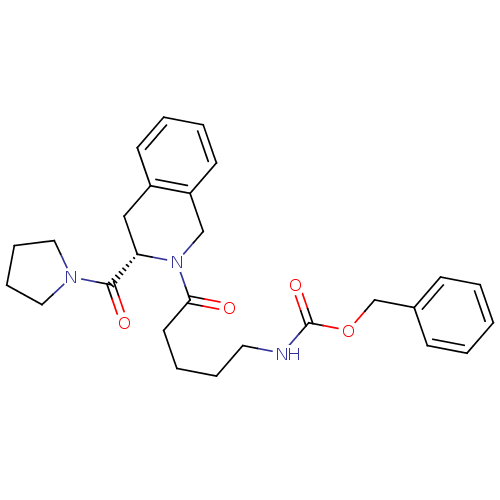 (CHEMBL136325 | {5-Oxo-5-[(S)-3-(pyrrolidine-1-carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille II Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase | Bioorg Med Chem Lett 9: 437-42 (1999) BindingDB Entry DOI: 10.7270/Q2348JJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [2420-3010] (Hepatitis C virus genotype 1b (isolate Con1) (HCV)) | BDBM243472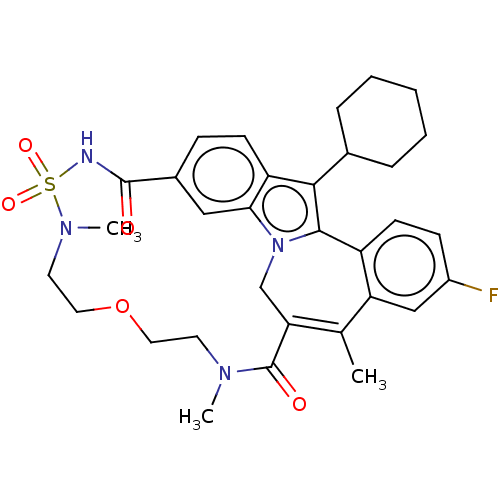 (US9427440, 20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen Sciences Ireland UC US Patent | Assay Description IC50 con1b were determined according to the method as described previously (Pauwels et al, 2007, J Virol 81:6909-19) using a primer-dependent transcr... | US Patent US9427440 (2016) BindingDB Entry DOI: 10.7270/Q2DN43Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [2420-3010] (Hepatitis C virus genotype 1b (isolate Con1) (HCV)) | BDBM243456 (US9427440, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen Sciences Ireland UC US Patent | Assay Description IC50 con1b were determined according to the method as described previously (Pauwels et al, 2007, J Virol 81:6909-19) using a primer-dependent transcr... | US Patent US9427440 (2016) BindingDB Entry DOI: 10.7270/Q2DN43Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [2420-3010] (Hepatitis C virus genotype 1b (isolate Con1) (HCV)) | BDBM243468 (US9427440, 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen Sciences Ireland UC US Patent | Assay Description IC50 con1b were determined according to the method as described previously (Pauwels et al, 2007, J Virol 81:6909-19) using a primer-dependent transcr... | US Patent US9427440 (2016) BindingDB Entry DOI: 10.7270/Q2DN43Z6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101339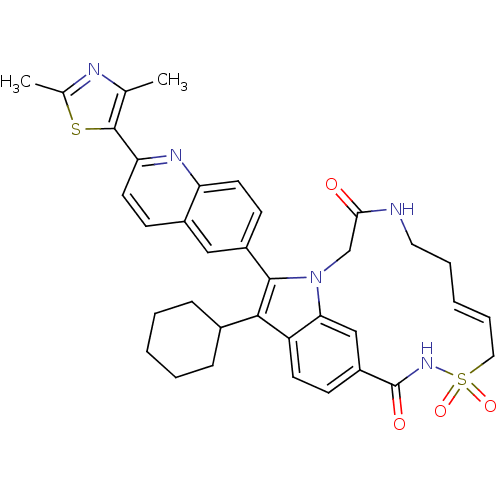 (US8524716, 18) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland US Patent | Assay Description Inhibition assay using HCV NS5B. | US Patent US8524716 (2013) BindingDB Entry DOI: 10.7270/Q22R3Q9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101334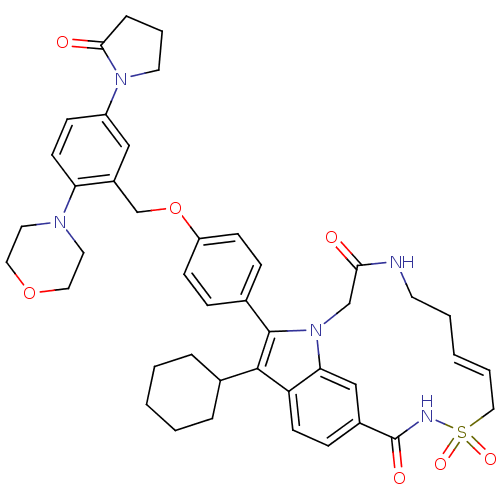 (US8524716, 13) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland US Patent | Assay Description Inhibition assay using HCV NS5B. | US Patent US8524716 (2013) BindingDB Entry DOI: 10.7270/Q22R3Q9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101312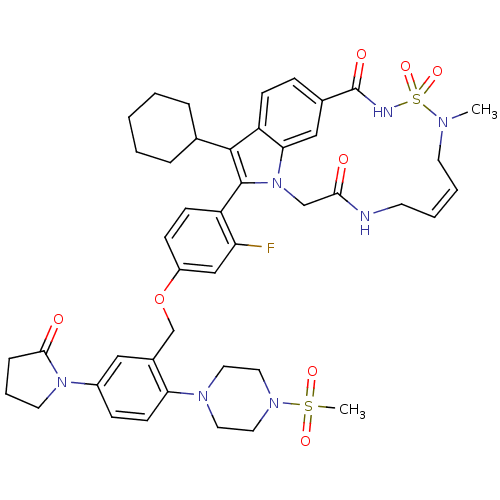 (US8524716, 19) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland US Patent | Assay Description Inhibition assay using HCV NS5B. | US Patent US8524716 (2013) BindingDB Entry DOI: 10.7270/Q22R3Q9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 1b (strain HC-J4) (HCV)) | BDBM101322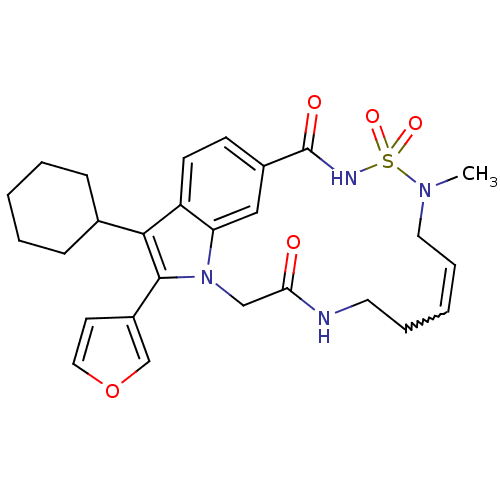 (US8524716, 29) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | 25 |
Janssen R&D Ireland US Patent | Assay Description Inhibition assay using HCV NS5B. | US Patent US8524716 (2013) BindingDB Entry DOI: 10.7270/Q22R3Q9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 264 total ) | Next | Last >> |